Abstract
The rotator cuff is a musculotendon unit responsible for movement in the shoulder. Rotator cuff tears represent a significant number of musculoskeletal injuries in the adult population. In addition, there is a high incidence of retear rates due to various complications within the complex anatomical structure and the lack of proper healing. Current clinical strategies for rotator cuff augmentation include surgical intervention with autograft tissue grafts and beneficial impacts have been shown, but challenges still exist because of limited supply. For decades, nanomaterials have been engineered for the repair of various tissue and organ systems. This review article provides a thorough summary of the role nanomaterials, stem cells and biological agents have played in rotator cuff repair to date and offers input on next generation approaches for regenerating this tissue.
Keywords: Rotator cuff regeneration, fatty infiltration, nanofibers, drug delivery vehicles, nanofibrous scaffolds
Lay Summary
The rotator cuff is a group of muscles and tendons that allow movement of the shoulder. Rotator cuff injuries occur often in athletes and in people that frequently perform overhead motion. The risk of injury also increases with age. Currently, implants from one part of the body to another are used to surgically repair the rotator cuff, but problems still rise because of limited supply in patients. To overcome this challenge and other concerns, nanomaterials have been used to help renew damaged tissues and organs. In this review article, we describe the role of nanomaterials, stem cells and biological agents in rotator cuff repair and provide insight on strategies for successful rotator cuff regeneration.
I. Introduction
Shoulder disorders have significantly impacted the adult population, in particularly causing the elderly debilitating pain, reduced function, and joint instability [1], [2]. Rotator cuff injuries are one of the most predominant soft tissue-related pathologies. As a result, more than 200,000 rotator cuff repair procedures per year are performed in the United States [3]–[5]. Clinical interventions are necessary because the rotator cuff does not heal properly as a result of the complex anatomical structure and range of motion in the shoulder [4], [6]. High retear rates (between 26% and 94%) still exist after rotator cuff surgical intervention due to infection, stiffness, patient age, tear size, muscle atrophy, and fatty infiltration [3], [7]. Clinical strategies to repair or regenerate the rotator cuff include suture techniques and tissue grafts. Large and massive tears owing to the short remnant tendon length cannot be repaired by suture techniques [8], [9] and need a reinforcement to help healing and regeneration. Rotator cuff augmentation with tissue grafts may promote mechanical integrity, tissue remodeling and surgical outcomes. Despite all the advantages of autograft compared to allograft and xenograft, in terms of immunological rejection, this strategy shows limitations that include donor-site morbidity and limited availability [3], [10], [11]. To overcome these challenges, researchers have investigated engineered scaffolds as an alternative approach for tissue regeneration. Nanomaterials (defined as those materials with constituent dimensions less than 100 nm) can mimic the native tissue and provide promise as a substitute for aiding tissue restoration [12]. Therefore, it is imperative to understand the impact of nanomaterials, specifically nanofibrous matrices, on the rotator cuff microenvironment presented in this paper. In addition, we must acknowledge the regenerative engineering approach, a new paradigm of converging developmental biology, stem cell science, physics and advanced material sciences, is warranted for successful rotator cuff regeneration [13]. This review provides a detailed overview on the role of nanomaterials, with focus on nanofibrous materials, stem cells and biological agents for rotator cuff regeneration. More specifically, the article illustrates the importance of muscle and tendon regeneration emphasizing fatty infiltration and repair strategies. Here, we also give insight and describe regenerative engineering strategies to overcome repair challenges towards promising rotator cuff regeneration techniques.
II. Anatomy of the Rotator Cuff: Musculotendon Structure
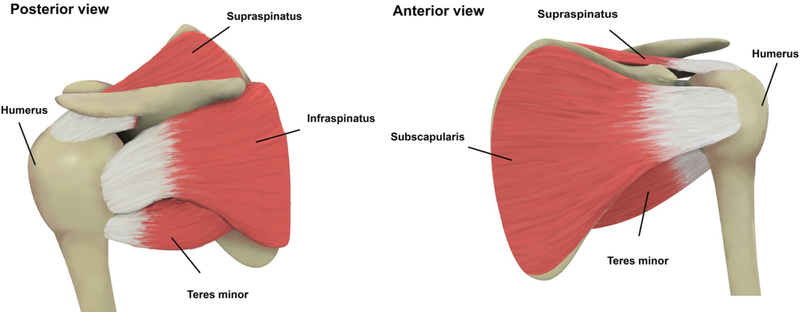
The stability and movement of the shoulder are controlled by four muscles, supraspinatus, subscapularis, infraspinatus and teres minor, whose tendons, named the rotator cuff, connect the muscles from scapula to humerus through a tendon-bone insertion (Figure 1) [3], [14]. The amalgamated structure and its surrounding form a capsule-cuff complex and together transmit force from muscle to bone [15]. The presence of collagens, elastins and proteoglycans impact the physical and chemical properties of the tendon. Type-I Collagen provides mechanical strength and is present in highest concentrations compared to other types of collagens and Type-III Collagen contributes to healing and repair.
Figure 1.
Rotator cuff anatomy and the involved tendons including Supraspinatus, Infraspinatus, Teres Minor and Subscapularis [3].
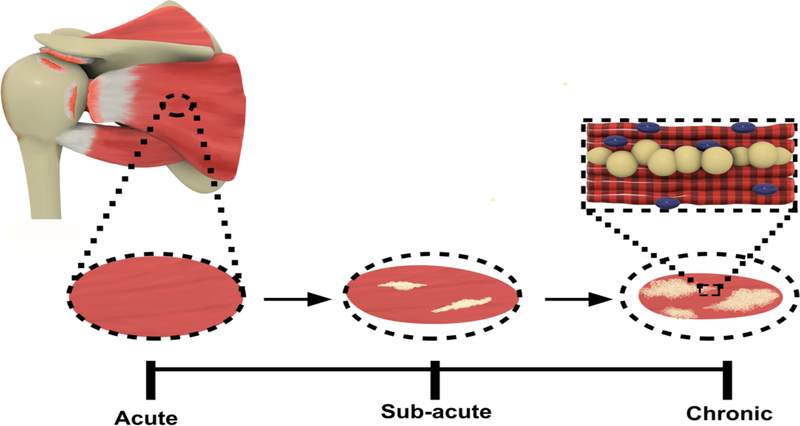
The delay between the time of rotator cuff injury and surgical repair is one of the most commonly observed challenges. This duration between repair is described by category, specifically “acute”, “sub-acute” and “chronic”. An acute rotator cuff injury involves the immediate repair of the injury, while sub-acute and chronic refer to a prolonged duration between time of injury and repair. A sub-acute or chronic rotator cuff injury can lead to fatty infiltration, also referred to as fatty degeneration, a condition leading to the damaging and weakening of rotator cuff muscles that occurs after rotator cuff tearing. Fatty degeneration is characterized by atrophy of muscle fibers, fibrosis and fatty accumulation within and around the muscles (Figure 2) [16], [17]. More importantly, fatty infiltration of the muscle has a significant influence on the high rate of rotator cuff retear after surgery. Significant research has focused on rotator cuff injury and repair, but there is still more to uncover about the regeneration of the soft tissue.
Figure 2.
Rotator cuff fatty infiltration in Sub-acute and Chronic injury.
III. Strategies for Rotator Cuff Regeneration
Clinically, surgical strategies have failed to gain a satisfactory treatment for massive rotator cuff tears, therefore, tissue regeneration strategies including growth factors, stem cell therapy, and biomaterial-based structures are being investigated to address current challenges. Platelet-rich plasma (PRP) which uses the patient’s own blood to supply growth factor that promotes a healing response, but research demonstrated conflicting evidence concerning the effectiveness and efficacy of the platelet-rich plasma [18], [19]. Studies including stem cell therapies in human trials have displayed some significance in reducing retear rates [20], [21]. Tissue engineering applications, utilizing scaffolds to augment rotator cuff repair, have demonstrated protection and assistance in tendon repair [22]. These strategies and therapies for rotator cuff regeneration, including PRP, stem cells and biological agents, have been reviewed in other manuscripts [3], [23]. Though some successes have been discovered with these approaches, the materials utilized still do not possess the material properties required for long-term rotator cuff repair. However, the development of nanotechnology has successfully enabled researchers to improve the material properties and overcome the limitations of traditional materials (i.e., poor mechanical properties of natural biomaterials, low biocompatibility of non-degradable polymers, and brittleness of osteoconductive materials) [10], [24]. Presently, nanomaterials such as polymeric/non-polymeric nanofibers, nanotubes, and nanoparticles are being investigated for several applications including drug delivery, tissue engineering, therapeutics, diagnostics and imaging [24]–[31]. Fabrication of nanomaterials has significantly increased surface roughness, surface area, and surface area to volume ratio which results in ideal physicochemical properties [32]. With this, nanomaterials have been extensively investigated in biomedical applications, specifically regenerative medicine. For example, Synthasome, Inc. is an advanced orthobiologics company that creates products to promote soft tissue and cartilage regeneration. Their first product, X-Repair, is a biocompatible, surgical mesh with high tensile stiffness and strength, similar to the native tendon. The X-Repair is clinically designed to reinforce surgical repair of soft tissue such as rotator cuff, patellar, and Achilles tendons. Studies with X-Repair augmentation of rotator cuff demonstrated that the device enhanced the mechanical properties of the surgical repair at time of surgery [33]. The X-Repair also displayed significant mechanical integrity of the repair site, and substantially reduced gap formation after surgery [34]. Among different nanostructures, nanofibers are one of the most interesting materials owing to their continuous nanoscale long fibers [3], [17], [35]–[37]. Since the first work by Laurencin and his colleagues [38], electrospun nanofibers have attracted attention in different fields of study including medicine and tissue regeneration [39], [40]. Nanofiber-based structures show the most similarity to native muscle/tendon tissue by providing the analogous structures and appropriate mechanical properties. Although, there are several technologies to fabricate nanofiber-based structures, electrospinning is the most widely used strategy that provides a layer of nanoscale fibers with small pore sizes and high surface area [3], [17], [35]–[38]. In the electrospinning process, the polymer-based solution ejects from a feed pump into an electric field applied between the feed pump and the collector to fabricate and stretch the nanofiber. Different orientations, including aligned and random in the scale of nanometer to micrometer, can be fabricated by changing the electrospinning parameters [3], [17], [35]–[38]. We recently reported a comprehensive review of the use of nanofiber-based matrices for rotator cuff regeneration [3]. The current review will discuss the regenerative potential of nanostructures not only as a matrix but also as a growth factor and stem cell delivery vehicle that can deliver cytokines to the incision site.
IV. Nanomaterials as Matrices
Bone-tendon interface tears occur most often in the shoulder and knee. The well-known strategies for repair depend on artificial grafts and implants such as TissueMend [41], Restore [42], GraftJacket [43], and Permacol [44]. But given the donor source of these transplants, patients may have severe immunogenic consequences. It has also been reported that the tendon regenerated through these scaffolds have poor mechanical properties due to the gradient structure when compared to native tissue [45]. Research has attempted to overcome this structural concern using different nanomaterials. For example, Moffat et al. used random poly(lactic-co-glycolic acid) PLGA nanofibers (material for bone) and aligned PLGA (material for tendon) in a nanofiber-based scaffold for rotator cuff repair [4]. They concluded that the mechanical properties and gradient composition were similar to native tissue, but mechanical properties were not analyzed after long-term in vitro studies. The purpose of the study was to show that fiber orientation affects the cell morphology with cells being well spread on aligned fibers and cells on random fibers displayed atypical morphology [4]. The regenerative potential of electrospun nanofibers for rotator cuff tendon regeneration in vivo was demonstrated by Taylor et al. in a rat model [46]. The electrospun PLGA nanofibers were investigated in vitro upon seeding with isolated tenocytes. According to the results, the electrospun nanofibers supported the cell viability and proliferation in vitro and improved the biomechanical properties in vivo [46]. PLGA nanofiber have also been engineered with mineral gradients of hydroxyapatite (HA) to create a controlled environment for the osteogenic differentiation of cells [47]. It was reported that a fibrous scaffold with mineralization patterns promote osteogenesis [48]. Nuss et al. investigated the healing properties of poly-N-acetyl glucosamine (sNAG) polymer nanofibers in a rat rotator cuff injury and repair model [49]. They found that in the presence of analgesics, tendons treated with the sNAG matrix had significantly increased max load and max stress at 4 weeks, but not at 8 weeks; however, at 14 days, ambulatory improvements were detected in stride with length and speed [49]. Given that a primary complication of rotator cuff repair surgery is early retear, they concluding that sNAG improve tendon-to-bone healing in the rat rotator cuff repair model [49]. Rotator cuff tears are usually a chronic injury, one acknowledged limitation of this study included that this group used an acute supraspinatus detachment. Another study evaluated the biomechanical efficacy of aligned-fiber polymer scaffolds for rotator cuff repair in a rat model [50]. PCL and PCL/poly(ethylene oxide) (PEO) co-electropsun scaffolds were implanted to augment supraspinatus repair. The study illustrated no adverse effects of surgical implantation of PCL/PEO polymer aligned scaffolds on the supraspinatus tendon structural properties in a rat rotator cuff model and validated this model as a potential platform for targeted delivery [50]. Besides the promising results of studies in the field of rotator cuff regeneration, the complex structure of enthesis remains to be a concern. In a newly conducted study, Sun et al. investigated poly(lactic-co-glycolic acid)-PCL (PLGA-PCL) and poly(lactic-co-glycolic acid)/collagen-I-PLC/nanohydroxyapatite (PLGA/Col-PCL/nHA) co-electrospun matrices to bridge massive rotator cuff tears in rabbit models [51]. The results showed that fibroblasts had higher viability and collagen secretion when seeded on PLGA/Col versus PLGA matrix. The osteoblasts demonstrated higher growth on the PCL/nHA matrix compared to the PCL in vitro. Bone formation was observed with the PCL only scaffold, but was less than the PCL/nHA construct [51]. In addition, the regenerated tendon in both groups displayed similar maximum load-to-failure and ultimate stress at 6 weeks; however, at 12 weeks, there was significantly higher maximum failure, ultimate stress and regenerated tendon in the PLGA/Col-PCL/nHA matrix. They concluded the incorporation of collagen and nHA strengthens tissue regeneration [51]. These studies demonstrate the potential of nanofiber-based matrix systems for functional rotator cuff repair as a results of the nanofibrous scaffold design and its profound effect on the cellular response.
V. Nanomaterials as Drug Delivery Vehicles of Biological Agents
Scaffolds, stem cells, growth factors and other biological molecules have played a significant role in the Regenerative Engineering paradigm. In addition, several cell sources have been isolated, characterized, and investigated for regenerating rotator cuff tendons. In this section, we describe and discuss scaffolds as drug delivery systems incorporated with biochemical cues for tissue regeneration.
a. Cell-based Therapies
Stem cells have been used for regenerating soft musculoskeletal tissue for several reasons including their innate ability to secrete paracrine factors (secretome) and to modulate the inflammation and the adaptive immune response [52]. Stem cells also have the specialized capability to differentiate into many different cellular and tissue phenotypes [53], [54]. These unique characteristics of stem cells are essential components in the convergence approach towards regenerating complex tissues including the rotator cuff. We will discuss stem-cell based methods used for soft tissue regeneration in this section.
The most widely used source of mesenchymal stem cells (MSCs) for soft tissue and rotator cuff regeneration is bone marrow, known as bone marrow derived mesenchymal stem cells (BMSCs). These cells are utilized for their ease of isolation and ability to undergo differentiation into multiple lineages such as osteogenic [55], adipogenic, chondrogenic, myogenic, neurogenic, and tenogenic/ligamentogenic [56]. Administration of BMSCs has been combined with other biological molecules and delivered with scaffolds to promote rotator cuff repair. For instance, new cartilage formation was observed at early time points when BMSCs were scaffold-delivered to promote proliferation, viability and differentiation [57]. BMSC administration in fibrin sealant was analyzed with respect to collagen fiber orientation and organization. Histological and biochemical reports at early stages in the study concluded no significant improvements [57]. However, the beneficial effects of BMSCs in a human clinical trial were evaluated on rotator cuff tears (1.5 – 3.5 cm) concluding that the BMSCs-treated groups demonstrated better healing and reduced retear rates 10 years post-surgical intervention compared to nontreated groups [20]. Like BMSCs, adipose derive stem cells (ADSCs) secrete many growth factors (GFs) and cytokines that influence cellular responses. But high numbers of ADSCs can be obtained by a minimally invasive procedure compared to BMSCs [52], [58]. BMSCs undergo senescence with prolonged tissue culture, and this has not been observed in ADSCs. In addition, the ease of harvest, genetic stability, and proliferative capacity of ADSCs synergize to provide these cells with a significant advantage over other types of stem cells [58]. Mora et al. investigated the effects of ADSCs on rotor cuff regeneration in a rat model. The study demonstrated statistical significance in absorbed energy and mechanical deformation at 2 weeks between the control groups and ADSCs-treated groups, yet at 4 weeks no significant differences were noted [59]. However, in a rabbit large rotator cuff tear model, Oh et al. illustrated substantial increase of muscle and tendon regeneration and low fatty degeneration in the injected ADSC treatment groups [60].
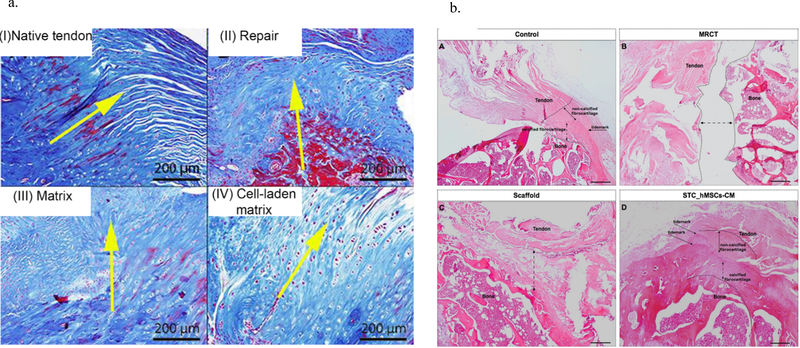
Studies using other stem cell types have also been reported for soft tissue regeneration therapies, including connective tissue progenitor stem cells (CTPSCs), muscle–derived satellite/stem cells (MDSCs), tendon-derived stem cells (TDSCs), synovium-derived stem cells, and umbilical cord blood-derived. In one study tenocyte-like cells from human patients were combined with bone morphogenetic protein-2 and −7 (BMP-2 and BMP-7) and showed significance on cellular proliferation and improvement in tendon-bone healing [61]. Experiments employing stem cell therapy report a wide range of results from positive results to no significance suggesting that stem cells are not an ideal individual treatment, but stem cells may be a promising component of a convergence approach towards rotator cuff regeneration. Further, to the beneficial effects of cell-based therapies, several studies investigated the efficacy of nanostructures as cell delivery vehicles [62]–[65]. Peach et al. evaluated the efficiency of a cell-laden matrix for tendon repair in a rotator cuff tear rat model in comparison with common surgical techniques and non-cell-laden matrices [62]. Poly (ε-caprolactone) (PCL) nanofibers were coated with polyphosphazene poly[(ethyl alanato)1(p-methyl phenoxy)1] phosphazene (PNEA-mPh) to improve the hydrophilicity of the nanofibers and support MSC adhesion and proliferation. Beside the improvement of mechanical and morphological properties of the nanofibrous structure, the cell-laden matrix modulated environment immunology and bioactivity by various strategies such as autocrine/paracrine signaling and cell homing mechanism. According to the results, the elongated cells with a lower cell number and a gradual transition from tendon to bone were shown in the cell-laden sample compared to the non-cell-laden sample (Figure 3a). Collagen immunohistochemistry- histological semi-quantitation was conducted and the ratio of Col I: Col III coverage on the tendons at 12-weeks post-injury was determined for all four groups using semi-quantitative histology. Monoclonal Col I (Cat. # ab6308, Abcam, MA) and Col III (Cat. # ab6310, Abcam, MA) antibodies were incubated with deparaffinized samples at a 1:2000 dilution overnight (37°C) and then imaged using a DAB peroxidase-based detection system. Concluding, the presence of MSCs led to a high Col I: Col III expression which is similar to the intact tendon [62].
Figure 3.
a) Masson trichrome staining of rotator cuff for different groups 12 weeks after surgery, I) Native tendon, II) Repaired group, III) Repaired group with matrix implantation without cell, IV) Repaired group with cell-seeded matrix implantation [56] (Reprinted from an open access article under terms Creative Commons Attribution License); b) Hematoxylin and eosin staining of rotator cuff for different groups 16 weeks after surgery, A) Uninjured group, B) Massive rotator cuff tear group 32 weeks after injury, C) Repaired group with matrix alone, and D) Repaired group with secretome-seeded matrix [59].
Secreted factors of stem cells are considered one of the major principles of cell therapeutic effects. Several reports including in vitro, in vivo and even clinical studies have demonstrated the therapeutic benefits of the secretome. Veronesi et al. reported an exhaustive study regarding the last 10 years of research on the usage of stem cell conditioned media and the high potential of cell-free therapy for musculoskeletal regeneration. Among all the tendon/muscle-related disorders, irreparable changes including fatty degeneration and atrophy are still untreatable. Recently, the stem cell secretome has been observed to functionally reduce the degeneration of muscle in the tendon/muscle lesions by promoting anti-inflammatory behavior, cell proliferation/differentiation and immune modulation. Sevivas et al. reported the regenerative efficacy of the secretome and cell-free therapy for rotator cuff muscle and tendon regeneration [65], [66]. The study investigated the role of secreted secretome on enthesis regeneration in a massive rotator cuff tear rat chronic model [65]. The matrices were fabricated through a combination of a keratin nanofibrous patch with human tendon cells and human MSC (hMSC) secretome. Based on the results, the secretome-seeded matrix significantly improved the proliferation and viability of tendon cells in vitro. Further, the implantation of secretome-seeded matrix in vivo showed histological and biomechanical improvement of the rotator cuff tendon repair by increasing the cell densities and fibrocartilage organization and when compared to untreated groups (Figure 3b) [65].
b. Growth-factors (GFs) and Small Drug Molecules
Regeneration requires a series of mechanisms to occur to achieve formation of mature tissue. As aforementioned, inflammatory cells release growth factors upon injury to promote cell proliferation, differentiation, ECM synthesis, and tissue regeneration [67], [68]. Several studies have investigated the impacts of growth factors on soft tissue regeneration including the rotator cuff [8], [69]–[72]. Growth factors can improve healing in rotator cuff repair. For instance, basic fibroblast growth factor (b-FGF) promotes tendon fibroblast proliferation and migration [73] and induces differentiation of MSCs into tenocytes [74]. Platelet derived growth factor (PDGF) has been shown to also have reached upregulation during the early phase of tendon healing [75], which is believed to onset the activation of other growth factors [64], and to stimulate cellular behavior (e.g. migration, proliferation, and matrix synthesis) at the healing site [76], [77]. There are several other growth factors and molecules such as platelet-rich plasma (PRPs) delivered for rotator cuff regeneration that display beneficial impact on soft tissue repair, but there are drawbacks. For example, local injection of growth factors presents a high risk of loss by degradation and circulation, in addition, the limited half-life of growth factors reduces efficacy of direct injection. Therefore, it is imperative to understand and develop a growth-factor, drug delivery vehicle as a therapy system for complex tissue and rotator cuff regeneration.
To overcome transport barriers of injected therapeutic drug molecules (e.g. growth factors) or stem cells for rotator cuff regeneration, nanomaterials has proven to be a promising carrier for drug delivery systems due to biocompatibility, biodegradability and mechanical integrity. Zhao et al. evaluated the impacts of b-FGF-loaded electrospun PLGA nanofiber for rotator cuff tear [69]. Biochemical and histological results showed that electrospun nanofibrous matrices aid in cell attachment and proliferation, and demonstrated that bFGF-loaded PLGA have a more pronounced effect on tendon-bone healing [69]. The favorable results of these studies and others summarized in Table 1 give promise for using nanostructures as drug delivery vehicles for rotator cuff repair and regeneration.
Table 1.
Nanostructures used as matrices or drug delivery vehicles for rotator cuff repair
| Material | Cell | Growth Factor | In vivo Model | Reference | |
|---|---|---|---|---|---|
| Matrices | Poly-N-Acetyl Glucosamine (sNAG) | - | - | Rat | [49] |
| PCL/PEO | - | - | Rat | [50] | |
| PLGA/COL-PCL/nHA | - | - | Rabbit | [51] | |
| PCL/chitosan | - | - | Rat | [78] | |
| Delivery vehicles | PCL-PNEA-mPh | rat Bone-derived mesenchymal stem cells (rBMSCs) | - | Rat | [62] |
| PCL | Juvenile tendon-derived cells | - | Rat | [63] | |
| PLGA | ASCs | BMP2 | Rat | [64] | |
| Keratin | Human tendon cell | hMSC secretome | Rat | [65] | |
| PLGA | - | bFGF | Rat | [69] | |
| PEU | - | PRP | Rat | [79] |
VI. Discussion and Future Outlook
Rotator cuff repair surgeries consist of greater than 200,000 procedures per year in the U.S. and retears are increasingly common. Current strategies for rotator cuff repair and regeneration show promise including those studies investigating growth factors and drug molecules, stem cells-based approaches, and nanomaterial structures, but there are still limitations and grand challenges that exist with regenerating complex tissue systems. For example, as previously mentioned, most studies reviewed are conducted in acute rotator cuff tears and clinically these injuries are chronic. Therefore, it is imperative to investigate rotator cuff repair and regeneration in large animal models such as rabbit or sheep to help determine the biochemical and mechanical necessity for human translation. Current cooperation is needed to address the clinical challenges regarding the rotator cuff repair and regeneration and the application of tissue regenerative engineering principles are vital to these efforts. The use of tissue regenerative engineering technologies remains to grow, [3], [13], [84]–[93], [17], [94]–[99], [38], [52], [53], [80]–[83]. In this mini review, we identified tissue engineered strategies for rotator cuff repair that used nanomaterials as matrices and as drug delivery vehicles. We believe that rotator cuff regeneration can be achieved using a regenerative engineering approach to influence stem cell behavior, modulate immune cell response, and promote angiogenesis guided by nanomaterial structures.
Acknowledgment
This work was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases (5DP1AR068147-04).
References
- [1].Urwin M et al. , “Estimating the burden of musculoskeletal disorders in the community: The comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation,” Ann. Rheum. Dis, vol. 57, no. 11, pp. 649–655, 1998, doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bishay V and Gallo RA, “The Evaluation and Treatment of Rotator Cuff Pathology,” Prim. Care Clin. Off. Pract, vol. 40, pp. 889–910, 2013, doi: 10.1016/j.pop.2013.08.006. [DOI] [PubMed] [Google Scholar]
- [3].Saveh-Shemshaki N, Nair LS, and Laurencin CT, “Nanofiber-based matrices for rotator cuff regenerative engineering,” Acta Biomater, vol. 94, pp. 64–81, Aug. 2019, doi: 10.1016/j.actbio.2019.05.041. [DOI] [PubMed] [Google Scholar]
- [4].Moffat KL, Kwei ASP, Spalazzi JP, Doty SB, Levine WN, and Lu HH, “Novel nanofiber-based scaffold for rotator cuff repair and augmentation,” Tissue Eng. - Part A, vol. 15, no. 1, pp. 115–126, Jan. 2009, doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meyer DC, Pirkl C, Pfirrmann CWA, Zanetti M, and Gerber C, “Asymmetric atrophy of the supraspinatus muscle following tendon tear,” J. Orthop. Res, vol. 23, no. 2, pp. 254–258, Mar. 2005, doi: 10.1016/j.orthres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- [6].Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, and Flatow EL, “Rotator cuff repair: An analysis of utility scores and cost-effectiveness,” J. Shoulder Elb. Surg, vol. 16, no. 2, pp. 181–187, Mar. 2007, doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- [7].Mather RC et al. , “The societal and economic value of rotator cuff repair,” J. Bone Jt. Surg. - Ser. A, vol. 95, no. 22, pp. 1993–2000, Nov. 2013, doi: 10.2106/JBJS.L.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Novakova SS et al. , “Tissue-engineered tendon constructs for rotator cuff repair in sheep,” J. Orthop. Res, Jul. 2017, doi: 10.1002/jor.23642. [DOI] [PubMed] [Google Scholar]
- [9].Burkhart SS, Diaz Pagàn JL, Wirth MA, and Athanasiou KA, “Cyclic loading of anchor-based rotator cuff repairs: Confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation,” Arthroscopy, vol. 13, no. 6, pp. 720–724, Dec. 1997, doi: 10.1016/S0749-8063(97)90006-2. [DOI] [PubMed] [Google Scholar]
- [10].Zhao S et al. , “Biomaterials based strategies for rotator cuff repair,” Colloids and Surfaces B: Biointerfaces, vol. 157. Elsevier B.V., pp. 407–416, Sep. 2017, doi: 10.1016/j.colsurfb.2017.06.004. [DOI] [PubMed] [Google Scholar]
- [11].Du Y, Ge J, Li Y, Ma PX, and Lei B, “Biomimetic elastomeric, conductive and biodegradable polycitrate-based nanocomposites for guiding myogenic differentiation and skeletal muscle regeneration,” Biomaterials, vol. 157, pp. 40–50, Mar. 2018, doi: 10.1016/j.biomaterials.2017.12.005. [DOI] [PubMed] [Google Scholar]
- [12].Zhang L and Webster TJ, “Nanotechnology and nanomaterials: Promises for improved tissue regeneration,” Nano Today, vol. 4, no. 1. Elsevier, pp. 66–80, Feb. 2009, doi: 10.1016/j.nantod.2008.10.014. [DOI] [Google Scholar]
- [13].Laurencin CT and Khan Y, “Regenerative engineering,” Science Translational Medicine, vol. 4, no. 160. Nov. 2012, doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- [14].Jeno SH and Schindler GS, Anatomy, Shoulder and Upper Limb, Arm Supraspinatus Muscle. 2019. [PubMed] [Google Scholar]
- [15].Camargo PR, Alburquerque-Sendín F, and Salvini TF, “Eccentric training as a new approach for rotator cufftendinopathy: Review and perspectives,” World J. Orthop, vol. 5, no. 5, pp. 634–644, 2014, doi: 10.5312/wjo.v5.i5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Osti L, Buda M, and Del Buono A, “Fatty infiltration of the shoulder: Diagnosis and reversibility,” Muscles, Ligaments and Tendons Journal, vol. 3, no. 4. pp. 351–354, Oct. 2013, doi: 10.11138/mltj/2013.3.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tang X, Saveh-Shemshaki N, Kan HM, Khan Y, and Laurencin CT, “Biomimetic Electroconductive Nanofibrous Matrices for Skeletal Muscle Regenerative Engineering,” Regen. Eng. Transl. Med, vol. 6, no. 2, pp. 228–237, Jun. 2020, doi: 10.1007/s40883-019-00136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pandey V, Bandi A, Madi S, … L. A.-J. of shoulder and, and undefined 2016, “Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff,” Elsevier. [DOI] [PubMed] [Google Scholar]
- [19].Randelli P et al. , “Regenerative Medicine in Rotator Cuff Injuries,” 2014, doi: 10.1155/2014/129515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hernigou P et al. , “Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study,” Int. Orthop, vol. 38, no. 9, pp. 1811–1818, 2014, doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- [21].Kim YS, Sung CH, Chung SH, Kwak SJ, and Koh YG, “Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study,” Am. J. Sports Med, vol. 45, no. 9, pp. 2010–2018, Jul. 2017, doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
- [22].Barber F, Burns J, Deutsch A, … M. L.-… : T. J. of, and undefined 2012, “A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair,” Elsevier. [DOI] [PubMed] [Google Scholar]
- [23].Dickinson M and Wilson SL, “A Critical Review of Regenerative Therapies for Shoulder Rotator Cuff Injuries,” SN Compr. Clin. Med, vol. 1, no. 3, pp. 205–214, Mar. 2019, doi: 10.1007/s42399-018-0019-2. [DOI] [Google Scholar]
- [24].Bagherzadeh R et al. , “Flexible and stretchable nanofibrous piezo- and triboelectric wearable electronics,” in Energy Harvesting Properties of Electrospun Nanofibers,. [Google Scholar]
- [25].Bagherzadeh R, Gorji M, Sorayani Bafgi MS, and Saveh-Shemshaki N, “Electrospun conductive nanofibers for electronics,” in Electrospun Nanofibers, Elsevier Inc., 2017, pp. 467–519. [Google Scholar]
- [26].U. K. R. Society, “Nanoscience and Nanotechnologies: Opportunities and Uncertainties,” 2004.
- [27].Lehner R, Wang X, Marsch S, and Hunziker P, “Intelligent nanomaterials for medicine: Carrier platforms and targeting strategies in the context of clinical application,” Nanomedicine: Nanotechnology, Biology, and Medicine, vol. 9, no. 6. Elsevier, pp. 742–757, Aug. 2013, doi: 10.1016/j.nano.2013.01.012. [DOI] [PubMed] [Google Scholar]
- [28].Wagner V, Dullaart A, Bock AK, and Zweck A, “The emerging nanomedicine landscape,” Nature Biotechnology, vol. 24, no. 10. Nature Publishing Group, pp. 1211–1217, Oct. 2006, doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- [29].Bhat S and Kumar A, “Biomaterials and bioengineering tomorrow’s healthcare,” Biomatter, vol. 3, no. 3, p. e24717, Jul. 2013, doi: 10.4161/biom.24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Jong WH and Borm PJA, “Drug delivery and nanoparticles: Applications and hazards,” International Journal of Nanomedicine, vol. 3, no. 2. Dove Press, pp. 133–149, 2008, doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yao J, Yang M, and Duan Y, “Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy,” Chemical Reviews, vol. 114, no. 12. American Chemical Society, pp. 6130–6178, Jun. 2014, doi: 10.1021/cr200359p. [DOI] [PubMed] [Google Scholar]
- [32].Golczak S, Kanciurzewska A, Fahlman M, Langer K, and Langer JJ, “Comparative XPS surface study of polyaniline thin films,” Solid State Ionics, vol. 179, no. 39, pp. 2234–2239, Dec. 2008, doi: 10.1016/j.ssi.2008.08.004. [DOI] [Google Scholar]
- [33].McCarron JA, Milks RA, Chen X, Iannotti JP, and Derwin KA, “Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model,” J. Shoulder Elb. Surg, vol. 19, no. 5, pp. 688–696, Jul. 2010, doi: 10.1016/j.jse.2009.12.008. [DOI] [PubMed] [Google Scholar]
- [34].Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, and Iannotti JP, “Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device,” J. Bone Jt. Surg. - Ser. A, vol. 91, no. 5, pp. 1159–1171, May 2009, doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saveh-Shemshaki N, Latifi M, Bagherzadeh R, Malekshahi Byranvand M, Naseri N, and Dabirian A, “Synthesis of mesoporous functional hematite nanofibrous photoanodes by electrospinning,” Polym. Adv. Technol, vol. 27, no. 3, pp. 358–365, Mar. 2016, doi: 10.1002/pat.3647. [DOI] [Google Scholar]
- [36].Saveh-Shemshaki N, Bagherzadeh R, and Latifi M, “Electrospun metal oxide nanofibrous mat as a transparent conductive layer,” Org. Electron, vol. 70, pp. 131–139, Jul. 2019, doi: 10.1016/j.orgel.2019.03.034. [DOI] [Google Scholar]
- [37].Saveh-Shemshakia A, N., Latifia M, Bagherzadeh R, & Dabirianc, “Functional Fe2O3 Nanofiber Photoanodes for Photoelectrochemical Water Splitting Application,” 2016. [Online]. Available: https://www.researchgate.net/publication/308952787.
- [38].Li WJ, Laurencin CT, Caterson EJ, Tuan RS, and Ko FK, “Electrospun nanofibrous structure: A novel scaffold for tissue engineering,” J. Biomed. Mater. Res, vol. 60, no. 4, pp. 613–621, Jun. 2002, doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- [39].Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, and Juncker D, “Fiber-based tissue engineering: Progress, challenges, and opportunities,” Biotechnology Advances, vol. 31, no. 5. Elsevier, pp. 669–687, Sep. 2013, doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seidi A, Ramalingam M, Elloumi-Hannachi I, Ostrovidov S, and Khademhosseini A, “Gradient biomaterials for soft-to-hard interface tissue engineering,” Acta Biomaterialia, vol. 7, no. 4. Elsevier, pp. 1441–1451, Apr. 2011, doi: 10.1016/j.actbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [41].James KS, Cornwell KC, and Greenburg AG, “TissueMend™,” Expert Review of Medical Devices, vol. 7, no. 1. p. 9, Jan. 2010, doi: 10.1586/erd.09.59. [DOI] [PubMed] [Google Scholar]
- [42].Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, and Wood D, “Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation,” J. Biomed. Mater. Res. Part B Appl. Biomater, vol. 73B, no. 1, pp. 61–67, Apr. 2005, doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- [43].Bond JL, Dopirak RM, Higgins J, Burns J, and Snyder SJ, “Arthroscopic Replacement of Massive, Irreparable Rotator Cuff Tears Using a GraftJacket Allograft: Technique and Preliminary Results,” Arthrosc. - J. Arthrosc. Relat. Surg, vol. 24, no. 4, pp. 403.e1–403.e8, 2008, doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- [44].Harper C, “Permacol™: Clinical experience with a new biomaterial,” Hosp. Med, vol. 62, no. 2, pp. 90–95, 2001, doi: 10.12968/hosp.2001.62.2.2379. [DOI] [PubMed] [Google Scholar]
- [45].Derwin KA, Baker AR, Spragg RK, Leigh DR, and Iannotti JP, “Commercial Extracellular Matrix Scaffolds for Rotator Cuff Tendon Repair,” J. Bone Jt. Surg, vol. 88, no. 12, pp. 2665–2672, Dec. 2006, doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- [46].Taylor ED, Nair LS, Nukavarapu SP, McLaughlin S, and Laurencin CT, “Novel nanostructured scaffolds as therapeutic replacement options for rotator cuff disease,” J. Bone Jt. Surg. - Ser. A, vol. 92, no. SUPPL. 2, pp. 170–179, Dec. 2010, doi: 10.2106/JBJS.J.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cross LM, Thakur A, Jalili NA, Detamore M, and Gaharwar AK, “Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces,” Acta Biomaterialia, vol. 42. Elsevier Ltd, pp. 2–17, Sep. 2016, doi: 10.1016/j.actbio.2016.06.023. [DOI] [PubMed] [Google Scholar]
- [48].Liu W, Lipner J, Xie J, Manning CN, Thomopoulos S, and Xia Y, “Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis,” ACS Appl. Mater. Interfaces, vol. 6, no. 4, pp. 2842–2849, Feb. 2014, doi: 10.1021/am405418g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nuss CA et al. , “Poly-N-Acetyl Glucosamine (sNAG) Enhances Early Rotator Cuff Tendon Healing in a Rat Model,” Ann. Biomed. Eng, vol. 45, no. 12, pp. 2826–2836, Dec. 2017, doi: 10.1007/s10439-017-1923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Beason DP et al. , “Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model,” Journal of Shoulder and Elbow Surgery, vol. 21, no. 2. Mosby, pp. 245–250, Feb. 2012, doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- [51].Sun Y et al. , “A synthetic bridging patch of modified co-electrospun dual nano-scaffolds for massive rotator cuff tear,” J. Mater. Chem. B, vol. 4, no. 45, pp. 7259–7269, Nov. 2016, doi: 10.1039/C6TB01674J. [DOI] [PubMed] [Google Scholar]
- [52].Narayanan G, Bhattacharjee M, Nair LS, and Laurencin CT, “Musculoskeletal Tissue Regeneration: the Role of the Stem Cells,” Regenerative Engineering and Translational Medicine, vol. 3, no. 3. Springer International Publishing, pp. 133–165, Sep. 2017, doi: 10.1007/s40883-017-0036-9. [DOI] [Google Scholar]
- [53].Narayanan G, Nair LS, and Laurencin CT, “Regenerative Engineering of the Rotator Cuff of the Shoulder,” ACS Biomater. Sci. Eng, vol. 4, no. 3, pp. 751–786, Mar. 2018, doi: 10.1021/acsbiomaterials.7b00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Caplan AI and Dennis JE, “Mesenchymal stem cells as trophic mediators,” Journal of Cellular Biochemistry, vol. 98, no. 5. pp. 1076–1084, Aug. 2006, doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- [55].Friedenstein AJ, Chailakhjan RK, and Lalykina KS, “THE DEVELOPMENT OF FIBROBLAST COLONIES IN MONOLAYER CULTURES OF GUINEA‐PIG BONE MARROW AND SPLEEN CELLS,” Cell Prolif, vol. 3, no. 4, pp. 393–403, 1970, doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- [56].Caplan AI, “Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics,” in Tissue Engineering, Jul. 2005, vol. 11, no. 7–8, pp. 1198–1211, doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- [57].Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, and Rodeo SA, “Application of bone marrow-derived mesenchymal stem cells in a Rotator cuff repair model,” Am. J. Sports Med, vol. 37, no. 11, pp. 2126–2133, 2009, doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- [58].Leto Barone AA, Khalifian S, Lee WPA, and Brandacher G, “Immunomodulatory effects of adipose-derived stem cells: Fact or fiction?,” BioMed Research International, vol. 2013. 2013, doi: 10.1155/2013/383685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mora MV, “Stem cell therapy in the management of shoulder rotator cuff disorders,” World J. Stem Cells, vol. 7, no. 4, p. 691, 2015, doi: 10.4252/wjsc.v7.i4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Oh JH, Chung SW, Kim SH, Chung JY, and Kim JY, “2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model,” J. Shoulder Elb. Surg, vol. 23, no. 4, pp. 445–455, 2014, doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- [61].Klatte-Schulz F et al. , “Characteristics and Stimulation Potential with BMP-2 and BMP-7 of Tenocyte-Like Cells Isolated from the Rotator Cuff of Female Donors,” PLoS One, vol. 8, no. 6, Jun. 2013, doi: 10.1371/journal.pone.0067209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Peach MS et al. , “Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering,” PLoS One, vol. 12, no. 4, p. e0174789, Apr. 2017, doi: 10.1371/journal.pone.0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Huegel J et al. , “Autologous tendon-derived cell-seeded nanofibrous scaffolds improve rotator cuff repair in an age-dependent fashion,” J. Orthop. Res, vol. 35, no. 6, pp. 1250–1257, Jun. 2017, doi: 10.1002/jor.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lipner J et al. , “In Vivo Evaluation of Adipose-Derived Stromal Cells Delivered with a Nanofiber Scaffold for Tendon-to-Bone Repair,” Tissue Eng. Part A, vol. 21, no. 21–22, pp. 2766–2774, Nov. 2015, doi: 10.1089/ten.tea.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sevivas N et al. , “Mesenchymal Stem Cell Secretome Improves Tendon Cell Viability In Vitro and Tendon-Bone Healing In Vivo When a Tissue Engineering Strategy Is Used in a Rat Model of Chronic Massive Rotator Cuff Tear,” Am. J. Sports Med, vol. 46, no. 2, pp. 449–459, Feb. 2018, doi: 10.1177/0363546517735850. [DOI] [PubMed] [Google Scholar]
- [66].Sevivas N et al. , “Mesenchymal Stem Cell Secretome,” Am. J. Sports Med, vol. 45, no. 1, pp. 179–188, Jan. 2017, doi: 10.1177/0363546516657827. [DOI] [PubMed] [Google Scholar]
- [67].Juhas M and Bursac N, “Engineering skeletal muscle repair,” Current Opinion in Biotechnology, vol. 24, no. 5. pp. 880–886, Oct. 2013, doi: 10.1016/j.copbio.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Würgler-Hauri CC, Dourte LAM, Baradet TC, Williams GR, and Soslowsky LJ, “Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model,” J. Shoulder Elb. Surg, vol. 16, no. 5 SUPPL., Sep. 2007, doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhao S et al. , “Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fbrous membranes,” Int. J. Nanomedicine, vol. 9, no. 1, pp. 2373–2385, May 2014, doi: 10.2147/IJN.S59536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Uggen C, Dines J, McGarry M, Grande D, Lee T, and Limpisvasti O, “The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model,” Arthrosc. - J. Arthrosc. Relat. Surg, vol. 26, no. 11, pp. 1456–1462, Nov. 2010, doi: 10.1016/j.arthro.2010.02.025. [DOI] [PubMed] [Google Scholar]
- [71].Hee CK et al. , “Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model.,” Am. J. Sports Med, vol. 39, no. 8, pp. 1630–9, Aug. 2011, doi: 10.1177/0363546511404942. [DOI] [PubMed] [Google Scholar]
- [72].Tokunaga T et al. , “FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin -Positive Tenocytes in a Rat Rotator Cuff Healing Model,” Am. J. Sports Med, vol. 43, no. 10, pp. 2411–2422, Oct. 2015, doi: 10.1177/0363546515597488. [DOI] [PubMed] [Google Scholar]
- [73].Chan BP, Chan KM, Maffulli N, Webb S, and Lee KK, “Effect of basic fibroblast growth factor. An in vitro study of tendon healing.,” Clin. Orthop. Relat. Res, no. 342, pp. 239–47, Sep. 1997. [PubMed] [Google Scholar]
- [74].Cai TY, Zhu W, Chen XS, Zhou SY, Jia LS, and Sun YQ, “Fibroblast growth factor 2 induces mesenchymal stem cells to differentiate into tenocytes through the MAPK pathway,” Mol. Med. Rep, vol. 8, no. 5, pp. 1323–1328, Nov. 2013, doi: 10.3892/mmr.2013.1668. [DOI] [PubMed] [Google Scholar]
- [75].Kobayashi M et al. , “Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits,” J. Shoulder Elb. Surg, vol. 15, no. 3, pp. 371–377, May 2006, doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [76].Lynch SE, Colvin RB, and Antoniades HN, “Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds,” J. Clin. Invest, vol. 84, no. 2, pp. 640–646, 1989, doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tsuzaki M et al. , “Insulin-like growth factor-I is expressed by avian flexor tendon cells,” J. Orthop. Res, vol. 18, no. 4, pp. 546–556, Jul. 2000, doi: 10.1002/jor.1100180406. [DOI] [PubMed] [Google Scholar]
- [78].Zhao S et al. , “A hierarchical, stretchable and stiff fibrous biotemplate engineered using stagger-electrospinning for augmentation of rotator cuff tendon-healing,” J. Mater. Chem. B, vol. 3, no. 6, pp. 990–1000, Feb. 2015, doi: 10.1039/c4tb01642d. [DOI] [PubMed] [Google Scholar]
- [79].Childers EP et al. , “Enhanced Rotator-Cuff Repair Using Platelet-Rich Plasma Adsorbed on Branched Poly(ester urea)s,” Biomacromolecules, vol. 19, no. 7, pp. 3129–3139, Jul. 2018, doi: 10.1021/acs.biomac.8b00725. [DOI] [PubMed] [Google Scholar]
- [80].Sethuraman S et al. , “Mechanical properties and osteocompatibility of novel biodegradable alanine based polyphosphazenes: Side group effects,” Acta Biomater, vol. 6, no. 6, pp. 1931–1937, Jun. 2010, doi: 10.1016/j.actbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lucas PA et al. , “Ectopic induction of cartilage and bone by water-soluble proteins from bovine bone using a polyanhydride delivery vehicle,” J. Biomed. Mater. Res, vol. 24, no. 7, pp. 901–911, Jul. 1990, doi: 10.1002/jbm.820240708. [DOI] [PubMed] [Google Scholar]
- [82].El-Amin SF et al. , “Integrin expression by human osteoblasts cultured on degradable polymeric materials applicable for tissue engineered bone,” J. Orthop. Res, vol. 20, no. 1, pp. 20–28, Jan. 2002, doi: 10.1016/S0736-0266(01)00062-6. [DOI] [PubMed] [Google Scholar]
- [83].Wang J, Valmikinathan CM, Liu W, Laurencin CT, and Yu X, “Spiral-structured, nanofibrous, 3D scaffolds for bone tissue engineering,” J. Biomed. Mater. Res. Part A, vol. 9999A, no. 2, p. NA–NA, May 2009, doi: 10.1002/jbm.a.32591. [DOI] [PubMed] [Google Scholar]
- [84].Jiang T, Deng M, James R, Nair LS, and Laurencin CT, “Micro- and nanofabrication of chitosan structures for regenerative engineering,” Acta Biomaterialia, vol. 10, no. 4. Elsevier BV, pp. 1632–1645, Apr. 2014, doi: 10.1016/j.actbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- [85].Laurencin CT, Christensen DM, and Taylor ED, “HIV/AIDS and the African-American community: A state of emergency,” J. Natl. Med. Assoc, vol. 100, no. 1, pp. 35–43, Jan. 2008, doi: 10.1016/S0027-9684(15)31172-X. [DOI] [PubMed] [Google Scholar]
- [86].Butler DL et al. , “The Impact of Biomechanics in tissue engineering and Regenerative medicine,” Tissue Engineering - Part B: Reviews, vol. 15, no. 4. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA, pp. 477–484, Dec. 2009, doi: 10.1089/ten.teb.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Abdel-Fattah WI, Jiang T, El-Bassyouni GET, and Laurencin CT, “Synthesis, characterization of chitosans and fabrication of sintered chitosan microsphere matrices for bone tissue engineering,” Acta Biomater, vol. 3, no. 4, pp. 503–514, Jul. 2007, doi: 10.1016/j.actbio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [88].Deng M et al. , “Miscibility and in vitro osteocompatibility of biodegradable blends of poly[(ethyl alanato) (p-phenyl phenoxy) phosphazene] and poly(lactic acid-glycolic acid),” Biomaterials, vol. 29, no. 3, pp. 337–349, Jan. 2008, doi: 10.1016/j.biomaterials.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lv Q, Nair L, and Laurencin CT, “Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors,” J. Biomed. Mater. Res. Part A, vol. 91A, no. 3, pp. 679–691, Dec. 2009, doi: 10.1002/jbm.a.32302. [DOI] [PubMed] [Google Scholar]
- [90].Ibim SM, Uhrich KE, Bronson R, El-Amin SF, Langer RS, and Laurencin CT, “Poly(anhydride-co-imides): In vivo biocompatibility in a rat model,” Biomaterials, vol. 19, no. 10, pp. 941–951, May 1998, doi: 10.1016/S0142-9612(98)00019-2. [DOI] [PubMed] [Google Scholar]
- [91].Nelson C, Khan Y, and Laurencin CT, “Nanofiber/Microsphere Hybrid Matrices In Vivo for Bone Regenerative Engineering: A Preliminary Report,” Regen. Eng. Transl. Med, vol. 4, no. 3, pp. 133–141, Sep. 2018, doi: 10.1007/s40883-018-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Barajaa MA, Nair LS, and Laurencin CT, “Bioinspired Scaffold Designs for Regenerating Musculoskeletal Tissue Interfaces,” Regen. Eng. Transl. Med, 2019, doi: 10.1007/s40883-019-00132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ogueri KS, Jafari T, Escobar Ivirico JL, and Laurencin CT, “Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering,” Regenerative Engineering and Translational Medicine, vol. 5, no. 2. Springer International Publishing, pp. 128–154, Jun. 2019, doi: 10.1007/s40883-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sun Z, Nair LS, and Laurencin CT, “The Paracrine Effect of Adipose-Derived Stem Cells Inhibits IL-1$β$-induced Inflammation in Chondrogenic Cells through the Wnt/$β$-Catenin Signaling Pathway,” Regen. Eng. Transl. Med, vol. 4, no. 1, pp. 35–41, Mar. 2018, doi: 10.1007/s40883-018-0047-1. [DOI] [Google Scholar]
- [95].Ifegwu OC et al. , “Bone Regenerative Engineering Using a Protein Kinase A-Specific Cyclic AMP Analogue Administered for Short Term,” Regen. Eng. Transl. Med, vol. 4, no. 4, pp. 206–215, Dec. 2018, doi: 10.1007/s40883-018-0063-1. [DOI] [Google Scholar]
- [96].Moore EM and West JL, “Bioactive Poly(ethylene Glycol) Acrylate Hydrogels for Regenerative Engineering,” Regen. Eng. Transl. Med, vol. 5, no. 2, pp. 167–179, Jun. 2019, doi: 10.1007/s40883-018-0074-y. [DOI] [Google Scholar]
- [97].Heath DE, “A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications,” Regenerative Engineering and Translational Medicine, vol. 5, no. 2. Springer International Publishing, pp. 155–166, Jun. 2019, doi: 10.1007/s40883-018-0080-0. [DOI] [Google Scholar]
- [98].Bowers DT and Brown JL, “Nanofibers as Bioinstructive Scaffolds Capable of Modulating Differentiation Through Mechanosensitive Pathways for Regenerative Engineering,” Regenerative Engineering and Translational Medicine, vol. 5, no. 1. Springer International Publishing, pp. 22–29, Mar. 2019, doi: 10.1007/s40883-018-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Clegg JR, Wechsler ME, and Peppas NA, “Correction to: Vision for Functionally Decorated and Molecularly Imprinted Polymers in Regenerative Engineering (Regenerative Engineering and Translational Medicine, (2017), 3, 3, (166–175), 10.1007/s40883–017-0028–9),” Regenerative Engineering and Translational Medicine, vol. 5, no. 4. Springer, p. 450, Dec. 2019, doi: 10.1007/s40883-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]