Abstract
Background/aims
In this meta-analysis, we aimed to evaluate the prognostic value of fibrosis-4 index (FIB-4) in COVID-19.
Methods
We performed a comprehensive literature search of PubMed, Embase, and Scopus databases on 26 November 2020. FIB-4 was calculated by [age (years) × AST (IU/L)]/[platelet count (109/L) × √ALT (U/L)]. A value above cutoff point was considered high and a value below cutoff point was considered low. The main outcome was mortality, the association between high FIB-4 and mortality was reported in odds ratio (OR). Sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic OR (DOR), area under the curve (AUC) were generated.
Results
There were 963 patients from five studies included in this systematic review and meta-analysis. Meta-analysis showed that high FIB-4 was associated with increased mortality [OR 3.96 (2.16–7.27), P < 0.001; I2: 41.3%]. High FIB-4 was associated mortality with a sensitivity of 0.56 (0.40–0.70), specificity of 0.80 (0.72–0.86), PLR 2.8 (1.8–4.2), NLR 0.55 (0.39–0.78), DOR 5 (2–10), and AUC of 0.77 (0.73–0.81). Fagan’s nomogram indicated that for a pre-test probability (mortality) of 30%, a high FIB-4 was associated with 54% post-test probability and a low FIB-4 was associated with 19%, respectively. The funnel-plot analysis was asymmetrical, trim-and-fill analysis by imputation of a study on the left side using linear estimator resulted in an OR of 3.48 (1.97–6.14). Egger’s test showed no indication of small-study effects (P = 0.881).
Conclusion
High FIB-4 was associated with mortality in patients with COVID-19.
Keywords: COVID-19, death, fibrosis-4 index, prognosis, severe
Introduction
Coronavirus disease 2019 (COVID-19) has a broad spectrum of clinical manifestations, ranging from asymptomatic to mild and severe conditions, which could culminate into acute respiratory distress syndrome (ARDS) and lethal multi-organ failure. With the current COVID-19 pandemic, reports show approximately 2% of patients with COVID-19 had underlying chronic liver disease. As with other preexisting comorbidities such as obesity, diabetes, and heart failure, this condition is also thought to influence the outcome of COVID-19 [1–3]. Additionally, SARS-CoV-2 is thought to interact with liver tissue, as data from previous SARS family of virus outbreak shows liver damage as one of the complications of infections [4,5]. Liver injury is of concern because it is shown to be associated with severe COVID-19 and poor outcomes [6].
Using a simple noninvasive clinical scoring system that could help identify and predict disease severity is crucial in this pandemic. Early identification of high-risk patients could help clinicians in triage and respiratory support allocation. The fibrosis-4 index (FIB-4) scoring system was initially developed as an efficient and readily available tool to identify liver fibrosis in patients with chronic viral hepatitis [7]. This system uses routine laboratory values and clinical measurement to calculate the severity of liver impairment, the variables are age, AST, ALT, and platelet count [8]. This scoring system was superior to NFS and BARD scores in assessing NAFLD patients, with a 80% positive predictive value and 90% negative predictive value [9]. With these potential benefits, this scoring will serve as an ideal noninvasive test for assessment of hepatic fibrosis, which is sensitive, specific, and applicable across all chronic liver diseases. Interestingly, FIB-4 has been shown to be a useful risk stratification tool in patients with known liver disease and various extra-hepatic associated conditions, including atrial fibrillation and intracerebral hemorrhage [10,11]. Moreover, this simple scoring system has been recently reported to be associated with ICU admission in patients with COVID-19 [12].
This meta-analysis aimed to explore the association between the FIB-4 index and the risk of mortality among COVID-19 patients. We seek to evaluate whether the FIB-4 index could serve as a simple and effective prognostication tool in patients with COVID-19.
Material and methods
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines. The protocol for this study is registered in PROSPERO (CRD42020223023).
Eligibility criteria
The inclusion criteria were prospective and retrospective observational studies in the form of research articles or research letters, reporting (1) COVID-19 patients, (2) FIB-4, and (3) mortality as an outcome. The main outcome was mortality, defined as death/non-survivor. The key exposure was high FIB-4, and the control was low FIB-4.
The exclusion criteria were commentaries, review articles, preprints, non-research letters, case reports, studies that did not report the outcome/exposure, and articles in non-English Language. Preprints were excluded due to varying credibility [13].
Search strategy and study selection
We performed a comprehensive literature search of PubMed, Embase, and Scopus databases using keywords ‘SARS-CoV-2’ OR ‘COVID-19’ OR ‘2019-nCoV’ AND ‘fibrosis-4 index’ OR ‘FIB-4’ on 26 November 2020. The PubMed (MEDLINE) search strategy was [(SARS-CoV-2) OR (COVID-19) OR (2019-nCoV)] AND [(fibrosis-4) OR (FIB-4)]. Two independent authors screened the title/abstracts after the removal of duplicates. Ineligible studies were excluded, and the full-texts of potentially relevant articles were assessed.
Data extraction
Data extraction from the eligible studies was performed by two independent authors using standardized extraction forms containing information on first author, study design, year of publication, patients’ characteristics, FIB-4 cutoff value, and mortality.
FIB-4 was calculated by [age (years) × AST (IU/L)]/[platelet count (109/L) × √ALT (U/L)]. A value above cutoff point was considered high and a value below cutoff point was considered low. The main outcome was mortality, the association between FIB-4 and mortality was reported in odds ratio (OR). Sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic OR (DOR), area under the curve (AUC) were generated for the association between FIB-4 and mortality.
Risk of bias and quality assessment was performed using the Newcastle–Ottawa Scale by two independent authors. Any discrepancies were resolved by discussion.
Statistical analysis
STATA 16 (StataCorp LLC, College Station, Texas, USA) was used to perform meta-analysis. A meta-analysis of proportion was performed for mortality in the included studies. We pooled the ORs using the DerSimonian and Laird random-effects model, regardless of heterogeneity, and the effect estimate was reported in ORs and its 95% confidence interval. A P-value of ≤0.05 was considered statistically significant. Heterogeneity among the included studies was assessed using the I2 and Cochrane Q test, where a value of >50% or P-value <0.10 indicates heterogeneity. Publication bias and small-study effects were assessed by funnel-plot analysis and Egger’s test. Trim-and-fill analysis was performed using linear estimator. Sensitivity analysis was performed by excluding studies with 100% liver disease. Sensitivity, specificity, PLR, NLR, DOR, AUC, and corresponding Fagan’s nomogram were generated.
Results
There were 963 patients from five studies included in this systematic review and meta-analysis (Fig. 1) [14–18]. Baseline characteristics of the included studies are displayed in Table 1. All studies reported mortality as their outcome. The mortality rate in the included studies was 30%.
Fig. 1.
PRISMA flowchart. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Table 1.
Baseline characteristics of the included studies
| Authors | Design | Population | Sample | FIB-4 cutoff value | Mean/median age (years) | Male (%) | CLD (%) | NOS |
|---|---|---|---|---|---|---|---|---|
| Forlano et al. (2020) | RO | OR was calculated for NAFLD Cohort | 61 (NAFLD) | >3.25 | 60 | 60 | NAFLD | 8 |
| Li et al. (2020) | RO | COVID-19 patients | 202 | >2.67 | 58 | 54 | 32.2 | 8 |
| Lopez-Mendez et al. (2020) | RO | COVID-19 >18 years old | 155 | >3.25 | 51 | 72 | 1.3 | 6 |
| Park et al. (2020) | RO | COVID-19 receiving respiratory support (low or high dose oxygen) | 289 | >4.95 | 72 | 46 | 5.2 | 8 |
| Sterling et al. (2020) | RO | COVID-19 patients | 256 | >2.67 | 58 | 43 | 5.9 | 8 |
CLD, chronic liver disease; FIB-4, fibrosis-4 index; NAFLD, nonalcoholic fatty liver disease; NOS, Newcastle–Ottawa Scale; RO, retrospective observational.
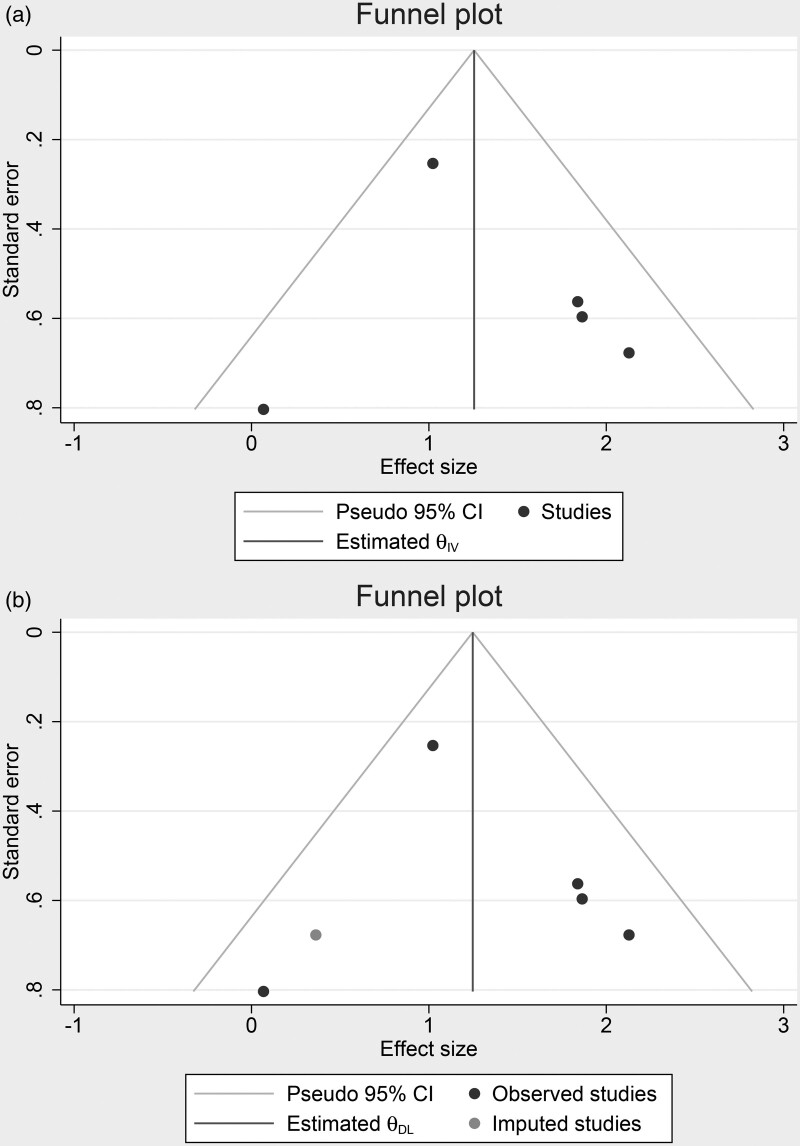
Meta-analysis showed that high FIB-4 was associated with increased mortality [OR 3.96 (2.16–7.27), P < 0.001; I2: 41.3%, P = 0.146] (Fig. 2). Sensitivity analysis by excluding Forlano et al., whose study include only patients with NAFLD, high FIB-4 remained significant [OR 4.49 (2.53–7.98), P < 0.001; I2: 33.1%, P = 0.214]. High FIB-4 was associated mortality with a sensitivity of 0.56 (0.40–0.70), specificity of 0.80 (0.72–0.86), PLR 2.8 (1.8–4.2), NLR 0.55 (0.39–0.78), DOR 5 (2–10), and AUC of 0.77 (0.73–0.81) (Fig. 3). Fagan’s nomogram indicate that for a pre-test probability (mortality) of 30%, a high FIB-4 was associated with 54% post-test probability and a low FIB-4 was associated with 19% post-test probability (Fig. 4). Deek’s asymmetry test was NS for publication bias (P = 0.26). The funnel-plot analysis showed asymmetrical shape, indicating possible publication bias (Fig. 5a). Trim-and-fill analysis by imputation of a study on the left side using linear estimator resulted in an OR of 3.48 (1.97–6.14) (Fig. 5b). Egger’s test showed no indication of small-study effects (P = 0.881).
Fig. 2.
Fibrosis-4 Index (FIB-4) and mortality in COVID-19.
Fig. 3.
SROC with prediction & confidence contours for high FIB-4 and mortality.
Fig. 4.
Fagan’s nomogram for high FIB-4 and mortality
Fig. 5.
Publication bias. Funnel-plot (a) and trim-and-fill analysis (b).
Discussion
This meta-analysis indicates that high FIB-4 was associated with increased mortality in patients with COVID-19 with 56% sensitivity and 80% specificity. The finding has a moderate heterogeneity possibly due to (1) different cutoff points and (2) different proportions of patients with comorbidities.
COVID-19 primarily causes organ damage due to the exaggerated immune response to the virus [19]. High liver function tests, including AST, ALT, GGT, and total bilirubin, are often reported in hospitalized patients with SARS-CoV-2 infection, reinforcing suspicion of varying degree of liver damage in patients with COVID-19 [20,21]. These simple parameters are among the most frequently offered on admission as well as complete blood count, in which a decrease in leukocyte and thrombocyte counts is commonly found in COVID-19 patients [22,23]. Such abnormalities in laboratory values are associated with increased severity and mortality in patients with COVID-19 diagnosis. Cytokine storm, characterized by the excessive release of inflammatory cytokines, such as interleukins, lactate dehydrogenase, C-reactive protein, and D-dimer, are postulated as an underlying mechanism for the development of life-threatening complications, such as ARDS, coagulopathy, and multi-organ dysfunction [6,19,24]. Elderly population is at a higher risk of developing severe SARS-CoV-2 infection, partly because of increasing age and the high prevalence of comorbidities and frail conditions [25–27].
The use of various scoring systems has been increasingly popular to predict the outcomes of COVID-19. Considering the high incidence of liver injury associated with SARS-CoV-2, simple parameters such as De Ritis Ratio and FIB-4 are often utilized to predict the need for mechanical ventilation or ICU stay [12,14,28].
The results of this meta-analysis confirm that the presence of liver impairment is associated with a poorer prognosis in COVID-19 patients. This impairment can be further quantified and staged using FIB-4 scoring system, even though this scoring system was initially designed for chronic liver disease population secondary to viral infection. In this study, we repurpose the use of FIB-4 as a prognostication tool in patients with COVID-19. Despite the limited amount of studies available for our meta-analysis and difference in cutoff points, we did not encounter a significant amount of heterogeneity in our analyses.
We found that the study by Forlano et al. displays unequivocal results related to mortality. It is interesting to note that all of the subjects included in this study have NAFLD. Due to the differing characteristic in Forlano et al., we performed sensitivity analysis by excluding this study. An in-depth assessment of this particular study reveals that NAFLD was not associated with mortality in COVID-19 patients. FIB-4 score >1.45 and >3.25 were also not associated with mortality in COVID-19 patients. However, data for both of these scoring results were impaired by the significant amount of missing data for FIB-4 scores in subjects of this study (37%) [18]. The amount of missing data in this variable exceeded the threshold of missing data of 10% in which there is a high possibility that the results of this analysis will be statistically biased [29]. In patients with chronic liver disease, an inherent perpetual inflammation occurs in the liver, which ultimately causes fibrosis in the end-stage of the disease. This inflammation is further exaggerated with SARS-CoV-2 virus, which might explain liver damage in some patients, and poorer prognosis in COVID-19 patients with chronic liver disease [30]. However, the study by Forlano et al. indicates that the COVID-19 severity in NAFLD patients was not due to the underlying liver disease’s severity but instead attributed to other factors. FIB-4 use was applicable in studies with a low proportion of chronic liver disease patients. The strongest association between FIB-4 and mortality is found in Sterling et al., who enrolled only 5.9% of patients with chronic liver disease. One of the excluded studies was Xiang et al. which was excluded due to composite endpoint (death or prolonged hospitalization), rather than death/mortality alone. The study indicated that FIB-4 was independently associated with progression to severe disease and death or prolonged hospitalization [31]. Thus FIB-4 might be repurposed for COVID-19 prognostication, regardless of the presence or absence of liver diseases.
Clinical implications
FIB-4 is a marker of liver fibrosis, however, it can be repurposed into a prognostication tool for patients with COVID-19 regardless of prior history of liver disease. Determining the optimal FIB-4 cutoff point requires further investigation. The studies included in this meta-analysis indicates that a cutoff point of as low as >2.67 is adequate for prognostication in patients with COVID-19. Despite varying cutoff points, the heterogeneity was not substantial. There is a potential difficulty in interpreting FIB-4 because platelet count often varies in patients with COVID-19 due to other causes than liver fibrosis, nevertheless, this repurposed score has shown to be useful regardless of prior history of liver disease.
Limitations
Our analysis has several limitations, we were unable to dismiss the possibility of publication bias using our funnel-plot analysis, however, our egger’s test showed no indication of small-study effects. Trim-and-fill analysis indicates that hypothetical studies’ imputation to achieve a symmetrical funnel-plot resulted in a significant association, albeit slightly weaker. We also acknowledge the limited data and studies available to use in this meta-analysis.
Conclusion
High FIB-4 was associated with mortality in patients with COVID-19.
Acknowledgements
R.P. helped in concept development, manuscript drafting, data acquisition, data analysis, and statistical analysis. E.Y. contributed to concept development, manuscript drafting, data acquisition, and data analysis. I.H. contributed to data acquisition, investigation, extensive review, and editing of the manuscript. M.A.L. contributed to data acquisition, data analysis, and manuscript drafting. S.A.N. contributed to investigation, extensive review, and editing. R.A.T.K. contributed to investigation, extensive review, and editing.
Data availability: Available on reasonable request.
The guidelines of the PRISMA 2009 Statement have been adopted; the protocol for this review is registered in PROSPERO (CRD42020223023).
All data generated or analyzed during this study are included in this published article.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr 2020; 14:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Gutierrez EJ, et al. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am J Emerg Med 2020. doi: 10.1016/j.ajem.2020.07.009 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, Meyer M. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab 2020. doi: 10.1016/j.diabet.2020.07.005 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 1091:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arabi YM, Al-Omari A, Mandourah Y, Al-Hameed F, Sindi AA, Alraddadi B, et al.; Saudi Critical Care Trial Group. Critically ill patients with the middle east respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med 2017; 45:1683–1695. [DOI] [PubMed] [Google Scholar]
- 6.Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis 2020; 7:2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 8.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol 2012; 12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito Y, Okumura Y, Nagashima K, Fukamachi D, Yokoyama K, Matsumoto N, et al. Impact of the fibrosis-4 index on risk stratification of cardiovascular events and mortality in patients with atrial fibrillation: findings from a Japanese Multicenter Registry. J Clin Med 2020; 9:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh NS, Kamel H, Navi BB, Iadecola C, Merkler AE, Jesudian A, et al. Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke 2020; 51:830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibáñez-Samaniego L, Bighelli F, Usón C, Caravaca C, Carrillo CF, Romero M, et al. Elevation of liver fibrosis index FIB-4 is associated with poor clinical outcomes in patients with COVID-19. J Infect Dis 2020; 222:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrina J, Lim MA, Pranata R. COVID-19 and misinformation: how an infodemic fuelled the prominence of vitamin D. Br J Nutr 2020:1–2. doi: 10.1017/S0007114520002950 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JG, Kang MK, Lee YR, Song JE, Kim NY, Kweon YO, et al.; Daegu-Gyeongbuk Liver Study Group (DGLSG). Fibrosis-4 index as a predictor for mortality in hospitalised patients with COVID-19: a retrospective multicentre cohort study. BMJ Open 2020; 10:e041989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Mendez I, Aquino-Matus J, Gall SMB, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol 2020; 20:100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterling RK, Oakes T, Gal TS, Stevens MP, deWit M, Sanyal AJ. The fibrosis-4 index is associated with need for mechanical ventilation and 30-day mortality in patients admitted with coronavirus disease 2019. J Infect Dis 2020; 222:1794–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Regan J, Fajnzylber J, Coxen K, Corry H, Wong C, et al. Liver fibrosis index FIB-4 is associated with mortality in COVID-19. Hepatol Commun 2020. doi: 10.1002/hep4.1650 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One 2020; 15:e0240400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Yamin M, et al. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr 2020; 14:2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: A pooled analysis. Liver Int 2020; 40:1787–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care 2020; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao C, Tao X, Cui W, Yi B, Pan T, Young KH, Qian W. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol 2020; 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis 2020; 14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr 2020; 14:2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pranata R, Henrina J, Lim MA, Lawrensia S, Yonas E, Vania R, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr 2021; 93:104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.July J, Pranata R. Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. Geriatr Gerontol Int 2020:ggi.14107. doi: 10.1111/ggi.14107 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Zinellu A, Arru F, De Vito A, Sassu A, Valdes G, Scano V, et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest 2021; 51:e13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Y, Peng CY. Principled missing data methods for researchers. Springerplus 2013; 2:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Invest 2020; 50:e13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang F, Sun J, Chen P-H, et al. Early elevation of FIB-4 liver fibrosis score is associated with adverse outcomes among patients with COVID-19. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1710 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]