Abstract
The α9-containing nicotinic acetylcholine receptors (nAChRs) are key targets for the treatment of neuropathic pain. α-Conotoxin RgIA4 is a peptide antagonist of human α9α10 nAChRs with high selectivity. However, structural rearrangement reveals a potential liability for clinical applications. We herein report our designer RgIA analogues stabilized by methylene thioacetal as non-opioid analgesic agents. We demonstrate that replacing disulfide loop I [CysI-CysIII] with methylene thioacetal in the RgIA skeleton results in activity loss whereas substitution of loop II [CysII-CysIV] can be accommodated. The lead molecule, RgIA-5524, exhibits highly selective inhibition of α9α10 nAChRs with an IC50 of 0.9 nM and much reduced degradation in human serum. In vivo studies showed that RgIA-5524 relieves chemotherapy-induced neuropathic pain in wild type but not α9 knockout mouse models, demonstrating that α9-containing nAChRs are necessary for the therapeutic effects. This work highlights the application of methylene thioacetal as disulfide surrogate in conotoxin-based, disulfide-rich peptide drugs.
Keywords: conotoxins, human α9α10 nAChRs, methylene thioacetal, disulfide surrogate, peptide drug stabilization, NMR structures, neuropathic pain
Graphical Abstract

Introduction
Neuropathic pain is a highly prevalent but poorly managed health issue which largely lacks adequate medications and in-depth understanding of its molecular mechanisms.1, 2 Opioids are first-line drugs for the treatment of moderate to severe neuropathic pain; however, the broad and less-regulated clinical application of these drugs has resulted in a critical public health emergency, the opioid epidemic crisis.3-5 To tackle this issue, new medications that can act through alternative molecular mechanisms with diminished side effects and toxicity are urgently needed. Intense research efforts have been devoted to the discovery of lead compounds that can target pain-related ion channels and receptors including NMDAR, Cav2.2, Nav1.7, TRPV1.6-10 Nicotinic acetylcholine receptors (nAChRs), distributed throughout both peripheral and central nervous systems, are a group of transmembrane ligand-gated cationic channels that mediate fast synaptic transmission and are involved in a wide range of nervous system disorders including neuropathic pain, Parkinson’s disease, schizophrenia, alcohol and drug addiction.11 Different nAChR subunits including α, β, γ, δ, and ε associate in various combinations within these homo- or hetero-pentameric receptors, leading to a complex variety of nAChR subtypes with distinct pharmacological and biophysical functions.12, 13 The nAChRs have previously been targeted for analgesic drug discovery albeit with progress being hindered by a narrow therapeutic window and side effects caused by indiscriminate subtype targeting.14, 15 Recent studies indicate that antagonists of α9-containing nAChRs are analgesic in animal models of neuropathic pain, which makes this receptor a potential new target for non-opioid analgesic medication research.16-18
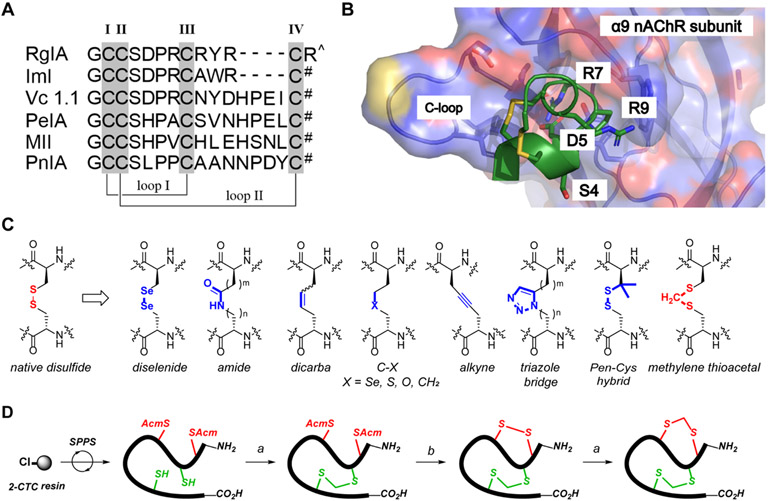
Nature provides a practical means to discover drugs for the treatment of refractory diseases.19, 20 The first FDA approved conotoxin-based drug, ω-conotoxin MVIIA (ziconotide) is clinically used in the treatment of chronic pain.21, 22 α-Conotoxins (CTxs), derived from the venom of predatory marine snails, are peptides that are active at nAChR subtypes.23, 24 Recent studies identified α-CTxs including RgIA, Vc1.1, and PeIA as promising candidates for non-opioid analgesic development (Figure 1A and 1B).25-29 RgIA exhibits high potency and selectivity on rodent α9α10 nAChRs but has low activity at the human variant. Based on the original sequence of RgIA, the second generation candidate RgIA4 was developed which exhibits high potency for both human and rodent α9α10 nAChRs.30-33 Structurally, CTxs rely on highly conserved cysteine frameworks to maintain rigid structures, which are crucial for receptor recognition, potency, and selectivity.34 Unfortunately, like other disulfide-rich peptides, RgIA4 is susceptible to disulfide-scrambling in reducing physiological environments that can lead to concomitant alternation of three-dimensional structures, aggregation, decreased therapeutic efficacy, and increased immunogenic side effects. Designer disulfide mimetics can prevent disulfide bond scrambling and therefore represent powerful tools in disulfide-rich peptide drug discovery.35 Strategies including dicarba, saturated dicarba, alkyne, thioether, ether, diselenide (Sec), penicillamine-cysteine (Pen-Cys) hybrid and triazole bridge replacement have been extensively applied in CTxs modifications (Figure 1C).35-46 However, such strategies lack broad applicability owing to incompatibility of the mimetic moiety with bioactivity as well as toxicity. Head-to-tail backbone cyclization is another investigated strategy to improve peptide stability through prevention of degradation as it stabilized the structure and reduced proteolysis susceptibility.47-50 Nevertheless, the application of this method to RgIA resulted in obvious potency drop on α9α10 nAChR binding affinity.51-54
Figure 1.
(A) Amino acid sequence alignments of α-Ctx RgIA, ImI, Vc1.1, PeIA, MII, and PnIA. Disulfide connectivity is CysI-CysIII (loop I) and CysII-CysIV (loop II). # = C-terminal amide; ^ = C-terminal carboxylate acid. (B) The binding surface of RgIA bound to α9 nAChR subunit crystal structure (PDB 6HY7). Key binding residues (Ser4, Asp5, Arg7, and Arg9) are labeled in black font and shown as stick representation with oxygen, nitrogen, and sulfur atoms in red, blue, and yellow, respectively. (C) Chemical structures of native disulfide and developed disulfide mimetics. Pen = L-penicillamine. (D) Chemical syntheses of methylene thioacetal RgIA analogues in this study. All linear peptides were synthesized through Fmoc-SPPS on the automated synthesizer. Fully folded peptides were obtained via a two-step procedure. Reaction conditions a) TCEP·HCl, K2CO3, H2O; then Et3N, CH2I2, THF. b) I2, AcOH, H2O.
By inserting a minimal functional carbon unit (CH2) into native disulfide, the unreducible methylene thioacetal is an efficient disulfide surrogate, which has been introduced into some bioactive peptides and proteins to improve their biophysical properties.55-58 Compared to the previous approaches requiring the use of unnatural amino acids and metallic catalysts, the methylene thioacetal bond is directly derived from two natural cysteines under simple basic condition, which is a major advancement for future therapeutic development. In this study, we applied the strategic replacement of native disulfide with methylene thioacetal to stabilize the globular active conformation of RgIA analogues. The best candidate RgIA-5524 exhibited high potency (IC50 = 0.9 nM) on human α9α10 nAChRs with superb selectivity against other pain-related ion channels and receptors.
There is an ongoing controversy regarding the therapeutic mechanism of action of α-CTxs, with a number of studies suggesting that stimulation of GABAB receptors is necessary yet others indicating that blockade of α9 nAChRs is key.30, 59-62 The present study, utilizing wildtype and knockout (KO) mice demonstrates not only that a selective α9α10 nAChR antagonist is analgesic, but also that the presence of the α9-nAChR subunit is required for analgesic activity.
Results and Discussion
Chemical Synthesis and Characterization of RgIA Methylene Thioacetal Analogues.
The chemical synthesis of RgIA analogues was achieved by using 9-fluorenylmethyloxycarbonyl (Fmoc) solid-phase peptide synthesis (SPPS) on 2-chlorotrityl chloride (2-CTC) resin followed by a two-step and regioselective intramolecular bond formation reactions (Figure 1D). The correct scaffold folding is CysI-CysIII, CysII-CysIV or its corresponding methylene thioacetal replacement with the same connectivity. Bonds were explicitly formed in an order of 1) methylene thioacetal formation on free Cys after trityl (Trt) removal through cleavage, 2) disulfide bond formation via in situ oxidative acetamidomethyl (Acm) deprotection-coupling process, and 3) repeating methylene thioacetal formation to generate the bis-methylene thioacetal replaced analogue. In detail, after cleavage of the assembled peptide chain from 2-CTC resin, the Trt protections were removed and the target methylene thioacetal bond was formed by treatment with diiodomethane in the presence of tris(2-carboxyethyl)phosphine hydrochloride (TCEP·HCl), potassium carbonate and trimethylamine (Et3N).54, 58 This key-step conversion can be conducted in as large as 300 mg scale in one batch, which enables large preparation of target peptides for further studies. The second disulfide bridge was formed after Acm deprotection by the treatment of excess iodine in 25% aqueous acetic acid (AcOH) to yield fully folded peptides. RgIA and RgIA4 were synthesized according to previous reports.30 All peptides were purified as ≥ 95% purity indicated by RP-HPLC and final products were analyzed by ESI-MS before NMR studies and biological assays. (Figure S1, Table S1 and LC chromatography is shown in Supporting Information).
In Vitro Biological Evaluation of RgIA Methylene Thioacetal Analogues.
The bioactivities of all synthesized analogues were tested by two-electrode-voltage-clamp (TEVC) electrophysiology on human α9α10 nAChRs heterologously expressed in Xenopus laevis oocytes (cRNA injection ratio 1:1). To determine the compatibility of methylene thioacetal as a disulfide surrogate in the RgIA series, we synthesized and tested a suite of analogues with different methylene thioacetal replacements. IC50 values determined by concentration-response analysis (Figure 2B) are shown in Figure 2A. By comparison, native RgIA inhibits ACh-evoked currents mediated by human α9α10 nAChRs with an IC50 value of 510 nM due to the low affinity for the human receptor.33, 63
Figure 2.
(A) Amino acid sequences and potencies of synthesized methylene thioacetal RgIA analogues on hα9α10 nAChRs. aBond connectivity is CI-CIII (loop I) and CII-CIV (loop II) or their corresponding methylene thioacetal surrogates XI-XIII and XII-XIV. X represents methylene thioacetal replacement; ^ represents C-terminal carboxylic acid; Cit = L-citrulline; iY = L-3-iodo-tyrosine; bA = β-alanine; bhY = L-β-homotyrosine. bCalculated from concentration-response curves. Numbers in parenthesis are 95% confidence intervals. (B) Concentration-response analysis for inhibition of human α9α10 nAChR by synthesized peptides on blocking ACh-induced current on human nAChR currents expressed in Xenopus laevis oocytes. (C) IC50 for inhibition of nAChR subtypes by RgIA-5524 and RgIA-5533 on blocking ACh-induced current on human nAChR currents expressed in Xenopus laevis oocytes. (D) Concentration-response analysis for inhibition of hα9α10 versus hα7 nAChR by RgIA-5524 and RgIA-5533. Data points represent the mean ± SEM from 3-4 independent experiments.
Differential effects were produced when methylene thioacetal was introduced to the sequence of a modified RgIA analogue, RgIA4. Specifically, RgIA-5533, which had the loop II [CysII-CysIV] disulfide exchanged for methylene thioacetal, had low nanomolar potency (IC50 = 6.1 nM). In contrast, as for analogue RgIA-5617, with the methylene thioacetal functionality moved to loop I [CysI-CysIII], there was a tremendous decrease in potency (IC50 = 880 nM). Activity was further eliminated when both disulfides were replaced in RgIA-5618 (IC50 > 10 μM). These data show that the loop II disulfide [CysII-CysIV] in RgIA is amenable to modification with methylene thioacetal whereas substitution at the other position [CysI-CysIII] abolishes activity for human α9α10 nAChRs. Our results are consistent with the pioneering research of dicarba modified analogues of RgIA in which both the trans/cis isomers of [CysII-CysIV] -dicarba RgIA maintained some (albeit much reduced) activity for the α9α10 nAChR whereas the [CysI-CysIII]-dicarba analogues were totally inactive.36 Similarly, the importance of the [CysI-CysIII] disulfide on the structure and activity was also demonstrated in another α-4/3-CTx ImI by analyzing disulfide-deficient analogues.63, 64 We then modified RgIA-5533 with mutants based on RgIA5 as well as a non-canonical amino acid, β-homotyrosine (bhTyr), to afford the most potent analogue RgIA-5524 with an IC50 value of 0.9 nM.30, 46 Single residue mutation of bhTyr with β-Alanine (bAla) yielded an analogue, RgIA-5573, which was less potent (IC50 = 2.9 nM) indicating the importance of a phenolic moiety at residue position 13, consistent with our recent research of critical residues in RgIA.63
We next investigated the subtype selectivity of RgIA-5533 and RgIA-5524. Two-electrode-voltage-clamp (TEVC) electrophysiology showed that both analogues failed to inhibit a wide range of nAChR subtypes at 10 μM (IC50 > 10 μM) including α1β1δε, α2β2, α2β4, α3β2, α3β4 α4β2, α4β4, α6/α3β2β3 and α6/α3β4 (Figure 2C, Figure S2). Concentration-response analysis indicated that RgIA-5533 had low activity (IC50 > 10 μM) on hα7 nAChRs and RgIA-5524 exhibited nanomolar activity (IC50 = 186 nM) which is greater than 200-fold higher than the IC50 for hα9α10 nAChRs (Figure 2D). To further assess the binding affinity of RgIA-5524 on hα7 nAChRs, we carried out a competition binding assay using [125I]α-bungarotoxin (α-Btx). RgIA-5524 produced only 41% inhibition at 10 μM (Figure 5A), consistent with its low potency in functional assays on the hα7 nAChR subtype
Figure 5,
(A) Binding activity of RgIA-5524 on other pain-related ion channels and receptors at 10 μM. aEach experiment was conducted with duplicate wells. Binding was calculated as % inhibition of the binding of a radioactively labeled ligand specific for each target and the enzyme inhibition effect was calculated as % inhibition of control enzyme activity. A secondary, concentration-response analysis was conducted when the primary screening assay indicated ≥50% inhibition. bnicotinic neuronal type. cstrychnine sensitive. dtrychnine insensitive; AR, adenosine receptor; AT, angiotensin; BK2, bradykinin receptor; CB, Cannabinoid receptor; CCK, cholecystokinin receptor; CRF, Corticotropin-releasing factor; D, dopamine; ET, endothelin receptor; GABA, γ-aminobutyric acid; GAL, galanin receptor; mGluR, metabotropic glutamate receptor; GlyR, Glycine receptor (strychnine-sensitive); H, histamine receptor; CysLT, cysteinyl leukotriene; M, muscarinic acetylcholine receptor; NK, neurokinin receptor; DOP, δ-opioid receptor; KOP, κ-opioid receptor; MOP, μ-opioid receptor; NOP, nociceptin/orphanin FQ receptor; GR, glucocorticoid receptor; ER, estrogen receptor; AR, androgen receptor; PAFR, platelet-activating factor receptor; TRH1, thyrotropin-releasing hormone; VPAC, vasoactive intestinal peptide receptor; V, vasopressin receptor; LTCC, L-type Ca2+ channel; NTCC, N-type Ca2+ channel; BZD, benzodiazepine; PCP, phencyclidine. (B) Functional activity of RgIA-5524 on GABAB receptors and hERG K+ channel. eTwo separate experiments were conducted with duplicate wells for the IC50 and EC50 studies. Cellular agonist and antagonist effect were calculated as % of control response and % inhibition to a known reference agonist or antagonist, respectively; fMeasured at 100 μM of RgIA-5524. (C) Enzyme and uptake assays of RgIA-5524. gEach experiment was conducted with duplicate wells. The antagonist effect was calculated as % inhibition to the measured component. TXA2 synthetase, Thromboxane A2 synthetase; constitutive NOS, constitutive NO synthase; MAO, monoamine oxidase. Concentration-response analysis of (D) agonist and (E) antagonist effect of RgIA-5524 on GABAB receptors. (F) Concentration-response analysis of RgIA-5524 on hERG K+ channel measured by tail current inhibition. (G) Inhibition of CYP enzyme isoforms by RgIA-5524 at 100 nM and 10 μM. Duplicated experiments were conducted for each concentration and data are expressed as means ± SEM.
In Vivo Pain-relieving Efficacy of RgIA-5524.
Chemotherapy-induced neuropathic pain is a major dose-limiting side effect of platin-based drugs.66 Currently, the pathophysiology of oxaliplatin-induced neuropathic pain remains poorly investigated and there are no approved drugs for the prevention of this dose-limiting adverse outcome. The in vivo analgesic activity of RgIA-5524 was assessed using a model of oxaliplatin-induced peripheral neuropathic pain in mice. Cold-allodynia is a disabling side effect of oxaliplatin. The magnitude and time course of this side effect is dose-dependent. Repeated daily injections of RgIA-5524 prevented the development of neuropathic pain caused by chemotherapy. Oxaliplatin (i.p. 3.5 mg /kg, 5 days each week) created substantial cold allodynia by day 21 of treatment as indicated by significantly reduced paw withdrawal latency on the cold plate. In contrast, oxaliplatin-treated mice that received 40 μg/kg of RgIA-5524 did not develop allodynia (Figure 3).
Figure 3.
In vivo pain-relieving effects of RgIA-5524 in chronic chemotherapy-induced neuropathic pain. RgIA-5524 relieves pain induced by repeated oxaliplatin dosing. Mice were injected once per day, 5 days per week with the chemotherapeutic agent oxaliplatin (3.5 mg/kg, i.p.) over a three-week period. On the days of oxaliplatin injection, mice also received either saline or RgIA-5524 (40 μg/kg). Once per week, twenty-four hours after last RgIA-5524 injection, mice were assessed for cold allodynia using a cold plate as described in Experimental Sections. Allodynia reached statistical significance by day 21 and was effectively reversed by RgIA-5524. Statistical evaluations of the data were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Data are expressed as means ± SEM n = 8 mice per group. xxxP < 0.001 for significant difference from Ox/Sal vs Sal/Sal treated mice; ***P < 0.001 for significant difference from Ox/Sal vs Ox/RgIA-5524 treated mice. Ox, oxaliplatin; Sal, 0.9% saline; s, seconds.
We next performed the single-injection oxaliplatin treatment study in both wild-type and α9 KO mice. The results show that RgIA-5524 was effective in reversing the acute cold allodynia 5 days after oxaliplatin treatment (s.c. 5.0 mg/kg and 10.0 mg/kg) as indicated by significant differences between the WT group of Sal/Sal vs Ox/Sal and Ox/RgIA-5524 vs Ox/Sal (Figure 4A, 4C). However, this effect did not occur in the α9 KO mice group, where no significance was observed between the α9 KO group of Ox/RgIA-5524 vs Ox/Sal (Figure 4B, 4D). This KO experiment demonstrated that the blockade of α9α10 nAChR by RgIA-5524 enabled the prevention or attenuation of chemotherapy-induced neuropathic pain. It is of interest to note that in the single dose oxaliplatin model, the latency of response to cold is the same in α9 KO and w.t. animals. This indicates that germline α9 knockout mice differ in the development of allodynia compared to adult mice in which the function of the a9 gene is conditionally silenced by an antagonist.
Figure 4.
The α9 nAChR subunit is required for analgesic effects of RgIA-5524. (A, B) On day 1, a single dose of oxaliplatin 5 mg/kg i.p. was given along with either RgIA 5524 (40 ug/kg, s.c.) or 0.9% saline. Mice were assessed for cold allodynia on day 5. RgIA-5524 prevented the development of allodynia in (A) wildtype mice but not in (B) α9-subunit null mice (n = 12 mice/group). (C, D) On day 1, mice were administered a higher dose of oxaliplatin (10 mg/kg i.p.) and either RgIA-5524 (40 ug/kg s.c.) or saline. Oxaliplatin-induced cold allodynia was prevented by the single dose of RgIA-5524 administered on day 1 in (C) wildtype mice but not in (D) α9-subunit null mice (n = 8 mice/group). Statistical evaluations of the data were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. All results are expressed as means ± SEM *P <0.05, **P <0.01, and ***P < 0.001 for significant difference from Sal/Sal treated mice. oP <0.05, ooP <0.01, and oooP < 0.001 for significant difference from oxaliplatin/RgIA-5524 treated mice. Ox, oxaliplatin; Sal, 0.9% saline; s, seconds., α9−/−, α9 knockout mice.
In Vitro Pharmacological, Toxicity and Metabolism Assays of RgIA-5524.
We further demonstrated that the most potent analogue RgIA-5524 was a promising non-opioid analgesic candidate via a broad suite of in vitro pharmacology assays. First, we tested RgIA-5524 on a wide range of various pain-associated receptors and ion channels. As summarized in Figure 5A, at 10 μM level, RgIA-5524 showed low or no activity (< 50% inhibition) on these potential targets including the opioid receptors, NMDAR, BZD, OCT receptors, and various voltage-gated ion channels (Na+, K+ & Ca2+). Testing RgIA-5524 on N-type Ca2+ channel showed low potency with only 58.4% inhibition at 10 μM, whereas further concentration-response analysis demonstrated only micromolar affinity, too low to account for the analgesic activity. Utilizing a cellular dielectric spectroscopy assay, we also demonstrated that RgIA-5524 failed to display any concentration-dependent agonist or antagonist effects at GABAB1b receptors, which has been a postulated mechanism for RgIA analgesia (Figure 5B, 5D and 5E).59, 66 Taken together with the above in vivo α9 KO mice study, the results firmly demonstrate that antagonizing α9-containing nAChRs is the dominant mechanism of the observed RgIA-5524 analgesic effects.
Drug-induced cardiotoxicity has become one of the major reasons leading to drug withdrawal in the past decades, which is closely related to the blockade of the human ether-a-go-go-related gene (hERG) K+ channel.67 No evidence for cardiovascular liability was indicated from an automated-whole cell patch-clamp assay in which RgIA-5524 caused < 25% inhibition at a high concentration of 100 μM (Figure 5B and 5F). Meanwhile, RgIA-5524 is inactive in a set of enzyme and uptake assays including acetylcholinesterase and MAO, which are potentially important in several neurodegenerative disorders (Figure 5C). Finally, we assessed the potential of RgIA-5524 to influence drug-drug interactions; no inhibition was observed against a wide panel of CYP enzyme isoforms at 10 μM (Figure 5G).
NMR Spectroscopy and Structural Analysis.
NMR studies of the modified analogues were performed to compare and contrast the structural features. Analogues including RgIA-5533, RgIA-5617 and RgIA-5524 were analyzed by homo-nuclear 2D 1-NMR spectroscopy including TOCSY, NOESY, COSY and HSQC. Together with our previous study of RgIA470, assignments on all residues except for N-terminal Gly1 primary amine were achieved (Supporting Information). In all molecules studied, Pro6 was identified as trans conformation by strong NOEs observed from Asp5 Hα to Pro6 Hδ. Hα secondary-shift analysis was used to assess any changes in the secondary structure elements. In general, secondary Hα shift confirmed that RgIA-5533, 5524 and 5617 all maintained the globular conformations. Subtle changes were observed in the critical residues including Asp5-Pro6-Arg7 and Arg/Cit9-Tyr/iTyr10-Gln/Arg11 segments. No obvious differences among the potent analogue RgIA-5533, RgIA-5524 or inactive RgIA-5617 compared with that of native disulfides bonded RgIA and RgIA4 were observed from Ha secondary chemical shifts. Slight deviations existed at C-termini of individual peptides primarily due to terminal flexibility (Figure 6A).
Figure 6.
(A) Overlay of the Secondary-chemical-shifts of RgIA (black), RgIA4 (grey), RgIA-5617 (pink), RgIA-5533 (green) and RgIA-5524 (blue). The x-axis shows the peptide sequences with substituted residue for the mutant at residue 4, 9, 10, 13 and 14 calculated based on their corresponding standard chemical shifts. Representative NMR structures and distance measurements (Å) between Cα in two intramolecular bridges. (B) RgIA bound to hα9 nAChR subunit crystal structure (PDB 6HY7); Representative NMR solution structures of (C) RgIA (PDB 2JUQ); (D) RgIA4; (E) RgIA-5533; (F) RgIA-5617 and (G) RgIA-5524. Structures are shown as stick representation with atom oxygen, nitrogen, sulfur and iodine colored in red, blue, yellow and purple, respectively. Cα distances were measured by PyMOL program.
Three-dimensional NMR solution structures of these analogues were calculated using CYANA 3.0 with atom distance and dihedral angle restraints generated from g11-NOESY and NOESY (200ms) spectra. Backbone dihedral angle restraints including φ, ψ, and χ1 were predicted by the TALOS program based on the Hα, Cα, Cβ and amide hydrogen (HN) chemical shifts. The 20 lowest energy state ensembles were obtained with low RMSD (Supporting Information). Together with previously reported structures of RgIA (NMR solution structure PDB 2JUQ68 and co-crystal extract PDB 6HY769) and RgIA470, the “closest to mean” energy states were chosen to represent each peptide and shown as Figure 6B, 6C, 6D, 6E, 6F, and 6G with average Cα distance of cysteine pairs measured by PyMOL program. All methylene thioacetal modified peptides maintained globular conformations closely resembling that of RgIA and RgIA4. The most significant difference between RgIA-5617 and the potent analogues (RgIA4, RgIA-5533, and 5524) were the Cα distances of cysteine pairs. Both the Cα distance of the two cysteine pairs in RgIA-5617 are apparently shortened (average of 4.8 and 4.7 Å in cysteine loop I and loop II, receptively) compared with the other molecules (average of 5.4-6.1 Å). One potential reason for the potency loss of analogue RgIA-5617 and RgIA-5618 is that the insertion of CH2 group in loop I disulfide [CysI-CysIII] forced a conformation “shrink” in these loop I modified analogues to accommodate the dihedral and torsional angle changes, which reduced their binding affinities. On the other hand, it has also been proposed by MD stimulation that the loop I disulfide in RgIA analogues might provide stacking interaction towards the receptor by directly contacting with the C-loop disulfide of the α9(+) surface.36 Therefore, methylene thioacetal replacement at this loop could cause potency loss by interfering with this binding site, which could be another contributing factor despite their minor secondary structure perturbations.
In Vitro Stability Assays.
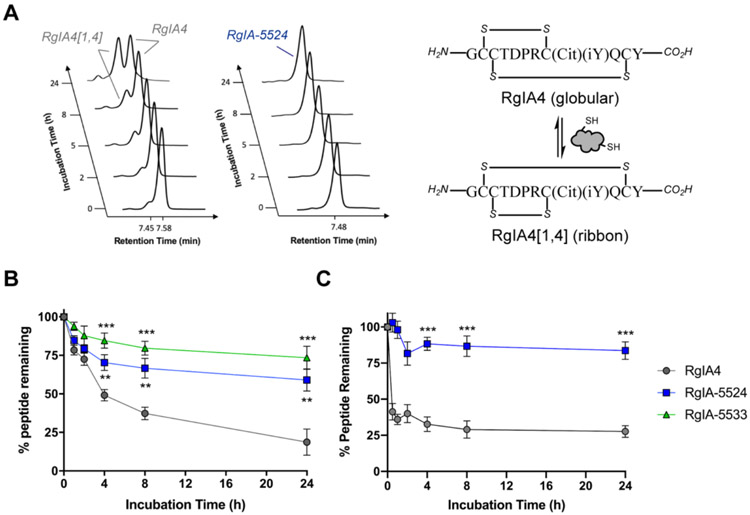
Generally, disulfide-folded peptides and proteins have rigid structures which result in relatively enhanced stability against proteases. However, free reducing thiols in human serum can interfere with the disulfide connectivity of cysteine-rich peptides by scrambling and thus lead to enzymatic degradation and potency loss.71 To determine how methylene thioacetal influenced the metabolic stability of RgIA4, in vitro human serum stability assay of RgIA-5524 and RgIA-5533 were performed. Peptides (0.1 mg/mL in 90% human serum AB type) were continuously incubated in human serum at 37 °C for 24 h and the amount of remaining peptide was determined by RP-HPLC at time point 0, 1, 2, 4, 8 and 24 h post-incubation. As shown in Figure 7A, RgIA4 scrambled rapidly into its isomer RgIA4[1,4] and ended up with less than 25% of the globular RgIA4, which is consistent with our previous observations.70 RgIA-5533 was significantly more stable than RgIA4 where above 70% of the peptide was intact even after 24 h incubation. RgIA-5524 was slightly less stable compared with RgIA-5533 possibly due to its higher arginine-rich sequence which can be cleaved by trypsin (Figure 7B). More importantly, complete disulfide scrambling inhibition was achieved when methylene thioacetal was introduced. We also assessed the stability of RgIA-5524 against RgIA4 in the presence of reduced glutathione (GSH) at physiological pH. Similar to human serum degradation results, single methylene thioacetal replacement in RgIA-5524 was able to largely suppress disulfide scrambling (Figure 7C). The concentration of RgIA4 was rapidly reduced to less than 50% of the initial level; after 8h, the concentration remained nearly constant which may represent equilibration between isomers under such reducing conditions.39 Overall, RgIA-5524 exhibited significantly improved stability which makes it a more attractive and promising candidate for further development.
Figure 7.
RgIA-5524 shows greatly improved stability compared with RgIA4. (A) Complete disulfide scrambling prevention was observed indicated by HPLC traces at certain time points post peptide incubation in 90% human serum at 37 °C. The front peak in left panel is the scrambled ribbon isomer RgIA4[1,4]. (B) Stability assay of RgIA-5524 and RgIA-5533 vs. RgIA4 in human serum. Peptides were incubated in 90% human serum AB type (0.1 mg/mL) at 37 °C. (C) Reductive stability assay of RgIA-5524 vs. RgIA4. Peptide samples were dissolved at 0.1 mg/mL in the presence of reduced GSH (10 equiv.) in pH 7.4 PBS and incubated at 37 °C. Statistical evaluations of the data were performed by student t (unpaired) test. All results are expressed as means ± SD (n = 3), **P<0.01, ***P < 0.001.
Conclusion
We report the design, synthesis, and pharmacological characterization of a suite of α-CTx RgIA analogues with the native disulfide bonds replaced by methylene thioacetal, which provided stabilized ligands targeting human α9α10 nAChRs as potential non-opioid based analgesic agents for the treatment of neuropathic pain. We identified RgIA-5524 as a highly selective and potent candidate which relieved chemotherapy-induced neuropathic pain via block of α9-containing nAChRs. Notably, RgIA-5524 demonstrated enhanced stability as well as complete disulfide scrambling suppression. This works also highlighted the power of methylene thioacetal as a native disulfide surrogate in disulfide-rich peptide stabilization. RgIA-5524 thus represents an advanced template for the development of peptidic non-opioid analgesics.
Experimental Section
Materials.
Chemicals. All chemicals were purchased and used directly without further purification. Fmoc-protected amino acids and reagents were purchased from Chemimpex, Thermal Fischer and Sigma Aldrich. Oocytes. Xenopus leavis frog oocytes used for two-electrode voltage clamp experiments were purchased from Xenopus One; Mice. CBA/CaJ inbred strain mice (2-3 weeks, male) used for in vivo assays were obtained from Jackson Laboratory.
Conotoxin Analogue Synthesis.
Solid-Phase Peptide Synthesis. Linear peptides were synthesized by using automated Fmoc-SPPS chemistry on synthesizer (Syzo I) as previously described using 2-CTC resin.70 Cleavage and Purification. Peptides were cleaved off from resin by treatment with a cocktail buffer (TFA:H2O:TIPS:EDT = 95:2:2:1, 3.0 mL/0.1 mmol) for 2.5 h. The obtained Peptide-TFA solution was then filtered via plastic filter and precipitated out into cold ether (40 mL) and cooled at −20 °C for 30 min before pelleted by centrifugation. The crude peptide was washed with cold ether (30 mL) to remove residue TFA and dried in vacuum. The crude product was then purified by RP-HPLC performed on Jupiter 5 μ C18 300 Å (250 x 10 mm) column at 3.0 mL/min with a H2O/ACN gradient containing 0.1% TFA from 5% to 45% ACN over 40 minutes on an Agilent 1260 HPLC system. The purified fractions containing targeted product were collected and lyophilized by Freeze Dryer (Labconco). LC/MS Analysis. Characterization of peptides was performed by LC/MS on a Phenomenex Gemini C18 3.0 μm (110 Å 150 x 3 mm) column at 0.4 mL/min flow speed with a H2O/ACN gradient containing 0.1% formic acid on Agilent 1260 Quadrupole LC/MS system. HPLC purification fractions, purity check for final products, stability assays were also analyzed by LC/MS. Methylene Thioacetal Formation. The reaction was carried out using a protocol reported by Cramer.55 The purified linear peptide was dissolved in dd H2O (6.4 mM) and treated with a pre-mixed TCEP·HCl (2.0 eq.) and K2CO3 (4.0 eq.) in H2O (19.0 mM). The mixture was gently stirred at room temperature for 2 h. Then Et3N (10.0 eq. 380 mM in THF) was added to the mixture followed by CH2I2 (6.0 eq. 230 mM dissolved in THF). This mixture was allowed to react at room temperature until a complete conversion of linear peptide in about 6 h (Note: Longer reaction time may lead to broad peak on RP-HPLC which is probably caused by amino acid racemization; 5% DMSO could be added in large-scale preparation). I2 Mediated Disulfide Formation. To the stirred bis-Acm-protected peptide solution in AcOH (aq. 25%, 1.0 mM) was added I2 (10.0 eq.) in AcOH (5.0 mg/mL). The reaction was stirred at room temperature for 10 min and monitored by LC-MS. The excess I2 was quenched by adding 1.0 M solution of ascorbic acid untill colorless and the mixture was then purified by RP-HPLC to afford peptides. All fully folded peptides were identified as ≥ 95% purity by RP-HPLC before NMR analysis and biological assays. Peptide Characterization. Molecular weights were measured by ESI-MS [M+H]+ and [M+2H]2+, RgIA-5617:Calc 1705.6 853.3, Found 1705.4 853.4; RgIA-5533: Calc 1705.6 853.3, Found 1705.4 853.4; RgIA-5618, Calc 1719.7 860.4, Found 1719.6 860.4; RgIA-5524, Calc 937.9, Found 937.5. MALDI-TOF [M+H]+ Calc 1873.5886, Found 1873.5533; RgIA-5573, Calc 1768.8 884.9, Found 1768.5 884.9.
Two-Electrode Voltage-Clamp (TEVC) Recording.
We followed a standard method as previously described.30, 69 Briefly, Xenopus laevis oocytes were used to express cloned human nAChR subtypes heterologously. For all of nAChRs, oocytes were injected with a 1:1 ratio (15-25 ng in equal parts) of each subunit cRNA. For the homologous human α7 nAChRs, oocytes were injected with 50 ng of α7 encoding cRNA. Recordings were performed 1-3 days postinjection. Oocytes were voltage-clamped at a membrane potential of −70 mV in a 30 μL oocyte chamber which was gravity perfused at a flowrate of 2-4 mL/min with ND-96 buffer containing 0.1 mg/mL BSA. A 1 s ACh (100 μM for all subtypes, with the exception of 200 μM for α7 and 10 μM for the muscle subtype) pulse per minute was applied to establish a baseline. Then ND96 solution containing the various concentrations of test peptides was switched and the ACh responses were measured until a steady state reached. All recordings were generated at room temperature and repeated as 3-6 independent experiments. Data analyses were performed with GraphPad Prism software and values including the resulting IC50 were calculated using a nonlinear regression sigmoidal dose-response.
In Vitro Pharmacology Assays.
Generally, RgIA-5524 was initially tested in quadruplicate at a default concentration of 10 μM in primary assays. A secondary assay was performed to determine a concentration-response curve when RgIA-5524 blocked higher than 50% of the radioligand binding. Competition Binding and Enzyme Assays. Cell membrane homogenates were incubated with the radioligand in the absence or presence of RgIA-5524. Nonspecific binding was determined in the presence of a specific agonist or antagonist at the target. Following incubation, the samples were filtered rapidly under vacuum through glass fiber filters presoaked in a buffer and rinsed several times with an ice-cold buffer using a 48-sample or 96-sample cell harvester. The filters were then counted for radioactivity in a scintillation counter using a scintillation cocktail. hERG K+ Channel Inhibition Assay. Automated whole cell patch-clamp (Qpatch 16) on human hERG transfected CHO-K1 cells was used to record outward potassium currents. After whole cell configuration was achieved at 22 °C, the cell was held at −80 mV. A 50 ms pulse to −40 mV was delivered to measure the leaking current. Then the cell is depolarized to +20 mV for 2 s followed by a 1 s pulse to −40 mV to reveal the hERG K+ tail current. This paradigm is delivered every 5 s to monitor current amplitude. The exocellular solution is applied first followed by RgIA-5524 solution sequentially on the same cell. E-4031 was tested as reference ligand. Functional Study of RgIA-5524 at GABAB1b Receptors (performed by Eurofins Cerep, France). RBL cells expressing human GABAB1b receptors were suspended in DMEM buffer and then distributed in microplates. Fluo4 NW mixed with probenicid in HBSS buffer complemented with 20 mM Hepes (pH 7.4) was then added into each well and equilibrated with the cells for 60 min at 37 °C then 15 min at 22 °C. For testing of agonist effect, the assay plates were positioned in a microplate reader, which was used for the addition of HBSS buffer (basal control), the reference agonist 3-APMPA at 100 μM (stimulated control) or various concentrations (EC50 determination) or RgIA-5524. For testing of antagonist effect, HBSS, CGP55845A at various concentrations (reference antagonist) or RgIA-5524 was added; then 5 min later, 3000 nM 3-APMPA (agonist reference compound, EC80) or HBSS buffer (basal control) was added. For both agonist and antagonist assays, the changes in fluorescence intensity, which varies proportionally to the free cytosolic Ca2+ ion concentration, was calculated as % of control response. CYP Enzyme Isoform Inhibition Assays. RgIA-5524 is pre-incubated with NADPH-generating system in PBS 7.4 for 5 min in a 37 °C dry incubator. The reaction is initiated by adding a mixture of a CYP enzyme isoform, a substrate, and BSA. The fluorescence in each well is read before and after the incubation period. The percent inhibition is calculated by subtracting the percent of control.
In Vivo Antinociceptive Activity Evaluation.
Neuropathic Pain Model. All experimental procedures on animals were performed in accordance with the NIH guidelines for the care and use of laboratory animals and were performed under Institutional Animal Care and Use Committees’ (IACUC) approved protocols at the University of Utah. All efforts were made to minimize suffering. Male CBA/CaJ mice (2-3 months old,) were injected with oxaliplatin. For the chronic administration group, oxaliplatin was administered i.p., at 3.5 mg/kg 5 days per week over a period of 21 days. For acute administration groups either 5.0 mg/kg oxaliplatin or 10.0 mg/kg oxaliplatin was given as a single dose. 0.9% Saline was used as vehicle control. Cold Plate Test. A cold plate test was performed using a hot/cold plate (IITC Life Science). Mice were allowed to acclimatize to the testing chamber until investigative behavior subsided. Then the plate temperature lowered from room temperature using a linear ramp (10 °C/min). The time and temperature of the first pain-related behavior (lifting and licking of the hind paw) were recorded. Raters were blind to drug and mouse genotype. Statistical evaluations of the data were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. All results are expressed as means ± SEM (n = 8-12). P values were *P <0.05, **P <0.01, and ***P < 0.001 for significant difference.
Structural Analysis.
NMR Spectroscopy. Peptide samples (prepared at concentration of 2.0 mM dissolved in a 10% D2O containing buffer pH 3.5 with 20 mM Na2HPO4, 50 mM NaCl, 50 μM NaN3, and 0.1 mM EDTA, uncorrected for isotope effects) were recorded at 298 K on an Inova 600 MHz spectrometer. Secondary structure determination was achieved using TOCSY (80 ms), NOESY (200 ms), g11-NOESY, gCOSY, and HSQC. Excitation sculpting schemes were used for water suppression. Spectra were analyzed using NMRPipe and SPARKY Structure Calculations. Three dimensional structures were calculated from the two-dimensional spectra using CYANA 3.0 with backbone dihedral angle constrains predicted by TALOS program. Non-canonical amino acids (L-citrulline, L-3-iodo-tyrosine, L-S-methylene-cysteine and L-β-homotyrosine) were built based on their corresponding natural amino acids using CYANA 3.0 as previously described.69 The 20 lowest-energy ensembles out of total 200 calculated structures were chosen for further analysis of the Cα distance measurement. Molecular representations were prepared using PyMOL program.
Stability Assays.
Testing peptides were dissolved in PBS 7.4 at concentration of 1.0 mg/mL for stock solution and were further diluted with either human serum (AB type, Sigma-Aldrich), or PBS 7.4 containing reduced glutathione (10 equiv.) to a final testing peptide concentration of 0.1 mg/mL. Then the diluted solutions were incubated at 37 °C and portions of the mixture was taken up at predetermined time points for RP-HPLC analysis. Serum protein were removed by denaturation with addition of equal volume of ACN, cooled on ice for 10 min and followed by centrifugation at 13,000 g for 10 min. The supernatant was collected and analyzed by RP-HPLC. The stability at each time point was calculated as the area of the treated peptide peaks (220 nm) on RP-HPLC as percentage of the area of the 0 h treated peptides. Each experiment was performed in triplicate. Data were analyzed by student t (unpaired) test. P values were **P < 0.01, ***P < 0.001 for significant difference at each time point.
Supplementary Material
Acknowledgements
We would like to acknowledge the support of Dr. David White and NIDA’s Addiction Treatment Discovery Program for data provided for use in this manuscript. We thank Prof. Eric Schmidt and Alan Blakely for helpful discussions. This research was supported financially by the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-17-1-0413 (to J. M. M and D. H.-C. C.), National Institutes of Health GM136430 (to J. M. M.) and National Institute of General Medical Science GM125001 (to D. H.-C. C.).
Abbreviations
- ACh
acetylcholine
- Acm
acetamidomethyl
- ACN
acetonitrile
- CTxs
conotoxins
- 2-CTC
2-chlorotrityl chloride
- DCM
dichloromethane
- DIEA
diisopropylethylamine
- EDT
1,2-ethanedithiol
- ESI-MS
electrospray ionization mass spectrometry
- FA
formic acid
- HATU
O-benzotriazole-N,N,N’,N’-tetramethyluronium hexafluorophosphate
- IC50
half-maximal inhibitory concentration
- i.p.
intraperitoneal
- nAChRs
nicotinic acetylcholine receptors
- NMR
nuclear magnetic resonance
- Ox
Oxaliplatin
- RP-HPLC
reversed-phase high performance liquid chromatography
- Sal
Saline
- s.c.
subcutaneous injection
- SPPS
solid-phase peptide synthesis
- TCEP·HCl
tris(2-carboxyethyl)phosphine hydrochloride
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TIPS
triisopropylsilane
- Trt
trityl
Footnotes
Conflicts of Interest
Patent application has been filed by the University of Utah for peptides described herein. N. Z., J. M. M. and D. H.-C. C are listed as inventors.
Supporting Information
Supporting Figures and Tables; LC-chromatogram and MS-spectrum for purified peptides; NMR chemical shift assignments; NMR solution structure ensemble; NMR spectra of compounds (PDF)
Twenty lowest energy states ensemble of NMR solution structures for RgIA-5533 (PDB), RgIA-5617(PDB) and RgIA-5524 (PDB).
References
- 1.Colloca L; Ludman T; Bouhassira D; Baron R; Dickenson AH; Yarnitsky D; Freeman R; Truini A; Attal N; Finnerup NB; Eccleston C; Kalso E; Bennett DL; Dworkin RH; Raja SN Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murnion BP Neuropathic Pain: Current Definition and Review of Drug Treatment. Aust. Prescr 2018, 41, 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady KT; McCauley JL; Back SE Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am. J. Psychiatry 2016, 173, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke DS, Forecasting the Opioid Epidemic. Science. 2016, 354, 529. [DOI] [PubMed] [Google Scholar]
- 5.Skolnick P The Opioid Epidemic: Crisis and Solutions. Annu. Rev. Pharmacol. Toxicol 2018, 58, 143–159. [DOI] [PubMed] [Google Scholar]
- 6.Wu LJ; Zhuo M Targeting the NMDA Receptor Subunit NR2B for the Treatment of Neuropathic Pain. Neurotherapeutics. 2009, 6, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagal SK; Brown AD; Cox PJ; Omoto K; Owen RM; Pryde DC; Sidders B; Skerratt SE; Stevens EB; Storer RI; Swain NA Ion Channels as Therapeutic Targets: A Drug Discovery Perspective. J. Med. Chem 2013, 56, 593–624. [DOI] [PubMed] [Google Scholar]
- 8.de Lera Ruiz M; Kraus RL Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem 2015, 58, 7093–7118. [DOI] [PubMed] [Google Scholar]
- 9.Mulcahy JV; Pajouhesh H; Beckley JT; Delwig A; Du Bois J; Hunter JC Challenges and Opportunities for Therapeutics Targeting the Voltage-Gated Sodium Channel Isoform Nav1.7. J. Med. Chem 2019, 62, 8695–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szallasi A; Cortright DN; Blum CA; Eid SR The Vanilloid Receptor TRPV1: 10 Years from Channel Cloning to Antagonist Proof-of-Concept. Nat. Rev. Drug. Discov 2007, 6, 357–372. [DOI] [PubMed] [Google Scholar]
- 11.Albuquerque EX; Pereira EF; Alkondon M; Rogers SW Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev 2009, 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson AJ; Lester HA; Lummis SC The Structural Basis of Function in Cys-loop Receptors. Q. Rev. Biophys 2010, 43, 449–499. [DOI] [PubMed] [Google Scholar]
- 13.Anand R; Conroy WG; Schoepfer R; Whiting P; Lindstrom J Neuronal Nicotinic Acetylcholine Receptors Expressed in Xenopus Oocytes have a Pentameric Quaternary Structure. J. Biol. Chem 1991, 266, 11192–11198. [PubMed] [Google Scholar]
- 14.Umana IC; Daniele CA; McGehee DS Neuronal Nicotinic Receptors as Analgesic Targets: It's a Winding Road. Biochem. Pharmacol 2013, 86, 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincler M Neuronal Nicotinic Receptors as Targets for Novel Analgesics. Expert. Opin. Inv Drugs 2005, 14, 1191–1198. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh JM; Absalom N; Chebib M; Elgoyhen AB; Vincler M α9 Nicotinic Acetylcholine Receptors and the Treatment of Pain. Biochem. Pharmacol 2009, 78, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hone AJ; Servent D; McIntosh JM α9-Containing Nicotinic Acetylcholine Receptors and the Modulation of Pain. Br. J. Pharmacol 2018, 175, 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X; Tae H-S; Chu Y; Jiang T; Adams DJ; Yu R Medicinal Chemistry, Pharmacology, and Therapeutic Potential of α-Conotoxins Antagonizing the α9α10 Nicotinic Acetylcholine Receptor. Pharmacol. Therapeut 2021, 222, 107792. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues T; Reker D; Schneider P; Schneider G Counting on Natural Products for Drug Design. Nat. Chem 2016, 8, 531–541. [DOI] [PubMed] [Google Scholar]
- 20.Patridge E; Gareiss P; Kinch MS; Hoyer D An Analysis of FDA-approved Drugs: Natural Products and Their Derivatives. Drug. Discov. Today 2016, 21, 204–207. [DOI] [PubMed] [Google Scholar]
- 21.Bjorn-Yoshimoto WE; Ramiro IBL; Yandell M; McIntosh JM; Olivera BM; Ellgaard L; Safavi-Hemami H Curses or Cures: A Review of the Numerous Benefits Versus the Biosecurity Concerns of Conotoxin Research. Biomedicines. 2020, 8, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanford M Intrathecal Ziconotide: A Review of its Use in Patients with Chronic Pain Refractory to Other Systemic or Intrathecal Analgesics. CNS Drugs. 2013, 27, 989–1002. [DOI] [PubMed] [Google Scholar]
- 23.Wulff H; Christophersen P; Colussi P; Chandy KG; Yarov-Yarovoy V Antibodies and Venom Peptides: New Modalities for Ion Channels. Nat. Rev. Drug. Discov 2019, 18, 339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safavi-Hemami H; Brogan SE; Olivera BM Pain Therapeutics from Cone Snail Venoms: From Ziconotide to Novel Non-opioid Pathways. J. Proteomics 2019, 190, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Layer RT; McIntosh JM Conotoxins: Therapeutic Potential and Application. Mar. Drugs 2006, 4, 119–142. [Google Scholar]
- 26.Livett BG; Sandall DW; Keays D; Down J; Gayler KR; Satkunanathan N; Khalil Z Therapeutic Applications of Conotoxins that Target the Neuronal Nicotinic Acetylcholine Receptor. Toxicon. 2006, 48, 810–829. [DOI] [PubMed] [Google Scholar]
- 27.Akondi KB; Muttenthaler M; Dutertre S; Kaas Q; Craik DJ; Lewis RJ; Alewood PF Discovery, Synthesis, and Structure-Activity Relationships of Conotoxins. Chem. Rev 2014, 114, 5815–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin AH; Muttenthaler M; Dutertre S; Himaya SWA; Kaas Q; Craik DJ; Lewis RJ;Alewood PF Conotoxins: Chemistry and Biology. Chem. Rev 2019, 119, 11510–11549. [DOI] [PubMed] [Google Scholar]
- 29.Cai F; Xu N; Liu Z; Ding R; Yu S; Dong M; Wang S; Shen J; Tae HS; Adams DJ; Zhang X; Dai Q, Targeting of N-Type Calcium Channels via GABAB-Receptor Activation by α-Conotoxin Vc1.1 Variants Displaying Improved Analgesic Activity. J. Med. Chem 2018, 61, 10198–10205. [DOI] [PubMed] [Google Scholar]
- 30.Romero HK; Christensen SB; Di Cesare Mannelli L; Gajewiak J; Ramachandra R; Elmslie KS; Vetter DE; Ghelardini C; Iadonato SP; Mercado JL; Olivera BM; McIntosh JM Inhibition of α9α10 Nicotinic Acetylcholine Receptors Prevents Chemotherapy-induced Neuropathic Pain. Proc. Natl. Acad. Sci. U. S. A 2017, 114, E1825–E1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen SB; Hone AJ; Roux I; Kniazeff J; Pin JP; Upert G; Servent D; Glowatzki E; McIntosh JM RgIA4 Potently Blocks Mouse α9α10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front. Cell. Neurosci 2017, 11, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huynh PN; Giuvelis D; Christensen S; Tucker KL; McIntosh JM RgIA4 Accelerates Recovery from Paclitaxel-Induced Neuropathic Pain in Rats. Mar. Drugs 2019, 18, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellison M; Haberlandt C; Gomez-Casati ME; Watkins M; Elgoyhen AB; McIntosh JM; Olivera BM α-RgIA: A Novel Conotoxin that Specifically and Potently Blocks the α9α10 nAChR. Biochemistry. 2006, 45, 1511–1517. [DOI] [PubMed] [Google Scholar]
- 34.Bulaj G; Olivera BM Folding of Conotoxins: Formation of the Native Disulfide Bridges during Chemical Synthesis and Biosynthesis of Conus Peptides. Antioxid. Redox. Signal 2008, 10, 141–155. [DOI] [PubMed] [Google Scholar]
- 35.Gori A; Gagni P; Rinaldi S Disulfide Bond Mimetics: Strategies and Challenges. Chem. Eur. J 2017, 23, 14987–14995. [DOI] [PubMed] [Google Scholar]
- 36.Chhabra S; Belgi A; Bartels P; van Lierop BJ; Robinson SD; Kompella SN; Hung A; Callaghan BP; Adams DJ; Robinson AJ; Norton RS Dicarba Analogues of α-Conotoxin RgIA. Structure, Stability, and Activity at Potential Pain Targets. J. Med. Chem 2014, 57, 9933–9944. [DOI] [PubMed] [Google Scholar]
- 37.MacRaild CA; Illesinghe J; van Lierop BJ; Townsend AL; Chebib M; Livett BG; Robinson AJ; Norton RS Structure and Activity of (2,8)-dicarba-(3,12)-cystino α-ImI, An α-Conotoxin Containing a Nonreducible Cystine Analogue. J. Med. Chem 2009, 52, 755–762. [DOI] [PubMed] [Google Scholar]
- 38.Belgi A; Burnley JV; MacRaild CA; Chhabra S; Elnahriry KA; Robinson SD; Gooding SG; Tae H-S; Bartels P; Sadeghi M/; Zhao F-Y; Wei H; Spanswick D; Adams DJ; Norton RS; Robinson AJ Alkyne-Bridged α-Conotoxin Vc1.1 Potently Reverses Mechanical Allodynia in Neuropathic Pain Models. J. Med. Chem 2021, 64, 3222–3233. [DOI] [PubMed] [Google Scholar]
- 39.Qu Q; Gao S; Wu F; Zhang MG; Li Y; Zhang LH; Bierer D; Tian CL; Zheng JS; Liu L Synthesis of Disulfide Surrogate Peptides Incorporating Large-Span Surrogate Bridges Through a Native-Chemical-Ligation-Assisted Diaminodiacid Strategy. Angew. Chem., Int. Ed 2020, 59, 6037–6045. [DOI] [PubMed] [Google Scholar]
- 40.Zhao R; Shi P; Chen J; Sun S; Chen J; Cui J; Wu F; Fang G; Tian C; Shi J; Bierer D; Liu L; Li Y-M Chemical Synthesis and Biological Activity of Peptides Incorporating an Ether Bridge as a Surrogate for a Disulfide Bond. Chem. Sci 2020, 11, 7927–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekan Z; Vetter I; Daly NL; Craik DJ; Lewis RJ; Alewood PF α-Conotoxin ImI Incorporating Stable Cystathionine Bridges Maintains Full Potency and Identical Three-dimensional Structure. J. Am. Chem. Soc 2011, 133, 15866–15869. [DOI] [PubMed] [Google Scholar]
- 42.Muttenthaler M; Nevin ST; Grishin AA; Ngo ST; Choy PT; Daly NL; Hu SH; Armishaw CJ; Wang CI; Lewis RJ; Martin JL; Noakes PG; Craik DJ; Adams DJ; Alewood PF Solving the α-Conotoxin Folding Problem: Efficient Selenium-directed On-resin Generation of More Potent and Stable Nicotinic Acetylcholine Receptor Antagonists. J. Am. Chem. Soc 2010, 132, 3514–3522. [DOI] [PubMed] [Google Scholar]
- 43.Walewska A; Zhang MM; Skalicky JJ; Yoshikami D; Olivera BM; Bulaj G Integrated Oxidative Folding of Cysteine/Selenocysteine Containing Peptides: Improving Chemical Synthesis of Conotoxins. Angew. Chem., Int. Ed 2009, 48, 2221–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knuhtsen A; Whitmore C; McWhinnie FS; McDougall L; Whiting R; Smith BO; Timperley CM; Green AC; Kinnear KI; Jamieson AG α-Conotoxin GI Triazole-peptidomimetics: Potent and Stable Blockers of a Human Acetylcholine Receptor. Chem. Sci 2019, 10, 1671–1676. [Google Scholar]
- 45.Gori A; Wang CI; Harvey PJ; Rosengren KJ; Bhola RF; Gelmi ML; Longhi R; Christie MJ; Lewis RJ; Alewood PF; Brust A Stabilization of the Cysteine-rich Conotoxin MrIA by Using a 1,2,3-Triazole as a Disulfide Bond Mimetic. Angew. Chem., Int. Ed 2015, 54, 1361–1364. [DOI] [PubMed] [Google Scholar]
- 46.Gajewiak J; Christensen SB; Dowell C; Hararah F; Fisher F; Huynh PN; Olivera BM; McIntosh JM Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide that Acts Through a Non-Opioid Based Mechanism. J. Med. Chem 2021, in press (doi.XXXX). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato AK; Viswanathan M; Kent RB; Wood CR Therapeutic Peptides: Technological Advances Driving Peptides into Development. Curr. Opin. Biotechnol 2006, 17, 638–642. [DOI] [PubMed] [Google Scholar]
- 48.Wang CK; Craik DJ Designing Macrocyclic Disulfide-rich Peptides for Biotechnological Applications. Nat. Chem. Biol 2018, 14, 417–427. [DOI] [PubMed] [Google Scholar]
- 49.Armishaw CJ; Jensen AA; Balle LD; Scott KC; Sorensen L; Stromgaard K Improving the Stability of α-Conotoxin AuIB through N-to-C Cyclization: the Effect of Linker Length on Stability and Activity at Nicotinic Acetylcholine Receptors. Antioxid. Redox. Signal 2011, 14, 65–76. [DOI] [PubMed] [Google Scholar]
- 50.Giribaldi J; Haufe Y; Evans ERJ; Amar M; Durner A; Schmidt C; Faucherre A; Moha Ou Maati H; Enjalbal C; Molgo J; Servent D; Wilson DT; Daly NL; Nicke A; Dutertre S Backbone Cyclization Turns a Venom Peptide into a Stable and Equipotent Ligand at Both Muscle and Neuronal Nicotinic Receptors. J. Med. Chem 2020, 63, 12682–12692. [DOI] [PubMed] [Google Scholar]
- 51.Clark RJ; Fischer H; Dempster L; Daly NL; Rosengren KJ; Nevin ST; Meunier FA; Adams DJ; Craik DJ Engineering Stable Peptide Toxins by Means of Backbone Cyclization: Stabilization of the α-Conotoxin MII. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 13767–13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halai R; Callaghan B; Daly NL; Clark RJ; Adams DJ; Craik DJ Effects of Cyclization on Stability, Structure, and Activity of α-Conotoxin RgIA at the α9α10 Nicotinic Acetylcholine Receptor and GABA(B) Receptor. J. Med. Chem 2011, 54, 6984–6992. [DOI] [PubMed] [Google Scholar]
- 53.Sadeghi M; Carstens BB; Callaghan BP; Daniel JT; Tae HS; O'Donnell T; Castro J; Brierley SM; Adams DJ; Craik DJ; Clark RJ Structure-Activity Studies Reveal the Molecular Basis for GABAB-Receptor Mediated Inhibition of High Voltage-Activated Calcium Channels by α-Conotoxin Vc1.1. ACS Chem. Biol 2018, 13, 1577–1587. [DOI] [PubMed] [Google Scholar]
- 54.Clark RJ; Jensen J; Nevin ST; Callaghan BP; Adams DJ; Craik DJ The Engineering of an Orally Active Conotoxin for the Treatment of Neuropathic Pain. Angew. Chem., Int. Ed 2010, 49, 6545–6548. [DOI] [PubMed] [Google Scholar]
- 55.Kourra C; Cramer N Converting Disulfide Bridges in Native Peptides to Stable Methylene Thioacetals. Chem. Sci 2016, 7, 7007–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mousa R; Lansky S; Shoham G; Metanis N BPTI Folding Revisited: Switching a Disulfide into Methylene Thioacetal Reveals a Previously Hidden Path. Chem. Sci 2018, 9, 4814–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer J-P; Schönauer R; Els-Heindl S; Bierer D; Koebberling J; Riedl B; Beck-Sickinger AG Adrenomedullin Disulfide Bond Mimetics Uncover Structural Requirements for AM1 Receptor Activation. J. Pept. Sci 2019, 25, e3147. [DOI] [PubMed] [Google Scholar]
- 58.Zheng N; Karra P; VandenBerg MA; Kim JH; Webber MJ; Holland WL; Chou DH, Synthesis and Characterization of an A6-A11 Methylene Thioacetal Human Insulin Analogue with Enhanced Stability. J. Med. Chem 2019, 62, 11437–11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callaghan B; Haythornthwaite A; Berecki G; Clark RJ; Craik DJ; Adams DJ Analgesic α-Conotoxins Vc1.1 and Rg1A Inhibit N-type Calcium Channels in Rat Sensory Neurons via GABAB Receptor Activation. J. Neurosci 2008, 28, 10943–10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuny H; de Faoite A; Huynh TG; Yasuda T; Berecki G; Adams DJ γ-Aminobutyric Acid Type B (GABAB) Receptor Expression is Needed for Inhibition of N-type (Cav2.2) Calcium Channels by Analgesic α-Conotoxins. J. Biol. Chem 2012, 287, 23948–23957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huynh TG; Cuny H; Slesinger PA; Adams DJ Novel Mechanism of Voltage-gated N-type (Cav2.2) Calcium Channel Inhibition Revealed through α-Conotoxin Vc1.1 activation of the GABAB Receptor. Mol. Pharmacol 2015, 87, 240–250. [DOI] [PubMed] [Google Scholar]
- 62.Luo S; Zhangsun D; Harvey PJ; Kaas Q; Wu Y; Zhu X; Hu Y; Li X; Tsetlin VI; Christensen S; Romero HK; McIntyre M; Dowell C; Baxter JC; Elmslie KS; Craik DJ; McIntosh JM Cloning, Synthesis, and Characterization of αO-Conotoxin GeXIVA, A Potent α9α10 Nicotinic Acetylcholine Receptor Antagonist. Proc. Natl. Acad. Sci. U. S. A 2015, 112, E4026–E4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huynh PN; Harvey PJ; Gajewiak J; Craik DJ; McIntosh JM Critical Residue Properties for Potency and Selectivity of α-Conotoxin RgIA Towards α9α10 Nicotinic Acetylcholine Receptors. Biochem. Pharmacol 2020, 181, 114124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabassum N; Tae HS; Jia X; Kaas Q; Jiang T; Adams DJ; Yu R Role of CysI-CysIII Disulfide Bond on the Structure and Activity of α-Conotoxins at Human Neuronal Nicotinic Acetylcholine Receptors. ACS Omega. 2017, 2, 4621–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamthanh H; Jegou-Matheron C; Servent D; Menez A; Lancelin JM Minimal Conformation of the α-Conotoxin ImI for the α7 Neuronal Nicotinic Acetylcholine Receptor Recognition: Correlated CD, NMR and Binding Studies. FEBS Lett. 1999, 454, 293–298. [DOI] [PubMed] [Google Scholar]
- 66.Staff NP; Grisold A; Grisold W; Windebank AJ Chemotherapy-induced Peripheral Neuropathy: A Current Review. Ann. Neurol 2017, 81, 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Noord C; Eijgelsheim M; Stricker BH Drug- and Non-drug-associated QT Interval Prolongation. Br. J. Clin. Pharmacol 2010, 70, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellison M; Feng ZP; Park AJ; Zhang X; Olivera BM; McIntosh JM; Norton RS α-RgIA, A Novel Conotoxin that Blocks the α9α10 nAChR: Structure and Identification of Key Receptor-binding Residues. J. Mol. Biol 2008, 377, 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zouridakis M; Papakyriakou A; Ivanov IA; Kasheverov IE; Tsetlin V; Tzartos S; Giastas P Crystal Structure of the Monomeric Extracellular Domain of α9 Nicotinic Receptor Subunit in Complex With α-Conotoxin RgIA: Molecular Dynamics Insights Into RgIA Binding to α9α10 Nicotinic Receptors. Front. Pharmacol 2019, 10, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng N; Christensen SB; Blakely A; Dowell C; Purushottam L; McIntosh JM; Chou DH Development of Conformationally Constrained α-RgIA Analogues as Stable Peptide Antagonists of Human α9α10 Nicotinic Acetylcholine Receptors. J. Med. Chem 2020, 63, 8380–8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gongora-Benitez M; Tulla-Puche J; Albericio F Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev 2014, 114, 901–926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.