Supplemental Digital Content is available in the text.
Keywords: biologics, COVID-19, inflammatory bowel disease (IBD), immunosuppression
Abstract
Objectives
COVID-19 has evolved into a global health crisis, variably affecting the management of patients with chronic illnesses. Patients with inflammatory bowel disease (IBD) may represent a vulnerable population due to frequent administration of immune-modifying treatments. We aimed to depict the natural history of COVID-19 infection in Greek patients with IBD at a nationwide level via unbiased reporting of all cases that were registered during the sequential waves of the pandemic.
Methods
Following a national call from the Hellenic Society for the study of IBD, we enrolled all IBD patients with established diagnoses of COVID-19. Clinical and epidemiological data, including COVID-19 modifying factors and IBD-associated therapies, were analyzed against adverse outcomes (hospitalization, ICU admission and death).
Results
We identified 154 IBD patients who were diagnosed with COVID-19 (men: 58.4%; mean age=41.7 years [SD = 14.9]; CD: 64.3%). Adverse outcomes were reported in 34 patients (22.1%), including 3 ICU admissions (1.9%) and two deaths (1.3%). Multivariate logistic regression analysis showed that age (OR = 1.04, 95% CI, 1–1.08) and dyspnea at presentation (OR = 7.36, 95% CI, 1.84–29.46) were associated with worse outcomes of COVID-19 infection. In contrast, treatment with biologics, in particular anti-TNF agents, exerted a protective effect against an unfavorable COVID-19 disease course (OR = 0.4, 95% CI, 0.16–0.99). Patients on subcutaneous biologics were more likely to halt treatment due to the infection as compared to those on intravenous biologics.
Conclusions
IBD patients who developed COVID-19 had a benign course with adverse outcomes being infrequent. Treatment with anti-TNF biologics had a protective effect, thus, supporting continuation of therapy during the pandemic.
Introduction
Coronavirus disease 2019 (COVID-19) is an acute respiratory syndrome that is caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), a member of the coronaviruses family [1]. The disease was first described in December 2019, and it soon spread worldwide, resulting in its upgrade to pandemic status by the WHO on 30 January2020 [2]. This led to the rapid implementation of preventive strategies to contain the spread of the virus, consisting of various local and global measures, which culminated in generalized lockdowns in several parts of the world. Such measures were deemed necessary due to considerable virus-associated morbidity and mortality, particularly in populations with specific unfavorable prognostic factors, such as increasing age, obesity, hypertension and other comorbidities [3].
Since the beginning of the pandemic, there have been substantial concerns regarding the potential for increased risk of patients with IBD from COVID-19. Previous data had reported that the viral receptor for entry into host cells, angiotensin-converting enzyme 2 (ACE2) receptor, is highly expressed across the GI tract [4]. This raised the possibility that, besides the respiratory system, the human intestine could be an additional target for the virus, a risk that could also be amplified by the presence of inflammation in patients with IBD. Furthermore, a large proportion of patients with Crohn’s disease (CD) or ulcerative colitis (UC) receive immunomodulatory treatments, which may cause various degrees of immunological compromise [5]. In fact, increased susceptibility to opportunistic and viral infections have been reported for patients under longitudinal administration of such therapies [6], which include steroids, thiopurines and methotrexate, as well as biological agents and small molecules [7]. Consequently, on-therapy patients with IBD could be at increased risk for both acquiring SARS-CoV-2 and experiencing a more severe course of COVID-19.
Although such concerns prompted a decisive response from national and international organizations that resulted in published guidelines on how to optimize management of IBD during the pandemic [8–10], actual data on the natural history and outcomes of COVID-19 in this selected population of patients are clearly needed. Published data so far mainly describe the experience of single IBD centers [11] or selected geographical regions [12,13]. The most valuable effort so far is the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD), an international registry that aims at depicting the overall behavior of COVID-19 in IBD patients in a broader manner [14–16]. In the present study, we prospectively identified all patients with IBD from the public, academic and private settings in Greece, who have been infected with SARS-CoV-2, since the beginning of the pandemic. We report, herein, their clinical and epidemiological parameters, present the outcomes of COVID-19 and also analyze patient characteristics that predicted an increased severity of the infection in this population, including the effects of various medical therapies.
Materials and methods
Study population
In October 2020, our IBD Society (Hellenic Society for the study of IBD) issued a National invitation for the reporting of all COVID-19 cases in patients with CD or UC (henceforth referred to as COVID-19/IBD) that had been diagnosed at that point or would occur thereafter. This was considered a priority, as the second (and any subsequent) wave of the pandemic was expected to be much more severe than the first one (March–May 2020), which was effectively managed in Greece, through early implementation of social distancing and lockdown measures [17]. The few COVID-19/IBD cases that had occurred at that moment were retrospectively inserted into the database (Fig. 1). The vast majority of patients, however, were prospectively reported between October 2020 and March 2021. Adult patients with an established IBD diagnosis and confirmed SARS-CoV-2 infection from January of 2020 through March 2021 were enrolled by their treating IBD specialists in the national registry. Diagnosis of SARS-CoV-2 infection was on the basis of either a positive PCR obtained through a nasopharyngeal swab or an anti-SARS-CoV-2 antibody on a symptomatic patient. The recorded symptoms included fever, cough, dyspnea, sore throat, headache, fatigue, arthralgia/myalgia, diarrhea, abdominal pain, nausea/vomiting and ageusia/anosmia.
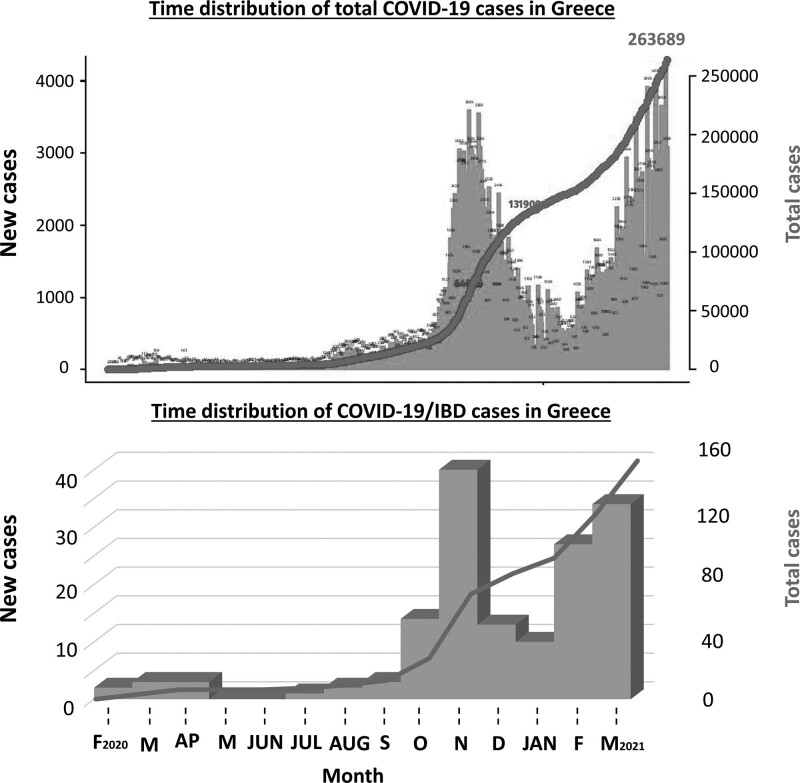
Fig. 1.
Total COVID-19 cases and COVID-19/IBD cases during the sequential waves of the pandemic in Greece. Blue bars indicate the number of daily cases and red lines indicate the cumulative number at each day.
Data collection and outcomes
Medical history (comorbidities, drugs), epidemiological characteristics (age, sex, height, weight, smoking status) and IBD-related data (disease type, treatment) were collected from patients’ records. Active disease was defined as Harvey–Bradshaw index ≥5 and simple clinical colitis activity index ≥5 for CD and UC, respectively. These clinical scores at the time of infection were collected from the patients’ medical records. When such information was not available, reporting was done via direct contact with the patient at the time of case recording. Recorded comorbidities included cardiovascular (coronary heart disease, hypertension, cerebrovascular disease) and respiratory diseases (asthma, COPD), diabetes mellitus, chronic renal and liver disease and history of malignancies. COVID-19-related data included symptoms and the diagnostic method used for COVID-19 confirmation. When there was a history of close contact with a known COVID-19 case that patients could definitively report, this was also recorded. Adverse events (hospital admission, ICU admission and death) due to COVID-19 were the primary study outcome. IBD treatment cessation following COVID-19 diagnosis was also examined. To identify prognostic factors for the course of COVID-19/IBD disease, we examined the correlations between epidemiological, clinical or treatment-related factors to the occurrence of adverse events due to COVID-19. The study was approved by the ‘Sotiria’ Hospital’s Ethical Board and has been performed in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards.
Statistical analysis
Statistical analysis was performed with the statistical package SPSS 23 (IBM, Armonk, New York, USA). For categorical variables total count and percentages are presented. For continuous variables that are normally distributed ‘mean value’ and ‘SD’ while for those not normally distributed ‘Median’ and ‘Interquartile range’ are presented. For the comparison of continuous variables, the parametric paired-sample t test and the nonparametric Mann–Whitney U were performed. The nonparametric X2 test, Fischer’s exact test and univariate logistic regression models were used for the comparison of categorical outcomes. The possible cofounders and prognostic factors were later added to a multivariate logistic regression model. A P value=0.05 was used as the threshold of statistical significance.
Results
Case identification and patient characteristics
We identified 154 patients with IBD who were diagnosed with COVID-19 between the beginning of the pandemic in Greece (26 January 2020) and the termination of our combined retrospective/prospective cohort study (March 2021). The distribution of cases along the study period followed the occurrence of cases in the general population and is depicted in Fig. 1. Only seven cases (4.5%) occurred during the first wave of the pandemic (January–June 2020), whereas 147 patients (95.5%) were infected after the recurrence of the pandemic in the late summer of 2020. Among all verified cases, 50 (32.5%) reported exposure to a known COVID-19 case, whereas the remaining (67.5%) had no such recollection. The diagnosis was made by PCR amplification of nasal swabs in 139 (90.3%) cases, whereas 15 (9.7%) patients were diagnosed via antibody testing and compatible clinical and radiological findings.
Table 1 depicts the characteristics of our COVID-19/IBD cohort. Most patients had a diagnosis of CD (n = 99, 64.3%), 48 of UC (31.2%), whereas there were also six cases of pouchitis and one unclassified colitis. Regarding known or potential adverse prognostic factors for COVID-19, smoking was reported by 22.7%, obesity (BMI> 30 kg/m2) by 13.6%, and hypertension by 14.9%. Overall, 35.7% of patients reported a history of at least one comorbidity. History of malignancy was reported by five patients (3.2%). As expected, the prevalence for COPD, cerebrovascular or coronary heart disease, diabetes, asthma, as well as for renal or liver disease was low among our IBD patients, given their young age (mean age=41.7 [SD = 14.9] years). No differences were observed between patients with CD or UC regarding those characteristics.
Table 1.
Epidemiological data, comorbidities, symptoms and IBD-related treatment of 80 patients diagnosed with SARS-COV2 infection
| All patients, n = 154 | Group with adverse event(s), n = 34 | Group without adverse event, n = 120 | P value | |
|---|---|---|---|---|
| Age in years [mean (SD)] | 41.7 (14.9) | 49.4 (16.1) | 39.6 (13.9) | 0.001a |
| >65 years old [n (%)] | 11 (7.1) | 6 (17.6) | 5 (4.2) | 0.007b |
| Men [n (%)] | 90 (58.4) | 22 (64.7) | 68 (56.7) | NSb |
| Disease [n (%)] | ||||
| Crohn’s disease | 99 (64.3) | 22 (64.7) | 77 (64.2) | NSb |
| Ulcerative colitis | 48 (31.2) | 11 (32.4) | 37 (30.8) | NSb |
| Pouchitis | 6 (3.9) | 1 (2.9) | 5 (4.2) | NSc |
| Unclassified | 1 (0.6) | 0 (0) | 1 (0.8) | NSc |
| Active disease | 24 (15.6) | 7 (20.6) | 17 (14.2) | NSb |
| Smoking status | NSb | |||
| Active | 35 (22.7) | 7 (20.6) | 28 (23.3) | |
| Former | 42 (27.3) | 13 (38.2) | 29 (24.2) | |
| Never | 76 (49.4) | 14 (41.2) | 62 (51.7) | |
| BMI, kg/m2 [mean (SD)] | 25.8 (4.5) | 27.6 (5.6) | 25.3 (4) | 0.009a |
| Obesity [n (%)] | 21 (13.6) | 9 (26.5) | 12 (10) | 0.013b |
| Comorbidities | 55 (35.7) | 20 (58.8) | 35 (29.2) | 0.001b |
| Coronary heart disease | 4 (2.6) | 2 (5.9) | 2 (1.6) | NSc |
| Diabetes | 4 (2.6) | 3 (8.8) | 1 (0.8) | 0.034c |
| Asthma | 4 (2.6) | 2 (5.9) | 2 (1.7) | NSc |
| COPD | 4 (2.6) | 4 (11.7) | 0 (0) | 0.002c |
| Hypertension | 23 (14.9) | 10 (29.4) | 13 (12.5) | 0.007b |
| Cerebrovascular disease | 2 (1.3) | 1 (2.9) | 1 (0.8) | NSc |
| Renal disease | 2 (1.3) | 1 (2.9) | 1 (0.8) | NSc |
| Liver disease | 8 (5.2) | 4 (11.7) | 4 (3.3) | NSc |
| Malignancy | 5 (3.2) | 2 (12.5) | 3 (2.5) | NSc |
| Other | 36 (23.4) | 12 (35.3) | 24 (20) | NSb |
| IBD treatment | ||||
| Biologic | 112 (72.7) | 20 (58.8) | 92 (76.7) | 0.039b |
| Infliximab | 56 (36.4) | 11 (32.4) | 45 (37.5) | NSb |
| Adalimumab | 34 (22.1) | 3 (8.8) | 31 (25.8) | 0.036c |
| Golimumab | 6 (3.9) | 2 (5.9) | 4 (3.3) | NSc |
| Vedolizumab | 12 (7.8) | 3 (8.8) | 9 (7.5) | NSc |
| Ustekinumab | 4 (2.6) | 1 (2.9) | 3 (2.5) | NSc |
| Tofacitinib | 2 (1.3) | 0 (0) | 2 (1.7) | NSc |
| Azathioprine | 18 (11.7) | 6 (17.6) | 12 (10) | NSc |
| Methotrexate | 11 (7.1) | 3 (8.8) | 8 (6.7) | NSc |
| 5-asa | 56 (36.4) | 14 (41.2) | 42 (35) | NSb |
| Corticosteroids | 8 (5.2) | 2 (5.9) | 6 (5) | NSc |
| No treatment | 6 (3.9) | 3 (8.8) | 3 (2.5) | NSc |
| Symptoms | ||||
| Fever | 113 (73.4) | 30 (88.2) | 83 (69.2) | 0.026b |
| Cough | 71 (46.1) | 19 (55.9) | 52 (43.3) | NSb |
| Dyspnea | 16 (10.4) | 10 (29.4) | 6 (5) | <0.001c |
| Fatigue | 97 (63) | 25 (73.5) | 72 (60) | NSb |
| Sore throat | 42 (27.3) | 8 (23.5) | 34 (28.3) | NSb |
| Headache | 72 (46.8) | 17 (50) | 55 (45.8) | NSc |
| Diarrhea | 30 (19.5) | 7 (20.6) | 23 (19.2) | NSc |
| Abdominal pain | 14 (9.1) | 6 (17.6) | 8 (6.7) | 0.049b |
| Nausea-vomiting | 6 (3.9) | 2 (5.9) | 4 (3.3) | NSc |
| Asymptomatic | 17 (11) | 0 (0) | 17 (14.2) | 0.025c |
Obesity, BMI >30 kg/m2. P values refer to comparisons between cases that developed and that did not develop any adverse event.
Independent-samples t test.
χ2.
Fischer’s exact.
The majority of patients (84.6%) were in remission at the time of SARS-CoV-2 acquisition, among whom six (3.9%) were not receiving any therapy. Overall, 112 patients with COVID-19/IBD (72.7%) were on treatment with biologics. Τhe majority received anti-TNF-α therapies, including infliximab (56 patients), adalimumab [34] and golimumab [6]. Twelve patients were on vedolizumab and four on ustekinumab, whereas two patients were receiving therapy with the JAK inhibitor tofacitinib. Among non-biological medications, 56 (36.4%) patients were on 5-ASA therapy and 29 (18.8%) on immunomodulators (18 thiopurines and 11 methotrexate). Finally, at the time of COVID-19 infection, eight patients (5.2%) were receiving steroids. Combination therapy with biologics and immunomodulators was administered to 14 (9.1%) patients.
Characteristics of COVID-19 infection in patients with IBD
The most common symptoms reported by our patients during the clinical presentation of the COVID-19 infection included fever (73.4%), fatigue (63%), headache (46.8%) and cough (46.1%) (Table 1). Interestingly, 22.7% of patients reported at least one GI symptom at presentation. The most prevalent was diarrhea (19.5%) followed by abdominal pain (9.1%) and nausea with or without vomiting (3.9%). The presentation was similar between patients with CD and UC.
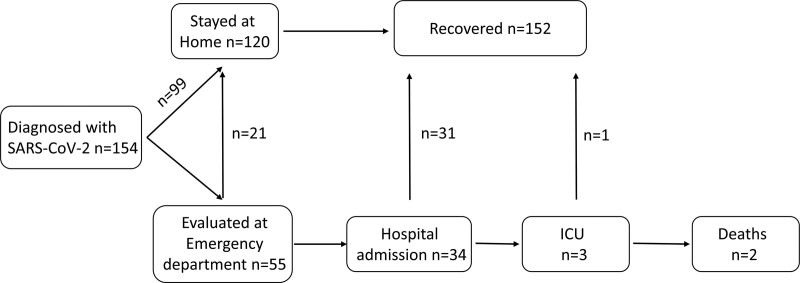
Figure 2 demonstrates the outcome of COVID-19 infection in our patients with IBD. Among the 154 patients who tested positive for SARS-Cov-2, 99 remained at home isolation, following advice by the National Health System authorities and/or their treating gastroenterologist. The remaining patients were evaluated at an ER COVID-reception area, where triage of positive cases has been taking place since the beginning of the pandemic. An additional 21 patients were released; thus, eventually, 34 patients were admitted to the hospital for further follow-up and treatment. Admission to an ICU and mechanical ventilation was applied in three cases, among whom two patients died. In all, an adverse outcome (hospitalization ± ICU admission ± death) occurred in 34/154 (22.1%). The rates of ICU admission and death were 1.9% and 1.3%, respectively.
Fig. 2.
Flow chart depicting the outcomes of SARS-CoV-2 infection of COVID-19/IBD patients.
Patients with adverse outcomes from COVID-19: characteristics and prognostic factors
Patients with adverse outcomes had a mean age of 49.4 years, 22 (64.7%) were male and 22 (64.7%) were diagnosed with CD (Table 1). Τhere were seven active smokers (20.6%) and 13 (38.2%) former smokers. Comorbidities existed in 20 (58.8%) with the most common being hypertension (29.4%). Twelve patients (35.3%) reported exposure to a known COVID-19 case. Regarding the IBD therapies at the time of admission, 11 (32.4%) were on infliximab, three (8.8%) on adalimumab, two (5.9%) on golimumab, three (8.8%) on vedolizumab, one (2.9%) on ustekinumab and nine (26.5%) on thiopurines. Three patients were on combination therapy (8.8%), and two (5.9%) were receiving corticosteroids for their IBD at the time of admission. Among hospitalized patients, three were admitted to ICU and received mechanical ventilation. Two of those patients died (1.3%). The first was an 81-years-old woman patient with morbid obesity (ΒΜΙ: 43) and hypertension who was treated with adalimumab. The second was a 71-years-old man with obesity (BMI: 30.6) and hypertension who was on azathioprine. Finally, a 50-years-old woman patient required mechanical ventilation. She was obese (BMI: 38.1), with COPD and hypertension and was receiving 5-ASA at the time of admission.
We next sought to identify potential predictors for adverse outcomes in our population of COVID-19/IBD patients. To accomplish this, we performed a regression analysis (Table 2) which showed that the possibility for adverse outcomes was significantly increased with age (OR = 1.05, 95% CI, 1.02–1.08), BMI (OR = 1.12, 95% CI, 1.02–1.21), diabetes mellitus (OR = 11.42, 95% CI, 1.15–113.6), hypertension (OR = 3.4, 95% CI, 1.33–8.66), fever (OR = 3.34, 95% CI 1.1–10.17) and dyspnea (OR = 7.92, 95% CI, 2.63–23.87). In contrast, anti-TNF treatment (OR = 0.45, 95% CI, 0.21–0.98) was associated with decreased incidence of hospitalization, ICU admission or death. Those factors along with sex as potential cofounder were then added to a multivariate model. Age (OR = 1.04, 95% CI, 1–1.08) and dyspnea (OR = 7.36, 95% CI, 1.84–29.46) were found to be independently associated with increased possibility for adverse outcome, while there was also a trend for fever (OR = 3.11, 95% CI, 0.88–10.97). Anti-TNF treatment was independently associated with decreased possibility for an adverse outcome (OR = 0.4, 95% CI, 0.16–0.99). Further analysis showed that, in our cohort of patients, death from COVID-19 was associated with older age (Mann–Whitney U test P = 0.001), increased BMI (Mann–Whitney U test P = 0.016), hypertension (Fischer’s exact test P = 0.022) and dyspnea (Fischer’s exact test P = 0.01).
Table 2.
Prognostic factors for adverse events due to COVID-19 in IBD patients
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Men | 1.40 | 0.64–3.09 | 0.402 | 1.49 | 0.58–3.83 | 0.410 |
| Age in years | 1.05 | 1.02–1.08 | 0.001 | 1.04 | 1.00–1.08 | 0.038 |
| BMI (kg/m2) | 1.12 | 1.02–1.21 | 0.012 | 1.00 | 0.89–1.12 | 0.971 |
| DM | 11.42 | 1.15–113.6 | 0.038 | 6.16 | 0.51–75.08 | 0.154 |
| Hypertension | 3.40 | 1.33–8.66 | 0.010 | 1.36 | 0.36–5.19 | 0.654 |
| anti-TNF | 0.45 | 0.21–0.98 | 0.043 | 0.40 | 0.16–0.99 | 0.049 |
| Fever | 3.34 | 1.10–10.17 | 0.034 | 3.11 | 0.88–10.97 | 0.077 |
| Dyspnea | 7.92 | 2.63–23.87 | <0.001 | 7.36 | 1.84–29.46 | 0.005 |
CI, confidence intervals; DM, diabetes mellitus; OR, odds ratio.
Cessation of IBD therapy was reported by 66 patients (42.9%). Ιn general, by univariate logistic regression, patients on subcutaneous treatments (OR = 4.06, 95% CI, 1.96–8.43) were more likely to stop therapy, whereas patients on intravenous treatment (OR = 0.36, 95% CI, 0.18–0.72) were more likely to continue their therapy without interruption (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A713).
Discussion
Herein, we present the results from our nationwide, combined retrospective/prospective cohort study of COVID-19 cases in patients with an established diagnosis of IBD during the first and second waves of the pandemic in Greece. We accomplished reporting of consecutive cases since the beginning of the pandemic and throughout the study period without interruption. Given the universal National reporting, our study allowed us to recognize the main characteristics and outcomes of COVID-19/IBD cases, which may have important clinical implications.
Firstly, a significant proportion of COVID-19/IBD patients have GI complaints at presentation, mostly diarrhea (20% of cases) and abdominal pain (9%). This is in line with previous studies in both the general population and IBD cohorts [12,18–21]. In a recent systematic review and metanalysis, pooled ratios for diarrhea and abdominal pain among COVID-19/IBD patients were 27% and 13%, respectively [22]. Importantly, Lukin et al. found significantly higher percentages of GI symptoms at COVID-19 presentation in IBD than non-IBD cases [13]. Furthermore, worsening of IBD symptoms may occur in as high as 40% of cases during the COVID-pandemic [11]. A pathophysiological basis for such an association is supported by studies showing expression of the viral entry proteins of the host, ACE2 receptor and transmembrane serine protease 2 (TMPRSS2), across the GI tract [4,23]. Nevertheless, IBD-related intestinal inflammation does not increase the local expression of those proteins [24]. Interestingly, medical therapy with steroids or biologics decreases the mucosal expression of ACE2 receptor, indicating that IBD patients do not constitute a group of increased susceptibility to SARS-CoV-2. From the clinical standpoint, however, the presence of GI manifestations may easily be considered as a typical flare of CD or UC and lead to delayed diagnosis of COVID-19. This, in turn, may result in the commencement or intensification of immunosuppressive therapies, including high doses of systemic steroids or thiopurine/anti-TNF combinations, which have been associated with adverse outcomes of COVID-19 [14,15]. Delayed testing for COVID-19 may also facilitate virus spreading. Therefore, gastroenterologists should have a very low threshold for testing patients with new-onset of IBD-related symptoms for possible COVID-19 infection, and persistently seek the presence of more typical manifestations such as fever, cough and/or dyspnea. Nevertheless, it should also be noted that COVID-19 may also present with atypical manifestations, which include the sole presence of GI symptoms without concurring respiratory findings [25].
Second, we confirm, herein, the overall good outcome of COVID-19 infection in IBD patients. Among the eighty COVID-19/IBD cases in our cohort, there were three admissions in ICU (1.9%), of whom two patients died (1.3%). These percentages are similar to the respective values in the SECURE-IBD database [26] and lower than the total death rate from COVID-19 in Greece, which was 3.1%, at the time of present manuscript preparation [27]. Nevertheless, within our population of COVID-19/IBD, certain patient characteristics increased the risk of adverse outcomes; those, however, were not unique for the IBD population but constituted of recognized bad prognostic factors for COVID-19, in particular old age and dyspnea at presentation [3]. Taken together, ours and previous data clearly indicate that IBD is not per se an indicator of a bad prognosis for individuals infected with SARS-Cov-2. Nevertheless, caution is required for those IBD patients with additional risk factors, as shown herein, since all three patients who either died or required mechanical ventilation were obese, and had hypertension or COPD. Along that line, guidelines from the British Society for Gastroenterology suggest that IBD patients with bad prognostic factors should exercise strict social distancing during the endemic phases of COVID-19 [8].
Finally, in our study, we clearly demonstrate the safety of biologic therapy in patients with IBD in the concurrence of infection with SARS-CoV-2. In fact, patients who were on biological therapy had a significantly lower probability for adverse outcomes, namely hospital or ICU admission and death. In our cohort, only four patients were on ustekinumab and two on tofacitinib; hence, no conclusions can be drawn regarding those therapies. However, ninety-six patients were receiving TNF inhibitors at the time of COVID-19 infection, among whom sixteen were admitted to the Hospital. Our statistical analysis showed that anti-TNF treatment exerted a significant protective effect on the outcome of COVID-19 infection. From a pathogenetic perspective, there may be various explanations for this finding, on the basis of the potential participation of TNF-α in the pathophysiology of COVID-19 infection. One such scenario may be that TNF-α can facilitate virus entry via regulating the spike protein-induced, TNF-α-converting enzyme (TACE)-dependent shedding of the ACE2 ectodomain [28]. An alternative explanation may be that TNF-α is an essential component of the SARS-CoV-2-associated cytokine storm and subsequent immune-mediated lung injury, insinuating that its inhibition may be a potential therapeutic approach for COVID-19 [29,30]. Although such interventions have not been reported so far, our current findings and similar published evidence stand as definitive reassurance that anti-TNF medications do not pose a risk for adverse outcomes of COVID-19 [14].
The aforementioned findings provide a strong rationale for IBD patients to continue biological therapy during the pandemic, as disease flare may have deleterious effects. Although original reports from China advocated cessation of all immunosuppressive treatments in endemic areas [31], current guidelines and expert advice support the opposite approach [8–10]. Our National approach was similar, recommending the continuation of all therapies in patients with IBD, alongside strict adherence to preventive measures, particularly for those receiving immunosuppression, and temporary cessation of therapy at the event of COVID-19 [17]. The probability of treatment cessation was significantly higher for patients under oral or subcutaneous immunosuppressants than on scheduled infusion therapies. Most probably this reflects the fact that daily administration of oral therapies or short intervals between subcutaneous injections could easily fall within the timeframe of the COVID-19 infection. Thus, although it was originally stated that a switch from i.v. infusions to subcutaneous medications during the pandemic may be advisable, our study supports the notion that patients should maintain their regular therapies. At the same time, patients did not discontinue taking 5-ASA formulations, indicating that concerns regarding the safety of immunosuppressive treatments also interfere with therapy during the pandemic.
Our study has certain limitations. Although it followed a National call for COVID-19/IBD reporting, there still may be missed cases; thus, some degree of reporting bias cannot be excluded, as also indicated by the relatively high rates of treatment with biologics in our cohort. Nevertheless, all tertiary IBD centers around the country participated in our study. As those are located within COVID-assigned Institutions, we believe that only a few patients may have escaped reporting. In addition, as the decision for Hospital admission was made by local Infectious disease/pulmonary medicine specialists, practices may differ between different Hospitals. We believe, however, that the strengths of our study far outweigh those shortcomings. To the best of our knowledge, this is the only study, so far, to examine the natural history of COVID-19 disease in patients with IBD on a national scale, aiming to report all detected cases. Thus, our study is deprived of the biases that characterize single-center or single-location reports, which constitute the vast majority of published studies up to now. Similarly, large international databases like the SECURE-IBD, may also favor reporting from large tertiary centers, miss the majority of community cases from minor centers and private practices, and suffer from unequal reporting between countries. In contrast, our study was organized by our IBD society, whose members constitute the totality of IBD-caring gastroenterologists from all different health environments (Academic Institutions, National Health System Facilities and private practice). Finally, the diagnosis of COVID-19 was on the basis of a PCR-positive test, almost exclusively, thus unequivocally substantiating the diagnosis for all of our patients. This again is different from the majority of other cohorts that have included both confirmed and suspected cases (i.e. diagnosis probable on indirect evidence).
In conclusion, we report, herein, the natural history of COVID-19 infection in patients with CD or UC at the national level, accounting for almost every reported case in Greece, during the first and second waves of the pandemic. We confirm an overall benign course of the infection in patients with IBD with the exception of patients with serious comorbidities. We report a protective role of anti-TNF therapies regarding the occurrence of adverse outcomes in our cohort, a finding that supports the continuation of administered therapies during the pandemic. Our study adds to existing evidence on COVID-19/IBD disease and contributes to the evolution of best strategies for the management of IBD patients until the termination of the current health crisis.
Acknowledgements
This work was supported by a clinical grant from the Hellenic Society for the Study of IBD.
Conflicts of interest
G.B. is the advisor/lecturer for Janssen, Pfizer, Takeda, Abbvie, MSD, Mylan, Genesis Pharma, Adacyte Therapeutics, Amgen, Ferring, Cooper; Funding (Grants/Honoraria): Pfizer, Takeda, Abbvie, Aenorasis; Research/Clinical Trials: Abbvie, Takeda. Genesis Pharma, Cooper. K.K. is the advisor/lecturer for Abbvie, Aenorasis, Janssen, MSD, Pfizer and Takeda, Amgen, Ferring, Galenica, Genesis Pharma. S.M. is the advisory/lecturer for Pfizer, Takeda, Abbvie, Ferring, MSD, Janssen. G.P. is the advisor/lecturer for Abbvie, Dicerna, Elpen, Gilead, GlaxoSmithKline, Ipsen, Janssen, Merck Sharp &amp; Dohme, Roche, Spring Bank, Takeda; research grants Abbvie, Gilead, Takeda; clinical trials: Abbvie, Astellas, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, Merck Sharp &amp; Dohme, Noorik, Novartis, Novo Nordisk, Regulus, Roche, Takeda. K.T. is the lecturer for Takeda, Amgen. E.T. is the lecturer for Ferring, Takeda. M.T. is the advisor/lecturer for Janssen, Pfizer, Takeda, Abbvie, MSD, Mylan, Genesis Pharma, Amgen, Research/Clinical Trials: AbbVie, Gilead, Takeda. V.N. is the advisor/lecturer for Janssen, Pfizer, Aenorasis, Takeda, Abbvie, MSD, Mylan, Amgen, Genesis Pharma, Cooper. E.Z. is the advisor/lecturer for Pfizer, Takeda, Abbvie, Amgen, Genesis Pharma, Aenorasis, Janssen. G.M. is the advisor/lecturer for AbbVie, Celgene, Celtrion, Ferrirng, Genesis, Hospira, Janssen, Millennium Pharmaceuticals, MSD, Mylan, Pharmacosmos, Pfizer, Takeda, VIANEX, Angelini, Falk Pharma, Galenica, Omega Pharma; Consultancies for MSD and Takeda; Research support from AbbVie, Galenica, Genesis, Menarini Group, MSD, Pharmathen. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou B, Kojima S, Kawamoto A, Fukushima M. COVID-19 pathogenesis, prognostic factors, and treatment strategy: urgent recommendations. J Med Virol 2021; 93:2694–2704. [DOI] [PubMed] [Google Scholar]
- 4.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020; 14:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaugerie L, Kirchgesner J. Balancing benefit vs risk of immunosuppressive therapy for individual patients with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology. 2019Vol. 17W.B. Saunders; 370–379. [DOI] [PubMed] [Google Scholar]
- 6.Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, et al. ; European Crohn’s and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014; 8:443–468. [DOI] [PubMed] [Google Scholar]
- 7.Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018; 155:337–346.e10. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020; 69:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magro F, Rahier JF, Abreu C, MacMahon E, Hart A, van der Woude CJ, et al. Inflammatory bowel disease management during the covid-19 outbreak: the ten do’s and don’ts from the ECCO-COVID task force. J Crohn’s Colitis. 2020; 14(Suppl_3):S798–S806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology 2020; 159:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzello F, Calabrese C, Salice M, Calandrini L, Privitera H, Melotti L, et al. COVID-19 in IBD: the experience of a single tertiary IBD center. Dig Liver Dis 2021; 53:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Lago I, Ramírez de la Piscina P, Elorza A, Merino O, Ortiz de Zárate J, Cabriada JL. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the basque country (Spain). Gastroenterology 2020; 159:781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS; Jill Roberts Center Study Group Study Group; Weill Cornell Medicine-Gastrointestinal Study Group. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology 2020; 159:1541–1544.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020; 159:481–491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021; 70:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal M, Zhang X, Brenner EJ, Ungaro RC, Kappelman MD, Colombel J-F. The impact of vedolizumab on COVID-19 Outcomes Among Adult IBD Patients in the SECURE-IBD Registry. J Crohn’s Colitis 2021; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamias G, Lagou S, Gizis M, Karampekos G, Kyriakoulis KG, Pontas C, et al. The Greek response to COVID-19: a true success story from an IBD perspective. Inflamm Bowel Dis 2020; 26:1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubatan J, Levitte S, Balabanis T, Patel A, Sharma A, Habtezion A. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology 2020; 159:1141–1144.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a Multicenter Research Network Study. Gastroenterology 2020; 159:1575–1578.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar VCS, Mukherjee S, Harne PS, Subedi A, Ganapathy MK, Patthipati VS, et al. Novelty in the gut: a systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterology 2020; 7:e000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh AK, Jena A, Kumar-M P, Jha DK, Sharma V. Clinical presentation of COVID-19 in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res 2021[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020; 526:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgueño JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients With IBD. Inflamm Bowel Dis 2020; 26:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quraishi MN, Cooney R, Brookes MJ, Sharma N. An urgent need to institute COVID-19 testing in patients with IBD experiencing flares. Frontline Gastroenterol 2020; 11:330–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SECURE-IBD Database. Current Summary Data. https://covidibd.org/current-data/. [Accessed 31 March 2021]
- 27.Daily Reports COVID-19 - National Public Health Organisation. https://eody.gov.gr/epidimiologika-statistika-dedomena/ektheseis-covid-19/. [Accessed 31 March 2021]
- 28.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A 2008; 105:7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C.Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine and Growth Factor Reviews. 2020Vol. 54Elsevier Ltd; 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khadke S, Ahmed N, Ahmed N, Ratts R, Raju S, Gallogly M, et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J 2020; 17:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An P, Ji M, Ren H, Su J, Ding NS, Kang J, et al. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. The Lancet Gastroenterology and Hepatology. 2020Vol. 5, Elsevier Ltd; 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.