Abstract
Purpose of Review:
Caring for a woman with epilepsy requires familiarity with the implications of antiepileptic drugs (AEDs) for pregnancy and contraception as well as an understanding of the effects of female hormones on epilepsy.
Recent Findings:
AED pregnancy registries and prospective studies of cognitive development continue to confirm that valproate poses a significantly increased risk of structural and cognitive teratogenesis. In contrast, data thus far suggest that lamotrigine and levetiracetam are associated with a relatively low risk for both anatomic and developmental adverse effects, although further studies are needed for these and other AEDs. The intrauterine device is a good contraceptive option for many women with epilepsy as it is highly effective and not subject to the drug-drug interactions seen between hormonal contraception and many AEDs. Hormonal-sensitive seizures are common among women with epilepsy; however, highly effective treatments for refractory catamenial seizures are limited.
Summary:
Women with epilepsy should be counseled early and regularly about reproductive health as it relates to epilepsy. AED selection for women of childbearing age should take future pregnancies and contraceptive needs into consideration.
INTRODUCTION
The stigma faced by patients with epilepsy carries over to their reproductive health choices: Sterilization of people with epilepsy was legal in parts of the United States until the latter half of the 20th century, and until 1980, some states still had laws forbidding people with epilepsy from marrying.1 Even today, despite substantial growth in our understanding of pregnancy and epilepsy, most of which is reassuring, many women with epilepsy still receive explicit or implicit messages that they should not consider pregnancy, which can deter them from addressing reproductive health with their health care providers. Women are often unfamiliar with the teratogenic risks of the antiepileptic drugs (AEDs) and their interactions with contraception, and many pregnancies in women with epilepsy are unplanned.2 Because of fears about the effects of epilepsy and AEDs, women may avoid pregnancy because of their epilepsy or may decrease or stop medications on their own when they do become pregnant.3 Thus it is critical for neurologists treating women with epilepsy to discuss the important topics of contraception and pregnancy early on in their relationship with their patients and revisit these discussions regularly, communicating that they are appropriate and important topics to discuss openly. Unfortunately, a minority of women report that conversations about contraception and pregnancy are initiated by their health care providers, and over one-third say these topics are not discussed at all.4 Additionally, many women notice a hormonal component to their epilepsy and may feel dismissed if this is not acknowledged by their health care providers. This article covers several of the topics important to women with epilepsy, including pregnancy, contraception, and catamenial epilepsy, and provides the reader with tools to integrate these topics into their clinical care of women with epilepsy.
PREGNANCY
Most women with epilepsy will need to remain on AEDs during pregnancy. While more information is needed on the risks related to seizures during pregnancy, tonic-clonic seizures expose the fetus to anoxia and increase the risk of maternal injury.5 Uterine contractions and fetal heart rate changes have been demonstrated during focal seizures with loss of consciousness.5 Seizures during pregnancy may also increase the risk of preterm labor and small-for-gestational-age infants.6 The UK confidential inquiry into maternal deaths demonstrated a tenfold increase in mortality among women with epilepsy during pregnancy and the postpartum period. Most of these deaths were attributed to sudden unexpected death in epilepsy (SUDEP), which underscores the need for medication compliance and therapeutic monitoring during pregnancy as well as careful prepartum counseling.7
While risks related to AED use in pregnancy exist, such as increased chances of structural and cognitive teratogenesis, these risks can be minimized by early prepartum counseling. Because the majority of pregnancies are unplanned, prepartum counseling should start no later than the prescription of the first AED to a woman of childbearing age, which is often well before the patient expresses an interest in becoming pregnant. Discussion points and recommendations for prepartum counseling and pregnancy are listed in Table 11-1.8
TABLE 11-1.
Management of Women With Epilepsy From Diagnosis Through Conceptiona
| ► At Diagnosis |
| Careful history including all seizure types,frequency,risk factors,family history of epilepsy and fetal malformations, reproductive history (eg, menarche,frequency of menses, prior pregnancies) |
| Consider alternative diagnoses such as nonepileptic seizures |
| Perform basic epilepsy evaluation including MRI and EEG |
| Consider inpatient monitoring prior to antiepileptic drugs (AEDs) in patients with atypical histories |
| Start the AED most appropriate for patient and with low risk of teratogenesis |
| Discuss available data on teratogenic risk and the AED chosen |
| Discuss risks of seizures and AED noncompliance in general and in regard to pregnancy |
| Start folic acid 0.4–4 mg/d along with AED Ensure adequate contraception |
| ► More Than 1 Year Preconception |
| Inpatient monitoring for all patients in whom seizures have not responded to AEDs |
| Consider epilepsy surgery in appropriate patients |
| Consider medication change in patients taking any of the following |
| AEDs associated with higher rates of teratogenesis, such as valproate, topiramate, and phenobarbital |
| AEDs without substantial information on associated teratogenesis |
| AEDs that are not controlling seizures |
| Consider a trial of AED withdrawal in appropriate patients who have been seizure free for 2–4 years |
| Decrease dose of AEDs in patients whose seizures have been well controlled |
| Establish therapeutic drug levels (ideally trough levels) at least 2 times a year |
| Discuss balance between risks of AEDs and risks of seizures during pregnancy |
| Discuss value of minimizing dose/level prior to pregnancy if possible and need to adjust dose during pregnancy |
| Continue folic acid |
| Ensure adequate contraception |
| ► Less Than 1 Year Preconception |
| Inpatient monitoring for all patients in whom seizures have not responded to AEDs |
| Consider AED adjustment only in cases where benefits clearly outweigh risks, keeping in mind that seizure control 9–12 months prior to conception is the best predictor of seizure control during pregnancy; advise use of contraception during AED changes |
| Check AED levels (ideally trough levels) several times and establish target level for pregnancy |
| Discuss genetic screening/counseling with appropriate patients |
| Establish a pregnancy plan with patient |
| Patient should notify doctors on first sign of pregnancy |
| Patient should be aware that AED levels will need to be checked more frequently throughout pregnancy, at least every 4 weeks |
| Decide which seizures types or AED levels will prompt medication adjustment during pregnancy and prepartum period |
| Start prenatal vitamin and continue folic acid 0.4–4 mg/d |
| Discuss social support, seizure safety in caring for infants, and need to avoid sleep deprivation |
| Discuss breast-feeding |
| ► Periconception and Postconception |
| Confirm patient is taking prenatal vitamin/folic acid |
| Establish/review pregnancy plan |
| Adjust or lower AEDs only in exceptional cases where benefits clearly outweigh risks |
| Discuss appropriate prenatal screening with patient and other physicians |
| Genetic counseling, if appropriate |
| Targeted anatomic ultrasound (“level II ultrasound”) at 20 weeks |
| Discuss social support, seizure safety in caring for infants, and need to avoid sleep deprivation |
| Discuss breast-feeding |
EEG = electroencephalogram; MRI = magnetic resonance imaging.
Modified with permission from Gerard EE, Cambridge University Press.8
Structural Teratogenesis
Pregnancies exposed to AEDs in the first trimester are at increased risk for major congenital malformations. Major congenital malformations are defined as structural abnormalities of surgical, functional, or cosmetic significance. The majority of these structural abnormalities have already occurred by 8 to 10 weeks of pregnancy, underscoring the need for early preconception planning. Over the past 2 decades, several international prospective registries have assessed the rates of major congenital malformations associated with various AEDs. Individual registries have different means of assessment and follow-up periods, which accounts for some of the interstudy variability.9 Overall, however, the registries have begun to paint a consistent picture of the major congenital malformation risk with some of the most commonly prescribed AEDs. Absolute malformation rates seen with individual monotherapy exposures are reported in Table 11-2.10-21
TABLE 11-2.
Major Congenital Malformation Rates
| Rate of Major Congenital |
||||
|---|---|---|---|---|
| Registry | Study | Carbamazepine | Gabapentin | Lamotrigine |
| Australian Pregnancy Registry | Vajda et al, 201410 | 5.5% (346) | 0% (14) | 4.6% (307) |
| Danish Registry | Mølgaard-Nielsen, Hviid, 201111 | NA | 1.7% (59) | 3.7% (1019) |
| International Registry of Antiepileptic Drugs and Pregnancy | Tomson et al, 201112 | 5.6% (1402) | NA | 2.9% (1280) |
| Finland National Birth Registry | Artama et al, 200513 | 2.7% (805) | NA | NA |
| GlaxoSmithKline Lamotrigine Registry | Cunnington et al, 201114 | NA | NA | 2.2% (1558) |
| North American AED Pregnancy Registry | Hernández-Díaz et al, 201215 | 3.0% (1033) | 0.7% (145) | 2.0% (1562) |
| Norwegian Medical Birth Registry | Veiby et al, 201416 | 2.9% (685) | NA | 3.4% (833) |
| Swedish Medical Birth Registry | Tomson, Battino, 201217 | 2.7% (1430) | 0% (18) | 2.9% (1100) |
| UK/Ireland pregnancy registry | Campbell et al, 201418 Mawhinney et al, 201319 Hunt et al, 200820 Morrow et al, 200621 | 2.6% (1657) | 3.2% (32) | 2.3% (2098) |
AED = antiepileptic drug; NA = not applicable.
When explaining the risk of major congenital malformations to women with epilepsy, it is important to explain that major congenital malformations can occur in healthy women. Rates of major congenital malformations range from 1% to 3%, depending on the population in which they are studied and how they are assessed. Across all registries, valproate has been consistently associated with the highest rates of major congenital malformations, ranging from 4.7% to 13.8%.9 Large cohorts have also outlined relatively low malformation rates with lamotrigine and carbamazepine exposure. Levetiracetam looks promising, although bigger cohorts are needed to confirm this early trend. Phenytoin, phenobarbital, and topiramate likely confer an intermediate risk of congenital malformations, whereas data on most other AEDs are too limited to stratify.9
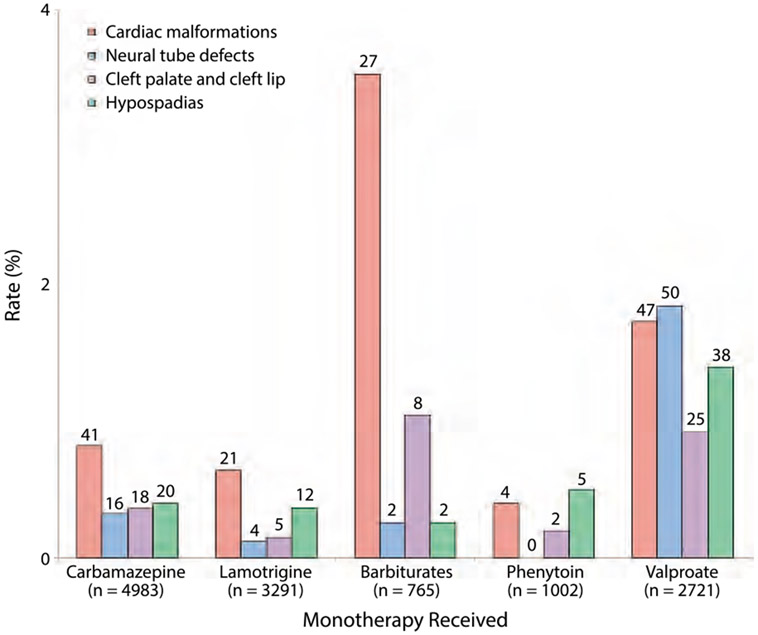
While in most studies malformations are presented as a single data point, major congenital malformations include a group of structural abnormalities ranging from surgically correctible hypospadias and oral clefts to more severe cardiac and neural tube defects. The pattern of malformation risk appears to be different for individual AEDs. In one compilation of the results from several pregnancy registries, neural tube defects were the most common major congenital malformation seen with valproate, while carbamazepine, lamotrigine, and barbiturates were most commonly associated with cardiac defects, and both hypospadias and cardiac defects were the most common malformations seen with phenytoin exposure (Figure 11-1).17 All the individual malformations (ie, cardiac, neural tube, oral clefts, and hypospadias) in this analysis, however, had higher rates for valproate than carbamazepine, lamotrigine, or phenytoin.
FIGURE 11-1.
Rates of several specific major congenital malformations associated with exposure to monotherapy with antiepileptic drugs. The number of fetuses with specific malformations are shown on top of the bars. The figure is based on combined data from 21 pregnancy registries. Note that the Y axis ranges from 0% to 4%. While it is important to explain the increased relative risks of major congenital malformations with certain drugs, it is also important to explain the difference between relative and absolute risk to patients.
Modified with permission from Tomson T, Battino D, Lancet Neurol.17 www.thelancet.com/journals/laneur/article/PIIS1474-4422(12)70103-5/abstract. ©2012 Elsevier Ltd.
In the United States, it is common practice to recommend a level II ultrasound at 18 to 20 weeks gestational age for women taking AEDs during pregnancy. This is a detailed anatomic evaluation, which provides very high sensitivity for structural abnormalities affecting the fetus (greater than 95% for neural tube defects in most laboratories).
Effect of dose.
For several AEDs, the risk of major congenital malformations has been shown to be dose related. The International Registry of Antiepileptic Drugs and Pregnancy (EURAP) reported a direct correlation between the dose at the time of conception and the risk of major congenital malformations for carbamazepine, lamotrigine, phenobarbital, and valproate. For valproate monotherapy, the risk of a major congenital malformation was 5.6% for preconception doses lower than 750 mg/d and 24.6% for doses higher than 1500 mg/d.12 Further research is needed to determine if serum concentrations, rather than dose, are a stronger prediction of teratogenic risk. In the meantime, part of preparing a woman with epilepsy for pregnancy involves trying to identify the minimum therapeutic dose (and corresponding level) that is able to control her seizures.
Polytherapy versus monotherapy.
It was previously thought that AED polytherapy should always be avoided during pregnancy if at all possible. This was based on prior studies that demonstrated a higher rate of major congenital malformations with polytherapy. A study from the North American Antiepileptic Drug Pregnancy Registry (NAAPR) suggested that polytherapy combinations that included valproate mostly drove prior data on polytherapy. The authors demonstrated that malformation rates seen with exposure to carbamazepine or lamotrigine in polytherapy with a drug other than valproate were similar to the rates seen with either drug in monotherapy, whereas if either was combined with valproate, malformation rates were much higher. The study also presented similar findings seen in two other registries.22 This raises the question of whether certain polytherapy combinations (eg, levetiracetam and lamotrigine) should be considered prior to initiating valproate for idiopathic or genetic generalized epilepsy, as illustrated in Case 11-1.
Case 11-1.
A 28-year-old woman with juvenile myoclonic epilepsy presented for prepartum counseling. She had a history of convulsive seizures and myoclonic jerks and had been on divalproex sodium extended-release (ER) 500 mg 2 times a day since age 14 with remission of both seizure types. A prior attempt to decrease divalproex sodium ER to 250 mg 2 times a day resulted in breakthrough convulsions. It was recommended that the patient stay on an antiepileptic drug (AED) through pregnancy but that switching to either levetiracetam or lamotrigine would minimize risk of teratogenesis. Levetiracetam was started, divalproex sodium was weaned, and the patient started on a daily prenatal vitamin as well as folic acid 1 mg daily. After a few weeks on levetiracetam 750 mg 2 times a day, the patient reported severe depressive symptoms. Lowering the dose to 500 mg 2 times a day resulted in a recurrence of myoclonic jerks and not much improvement in her mood. At this point, the patient was started on lamotrigine with a plan to wean levetiracetam. When the patient reached a lamotrigine level of 7 mcg/mL on a dose of 150 mg 2 times a day, levetiracetam was weaned. The patient felt emotionally better on the combination of lamotrigine 150 mg 2 times a day and levetiracetam 250 mg 2 times a day, but once levetiracetam was discontinued, she had daily myoclonus. Levetiracetam was resumed at 250 mg 2 times a day in addition to lamotrigine. She remained seizure free on this combination for 9 months before conceiving. Baseline levels of levetiracetam and lamotrigine were drawn to establish her target levels for pregnancy. She did well in pregnancy and the postpartum period with monthly monitoring and dose adjustment of both AEDs.
Comment.
This case illustrates the challenges involved in adjusting a woman’s AEDs prior to pregnancy. This can be a relatively long process, and it is important to observe a period of seizure freedom once the regimen is changed. Valproate should be avoided whenever possible in women planning pregnancy. If the patient has efficacy or tolerability issues with lamotrigine or levetiracetam, each of which are associated with lower teratogenic risks compared with valproate, the combination of the two drugs can be considered. New data suggest that teratogenic risk is not necessarily increased by polytherapy, especially if the polytherapy does not include valproate. Although the specific combination of levetiracetam and lamotrigine still needs to be studied, it is likely preferable to valproate monotherapy.
| Malformations With Individual Antiepileptic Drugs as Monotherapy (N = ) | |||||
|---|---|---|---|---|---|
| Levetiracetam | Oxcarbazepine | Phenobarbital | Phenytoin | Topiramate | Valproic Acid |
| 2.4% (82) | 5.9% (17) | 0% (4) | 2.4% (41) | 2.4% (42) | 13.8% (253) |
| 0% (58) | 2.8% (393) | NA | NA | 4.6% (108) | NA |
| 1.6% (126) | 3.3% (184) | 7.4% (217) | 5.8% (103) | 6.8% (73) | 9.7% (1010) |
| NA | NA | NA | NA | NA | 10.7% (263) |
| NA | NA | NA | NA | NA | NA |
| 2.4% (450) | 2.2% (182) | 5.5% (199) | 2.9% (416) | 4.2% (359) | 9.3% (323) |
| 1.7% (118) | 1.8% (57) | 7.4% (27) | NA | 4.2% (48) | 6.3% (333) |
| 0% (61) | 3.7% (27) | 14% (7) | 6.7% (119) | 7.7% (52) | 4.7% (619) |
| 0.7% (304) | NA | NA | 3.7% (82) | 9% (203) | 6.7% (1290) |
Cognitive Teratogenesis
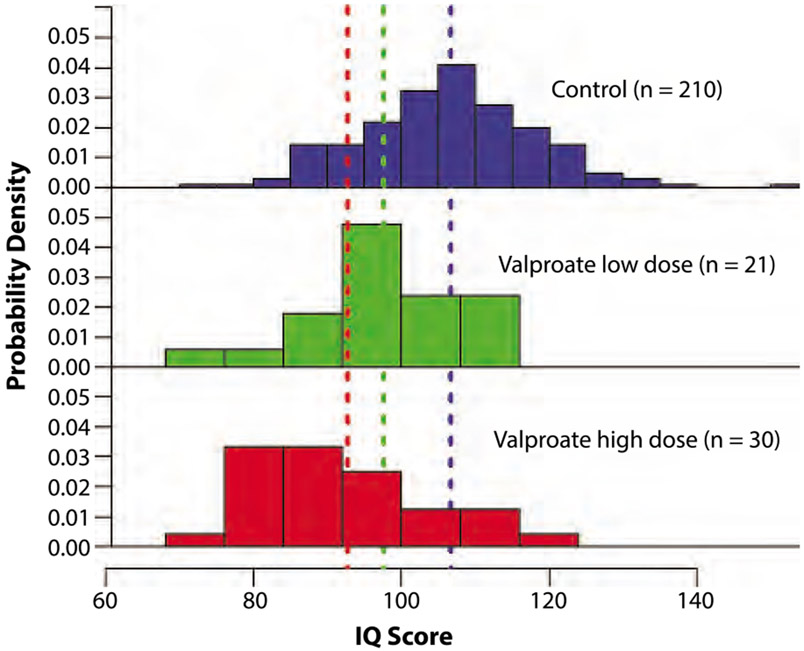
Until recently, much of the available data on cognitive development and AED exposure were based on case series and retrospective studies, most of which did not control for the contribution of maternal IQ, a critical factor in estimating a child’s IQ. Both the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study and a study from the Liverpool and Manchester Neurodevelopmental Group (LMNG) accounted for maternal IQ in addition to other important variables.23,24 Both studies were prospective, recruiting mothers antenatally and following their children for 6 years. Of note, 92 children from the LMNG cohort also participated in the NEAD study (making up approximately 30% of the NEAD sample). Each study confirmed the previously suspected adverse effects of valproate on cognitive outcomes: Exposure to valproate was associated with a decrease in full-scale IQ by approximately 10 points compared to children exposed to other AEDs and to a control group. This effect appears to be dose related (Figure 11-2).
FIGURE 11-2.
Distribution of full-scale IQ scores across the control and valproate-exposed groups in Liverpool and Manchester Neurodevelopmental Group (LMNG) prospective study of antiepileptic drug–exposed children at 6 years. Colored dashed lines represent the mean IQ for each group. The adjusted mean IQ score of children exposed to high-dose (more than 800 mg/d) valproate was significantly lower than that of controls (P<.0001), with an adjusted mean reduction of 9.7 points. The adjusted mean IQ score of the children exposed to low-dose (800 mg/d or less) valproate was also lower than that of controls, although the difference was not statistically significant (P=.09). This trend illustrates the dose-dependent effect of valproate on cognitive development.
Reprinted with permission from Baker G, et al, Neurology.24 www.neurology.org/content/84/4/382.short. © 2014 American Academy of Neurology.
The cognitive effects of other AEDs require further investigation in order to make firm conclusions about their developmental risk. In the NEAD study, which did not include a control group, IQs at age 6 were similar between children exposed to lamotrigine, phenytoin, or carbamazepine. The LMNG study included a control group and found that average full-scale IQs of the controls were similar to those of the lamotrigine- and carbamazepine-exposed children. In this study, however, carbamazepine exposure was associated with a higher risk of having an IQ lower than 85 and decreased verbal abilities.24
The cognitive effects of levetiracetam exposure have only been evaluated in one study by the LMNG, which reported that developmental scores of exposed children at 3 years were similar to controls but better than those of valproate-exposed children.25 In rodent models, levetiracetam also seems to be a promising agent. Several AEDs (eg, diazepam, phenobarbital, phenytoin, and valproate) have been shown to induce apoptosis in the brains of rat pups; this is thought to be one mechanism by which AED-induced cognitive changes occur.26 A few AEDs (ie, carbamazepine, lamotrigine, levetiracetam, and topiramate) do not produce apoptosis when given alone, but levetiracetam is the only AED that does not enhance apoptosis even when given with an AED that induces apoptosis.26
In addition to its effect on IQ, valproate has now been associated with adverse effects on behavioral development. In a population-based study, the risks of a formal diagnosis of autism or autism spectrum disorder with valproate exposure were 2.5% and 4.4%, respectively, compared to 0.5% and 1.5% in the general population.27
Obstetrical and Perinatal Outcomes
A 2014 population study demonstrated that women with epilepsy are not at increased risk for miscarriage.28 However, women with epilepsy may be at increased risk for obstetric complications, including gestational hypertension, preeclampsia, and postpartum hemorrhage. Preterm birth, intrauterine growth restriction, and small-for-gestational-age infants are also more common in women with epilepsy.29 Conflicting evidence exists on whether these complications are related to the effect of AEDs, seizures during pregnancy, or epilepsy itself.29 A Norwegian study found a particular association between topiramate and microcephaly as well as birth weight.16 The NAAPR also found an association between both topiramate exposure and zonisamide exposure and low birth weight.30 On the other hand, seizures during pregnancy have been shown to increase the risk of small-for-gestational-age infants and preterm delivery.6 Cesarean delivery may also be more common in women with epilepsy, but this is not a universal finding and the reasons for this association are unclear.29 Neither epilepsy nor AED use is usually an indication for a cesarean delivery.
Seizure Frequency During Pregnancy
Most women with epilepsy (54% to 80%) will not experience a change in the frequency of seizures during pregnancy. Seizure frequency increases in one-third or fewer of women with epilepsy (15.8% to 32%) and decreases in a minority of patients (3% to 24%).31-34 Seizure stability prior to pregnancy is one of the strongest predictors of seizure control during pregnancy31,35; women who are seizure free in the 9 months prior to pregnancy have an 84% to 92% chance of remaining seizure free during pregnancy on their current regimen.31 Patients with generalized epilepsy seem to have less of a risk of seizures during pregnancy than patients with focal seizures, although both groups are at increased risk of seizures in the peripartum and postpartum periods.31,33,35 One study has suggested that a history of catamenial epilepsy may reduce the risk of seizures during pregnancy.32 Refer to Appendix B for a summary of the American Academy of Neurology (AAN) evidence-based guideline for clinicians “Management Issues for Women With Epilepsy—Focus on Pregnancy: Obstetrical Complications and Change in Seizure Frequency.”
A 2014 study from the Australian Pregnancy Register (APR) suggested that seizure control during pregnancy might relate to the AED regimen used.36 The authors found that, among pregnancies managed with monotherapy, risk of seizures was lowest with valproate (27%), levetiracetam (31.8%), and carbamazepine (37.8%). A higher risk of seizures was seen with lamotrigine (51.3%). Phenytoin (51.2%) and topiramate (54.8%) were also associated with a higher risk of seizures; however, the sample sizes of these groups were small. EURAP also reported a higher risk of seizures in patients taking lamotrigine or oxcarbazepine.33,37 In contrast to the findings of the APR, one small study that compared seizure frequency in pregnancy to a woman’s 1-yearbaseline found a greater risk of increasing seizures in patients treated with levetiracetam monotherapy (47%) compared with lamotrigine monotherapy (38%)38 The NAAPR also published the rates of seizures during pregnancy, with levetiracetam having similar rates to lamotrigine.39
One possible explanation for the findings of the APR and EURAP is the variable metabolism of AEDs during pregnancy. For example, lamotrigine clearance depends heavily on glucuronidation, a process induced by the increases in estrogen during pregnancy. In the majority of women with epilepsy (77%), lamotrigine clearance increases by over 200% over the course of pregnancy.40 Lamotrigine doses need to be increased substantially over the course of a pregnancy to maintain prepregnancy levels. Doses of over 600 mg/d are not uncommon by the end of pregnancy. One study demonstrated that close monitoring of lamotrigine levels on a monthly basis with corresponding dose adjustments resulted in a relatively low rate of seizure deterioration (19%), similar to that seen in pregnancies managed with other AEDs.41 Oxcarbazepine clearance is also dependent on glucuronidation, and this may play a role in the increased seizure frequency observed in prior cohorts.42 In contrast, carbamazepine levels are relatively stable throughout pregnancy, which may account for observed seizure stability.43 It is not yet clear whether AED metabolism and the need for dose adjustments will completely explain the findings of the APR and EURAP cohorts. Both registries reported that lamotrigine dosing had been increased in less than 50% of cases,33,36 suggesting that many did not have levels monitored. In the APR, only 15.9% of levetiracetam pregnancies were noted to have dose adjustments during pregnancy,36 which is interesting given that levetiracetam metabolism appears to increase significantly during pregnancy as well.38 Future prospective studies during which appropriate dose adjustment is made will be necessary to resolve this question.
Therapeutic Drug Monitoring
In addition to the recognized changes in AED metabolism over the course of pregnancy, substantial interindividual variation in metabolism also exists.40 AED adjustments during pregnancy are an important part of caring for a woman with epilepsy and should be directed by AED levels whenever possible. AED levels should be checked monthly for most drugs, especially for women on lamotrigine or oxcarbazepine. As part of planning a pregnancy, it is ideal to obtain several baseline levels when the patient is at her optimal seizure control in order to set her target therapeutic level. It is also important to recognize that AED metabolism can return to baseline relatively quickly postpartum. In the case of lamotrigine, prepregnancy clearance rates are reached within 2 to 3 weeks.40 To avoid toxicity, the patient should be given a plan before delivery to reduce her AEDs postpartum. Depending on the patient and her baseline drug levels, the authors commonly leave patients at a slightly higher dose than baseline for the first 1 to 3 months postpartum to protect them from the effects of sleep deprivation.
Folic Acid
For women of childbearing age taking AEDs, the AAN practice guideline recommends folic acid supplementation of 0.4 mg/d to 4 mg/d.44 (Refer to Appendix C for a summary of the AAN evidence-based guideline for clinicians.) These recommendations are extrapolated from folic acid supplementation in the general population, which reduces risk for neural tube defects.45 Additionally, several AEDs have been associated with lower serum folic acid levels, and low first-trimester serum folic acid levels have been correlated with an increased risk for congenital malformations in women with epilepsy.46,47 Little direct evidence actually exists that folic acid reduces the risk of major congenital malformations in women taking AEDs, although the AAN practice parameter indicates that prior studies may have been underpowered to detect a benefit.44 Folic acid may also reduce the risk of miscarriage in women with epilepsy.48 Similar to some studies in the general population, the NEAD study found an association between periconceptual folic acid supplementation (0.4 mg/d or more) and higher IQs in children of women with epilepsy.23 In contrast, in the LMNG study of cognitive development in children of women with epilepsy, no specific association between folic acid and IQ was identified; however, LMNG assessed folate use in the first trimester rather than periconceptionally.24 The optimal dose of folic acid is not known. Recent concerns about the effect of high-dose folic acid have been raised by a study that found delayed psychomotor development in children of women exposed to doses greater than 5 mg/d compared to women who took doses of 0.4 mg/d to 1 mg/d.49 This study excluded women with epilepsy. More research is needed to determine the optimal dose of folic acid in women with epilepsy.
Vitamin K
Third-trimester vitamin K supplementation has been suggested for women taking enzyme-inducing AEDs (EIAEDs) (eg, carbamazepine, phenytoin, phenobarbital; refer to Table 11-3 for other EIAEDs). This suggestion is based on a concern for increased risk of intracranial neonatal hemorrhage associated with EIAED exposure that was reported in early case studies. In a large epidemiologic study of 662 women with epilepsy taking EIAEDs during pregnancy, no significant difference in bleeding complications of the newborns of mothers using EIAEDs and control subjects was shown when all children received vitamin K at birth (as is standard of care in the United States).50 The 2009 AAN guideline states that insufficient evidence exists to recommend for or against the practice of peripartum vitamin K supplementation.44
TABLE 11-3.
Interactions Between Antiepileptic Drugs and Hormonal Contraception
| Antiepileptic Drug | Effect on Hormonal Contraception |
Affected by Hormonal Contraception? |
|---|---|---|
| Clonazepam, diazepam, ethosuximide, ezogabine, gabapentin, lacosamide, lorazepam, levetiracetam, pregabalin, vigabatrin, zonisamide | None | No |
| Carbamazepine, clobazam, eslicarbazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, rufinamide | Decreased ethinyl estradiol levels Decreased progestin levels | Unknown |
| Topiramate | Decreased ethinyl estradiol levels (dose-dependent effect) | Unknown |
| Perampanel | Decreased progestin levels | Unknown |
| Lamotrigine | Decreased progestin levels | Decreased lamotrigine levels (with estrogen-containing contraception) |
| Valproate | No | Decreased valproate levels (with estrogen-containing contraception) |
Breast-feeding
The benefits of breast-feeding have been well established and include a decreased risk of infections, diabetes mellitus, leukemia, and sudden infant death syndrome in the infant and a decreased risk of breast and ovarian cancer as well as diabetes mellitus in the mother.51 Breastfeeding also promotes mother-infant bonding. In the NEAD study, children exposed to carbamazepine, lamotrigine, phenytoin, or valproate in breast milk as infants had higher IQs and language scores at 6 years of age when compared to those children whose mothers were taking AEDs and did not breast-feed.52 A transient improvement on parent-reported developmental abilities of children was noted in breast-fed infants in a Norwegian cohort of AED-exposed children at 6 and 18 months, although the effect was not sustained at 36 months.53 Neither study found adverse effects on developmental outcomes related to breast milk exposure to the studied drugs (carbamazepine, lamotrigine, phenytoin, and valproate). While further prospective studies of AED exposure via breast milk are necessary, for most AEDs, the theoretical concern of prolonged infant exposure likely does not outweigh the known benefits of breast-feeding.
Postpartum Safety
A key part of managing a pregnancy in a woman with epilepsy is discussing seizure safety with her and her family. While breast-feeding should be supported and encouraged, the sleep deprivation associated with trying to feed a newborn every 2 to 4 hours may lower the seizure threshold in many women with epilepsy. Family members should be asked to help with night feedings with either expressed breast milk or formula so the patient can get a stretch of uninterrupted sleep (typically 6 to 8 hours, depending on the patient). To maintain milk supply, the patient may have to pump milk additional times during the day. Other safety recommendations include giving the child baths only in the presence of another adult and changing diapers on a pad on the floor instead of on a changing table. Avoiding stairs when possible and using a stroller rather than an infant carrier strapped to the mother should also be considered. The importance of not having an infant sleep in bed with the parents should be stressed. Women with epilepsy are at increased risk for postpartum depression, and this should be discussed with the patient and her family both before and after delivery.54
CONTRACEPTION
Despite the importance of carefully anticipated pregnancies in women with epilepsy, 50% of women with epilepsy report that their pregnancies were unplanned, a number similar to that of the general population.2 Many patients are unaware of important drug-drug interactions between AEDs and hormonal contraception that can lead to contraceptive failure.55 Discussing appropriate contraception is an important part of managing a woman with epilepsy and should be addressed by both her neurologist and gynecologist. Both specialties should be aware of numerous different drug-drug interactions between AEDs and hormonal contraception. Moreover, the clinical significance of some pharmacologic interactions remains unclear and controversial, making firm recommendations difficult. Both the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) have released evidence-based reviews and opinions on the use of contraception in women with epilepsy.56,57
The most reliable form of reversible contraception for any woman is the intrauterine device (IUD) (Table 11-4), and this is considered the contraceptive method of choice for most women with epilepsy.58 While the IUD used to be reserved for women who have previously given birth, it is now clear that it can be used safely in nulliparous women and has been recommended as an optimal form of contraception for teenage girls by the American Academy of Pediatrics.60 Three types of IUDs are available in the United States, a copper IUD and two levonorgestrel-releasing IUDs. The copper IUD is approved for 10 years of use, the 52-mg levonorgestrel-releasing IUD is approved for 5 years, and the 13.5-mg levonorgestrel-releasing IUD is approved for 3 years. The 52-mg levonorgestrel-releasing IUD is approved for the treatment of heavy menstrual bleeding as well as contraception. Both the copper and levonorgestrel-releasing IUDs are relatively free from clinically significant drug-drug interactions. Although the levonorgestrel-releasing IUDs contain a progestin, levonorgestrel, serum concentrations of levonorgestrel are 200 pg/mL or less, and the device is thought to exert its contraceptive effects locally. A study of the efficacy of the 52-mg levonorgestrel-releasing IUD in 56 women taking EIAEDs found a failure rate of 1.1% per year.61 Although this failure rate is slightly higher than the failure rate of 0.2% seen in larger general population studies, it is still much lower than that of other contraceptive options. A comparable study of the 13.5-mg levonorgestrel-releasing IUD is not available.
TABLE 11-4.
Contraceptive Options, Efficacy, and Considerations for Women With Epilepsy
| Contraceptive Method | Pregnancies Per Yeara |
Considerations for Women With Epilepsy |
|---|---|---|
| Intrauterine device (IUD) | ||
| Copper IUD | <1% | No significant antiepileptic drug (AED) interactions |
| Copper IUD may preclude 3-tesla MRI at some institutions | ||
| Levonogestrel-releasing IUD | <1% | Levonorgestrel-releasing IUD reduces or eliminates menstrual bleeding |
| Combined hormonal contraception | ||
| Combined oral contraceptive pills | 9% | Not recommended with enzyme-inducing antiepileptic drugs (EIAEDs) |
| Vaginal ring | 9% | Will reduce lamotrigine levels |
| Transdermal patch | 9% | May be used to treat symptoms of polycystic ovary syndrome |
| Transdermal patch may worsen seizure control59 | ||
| Progesterone-only contraception | ||
| Etonogestrel implant | 0.05% | Efficacy of the etonogestrel implant may be reduced by EIAEDs |
| Depot medroxyprogesterone acetate injection | 6% | The depot medroxyprogesterone acetate injection may offer seizure-control benefit in some patients if amenorrhea achieved |
| The depot medroxyprogesterone acetate injection is associated with reversible bone loss | ||
| Progesterone-only pills | 9%b | Progesterone-only pills require excellent compliance Efficacy of progesterone-only pills is reduced by EIAEDs and possibly lamotrigine and perampanel |
| Barrier methods | ||
| Male condom | 18% | No AED interactions |
| Female condom | 21% | Condoms are the only method that reduces transmission of sexually transmitted disease Efficacy with regular use not ideal for women with epilepsy not planning pregnancy Can be used in addition to measures above to improve efficacy |
MRI = magnetic resonance imaging.
Pregnancy rates are from the general population assuming typical use and no drug-drug interactions. Pregnancy rates are from Trussell J, Contraception.58
Because of a lack of specific data, Trussell cites a 9% failure rate for progesterone-only pills based on the failure rate of combined oral contraceptive pills but acknowledges that the progesterone-only pills likely have an even higher failure rate.
Numerous forms of hormonal contraception are available (Table 11-4). Some use a combination of synthetic estrogens (typically ethinyl estradiol) and progestins; this group includes the most widely prescribed form of contraception, combined oral contraceptive pill (“The Pill”). Others use only synthetic progestins.
Enzyme-inducing Antiepileptic Drugs and Contraception
EIAEDs are inducers of hepatic P450 enzymes and induce the metabolism of both ethinyl estradiol and synthetic progestins (Table 11-3). Contraceptive failure has been reported when these medications have been used with combined hormonal contraception. Thus both the CDC and WHO advise against combining EIAEDs with combined oral contraceptive pills, the vaginal ring, or the transdermal patch.56,57 While some authors have previously recommended using combined oral contraceptive pills with a higher dose of ethinyl estradiol, no evidence exists to support this approach, and, in fact, early reports of contraceptive failure occurred in women taking higher-dose pills and EIAEDs.62
Depot medroxyprogesterone acetate is an injection typically administered as 150 mg every 12 weeks. Both the CDC and WHO state that depot medroxyprogesterone acetate can be used safely in patients taking EIAEDs.56,57 Officially, no adjustment of the depot medroxyprogesterone acetate dose or administration interval is recommended, as in patients not taking medications the depot medroxyprogesterone acetate dose at 3 months in most women is greater than that needed to suppress ovulation, but the potential effect of EIAEDs on depot medroxyprogesterone acetate, which is metabolized by the P450 system, has not been studied.
The etonogestrel subdermal implant is inserted into the arm and supplies a synthetic progestin for up to 3 years. The CDC and WHO state that the implant “can be considered” in women taking EIAEDs, but note that its efficacy may be reduced. Pregnancies have been reported in women using the implant while taking carbamazepine, phenytoin, or phenobarbital.57,63 Women using this combination should be strongly encouraged to use an additional form of contraception.
Topiramate and Contraception
The effect of topiramate on hormonal contraception is controversial. Topiramate is a weak inducer of P450 enzymes, and its effect appears to be dose-related. There may also be interindividual variability in susceptibility to this enzyme-inducing effect. When used in combination with combined oral contraceptive pills, topiramate at doses of 200 mg/d to 800 mg/d can lower ethinyl estradiol serum concentrations but not norethindrone concentrations64 In one study, however, topiramate doses of 200 mg or less did not significantly reduce ethinyl estradiol concentrations in women taking a pill containing 35 mcg of ethinyl estradiol and 1 mg of norethindrone, and the authors argued this combination should provide effective contraception.65 Nevertheless, the WHO and CDC consider topiramate an EIAED and advise against combining it with any form of hormonal contraception other than depot medroxyprogesterone acetate and possibly the etonogestrel implant.56,57 If topiramate is used in conjunction with combined oral contraceptive pills, condom use should also be encouraged and low-dose (less than 35 mcg ethinyl estradiol) pills should probably be avoided.
Lamotrigine and Contraception
Lamotrigine has complex bidirectional interactions with hormonal contraception. Ethinyl estradiol induces glucuronidation pathways, resulting in significant decreases in lamotrigine levels by over 50%, and can result in loss of seizure control if dosing is not adjusted (Case 11-2).66 The induction of glucuronidation by ethinyl estradiol manifests and disappears quickly, within a few days, thus lamotrigine levels can also rise during the hormone-free week built into most combined hormonal contraception.67,68 This may result in symptoms of lamotrigine toxicity in patients requiring higher baseline levels.
Case 11-2.
A 20-year-old woman presented for evaluation of worsening seizures. She was diagnosed with generalized epilepsy at age 14 after her second tonic-clonic seizure. She was started on lamotrigine but continued to have one tonic-clonic seizure every 2 to 4 months until her dose reached 200 mg 2 times a day, which corresponded to a level of 9 mcg/mL. On this dose, she remained seizure free for 4 years. Three months prior to presentation, she started a combined oral contraceptive pill for symptoms of polycystic ovary syndrome, including irregular menses, significant acne, and hirsutism. She also desired a second birth control measure in addition to consistent condom use. Within the first 2 months of starting the combined oral contraceptive pill, she had three more unprovoked tonic-clonic seizures. Her lamotrigine level at this time was 5.3 mcg/mL despite compliance with her prior lamotrigine regimen. An intrauterine device (IUD) was considered, but she preferred to stay on the combined oral contraceptive pill, if possible, as it had significantly improved her polycystic ovary syndrome symptoms. Her lamotrigine dose was increased to 300 mg in the morning and 250 mg at night, and she began taking her combined oral contraceptive pills continuously, without a placebo week. On this regimen, her lamotrigine level was 9.6 mcg/mL, and she has remained seizure free for another 3 years.
Comment.
This case illustrates the important interactions between estrogen-containing hormonal contraception and lamotrigine. Ethinyl estradiol is a potent inducer of lamotrigine metabolism and can cause lamotrigine levels to fall, which can lead to a loss of seizure control. This can be addressed by increasing the dose of lamotrigine when estrogen-containing combined oral contraceptive pills are started and by monitoring drug levels before and after initiation. During the placebo week of most combined oral contraceptive pills, lamotrigine levels can rise and cause side effects, thus taking the pills continuously, without a placebo week, is preferable. This also improves contraceptive efficacy. Because of a lack of drug-drug interactions and better contraceptive efficacy, an IUD would have been the ideal contraceptive option for this patient; however, this would not have addressed the symptoms of polycystic ovary syndrome, which was important to this patient.
Based on the risk of increased seizures, the WHO and CDC advise against the combination of lamotrigine and all combined hormonal contraceptive methods.56,57 However, a neurologist experienced in adjusting lamotrigine dosing usually can circumvent this issue. An understudied question is whether lamotrigine can have a clinically significant impact on the efficacy of hormonal contraception. In one study, lamotrigine 300 mg given in combination with a combined oral contraceptive pill containing 30 mcg of ethinyl estradiol and 150 mcg of levonorgestrel decreased levonorgestrel levels by 19%.69 An increase in follicle-stimulating hormone and luteinizing hormone levels and intermenstrual spotting was also seen, although ovulation was not thought to have occurred based on luteal progesterone levels. Many of the combined oral contraceptive pills prescribed today have low doses (less than 30 mcg) of ethinyl estradiol and rely heavily on the effect of the progestin component to suppress ovulation. It is not clear whether the effect of lamotrigine on the progestin component might compromise efficacy of low-dose pills. If lamotrigine and combined oral contraceptive pills must be combined, one strategy is to use a pill with at least 30 mcg of ethinyl estradiol and consider using an extended-cycle regimen with either a short or no placebo week as in Case 11-2. Eliminating the placebo week avoids fluctuations in lamotrigine levels and may increase the efficacy of the combined oral contraceptive pill. Triphasic pills, which vary the amount of ethinyl estradiol or the progestin throughout the month, should not be used.
More research on the effect of various AEDs on the progestins in hormonal contraception is needed. At the recommended dose of 12 mg, the new AED perampanel decreases levonorgestrel levels by 40%, and the manufacturer recommends using additional nonhormonal contraception if levonorgestrel-containing oral contraceptive pills or subdermal implants are used with perampanel.70
Emergency Contraception
In the United States, emergency contraception (also known as “the morning-after pill”) is available in the form of two 0.75-mg levonorgestrel pills taken 12 hours apart. The effect of EIAEDs on this levonorgestrel preparation is not known. A copper IUD inserted within 5 or more days of intercourse also provides highly effective emergency contraception as well as ongoing contraception.56 In the United Kingdom, the Royal College of Obstetricians and Gynaecologists (RCOG) recommends copper IUD insertion, when needed, as emergency contraception for women taking EIAEDs.71 If levonorgestrel is used for emergency contraception in women taking EIAEDs, the RCOG recommends that the dose of levonorgestrel should be doubled (3-mg total dose). Others recommend an initial dose of 1.5 mg followed 12 hours later by an additional 0.75 mg.72 It is not known if the effect of lamotrigine or perampanel on levonorgestrel levels would affect emergency hormonal contraception, and no specific emergency contraceptive recommendations for these medications have been made.
EFFECT OF SEX HORMONES ON SEIZURES
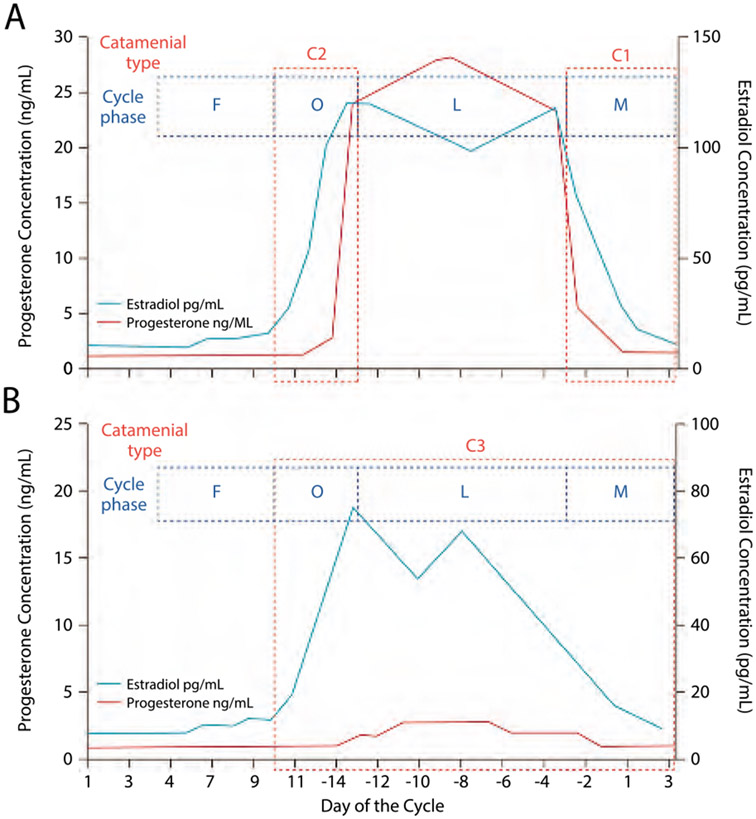
Many women notice an association between their seizures and menstrual cycles. Both preclinical and clinical data suggest that the female sex hormones estrogen and progesterone can alter seizure susceptibility. Catamenial epilepsy is defined by a pattern of increased seizures at specific times in the menstrual cycle that are characterized by an increased estrogen-to-progesterone ratio. The most commonly used definition of catamenial epilepsy was described by Herzog and is characterized by a twofold increase in seizure frequency during one of three vulnerable time periods compared to seizure frequency during the rest of the menstrual cycle. Based on this definition, catamenial epilepsy affects 42% of women with epilepsy.73 Within this group of patients, the most common pattern is the C1 pattern, a perimenstrual pattern in which seizures occur predominantly between day −3 and day +3 of the menstrual cycle (Figure 11-374). C2 is a periovulatory pattern in which seizures are seen mostly on days +10 to −13. In the C3 pattern, seizures are most common in the luteal phase, day +10 to day +3 of the next cycle. This pattern is most commonly seen in anovulatory cycles, but may also be seen in ovulatory cycles. A common feature of all patients with catamenial epilepsy is a relative decrease in seizures during the follicular phase (days 4 to 9).
FIGURE 11-3.
Three patterns of catamenial epilepsy. Day 1 is the first day of menstrual flow and day −14 is the day of ovulation. A, Normal cycle with normal ovulation. C1 pattern is associated with exacerbation of seizures in the perimenstrual phase (day −3 to day +3 of next cycle), and C2 pattern is associated with exacerbation of seizures in the periovulatory phase (day +10 to day −13). B, Inadequate luteal phase cycle typically seen with anovulation. The C3 pattern is associated with exacerbations during the luteal phase (day +10 to day +3 of the next cycle).
C = catamenial seizure pattern; F = follicular phase; L = luteal phase; M = perimenstrual phase; O = periovulatory phase.
Reprinted with permission from Harden CL, Pennell PB, Lancet Neurol.www.thelancet.com/journals/laneur/artide/PIIS1474-4422(12)70239-9/abstract. ©2013 Elsevier Ltd.
Careful tracking of seizure frequency, menstrual cycles, and, ideally, ovulation is essential to make a diagnosis of catamenial epilepsy. At-home methods of tracking ovulation include tracking first morning temperatures and over-the-counter ovulation tracking kits. Smartphone menstrual tracker applications can also be helpful. For many patients, tracking their seizure patterns gives them a better sense of control over an otherwise unpredictable condition. Although it is important to recognize and understand catamenial epilepsy, research on treatment options is still limited. At present, no unique treatment options for these patients have been proven to be more beneficial than standard therapies. Hormonal therapies or hormonally targeted treatment should never be offered as first-line therapy. Management should begin with standard AED therapies, appropriate for the patient’s epilepsy type, and epilepsy surgery, if appropriate, for patients with intractable epilepsy.
For patients in whom standard treatments have failed, several adjunctive treatments targeting catamenial patterns have been evaluated (Table 11-575-80), although most studies have been small case series. The most robust study of catamenial epilepsy was a multicenter prospective placebo-controlled trial of natural progesterone. Women received 200 mg natural progesterone lozenges 3 times a day for days +14 to +25 of their cycle and then tapered the dose over the next 3 days. Overall, the trial did not demonstrate a difference between placebo and progesterone therapy. A secondary analysis indicated that women with the C1 pattern were most likely to be responders, with 21% to 57% of them having a 50% or more reduction in seizures.80 Thus progesterone, as prescribed in the trial, may be an appropriate adjunctive treatment for patients with predominantly perimenstrual seizures. Further research is necessary to determine if a different progesterone regimen might be beneficial for women with other catamenial patterns.
TABLE 11-5.
Adjunctive Treatments for Catamenial Epilepsy
| Drug and Study | Dosing | Efficacy (Sample Size) | Comments and Side Effects |
|---|---|---|---|
| Acetazolamide Lim et al, 200175 | Start 4 mg/kg/d (up to 1 g/d) starting 5 days before menses, for 5–7 days | >50% decrease in seizures in 40% of patients (n = 20) | Tolerance developed in 15% of patients Study also included continuous-use and pulse-dose acetazolamide Polyuria, dizziness |
| Clobazam Feely et al, 198276 | 20–30 mg/d for 10 days beginning 2–4 days before menses | >50% reduction in seizures in 44% of patients (n = 18) | An additional 33% of patients responded to both placebo and clobazam Sedation, depression |
| Clomiphene citrate Herzog, 198877 | 25–100 mg/d on days 5–9; dose increase until regular menstrual cycle | >50% reduction in seizures in 66% of patients (n = 12) | Breast tenderness, pelvic pain, ovarian cysts/ovarian hyperstimulation |
| Gonadotropin-releasing hormone analogue (triptorelin) Bauer et al, 199278 | 3.75 mg IM every 4 weeks | Seizure freedom in 30% of patients; seizure improvement in an additional 50% (80% with benefit) (n = 10) | Menopausal symptoms, osteopenia |
| Medroxyprogesterone Mattson et al, 198479 | Medroxyprogesterone 10 mg orally, 2–4 times/d Or Depot medroxyprogesterone acetate 120–150 mg IM every 6–12 weeks |
Reduction in seizure frequency noted in 50% of treated subjects and 63% of those achieving amenorrhea Mean seizure reduction among responders was 52%(range 25%–71%) (n = 14, amenorrhea in 11) |
Dosing adjusted to achieve amenorrhea No patients were seizure free Weight gain, breast tenderness, menstrual spotting, osteopenia (reversible) |
| Progesterone lozenges Herzog et al, 201280 | 200 mg 3 times/d on days 14–25; 100 mg 3 times/d on days 6–27; 50 mg 3 times/d on day 28 | >50% reduction in seizures in 22% of patients (not different from placebo) (n = 85) | Primary end point not met, but patients with principally perimenstrual seizures were more likely to respond (26%–71% of these patients had >50% reduction) |
IM = intramuscular.
CONCLUSION
The selection of an AED for a woman of childbearing age should keep future pregnancies in mind. The goal should be to find the agent that best controls her seizures at the lowest possible dose. Given the significant risk of structural and cognitive teratogenesis, valproate is a poor first-choice AED in women of childbearing age, and if valproate is prescribed, it should be kept at the lowest dose needed. Pregnancy and contraception should be discussed with women with epilepsy early in their treatment and revisited regularly. AED changes, if necessary, should ideally be initiated 1 year before conception. Changing AED regimens once a woman is already pregnant offers little reduction in the risk of major congenital malformations. Whether in utero effects of AEDs on cognitive development, which are thought to largely occur in the third trimester, could be improved by switching appropriate patients off valproic acid during pregnancy is not known, and the risk of precipitating seizures has to be considered. Thus, addressing AED changes prior to pregnancy is preferable. For all AEDs, therapeutic drug monitoring during pregnancy should be performed; this is most critical for patients taking lamotrigine and oxcarbazepine.
Early discussion of the effectiveness of contraception and drug-drug interactions is also a critical part of caring for women with epilepsy and can help prevent unplanned pregnancy. An IUD is the most effective form of reversible contraception and a good option for many women with epilepsy. Finally, the effect of female hormones on seizure frequency is important to understand and discuss with patients. In some cases, adjunctive therapies for catamenial epilepsy are appropriate, although more research is needed to identify highly effective therapies.
KEY POINTS.
Up to 50% of pregnancies in women with epilepsy are unplanned.
Most women with epilepsy feel that their health care providers do not initiate conversations about pregnancy and contraception, and one-third say they have never discussed these topics with their physicians.
Risks related to antiepileptic drug use and seizures during pregnancy can be reduced by early prepartum counseling and planning.
Prepartum counseling should start no later than the prescription of the first antiepileptic drug to a woman of childbearing age.
Valproate has been consistently associated with the highest rates of major congenital malformations.
It is common practice to recommend a level II ultrasound at 18 to 20 weeks gestational age for women taking antiepileptic drugs during pregnancy. This is a detailed anatomic evaluation, which provides very high sensitivity for structural abnormalities affecting the fetus.
Part of preparing a woman with epilepsy for pregnancy involves trying to identify the minimum therapeutic dose (and corresponding level) that is able to control her seizures.
Polytherapy regimens that do not include valproate may not increase teratogenic risks as much as previously thought.
Valproate exposure during pregnancy has been associated with a decrease in mean IQ by 10 points as well as an increased risk of autism and autism spectrum disorders in exposed children.
Women with epilepsy do not appear to be at an increased risk of miscarriage. However, there are several other pregnancy complications that are more likely in women with epilepsy and may be related to the effect of seizures, antiepileptic drugs, or both.
Neither epilepsy nor antiepileptic drug use is usually an indication for a cesarean delivery.
Seizure freedom in the 9 months prior to pregnancy is a strong predictor of seizure freedom during pregnancy.
Lamotrigine metabolism can increase by over 200% during pregnancy, and frequent dose adjustment is necessary to maintain seizure control.
For most antiepileptic drugs, levels should be checked monthly during pregnancy.
Women whose antiepileptic drug doses have been increased over the course of pregnancy should be given a plan to reduce their dose over the first 1 to 3 weeks postpartum. In some cases, it may be appropriate to leave the dose a little higher than baseline to protect against postpartum seizures.
Breast-feeding is safe for most women taking antiepileptic drugs and should be encouraged.
The postpartum period can be a time of increased stress and sleep deprivation, as well as increased seizure frequency in women with epilepsy. Health care providers should engage members of the patient’s support system in creating a postpartum safety plan.
The intrauterine device is the most effective form of reversible contraception and is not limited by significant drug-drug interactions.
Both the Centers for Disease Control and Prevention and the World Health Organization advise against combining enzyme-inducing antiepileptic drugs with combined oral contraceptive pills, the vaginal ring, or the transdermal patch.
The World Health Organization and Centers for Disease Control and Prevention state that depot medroxyprogesterone acetate can be used in conjunction with enzyme-inducing antiepileptic drugs, although the efficacy of this combination has not been well studied.
Pregnancies have been reported with the combination of enzyme-inducing antiepileptic drugs and the etonogestrel implant.
The effect of topiramate on the efficacy of hormonal contraception is controversial; however, the World Health Organization and Centers for Disease Control and Prevention consider topiramate an enzyme-inducing antiepileptic drug and advise against combining it with any form of hormonal contraception other than depot medroxyprogesterone acetate and possibly the etonogestrel implant.
Hormonal birth control that contains synthetic estrogens can lower lamotrigine levels by up to 50%.
Hormonal therapies for catamenial epilepsy should not be used in lieu of standard epilepsy treatment.
Oral progesterone taken 3 times a day may reduce seizure frequency in patients with the most common catamenial epilepsy pattern where seizures typically occur 3 days before to 3 days after the onset of menses.
ACKNOWLEDGMENT
This work was supported by a grant (2U01NS038455; Drs Gerard and Meador) from the National Institute of Neurological Disorders and Stroke.
Footnotes
Relationship Disclosure:
Dr Gerard has received honoraria from the American College of Physicians and research support from the National Institute of Neurological Disorders and Stroke and SAGE Pharmaceuticals. Dr Gerard has served as a research consultant on a trial sponsored by UCB, Inc. Dr Meador serves as a consultant for the Epilepsy Study Consortium and on the editorial boards of Epilepsy and Behavior, Journal of Clinical Neurophysiology, and Neurology. Dr Meador has received travel, meeting, and accommodation compensation from UCB, Inc, and receives research support from the National Institute of Neurological Disorders and Stroke and the Patient-Centered Outcomes Research Institute.
Unlabeled Use of Products/Investigational Use Disclosure:
Drs Gerard and Meador report no disclosures.
REFERENCES
- 1.The history and stigma of epilepsy. Epilepsia 2003;44(suppl 6):12–14. doi: 10.1046/j.1528-1157.44.s.6.2.x.12558825 [DOI] [Google Scholar]
- 2.Davis AR, Pack AM, Kritzer J, et al. Reproductive history, sexual behavior and use of contraception in women with epilepsy. Contraception 2008;77(6):405–409. doi: 10.1016/j.contraception.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.May TW, Pfäfflin M, Coban I, Schmitz B. Fears, knowledge, and need of counseling for women with epilepsy. Results of an outpatient study. Nervenarzt 2009;80(2):174–183. doi: 10.1007/s00115-008-2632-x. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez B, Gibson P, Kustra R. Epilepsy and women's health issues: unmet needs—survey results from women with epilepsy. Epilepsy Behav 2007;10(1):163–169. [DOI] [PubMed] [Google Scholar]
- 5.Sveberg L, Svalheim S, Taubøll E. The impact of seizures on pregnancy and delivery. Seizure 2015;28:35–38. doi: 10.1016/j.seizure.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Chiou HY, Lin HC, Lin HL. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol 2009;66(8):979–984. doi: 10.1001/archneurol.2009.142. [DOI] [PubMed] [Google Scholar]
- 7.Edey S, Moran N, Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia 2014;55(7):e72–e74. doi: 10.1111/epi.12621. [DOI] [PubMed] [Google Scholar]
- 8.Gerard EE. Preconception counseling for women with epilepsy. In: Bui E, Klein A, eds. Women with epilepsy. Cambridge, United Kingdom: Cambridge University Press, 2014:141–156. [Google Scholar]
- 9.Tomson T, Xue H, Battino D. Major congenital malformations in children of women with epilepsy. Seizure 2015;28:40–44. doi: 10.1016/j.seizure.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Vajda FJ, O’Brien TJ, Lander CM, et al. The teratogenicity of the newer antiepileptic drugs—an update. Acta Neurol Scand 2014;130(4):234–238. doi: 10.1111/ane.12280. [DOI] [PubMed] [Google Scholar]
- 11.Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305(19):1996–2002. doi: 10.1001/jama.2011.624. [DOI] [PubMed] [Google Scholar]
- 12.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10(7):609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 13.Artama M, Auvinen A, Raudaskoski T, et al. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology 2005;64(11):1874–1878. [DOI] [PubMed] [Google Scholar]
- 14.Cunnington MC, Weil JG, Messenheimer JA, et al. Final results from 18 years of the International Lamotrigine Pregnancy Registry. Neurology 2011;76(21):1817–1823. doi: 10.1212/WNL.0b013e31821ccd18. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012; 78(21):1692–1699. doi: 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 16.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol 2014;261(3):579–588. doi: 10.1007/s00415-013-7239-x. [DOI] [PubMed] [Google Scholar]
- 17.Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol 2012;11(9):803–813. doi: 10.1016/S1474-4422(12)70103-5. [DOI] [PubMed] [Google Scholar]
- 18.Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland epilepsy and pregnancy registers. J Neurol Neurosurg Psychiatry 2014;85(9):1029–1034. doi: 10.1136/jnnp-2013-306318. [DOI] [PubMed] [Google Scholar]
- 19.Mawhinney E, Craig J, Morrow J, et al. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology 2013;80(4):400–405. doi: 10.1212/WNL.0b013e31827f0874. [DOI] [PubMed] [Google Scholar]
- 20.Hunt S, Russell A, Smithson WH, et al. Topiramate in pregnancy: Preliminary experience from the UK epilepsy and pregnancy register. Neurology 2008;71(4):272–276. doi: 10.1212/01.wnl.0000318293.28278.33. [DOI] [PubMed] [Google Scholar]
- 21.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK epilepsy and pregnancy register. J Neurol Neurosurg Psychiatry 2006; 77(2):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes LB, Mittendorf R, Shen A, et al. Fetal effects of anticonvulsant polytherapies: different risks from different drug combinations. Arch Neurol 2011;68(10):1275–1281. doi: 10.1001/archneurol.2011.133. [DOI] [PubMed] [Google Scholar]
- 23.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12(3):244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker GA, Bromley RL, Briggs M, et al. IQ at 6 years following in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology 2014;84(4):382–390. doi: 10.1212/WNL.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shallcross R, Bromley RL, Cheyne CP, et al. In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology 2014; 82(3):213–221. doi: 10.1212/WNL.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 26.Meador KJ. Cognitive effects of epilepsy and its treatments. In: Wyllie E, Gidal B, Goodkin H, et al. , editors. Wyllie's treatment of epilepsy: principles and practice. 6th ed. Philadelphia, PA: Wolters Kluwer, 2015:989–994. [Google Scholar]
- 27.Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309(16):1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bech BH, Kjaersgaard MI, Pedersen HS, et al. Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: population based cohort study. BMJ 2014;349:g5159. doi: 10.1136/bmj.g5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borthen I. Obstetrical complications in women with epilepsy. Seizure 2015;28:32–34. doi: 10.1016/j.seizure.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Hernández-Díaz S, Mittendorf R, Smith CR, et al. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet Gynecol 2014; 123(1): 21–28. doi: 10.1097/AOG.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 31.Harden CL, Hopp J, Ting TY, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73(2):126–132. doi: 10.1212/WNL.0b013e3181a6b2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cagnetti C, Lattanzi S, Foschi N, et al. Seizure course during pregnancy in catamenial epilepsy. Neurology 2014;83(4):339–344. doi: 10.1212/WNL.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 33.Battino D, Tomson T, Bonizzoni E, et al. Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia 2013;54(9):1621–1627. doi: 10.1111/epi.12302. [DOI] [PubMed] [Google Scholar]
- 34.La Neve A, Boero G, Francavilla T, et al. Prospective, case-control study on the effect of pregnancy on seizure frequency in women with epilepsy. Neurol Sci 2014;36(1):79–83. doi: 10.1007/s10072-014-1908-0. [DOI] [PubMed] [Google Scholar]
- 35.Thomas SV, Syam U, Devi JS. Predictors of seizures during pregnancy in women with epilepsy. Epilepsia 2012;53(5):e85–e88. doi: 10.1111/j.1528-1167.2012.03439.x. [DOI] [PubMed] [Google Scholar]
- 36.Vajda FJ, O'Brien T, Lander C, et al. The efficacy of the newer antiepileptic drugs in controlling seizures in pregnancy. Epilepsia 2014;55(8):1229–1234. doi: 10.1111/epi.12711. [DOI] [PubMed] [Google Scholar]
- 37.EURAP Study Group. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology 2006;66(3):354–360. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- 38.Reisinger TL, Newman M, Loring DW, et al. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav 2013;29(1):13–18. doi: 10.1016/j.yebeh.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78 (21):1692–1699. doi: 10.1212/WNL.0b013e3182574f39. [DOI] [PubMed] [Google Scholar]
- 40.Polepally AR, Pennell PB, Brundage RC, et al. Model-based lamotrigine clearance changes during pregnancy: clinical implication. Ann Clin Transl Neurol 2014;1(2):99–106. doi: 10.1002/acn3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabers A, Petrenaite V. Seizure frequency in pregnant women treated with lamotrigine monotherapy. Epilepsia 2009;50(9):2163–2166. doi: 10.1111/j.1528-1167.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 42.Petrenaite V, Sabers A, Hansen-Schwartz J. Seizure deterioration in women treated with oxcarbazepine during pregnancy. Epilepsy Res 2009;84(2–3):245–249. doi: 10.1016/j.eplepsyres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Johnson EL, Stowe ZN, Ritchie JC, et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav 2014;33(6):49–53. doi: 10.1016/j.yebeh.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73(2):142–149. doi: 10.1212/WNL.0b013e3181a6b325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 2010;39(suppl 1):i110–i121. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linnebank M, Moskau S, Semmler A, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol 2011;69(2):352–359. doi: 10.1002/ana.22229. [DOI] [PubMed] [Google Scholar]
- 47.Kjaer D, Horvath-Puhó E, Christensen J, et al. Antiepileptic drug use, folic acid supplementation, and congenital abnormalities: a population-based case-control study. BJOG 2008;115(1):98–103. doi: 10.1111/j.1471-0528.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 48.Pittschieler S, Brezinka C, Jahn B, et al. Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J Neurol 2008;255(12):1926–1931. doi: 10.1007/s00415-008-0029-1. [DOI] [PubMed] [Google Scholar]
- 49.Valera-Gran D, García de la Hera M, Navarrete-Muñoz EM, et al. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr 2014;168(11):e142611. doi: 10.1001/jamapediatrics.2014.2611. [DOI] [PubMed] [Google Scholar]
- 50.Kaaja E, Kaaja R, Matila R, Hiilesmaa V. Enzyme-inducing antiepileptic drugs in pregnancy and the risk of bleeding in the neonate. Neurology 2002;58(4):549–553. doi: 10.1212/WNL.58.4.549. [DOI] [PubMed] [Google Scholar]
- 51.Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med 2009;4(suppl 1):S17–S30. doi: 10.1089/bfm.2009.0050. [DOI] [PubMed] [Google Scholar]
- 52.Meador KJ, Baker GA, Browning N, et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr 2014;168(8):729–736. doi: 10.1001/jamapediatrics.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veiby G, Engelsen BA, Gilhus NE. Early child development and exposure to antiepileptic drugs prenatally and through breastfeeding: a prospective cohort study on children of women with epilepsy. JAMA Neurol 2013;70 (11):1367–1374. doi: 10.1001/jamaneurol.2013.4290. [DOI] [PubMed] [Google Scholar]
- 54.Bjørk MH, Veiby G, Reiter SC, et al. Depression and anxiety in women with epilepsy during pregnancy and after delivery: a prospective population-based cohort study on frequency, risk factors, medication, and prognosis. Epilepsia 2015;56(1):28–39. doi: 10.1111/epi.12884. [DOI] [PubMed] [Google Scholar]
- 55.Pack AM, Davis AR, Kritzer J, et al. Antiepileptic drugs: are women aware of interactions with oral contraceptives and potential teratogenicity? Epilepsy Behav 2009;14(4):640–644. doi: 10.1016/j.yebeh.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep 2010;59(RR-4):1–86. [PubMed] [Google Scholar]
- 57.Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception 2011;83(1):16–29. doi: 10.1016/j.contraception.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Trussell J. Contraceptive failure in the United States. Contraception 2011;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herzog AG. Differential impact of antiepileptic drugs on the effects of contraceptive methods on seizures: Interim findings of the epilepsy birth control registry. Seizure 2015;28:71–75. doi: 10.1016/j.seizure.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Contraception for adolescents. Pediatrics 2014;134(4):e1244–e1256. doi: 10.1542/peds.2014-2299. [DOI] [PubMed] [Google Scholar]
- 61.Bounds W, Guillebaud J. Observational series on women using the contraceptive Mirena concurrently with anti-epileptic and other enzyme-inducing drugs. J Fam Plann Reprod Health Care 2002;28(2):78–80. [DOI] [PubMed] [Google Scholar]
- 62.Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure 2015;28:66–70. doi: 10.1016/j.seizure.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Lange J, Teal S, Tocce K. Decreased efficacy of an etonogestrel implant in a woman on antiepileptic medications: a case report. J Med Case Rep 2014;8(1):43. doi: 10.1186/1752-1947-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenfeld WE, Doose DR, Walker SA, Nayak RK. Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 1997;38(3):317–323. doi: 10.1111/j.1528-1157.1997.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 65.Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia 2003;44(4):540–549. doi: 10.1046/j.1528-1157.2003.55602.x. [DOI] [PubMed] [Google Scholar]
- 66.Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia 2007;48(3):484–489. doi: 10.1111/j.1528-1167.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 67.Contin M, Albani F, Ambrosetto G, et al. Variation in lamotrigine plasma concentrations with hormonal contraceptive monthly cycles in patients with epilepsy. Epilepsia 2006;47(9):1573–1575. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 68.Sabers A, Wolf P, Møller A, et al. A prospective, randomized, multicentre trial for the treatment of refractory status epilepticus; experiences from evaluating the effect of the novel drug candidate, NS1209. Epilepsy Res 2013;106(1–2):292–295. doi: 10.1016/j.eplepsyres.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Sidhu J, Job S, Singh S, Philipson R. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol 2006;61(2):191–199. doi: 10.1111/j.1365-2125.2005.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.FDA approved labeling text. Highlights of prescribing information, initial U.S. approval, serious psychiatric and behavioral reactions. Fycompa prescribing information. U.S. Food and Drug Administration; 2012. www.accessdata.fda.gov/drugsatfda_docs/label/2012/202834lbl.pdf. Published October 22, 2012. Accessed December 1, 2015. [Google Scholar]
- 71.Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Clinical guidance: drug interactions with hormonal contraception. www.fsrh.org/pdfs/CEUGuidanceDrugInteractionsHormonal.pdf. Published January 2011. Accessed December 1, 2015.
- 72.Schwenkhagen AM, Stodieck SRG. Which contraception for women with epilepsy? Seizure 2008;17(2):145–150. doi: 10.1016/j.seizure.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Herzog AG. Catamenial epilepsy: update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure 2015;28:18–25. doi: 10.1016/j.seizure.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 74.Harden CL, Pennell PB. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neurol 2013;12(1):72–83. doi: 10.1016/S1474-4422(12)70239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim LL, Foldvary N, Mascha E, Lee J. Acetazolamide in women with catamenial epilepsy. Epilepsia 2001;42(6):746–749. [DOI] [PubMed] [Google Scholar]
- 76.Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy. A model for evaluating anticonvulsants. Lancet 1982;2(8289):71–73. [DOI] [PubMed] [Google Scholar]
- 77.Herzog AG. Clomiphene therapy in epileptic women with menstrual disorders. Neurology 1988;38(3):432–434. [DOI] [PubMed] [Google Scholar]
- 78.Bauer J, Wildt L, Flügel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol 1992;239(5):284–286. [DOI] [PubMed] [Google Scholar]
- 79.Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology 1984;34(9):1255–1258. [DOI] [PubMed] [Google Scholar]
- 80.Herzog AG, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology 2012;78(24):1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]