Abstract
Background
Venom immunotherapy (VIT) is commonly used for preventing further allergic reactions to insect stings in people who have had a sting reaction. The efficacy and safety of this treatment has not previously been assessed by a high‐quality systematic review.
Objectives
To assess the effects of immunotherapy using extracted insect venom for preventing further allergic reactions to insect stings in people who have had an allergic reaction to a sting.
Search methods
We searched the following databases up to February 2012: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library, MEDLINE (from 1946), EMBASE (from 1974), PsycINFO (from 1806), AMED (from 1985), LILACS (from 1982), the Armed Forces Pest Management Board Literature Retrieval System, and OpenGrey. There were no language or publication status restrictions to our searches. We searched trials databases, abstracts from recent European and North American allergy meetings, and the references of identified review articles in order to identify further relevant trials.
Selection criteria
Randomised controlled trials of venom immunotherapy using standardised venom extract in insect sting allergy.
Data collection and analysis
Two authors independently undertook study selection, data extraction, and assessment of risk of bias. We identified adverse events from included controlled trials and from a separate analysis of observational studies identified as part of a National Institute for Health and Clinical Excellence Health Technology Assessment.
Main results
We identified 6 randomised controlled trials and 1 quasi‐randomised controlled trial for inclusion in the review; the total number of participants was 392. The trials had some risk of bias because five of the trials did not blind outcome assessors to treatment allocation. The interventions included ant, bee, and wasp immunotherapy in children or adults with previous systemic or large local reactions to a sting, using sublingual (one trial) or subcutaneous (six trials) VIT. We found that VIT is effective for preventing systemic allergic reaction to an insect sting, which was our primary outcome measure. This applies whether the sting occurs accidentally or is given intentionally as part of a trial procedure.
In the trials, 3/113 (2.7%) participants treated with VIT had a subsequent systemic allergic reaction to a sting, compared with 37/93 (39.8%) untreated participants (risk ratio [RR] 0.10, 95% confidence interval [CI] 0.03 to 0.28). The efficacy of VIT was similar across studies; we were unable to identify a patient group or mode of treatment with different efficacy, although these analyses were limited by small numbers. We were unable to confirm whether VIT prevents fatal reactions to insect stings, because of the rarity of this outcome.
Venom immunotherapy was also effective for preventing large local reactions to a sting (5 studies; 112 follow‐up stings; RR 0.41, 95% CI 0.24 to 0.69) and for improving quality of life (mean difference [MD] in favour of VIT 1.21 points on a 7‐point scale, 95% CI 0.75 to 1.67).
We found a significant risk of systemic adverse reaction to VIT treatment: 6 trials reported this outcome, in which 14 of 150 (9.3%) participants treated with VIT and 1 of 135 (0.7%) participants treated with placebo or no treatment suffered a systemic reaction to treatment (RR 8.16, 95% CI 1.53 to 43.46; 2 studies contributed to the effect estimate). Our analysis of 11 observational studies found systemic adverse reactions occurred in 131/921 (14.2%) participants treated with bee venom VIT and 8/289 (2.8%) treated with wasp venom VIT.
Authors' conclusions
We found venom immunotherapy using extracted insect venom to be an effective therapy for preventing further allergic reactions to insect stings, which can improve quality of life. The treatment carries a small but significant risk of systemic adverse reaction.
Keywords: Adult; Animals; Child; Humans; Allergens; Allergens/administration & dosage; Allergens/adverse effects; Allergens/immunology; Ants; Ants/immunology; Bee Venoms; Bee Venoms/administration & dosage; Bee Venoms/adverse effects; Bee Venoms/immunology; Desensitization, Immunologic; Desensitization, Immunologic/adverse effects; Desensitization, Immunologic/methods; Insect Bites and Stings; Insect Bites and Stings/immunology; Insect Bites and Stings/prevention & control; Randomized Controlled Trials as Topic; Wasp Venoms; Wasp Venoms/administration & dosage; Wasp Venoms/adverse effects; Wasp Venoms/immunology
Plain language summary
Immunotherapy for preventing allergic reactions to insect stings
At least 1 in 200 people have suffered a severe allergic reaction to a sting from a bee, wasp, or ant, and insect stings are the second most common cause of fatal allergic reactions in some countries. Treatment with insect venom, usually given by a course of injections (called venom immunotherapy), is thought to reduce the risk of allergic reactions to an insect sting. In this review, we evaluated the effectiveness of venom immunotherapy for preventing allergic reactions to insect stings.
From analysis of 7 studies, which included 392 participants, we found that this treatment reduces the chance of having a serious allergic reaction to an insect sting by 90%, a consistent finding between studies. Venom immunotherapy also significantly improves the quality of life of people who have had a serious allergic reaction to an insect sting by reducing anxiety and possible limitation of activities caused by fear of insects. However, almost 1 in 10 people treated with venom immunotherapy during the trials had an allergic reaction to their treatment. We were unable to find out whether venom immunotherapy prevents fatal allergic reactions to insect stings, because these are so rare. The decision whether to start venom immunotherapy depends on an accurate diagnosis, followed by careful assessment of a person's risk of having another allergic reaction to a sting, the degree to which the insect sting allergy affects their quality of life, and the risk of an allergic reaction to their treatment.
Summary of findings
for the main comparison.
| Venom immunotherapy compared with no venom immunotherapy for insect sting allergy | ||||||
|
Patient or population: adults and children with a previous allergic reaction to an insect sting Setting: allergy referral units in USA (3 trials), Netherlands (2 trials), Italy, and Australia Intervention: venom immunotherapy Comparison: no venom immunotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No venom immunotherapy | Venom immunotherapy | |||||
|
Any systemic reaction to an insect sting Follow‐up: between 6 weeks and 4 years |
Low‐risk population |
RR 0.10 (0.03 to 0.28) |
206 (7) | ⊕⊕⊕⊕ higha | ‐ | |
| 83 per 1000 | 8 per 1000 (2 to 23) | |||||
| Medium‐risk population | ||||||
| 398 per 1000 | 40 per 1000 (12 to 111) | |||||
| High‐risk population | ||||||
| 724 per 1000 | 72 per 1000 (22 to 203) | |||||
|
Large local reaction Follow‐up: between 12 weeks and 4 years |
Low‐risk population |
RR 0.41 (0.24 to 0.69) |
112 (5) | ⊕⊕⊕⊝ moderateb | ‐ | |
| 700 per 1000 | 287 per 1000 (168 to 483) | |||||
| Medium‐risk population | ||||||
| 885 per 1000 | 363 per 1000 (212 to 611) | |||||
| High‐risk population | ||||||
| 1000 per 1000 | 410 per 1000 (240 to 690) | |||||
|
Vespid Quality of Life Questionnaire ‐ end of treatment Scale from 0 to 7, where a higher score indicates less quality of life impairment. Follow‐up: 1 year |
Mean VQLQ score in the control group was 4.2 (95% CI 3.8 to 4.6) | Mean VQLQ score in the intervention groups was 1.21 points higher (0.75 to 1.67 points higher) | ‐ | 112 (2) | ⊕⊕⊕⊝ moderatec | ‐ |
|
Any systemic reaction to treatment Follow‐up: between 6 weeks and 1 year |
Low‐risk population |
RR 8.16 (1.53 to 43.46) |
285 (6) | ⊕⊕⊕⊝ moderated | ‐ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium‐risk population | ||||||
| 7 per 1000 | 57 per 1000 (11 to 304) | |||||
| High‐risk population | ||||||
| 31 per 1000 | 253 per 1000 (47 to 1347) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; VQLQ: Venom Quality of Life Questionnaire | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Assumed risks are based on the total control group risk across all included studies (medium‐risk population) and the included studies with the lowest (low‐risk population) and highest (high‐risk population) control group risks. Where possible, a control group with a non‐zero risk was used for the low‐risk population estimate. a. Although two studies had significant methodological limitations, particularly with respect to blinding of outcome assessors, the strong treatment effect was consistent across all four trials which contributed to this estimate, and assessment of systemic reactions may be less susceptible to bias than other more subjective outcomes. b. Only 1 study blinded outcome assessors, and there was significant heterogeneity between the outcomes of individual studies (I² statistic = 43%). c. The estimate of treatment effect for this subjective outcome measure relied on the assessment of non‐blinded study participants. d. The estimate of treatment effect for this outcome measure largely relied on one study. Although the study was of very high quality, it is unclear whether the estimate obtained from a small number of adverse reactions to a single ant venom extract in this study is representative for other forms of venom immunotherapy.
Background
Description of the condition
Insect sting allergy is a common cause of severe allergic reactions (anaphylaxis) and may be fatal. Reported allergy to insect stings is confined to insects from the order Hymenoptera, which includes bees, wasps, and ants. The population‐based prevalence of a history of Hymenoptera sting systemic reactions (SRs; that is, reactions involving areas of the body beyond the site of the sting) is approximately 0.3% to 7.5% depending on the diagnostic criteria used (Bilo 2005). In children, and based on few studies, the prevalence rate of SRs is lower than in adults, ranging from 0.15% to 0.8% (Bilo 2009). The prevalence of SRs to sting exposure among beekeepers is high and ranges from 14% to 32% (Muller 2005).
The relative prevalence of SRs to different species within the Hymenoptera order varies according to the prevalence of species that deliver potentially allergenic venom in the local region. For example, in South‐Eastern Australia, the jack jumper ant (Myrmecia pilosula) is a common cause of SRs. In the Americas, imported fire ants (a species of the genus Solenopsis) are common, but in both Europe and North America, SRs are most commonly caused by wasps (especially species of the genus Vespula) or honey bees (Apis mellifera) (Bilo 2005). In Europe, the term 'wasp' is typically used to include all members of the Vespidae family (e.g. 'European wasp', 'paper wasp', 'hornets', 'yellowjacket'); in North America, species of Vespula and Dolichovespula genera are termed 'yellowjackets', and species of the genus Polistes are termed 'wasp'. In this review, we used the European terminology, with the exception of the description of the interventions in the 'Characteristics of included studies' tables (Characteristics of included studies), where we retained the authors' original descriptions.
Although the incidence of insect sting mortality is low, ranging from 0.03 to 0.48 fatalities per 1,000,000 inhabitants per year (Antonicelli 2002; Bilo 2005), this may be an underestimate due to miscoding of the cause of death. It is estimated that 40 to 100 fatal sting reactions occur each year in the USA (Pumphrey 2000).
Recent studies in the UK and Australia have demonstrated that sting allergy comes second only to drug allergy as a cause of fatal anaphylaxis, accounting for two to three times as many deaths as food anaphylaxis (Clark 2007; Liew 2009). Men over 40 years of age with concomitant cardiovascular disease and a previous history of Hymenoptera sting allergy are at the highest risk of severe and fatal anaphylactic sting reactions. Other factors that increase the risk of a severe or fatal sting reaction include the use of certain medications (e.g. beta blockers), stings in the head or neck, bee stings, a rare condition called mastocytosis (where there is an accumulation of mast cells or mastocytes in various organs), and delayed or lack of use of adrenaline to treat the reaction (Bilo 2005; Rueff 2009). Recently, high baseline serum tryptase concentrations and angiotensin‐converting enzyme (ACE) inhibitor medication were found to be associated with increased risk of severe anaphylactic reactions (Bonadonna 2009; Rueff 2009).
Hymenoptera sting allergy can manifest as large local reactions (LLRs) or SRs, which are mostly immunoglobulin (IgE)‐mediated. A LLR is commonly defined as a swelling exceeding 10 cm in diameter and lasting at least 24 hours. In SRs, the skin, gastrointestinal, respiratory, and cardiovascular systems may be involved. In children, about 60% of SRs are mild and restricted to the skin, whereas in adults, respiratory or cardiovascular symptoms occur in about 70% of SRs (Brown 2004).
Allergic sting reactions are responsible for significant morbidity, and they may have a detrimental effect on health‐related quality of life. They may also deter people from carrying on their normal daily activities (Oude Elberink 2003).
Identifying the insect species to which the person has reacted is an important step for effective management of Hymenoptera sting allergy (Golden 2005). Diagnosis of Hymenoptera venom allergy is based on a clear clinical history of allergic reaction to Hymenoptera sting and demonstration of a positive skin test, venom‐specific allergy antibodies (IgE) in serum, or both.
The current treatment for insect venom allergy includes advice to minimise exposure to further stings, prescription of self‐administered adrenaline, and venom immunotherapy (VIT) in selected people. Although both pure insect venom and whole body extract have been used for allergen immunotherapy of people with insect sting allergy, this review only considered trials of VIT using insect venom. Venom immunotherapy using whole body extract is thought to be ineffective for preventing allergic reactions to bee or wasp stings (Hunt 1978), but it is thought to be effective for preventing allergic reactions to imported fire ant (Solenopsis invicta) stings (Freeman 1992).
Description of the intervention
Venom immunotherapy is the only allergen‐specific treatment available to prevent future severe reactions in those who are allergic to insect stings. Adults who experience an anaphylactic reaction to an insect sting have a 40% to 60% risk of reacting to a further sting (Bilo 2009). This risk may be reduced by VIT. Venom immunotherapy involves the administration of gradually increasing doses of the insect sting venom to which the person is allergic, in order to induce a state of tolerance to subsequent stings. The standard form of VIT is subcutaneous injections given at intervals of one to eight weeks for a period of three to five years. However, some centres use accelerated injection protocols in the initial phase (called rush or ultra‐rush updosing), and the use of sublingual VIT is also being investigated. Treatment plans of various durations have been devised in an effort to maximise protection, minimise side‐effects, and optimise convenience for the affected person. In North America, VIT is recommended for all adults who have experienced a SR following an insect sting (Moffit 2004), whereas in many European centres, VIT is only recommended for the most severe reactions (Bonifazi 2005). Venom immunotherapy is usually given for three to five years, and it is thought to lead to a long‐term reduction in the risk of allergic reaction to an insect sting, even after the treatment course has finished (Golden 2011).
How the intervention might work
Venom immunotherapy is thought to change the way in which the body's immune system responds to insect venom when it is introduced into the body through a sting. It modulates both T‐ and B‐cell response to the allergen (Ewan 2001), with early production of interleukin‐10 (IL‐10) and induction of T‐cell "anergy" (Akdis 2007). Venom immunotherapy is also associated with an increase in venom‐specific IgG4 and a gradual fall in specific IgE (Wilson 1994). Although the majority of adverse effects of VIT are very mild, there is a risk of systemic allergic reaction to the treatment (Mosbech 2000). The success rate of VIT varies in different centres. Accidental (field) sting and sting challenge are the most reliable means of evaluating the effectiveness of VIT since there is a poor correlation between levels of allergen‐specific serum immunoglobulin and risk of reacting to a sting (Senti 2006). Sting challenges are commonly used in clinical trials, but they may elicit inconsistent responses, can carry a significant risk of severe allergic reaction, and may induce further sensitisation (Bilo 2005).
Why it is important to do this review
Despite its common use for the prevention of anaphylactic reactions to insect stings, there has been no high‐quality systematic evaluation of the effectiveness and safety of insect VIT. There is some variation in the use of VIT in clinical practice, and a precise estimate of the overall treatment efficacy and safety would be valuable to inform clinical decision‐making and policy.
Objectives
To assess the effects of immunotherapy using extracted insect venom for preventing further allergic reactions to insect stings in people who have had an allergic reaction to a sting.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing VIT with placebo, no treatment, or back‐up treatment, such as education and provision of self‐administered adrenaline, for prevention of insect sting reactions.
Types of participants
We included all participants with previous systemic reactions (SR) or large local reactions (LLR) to any insect sting, a positive skin test, serum‐specific IgE, or both, to insect venom in this review, regardless of age, gender, ethnicity, or duration of insect sting allergy.
Types of interventions
Interventions
We included studies using standardised venom extract in any form of immunotherapy (subcutaneous or sublingual). We included all appropriate allergens at all doses and all durations of treatment. We also planned to include studies that used a mix of different extracts, e.g. bee and wasp together. We did not include studies that used whole body extract.
Control
The control intervention took the form of a placebo, no treatment, or back‐up treatment for the prevention of fatal insect sting anaphylaxis, such as education and provision of self‐administered adrenaline. In trials comparing more than one treatment arm to a control group, we only included the treatment arm using standard venom extract compared to a control group.
Types of outcome measures
Primary outcomes
Systemic reaction to a 'field' insect sting or a sting challenge during treatment. Where studies reported multiple time points for this outcome measure, we used the closest time point to the end of VIT. Where possible, we graded SRs as mild, moderate, or severe using the scoring method of Brown (Brown 2004). Where SRs were graded using the method of Mueller (Mueller 1966), we classed grade 1 reactions as 'mild', grades 2 and 3 as 'moderate', and grade 4 as 'severe'.
Fatal SR due to a field or challenge insect sting over the same period.
Secondary outcomes
Large local reactions to a field sting or sting challenge during treatment or during the 10 years following treatment.
Quality of life or anxiety score, assessed using a published scale (Oude Elberink 2002).
Adverse events to immunotherapy (local reaction, systemic reaction).
Search methods for identification of studies
We aimed to identify all relevant trials regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 21 February 2012:
the Cochrane Skin Group Specialised Register using the following terms: allerg* and (bite* or sting*) and immuno*;
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library using the search strategy in Appendix 1;
MEDLINE (from 1946) using the strategy in Appendix 2;
EMBASE (from 1974) using the strategy in Appendix 3;
PsycINFO (from 1806) using the strategy in Appendix 4;
AMED (Allied and Complementary Medicine, from 1985) using the strategy in Appendix 5; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 6.
We also searched the Armed Forces Pest Management Board Literature Retrieval System (LRS) (http://lrs.afpmb.org/) on 21 February 2012, using the terms 'venom AND immunotherapy' and 'allergy AND [immunotherapy OR desensitization]'.
Trials registers
We searched for reports of trials in the following databases on 21 February 2012:
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The Ongoing Skin Trials Register (www.nottingham.ac.uk/ongoingskintrials).
Searching other resources
Handsearching
We searched the proceedings of the European Academy of Allergy and Clinical Immunology (EAACI) from 2008 to 2010 and the American Academy of Allergy, Asthma & Immunology (AAAAI) from 2008 to 2011 for relevant studies.
Reference lists
We searched the reference lists of studies and review articles for further references to relevant trials.
Grey literature
We searched for grey literature through the OpenGrey database (previously called 'System for Information on Grey Literature in Europe') at http://opensigle.inist.fr/ using the term 'venom immunotherapy' on 28th December 2010. This search was not run again as no new material has been added to the database since 2005.
Correspondence
We contacted the authors of all included trials for additional data and clarification of methodology. We contacted VIT product manufacturers for information on published or unpublished studies relevant to our review ‐ HollisterStier, Stallergenes, ALK‐Abello, and Allergy Therapeutics.
Language
We imposed no language restrictions on our search. We included studies published in languages other than English if they fulfilled the inclusion criteria.
Adverse effects
In addition to looking for adverse effects of VIT in the included studies, we included data from an adverse events analysis of observational studies, undertaken as part of a National Institute for Health and Clinical Excellence Health Technology Assessment of Pharmalgen VIT (Hockenhull 2012).
Data collection and analysis
Selection of studies
ME and RB independently reviewed the titles and abstracts of the studies identified by the literature search. We assessed included studies against the preset inclusion criteria.
Data extraction and management
ME and RB independently extracted data from the included studies onto a specially‐prepared data extraction form. RB and ME contacted all trial authors to clarify any questions about methodology and to obtain any missing data. ME entered all quantitative results into Review Manager 5 (RevMan), and RB checked this.
Assessment of risk of bias in included studies
We assessed and documented the methodological quality of the included studies. Two authors (ME and RB) independently assessed the risk of bias of each trial using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2009). We made judgments on the risk of bias in six domains: sequence generation, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. We planned to incorporate this assessment into the interpretation of results by performing sensitivity analyses, excluding studies with the highest risk of bias. Each domain is addressed in the 'Risk of bias' table for each study, which is part of the 'Characteristics of included studies' table. These judgments are categorised as 'low risk of bias', 'high risk of bias', or 'unclear risk of bias'.
Measures of treatment effect
We calculated the risk ratio (RR) with 95% confidence intervals (CI) for dichotomous (binary) data. We calculated the mean difference (MD) with 95% CI when continuous outcomes were measured on standard scales. We performed all analysis on the basis of intention‐to‐treat. For analysis of risk of systemic reaction, we used the number of participants stung during the follow‐up period as the denominator in each study. However, we performed a secondary analysis using the number of participants randomised and assessed for possible sting reaction during follow‐up as the denominator in each study.
Unit of analysis issues
For cross‐over trials we planned to use the first treatment period as a parallel‐group study and ignore the crossing‐over, since a cross‐over design might not be appropriate for immunotherapy studies. We listed non‐randomised controlled studies, but did not discuss them further. When studies reported more than one active intervention, we planned to analyse the active groups separately, but divide the number of participants in the control group. Where studies reported outcomes as non‐parametric statistics, we planned to include these in meta‐analysis where possible by asking authors for original datasets.
Dealing with missing data
If we noted significant dropout of participants, we planned to conduct an intention‐to‐treat analysis without imputing missing data. We contacted all authors for details about study design, descriptive statistics, or other information as necessary. We only analysed the available data and planned to discuss the impact of the missing data on our findings where large amounts of outcome data were missing.
Assessment of heterogeneity
We assessed heterogeneity among trials using the I² statistic (I² statistic > 30% was considered as significant heterogeneity, and I² statistic > 80% was considered as substantial heterogeneity). Where substantial heterogeneity existed between studies for the primary outcome, we planned to explore the reasons for heterogeneity and not pool trial results. However, there was a low level of heterogeneity between trials for the primary outcome.
Assessment of reporting biases
We planned to assess publication bias using a funnel plot for the primary outcome measures if at least 10 trials were included in these analyses; however, we had less than 10 included studies.
Data synthesis
We used Review Manager 5 to analyse data. Data were analysed using the intention‐to‐treat principle, i.e. all participants were analysed in the treatment group to which they were randomised, independent of their actual treatment. We combined all relevant data extracted from included studies in a meta‐analysis provided that there was sufficient homogeneity (i.e. I² statistic ≤ 80%) amongst studies. Where substantial heterogeneity (I² statistic > 80%) was found, we planned to explore this by prespecified sensitivity and subgroup analysis. We used a random‐effects meta‐analysis as an overall summary. Where meta‐analyses were not applicable, we used a narrative synthesis of outcomes from relevant studies.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses to explore potential sources of heterogeneity, with appropriate tests of interaction. Prespecified subgroups were as follows:
Type of immunotherapy: subcutaneous versus sublingual VIT.
Age of participants: 18 years or over and under 18 years.
Duration of immunotherapy: up to 1 year, 1 to 3 years, and over 3 years.
Type of insect sting allergy: bees, wasps, and ants.
Type of immunotherapy updosing regimen: rush/ultra rush versus standard weekly or 2‐weekly updosing.
Sensitivity analysis
Where substantial heterogeneity was found in meta‐analysis of primary outcomes (I² statistic > 80%), we planned to explore possible reasons for this in sensitivity analyses, including quality of included studies. However, we did not undertake sensitivity analyses because of the small number of trials contributing data to the analyses and the low level of heterogeneity between trial outcomes for most analyses.
Results
Description of studies
Results of the search
The search identified 316 titles. We reviewed the full articles for 31 titles, and these yielded 10 separate publications relating to 6 RCTs (Brown 2003; Hunt 1978; Oude Elberink 2006; Oude Elberink 2009; Severino 2008; Valentine 1990) and 1 quasi‐randomised controlled trial (Golden 2009), including 392 participants for inclusion in the review. A search of references from all included studies and a review of all identified review articles did not identify any new trial of VIT that could be included in the systematic review. We contacted the authors of all the trials for original data and clarification of methods; we received further details from the authors or their collaborators for all trials (Brown 2003; Golden 2009; Hunt 1978; Severino 2008; Oude Elberink 2006; Oude Elberink 2009; Valentine 1990).
For two RCTs, earlier publications described outcomes in a subset of participants that were also included in a later report (Oude Elberink 2006; Valentine 1990).
Included studies
Of the seven trials included in the review, one studied children (Valentine 1990); one studied both adults and children (Hunt 1978); and five studied adults (Brown 2003; Golden 2009; Oude Elberink 2006; Oude Elberink 2009; Severino 2008). Two studied participants with a history of LLR (Golden 2009; Severino 2008); two studied participants with a history of cutaneous SR to an insect sting (Oude Elberink 2009; Valentine 1990); and three studied participants with a history of either any SR or anaphylaxis (Brown 2003; Hunt 1978; Oude Elberink 2006). One study was restricted to participants allergic to ant stings (Brown 2003); two studies were restricted to participants allergic to yellowjacket stings (Oude Elberink 2006; Oude Elberink 2009); one, to honeybee (Severino 2008); and three studies included participants allergic to a range of insect stings. One trial studied sublingual immunotherapy (Severino 2008), and the other six studied subcutaneous immunotherapy. The duration of treatment was less than a year in four studies (Brown 2003; Golden 2009; Hunt 1978; Severino 2008) and a year or longer in three studies (Oude Elberink 2006; Oude Elberink 2009; Valentine 1990). Four studies (Brown 2003; Golden 2009; Hunt 1978; Severino 2008) assessed rates of SR by sting challenge, and three studies (Oude Elberink 2006; Oude Elberink 2009; Valentine 1990) assessed rates of SR by recording of accidental field stings.
Excluded studies
Reasons for rejecting the other 21 titles were as follows: no appropriate control group (15), not a randomised controlled trial (5), not VIT (1). Please see the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
Full details are shown in the 'Characteristics of included studies' tables. Please see the 'Risk of bias' graph (review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies) (Figure 1) and the 'Risk of bias' summary (review authors' judgements about each 'Risk of bias' item for each included study) (Figure 2).
1.
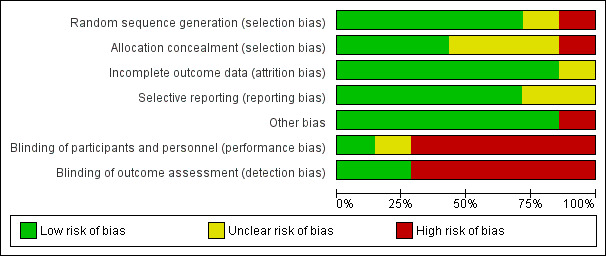
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
2.
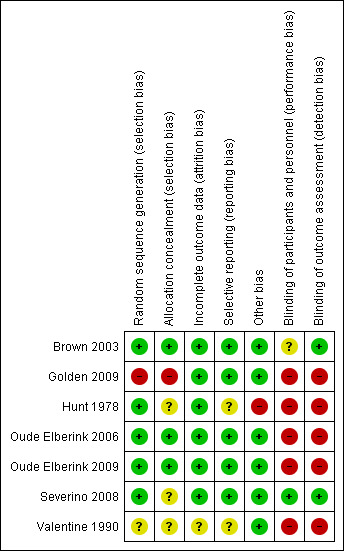
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Random sequence generation
There was a low risk of bias related to random sequence generation in five studies (Brown 2003; Hunt 1978; Oude Elberink 2006; Oude Elberink 2009; Severino 2008), unclear risk in one study (Valentine 1990), and high risk in one study (Golden 2009).
Allocation
There was a low risk of bias related to allocation concealment in three studies (Brown 2003; Oude Elberink 2006; Oude Elberink 2009), where a third party was used for treatment allocation. We judged there was an unclear risk in three studies (Hunt 1978; Severino 2008; Valentine 1990) due to insufficient information provided, and a high risk in one study (Golden 2009) due to use of a predictable sequence for randomisation.
Blinding
There was a low risk of bias related to blinding in one study (Severino 2008), an unclear risk in one study (Brown 2003), and a high risk in five studies (Golden 2009; Hunt 1978; Oude Elberink 2006; Oude Elberink 2009; Valentine 1990) due to absent blinding of physicians or outcome assessors.
In the study with unclear risk related to blinding, the reason was that 12 of 35 VIT‐treated participants were unblinded prior to the assessment of the primary outcome measure, following an interim analysis (Brown 2003). However, this did not clearly introduce a risk of bias into the study because outcomes were similar in this group to those who remained blinded.
Incomplete outcome data
There was a low risk of bias related to incomplete outcome data in six studies where loss to follow up rates were low (Brown 2003; Golden 2009; Hunt 1978; Oude Elberink 2006; Oude Elberink 2009; Severino 2008). In one study, loss to follow up rates were unclear (Valentine 1990).
Selective reporting
There was a low risk of bias related to selective reporting of outcomes in five studies where the specified outcomes in the methodology were reported in the results (Brown 2003; Golden 2009; Oude Elberink 2006; Oude Elberink 2009; Severino 2008), and for two studies this was unclear (Hunt 1978; Valentine 1990).
Other potential sources of bias
In 1 study, there was a significant risk of bias because the primary outcome measure was not assessed in 8 of 20 placebo‐treated participants, due to safety concerns after an interim analysis (Hunt 1978). In the other studies, there was a low risk of other potential bias.
Effects of interventions
See: Table 1
Primary outcomes
Systemic reaction to field or challenge sting
Meta‐analysis of data from 6 of our 7 included studies (with a total of 205 participants stung during follow‐up) showed that VIT significantly reduced our primary outcome measure, risk of SR to an insect sting (RR 0.10, 95% CI 0.03 to 0.28; Analysis 1.1), with no significant heterogeneity between studies (I² statistic = 0%). Data from just four of the studies (Brown 2003; Hunt 1978; Severino 2008; Valentine 1990) contributed to this effect estimate, because there were no systemic reactions to follow‐up stings at all in two studies (Golden 2009; Oude Elberink 2009).
1.1. Analysis.
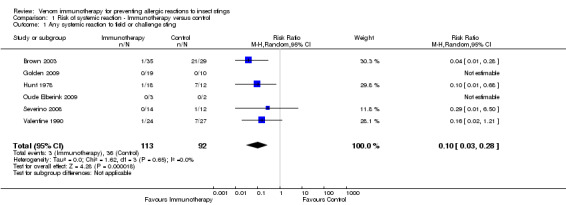
Comparison 1 Risk of systemic reaction ‐ Immunotherapy versus control, Outcome 1 Any systemic reaction to field or challenge sting.
For 124 participants who were stung in 5 studies information was available on severity of SR. Venom immunotherapy was significantly effective for reducing mild SRs (124 stung participants; RR 0.15, 95% CI 0.03 to 0.85; I² statistic = 0%; 2 studies contributed to effect estimate; Analysis 1.2) and severe SRs (RR 0.05, 95% CI 0.00 to 0.81; 1 study contributed to effect estimate; Analysis 1.4). The effect of VIT in reducing the risk of moderate SRs (RR 0.06, 95% CI 0.00 to 1.09; 1 study contributed to effect estimate; Analysis 1.3) or SR needing treatment with adrenaline (RR 0.07, 95% CI 0.00 to 1.12; I² statistic = 43%; 2 studies contributed to effect estimate; Analysis 1.5) was not statistically significant. The reason for the significant heterogeneity between study outcomes was not clear.
1.2. Analysis.
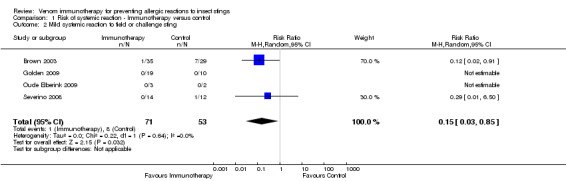
Comparison 1 Risk of systemic reaction ‐ Immunotherapy versus control, Outcome 2 Mild systemic reaction to field or challenge sting.
1.4. Analysis.
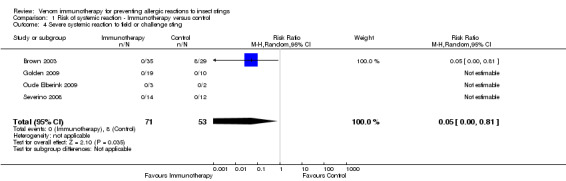
Comparison 1 Risk of systemic reaction ‐ Immunotherapy versus control, Outcome 4 Severe systemic reaction to field or challenge sting.
1.3. Analysis.
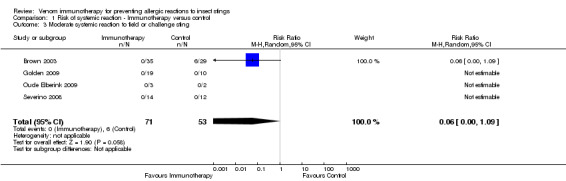
Comparison 1 Risk of systemic reaction ‐ Immunotherapy versus control, Outcome 3 Moderate systemic reaction to field or challenge sting.
1.5. Analysis.
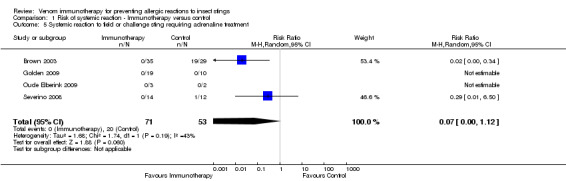
Comparison 1 Risk of systemic reaction ‐ Immunotherapy versus control, Outcome 5 Systemic reaction to field or challenge sting requiring adrenaline treatment.
In the study by Oude Elberink 2006, no participants treated with immunotherapy were stung during follow‐up, and one participant in the control group had a severe SR to a sting during follow‐up, requiring treatment with adrenaline. These data could not be included in a meta‐analysis due to absence of follow‐up stings in one treatment arm, but we included them in calculations of absolute risk of systemic reaction. These showed that 3/113 (2.7%) participants treated with VIT had a subsequent systemic allergic reaction to a sting, compared with 37/93 (39.8%) untreated participants. A summary of the major study findings, including estimates of treatment efficacy in populations at different baseline risk, is shown in Table 1.
In studies that relied on accidental field stings for assessment of risk of systemic reaction, we also analysed the data using the number of participants assessed as the denominator in each study. Meta‐analysis of data from all 7 studies (including 367 participants in this way) showed a similar reduction in risk of SR to an insect sting in the VIT‐treated group (RR 0.11, 95% CI 0.04 to 0.31; Analysis 2.1) with no significant heterogeneity between studies (I² statistic = 0%).
2.1. Analysis.
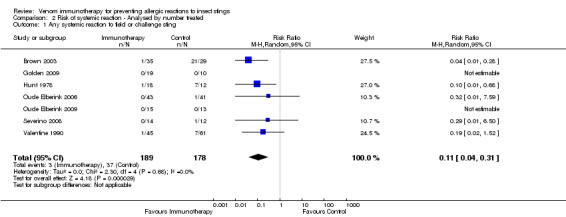
Comparison 2 Risk of systemic reaction ‐ Analysed by number treated, Outcome 1 Any systemic reaction to field or challenge sting.
Fatal systemic reaction to field or challenge sting
No study reported any fatal SR to a field or challenge sting, so it was not possible to assess whether VIT can prevent fatal sting anaphylaxis.
Secondary outcomes
Large local reactions
Five studies reported whether participants had developed a large local reaction (LLR) to an insect sting at follow‐up (Golden 2009; Oude Elberink 2006; Oude Elberink 2009; Severino 2008; Valentine 1990). Meta‐analysis of 4 of these studies with, in total, 111 participants who were stung showed that VIT significantly reduced the risk of a LLR to insect sting (RR 0.41, 95% CI 0.24 to 0.69; I² statistic = 43%; Analysis 3.1). In the fifth study (Oude Elberink 2006), no LLR occurred ‐ only one sting in the control group. The reason for the significant heterogeneity between study outcomes was not clear.
3.1. Analysis.
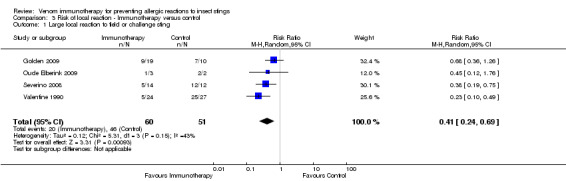
Comparison 3 Risk of local reaction ‐ Immunotherapy versus control, Outcome 1 Large local reaction to field or challenge sting.
One study (Severino 2008) also reported smaller LLRs in VIT‐treated participants than placebo‐treated participants following a sting challenge (median LLR size = 8.5 cm in VIT‐treated participants and 20.5 cm in placebo‐treated participants; P = 0.02).
Quality of life outcomes
Two studies reported quality of life using the Vespid Quality of Life Questionnaire (VQLQ) in 112 participants (Oude Elberink 2006; Oude Elberink 2009). Quality of life was significantly improved after 1 year of VIT compared with no VIT (mean difference [MD] 1.21 points on a 7‐point scale, 95% CI 0.75 to 1.67; I² statistic = 0%; Analysis 4.1). When analysed as change during treatment, there was a greater improvement in quality of life after VIT compared with no VIT (MD 1.30, 95% CI 0.97 to 1.63; I² statistic = 0%; Analysis 4.2). When analysed as a response rate, where an improvement of ≥ 0.5 points is regarded as a significant response to treatment, VIT increased the chance of a significant quality of life improvement (RR 7.11, 95% CI 3.02 to 16.71; I² statistic = 0%; Analysis 4.3). The same two studies (Oude Elberink 2006; Oude Elberink 2009) reported participant views of the burden of treatment in both VIT and control arms using a 7‐point scale where a score of 1 to 3 was a positive view of treatment and a score of 4 to 7 was a negative or neutral view of treatment. In participants with a history of anaphylactic reaction to yellowjacket sting, 41 of 43 immunotherapy‐treated participants had a positive overall assessment of their treatment after 1 year, compared with 19 of 41 participants in the control group (RR 2.01, 95% CI 1.43 to 2.82). In participants with a history of cutaneous SR to yellowjacket sting, 14 of 15 VIT‐treated participants and 5 of 12 participants in the control group had a positive overall assessment of their treatment at 1 year (RR 2.24, 95% CI 1.13 to 4.43).
4.1. Analysis.
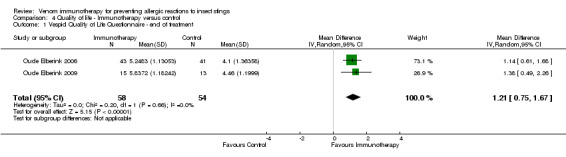
Comparison 4 Quality of life ‐ Immunotherapy versus control, Outcome 1 Vespid Quality of Life Questionnaire ‐ end of treatment.
4.2. Analysis.
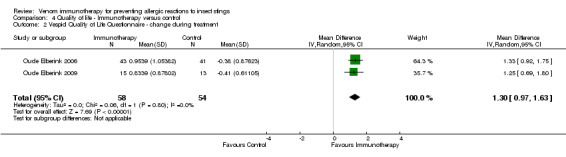
Comparison 4 Quality of life ‐ Immunotherapy versus control, Outcome 2 Vespid Quality of Life Questionnaire ‐ change during treatment.
4.3. Analysis.
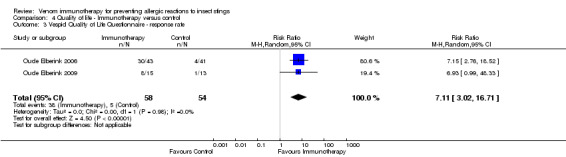
Comparison 4 Quality of life ‐ Immunotherapy versus control, Outcome 3 Vespid Quality of Life Questionnaire ‐ response rate.
Adverse effects
One study reported local reactions to subcutaneous VIT in 29 participants, but there was no statistically significant increase in risk of local reactions in immunotherapy versus no treatment (RR 9.35, 95% CI 0.59 to 147.09; Analysis 5.1). Six studies reported systemic reactions to treatment in 285 participants (Brown 2003; Golden 2009; Hunt 1978; Oude Elberink 2006; Oude Elberink 2009; Severino 2008). Immunotherapy increased the risk of systemic reactions (RR 8.16, 95% CI 1.53 to 43.46; I² statistic = 0%; 2 studies contributed to the effect estimate; Analysis 5.2). One study reported the number of systemic reactions per participant and per injection visit in 67 participants (Brown 2003), and it found a significantly increased risk of systemic reactions in participants treated with VIT (MD 1.00 reaction per participant; 95% CI 0.08 to 1.91; Analysis 5.3) (MD 0.04 systemic reactions per injection visit; 95% CI 0.01 to 0.07; Analysis 5.4).
5.1. Analysis.
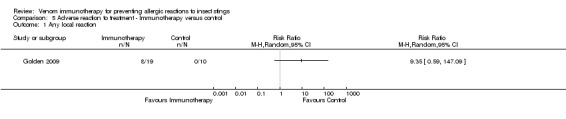
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 1 Any local reaction.
5.2. Analysis.
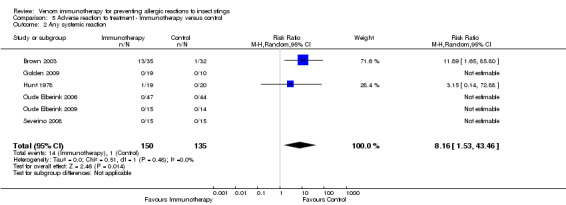
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 2 Any systemic reaction.
5.3. Analysis.
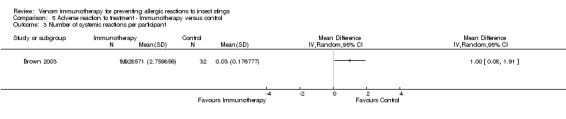
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 3 Number of systemic reactions per participant.
5.4. Analysis.
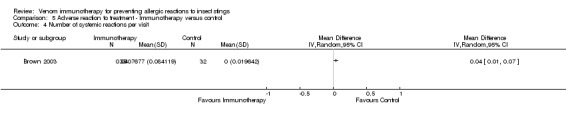
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 4 Number of systemic reactions per visit.
Five studies reported data on severity of systemic reactions to VIT in 246 participants (Brown 2003; Golden 2009; Oude Elberink 2006; Oude Elberink 2009; Severino 2008). When divided by severity of adverse reaction, VIT did not significantly increase the risk of mild systemic reaction to treatment (RR 15.58, 95% CI 0.94 to 259.56; Analysis 5.5), moderate systemic reaction to treatment (RR 2.74, 95% CI 0.30 to 25.05; Analysis 5.6), severe systemic reaction to treatment (RR 4.58, 95% CI 0.23 to 92.00; Analysis 5.7), or systemic reaction to treatment requiring adrenaline (RR 6.40, 95% CI 0.83 to 49.21; Analysis 5.8).
5.5. Analysis.
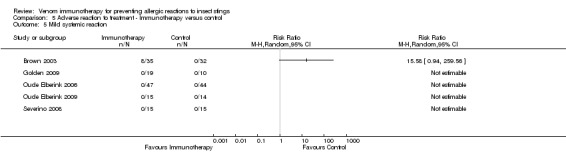
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 5 Mild systemic reaction.
5.6. Analysis.
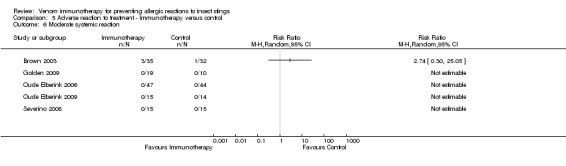
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 6 Moderate systemic reaction.
5.7. Analysis.
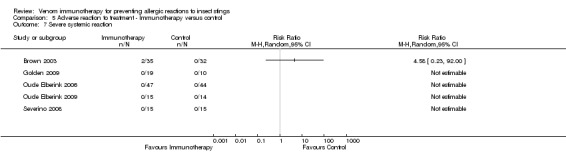
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 7 Severe systemic reaction.
5.8. Analysis.
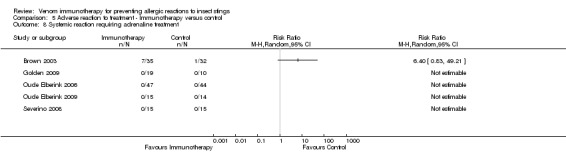
Comparison 5 Adverse reaction to treatment ‐ Immunotherapy versus control, Outcome 8 Systemic reaction requiring adrenaline treatment.
We also reviewed adverse events data from 11 observational studies (1446 participants) of Pharmalgen subcutaneous VIT (Carballada 2003; Carballada 2009; Carballada 2010; Fricker 1997; Haeberli 2003; Kalogeromitros 2010; Muller 1989; Muller 1992; Ramirez 1981; Sanchez‐Machin 2010; Schiavino 2004), identified as part of a National Institute for Health and Clinical Excellence Health Technology Assessment (Hockenhull 2012). Either Pharmalgen or a closely‐related product was used in at least three of the seven trials of subcutaneous VIT (Oude Elberink 2006; Oude Elberink 2009; Valentine 1990).
The adverse events data from observational studies are shown in Table 2.
1. Adverse effects ‐ observational studies (Pharmalgen).
| Study | Systemic reaction to treatment | Large local reaction to treatment |
| Carballada 2003 |
Bee = 37/3269 (1.1%) doses; 22/208 (10.6%) participants Wasp = 0/33 (0%) participants |
31/3269 (0.9%) doses; 26/208 (12.5%) participants 6/428 (1.4%) doses; 5/33 (15.2%) participants |
| Carballada 2009 | Bee = 2/21 (9.5%) participants | 3/21 (14.3%) participants |
| Carballada 2010 |
Bee = 50/438 (11.4%) participants (72 % grade 1 reactions; 4% grade 2; 10% grade 3; 1% grade 4) Wasp = 0/124 (0%) participants |
‐ |
| Fricker 1997 | Bee or wasp = 2/10 (20%) participants | ‐ |
| Haeberli 2003 |
Bee = 18/104 (17.3%) participants Wasp = 4/57 (7.0%) participants |
‐ |
| Kalogeromitros 2010 |
Bee = 7/29 (24.1%) participants Wasp = 2/18 (11.1%) participants 33/1520 (2.2%) doses overall |
‐ |
| Muller 1989 |
Bee = 30/67 (44.8%) participants; 31/148 (20.9%) doses (objective systemic reactions) |
‐ |
| Muller 1992 |
Wasp = 2/57 (4%) participants (objective systemic reactions) |
‐ |
| Ramirez 1981 | Bee or wasp = 0/22 (0%) participants | 36/859 (4.2%) doses |
| Sanchez‐Machin 2010 | Bee = 2/54 (3.7%) participants; 4/324 (1.2%) doses | 58/324 (17.9%) doses |
| Schiavino 2004 | Bee or wasp = 4/56 (7.1%) participants | 6/56 (10.7%) participants ('severe' local reactions) |
Systemic reactions to treatment occurred in 3.7% to 44.8% of participants treated with bee VIT. Overall, in the studies assessed, 131/921(14.2%) participants, or 72/3741 (1 in 52) doses, had a systemic reaction.
Systemic reactions to treatment occurred in 0% to 11.1% of participants treated with wasp VIT. Overall, 8/289 (2.8%) participants (per dose rate not available) had a systemic reaction.
Systemic reactions to treatment occurred in 0% to 20% of participants in reports of mixed groups of bee and wasp venom allergy treated with VIT. Overall, 6/88 (6.8%) participants, or 33/1520 (1 in 46) doses, had a systemic reaction. These compare with systemic reaction rates of 9.3% participants (Analysis 5.2) and 1 in 25 doses (95% CI 1 in 100, 1 in 14; Analysis 5.4) in the active arms of the trials.
Large local reactions occurred in 12.7%, 15.2%, and 11.5% of participants treated with VIT for bee, wasp, and a mixed group, respectively, at rates of 1 in 105, 1 in 71, and 1 in 13 doses, respectively.
No observational study or controlled trial reported any fatal adverse reaction, so it was not possible to estimate the fatality risk associated with VIT.
Subgroup analyses
We undertook 10 planned subgroup analyses and 2 unplanned subgroup analyses. We did not undertake further sensitivity analyses, because of the small number of trials contributing data to the analyses and the low level of heterogeneity between trial outcomes for most analyses.
First, we analysed our primary outcome measure, systemic reaction to a field or challenge sting, in five subgroup analyses. We found no evidence that the primary outcome differed according to the following:
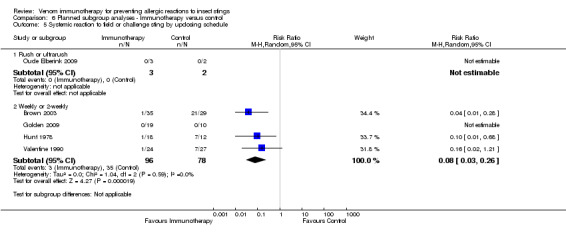
the route of administration of VIT, although the effect of sublingual immunotherapy when analysed alone was not statistically significant (subcutaneous VIT: RR 0.08, 95% CI 0.03 to 0.26) (sublingual VIT: RR 0.29, 95% CI 0.01 to 6.50) (test for subgroup differences: I² statistic = 0%; P = 0.46; Analysis 6.1);
age (for 0 to 18 years: RR 0.16, 95% CI 0.02 to 1.21) (for those aged > 18 years: RR 0.07, 95% CI 0.01 to 0.48) (test for subgroup differences: I² statistic = 0%; P = 0.58; Analysis 6.2);
duration of immunotherapy (under 1 year: RR 0.08, 95% CI 0.02 to 0.28) (1 to 3 years: RR not estimable) (over 3 years: RR 0.16, 95% CI 0.02 to 1.21) (test for subgroup differences: I² statistic = 0%; P= 0.56; Analysis 6.3);
insect species (for the honeybee: RR 0.29, 95% CI 0.01 to 6.50) (for the wasp: RR not estimable) (for the ant: RR 0.04, 95% CI 0.01 to 0.28) (test for subgroup differences: I² statistic = 11.5%; P = 0.29; Analysis 6.4);
or updosing schedule (for rush/ultra‐rush: RR not estimable) (for weekly/2‐weekly dosing: RR 0.08, 95% CI 0.03 to 0.26; Analysis 6.5).
6.1. Analysis.
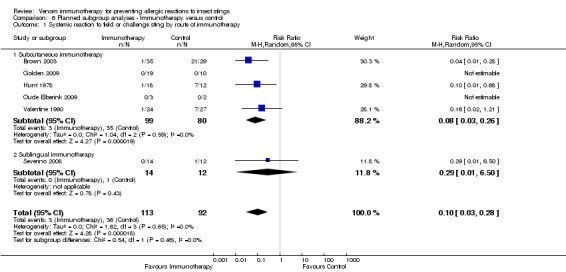
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 1 Systemic reaction to field or challenge sting by route of immunotherapy.
6.2. Analysis.
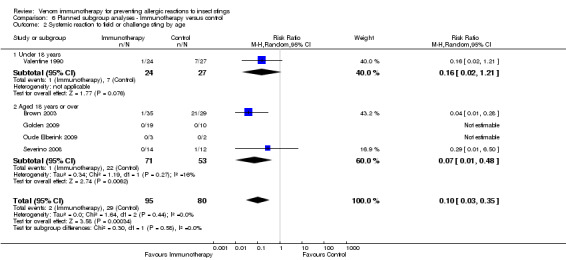
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 2 Systemic reaction to field or challenge sting by age.
6.3. Analysis.
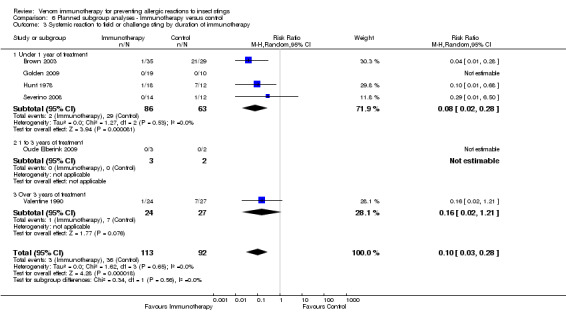
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 3 Systemic reaction to field or challenge sting by duration of immunotherapy.
6.4. Analysis.
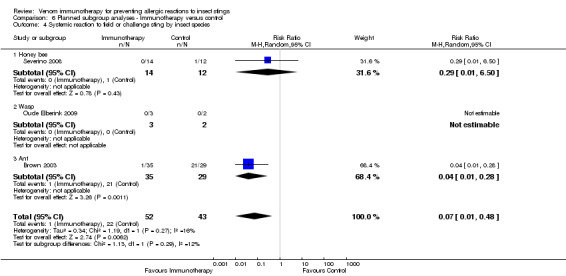
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 4 Systemic reaction to field or challenge sting by insect species.
6.5. Analysis.
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 5 Systemic reaction to field or challenge sting by updosing schedule.
Next, we analysed the risk of a systemic adverse reaction to VIT in the same five subgroups. We found no evidence that risk of systemic adverse reaction differed according to the following:
route of VIT (subcutaneous: RR 8.16, 95% CI 1.53 to 43.46; Analysis 6.6) (sublingual: RR not estimable);
age (0 to 18 year: RR not estimable) (aged > 18 years: RR 11.89, 95% CI 1.65 to 85.80; Analysis 6.7);
duration of immunotherapy (under 1 year: RR 8.16, 95% CI 1.53 to 43.46; Analysis 6.8) (1 to 3 years or over 3 years: RR not estimable);
insect species (for the honeybee and wasp: RR not estimable) (for the ant: RR 11.89, 95% CI 1.65 to 85.80; Analysis 6.9); or
updosing schedule (for rush/ultra‐rush: RR not estimable) (for weekly/2‐weekly dosing: RR 8.16, 95% CI 1.53 to 43.46; Analysis 6.10).
6.6. Analysis.
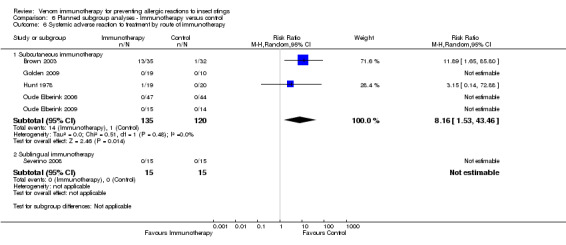
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 6 Systemic adverse reaction to treatment by route of immunotherapy.
6.7. Analysis.
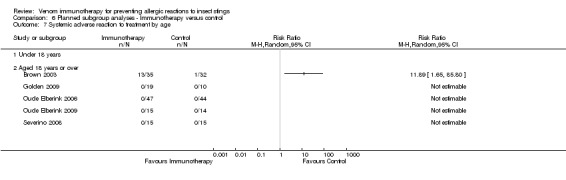
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 7 Systemic adverse reaction to treatment by age.
6.8. Analysis.
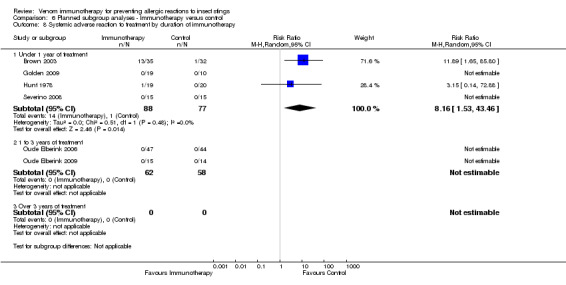
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 8 Systemic adverse reaction to treatment by duration of immunotherapy.
6.9. Analysis.
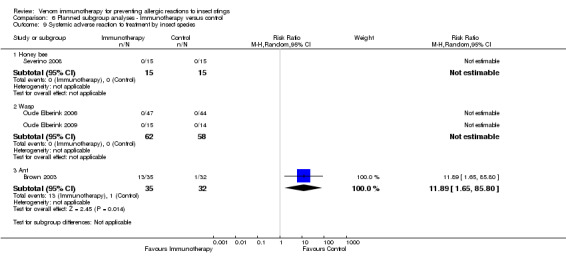
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 9 Systemic adverse reaction to treatment by insect species.
6.10. Analysis.
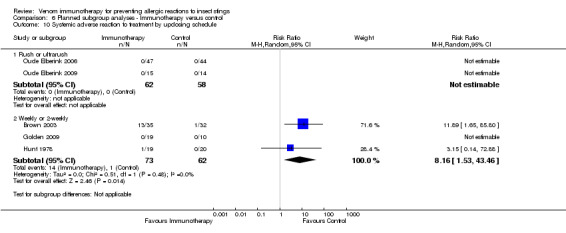
Comparison 6 Planned subgroup analyses ‐ Immunotherapy versus control, Outcome 10 Systemic adverse reaction to treatment by updosing schedule.
In a post‐hoc analysis, we found no evidence that the effect of VIT on risk of SR to an insect sting differed according to history of prior allergic reaction (RR 0.29, 95% CI 0.01 to 6.50), prior large local reaction (RR 0.08, 95% CI 0.03 to 0.26) or prior systemic reaction (test for subgroup differences: I² statistic = 0%; P = 0.46; Analysis 7.1).
7.1. Analysis.
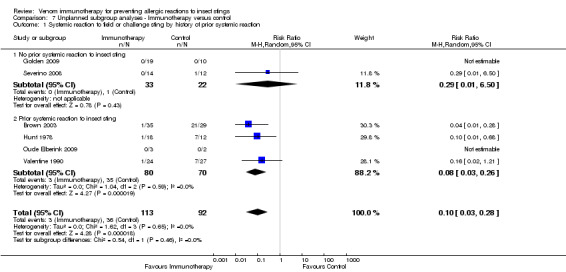
Comparison 7 Unplanned subgroup analyses ‐ Immunotherapy versus control, Outcome 1 Systemic reaction to field or challenge sting by history of prior systemic reaction.
We found a significant risk of systemic adverse reaction to VIT in those with a prior SR (RR 8.16, 95% CI 1.53 to 43.46; Analysis 7.2) and no reported occurrence of systemic adverse reaction to VIT in those with a prior LLR.
7.2. Analysis.
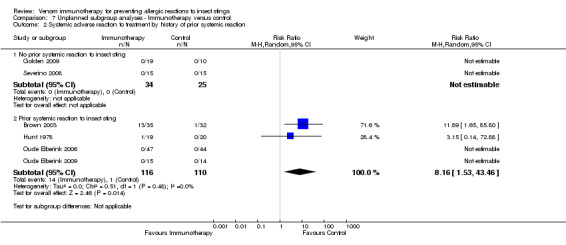
Comparison 7 Unplanned subgroup analyses ‐ Immunotherapy versus control, Outcome 2 Systemic adverse reaction to treatment by history of prior systemic reaction.
Discussion
Summary of main results
We identified 7 controlled clinical trials of venom immunotherapy (VIT) for preventing allergic reactions to insect stings; these trials included 392 participants with a prior history of allergic reaction to a sting. The studies included children and adult participants allergic to bee, wasp, and ant venom; those with severe systemic or simple large local reactions to stings; and both sublingual and subcutaneous treatment. Five studies were judged to have a significant risk of bias (Golden 2009; Hunt 1978; Oude Elberink 2006; Oude Elberink 2009; Valentine 1990), primarily due to outcome assessors not being blinded. We found that VIT significantly reduced the risk of a systemic allergic reaction to a future insect sting, a finding that was consistent between studies, although just four studies contributed to the effect estimate for the primary outcome. The treatment appeared to be effective for preventing allergic reactions of different severity, but we were unable to establish whether it can prevent fatal allergic reactions, because of their rarity.
In two non‐blinded studies, VIT resulted in significantly improved quality of life for participants, by reducing anxiety and limitations to activities caused by fear of insects, and in these two studies, almost all participants treated with VIT had a positive view of their treatment after one year (Oude Elberink 2006; Oude Elberink 2009). Very few participants in these two studies were stung following treatment, but they experienced an improved quality of life due to reduced anxiety and reduced restriction of activities related to fear of being stung. Immunotherapy was associated with a significant risk of a systemic adverse reaction to treatment, which occurred in 9.3% of participants treated with VIT in the controlled trials, reported in 1 trial as a rate of 1 systemic reaction per 25 injections. Analysis of adverse events data from non‐randomised studies of VIT found that 2.8% of participants treated with wasp VIT and 14.2% treated with bee VIT suffered a systemic adverse reaction to treatment, with wide variations according to the population studied. There were insufficient data to estimate the risk of fatal allergic reaction to treatment with VIT.
Subgroup analyses failed to identify a route of immunotherapy, participant age, duration of immunotherapy, insect species, or updosing schedule with a different efficacy or safety profile, although data were limited for many of these analyses by the small numbers of events.
Overall completeness and applicability of evidence
We found clear evidence for the efficacy of VIT in preventing further allergic reactions to insect stings. While the methodological quality of the studies was mixed, this finding was consistent across studies. It is uncertain whether this treatment can prevent fatal sting anaphylaxis, because of the rarity of that outcome, but there was an improved quality of life associated with treatment. We found adverse reaction rates were significant, and these varied in the observational studies according to insect species and patient population. For many people with a history of systemic reaction (SR) to an insect sting, the annual risk of a further sting from the same insect is relatively low (Hockenhull 2012). The decision to undertake VIT clearly needs careful assessment of the risk of a further insect sting occurring, a person's quality of life impairment and treatment preference, and the risk of a systemic adverse reaction to treatment for a particular insect species.
Quality of the evidence
The results of this review are based on analysis of relatively few trials and participants, but they are consistent for the primary outcome measure and some secondary outcomes.
We included one quasi‐randomised trial (Golden 2009), and the issue of whether to include quasi‐randomised trials was not specifically considered at the protocol stage. The quasi‐randomised nature of the trial only came to light in correspondence with the study authors after we had completed data analysis. Therefore, we elected to keep this study in the review, although its inclusion or exclusion does not significantly change the main study outcomes.
The value of the planned subgroup analyses was limited by small numbers of events in many cases. Further work is needed to establish whether there are differential benefits for VIT, in particular, patient groups or using particular modes of delivery. This may help to establish evidence‐based indications for initiating treatment. In particular, we only identified one trial of sublingual VIT; while this type of treatment may carry a reduced risk of systemic adverse reactions (Radulovic 2011), its clinical efficacy still needs to be investigated further. We excluded studies of whole body extract immunotherapy from the review, and a separate review of this intervention is warranted (Freeman 1992). We only identified two trials of VIT in participants with a prior history of LLR without systemic reaction to an insect sting, and it is not clear whether VIT is effective for preventing future systemic reactions in these participants. Other areas for further research are the optimal maintenance dose, dosing interval, and treatment duration.
Potential biases in the review process
The strengths of this review were the consistency of results over a wide variety of settings, patient groups, insect species, and to a limited extent, over different modes of treatment delivery and the high proportion of original data acquired from study authors. These meant that the review has clear‐cut findings, but the absence of blinding in some of the included studies and the relatively short follow‐up period in most of them should be noted.
Agreements and disagreements with other studies or reviews
One other systematic review of VIT has been undertaken (Watanabe 2010). It was limited to subcutaneous VIT and only identified three of the trials that we included. It was also complicated by considering two separate publications describing outcomes in participants from the same trial as two separate RCTs (Valentine 1990), which means their meta‐analysis was flawed. Nevertheless, they found that VIT reduces the risk of systemic reactions to an insect sting (odds ratio 0.29, 95% CI 0.10 to 0.87), and their conclusion that the risk‐benefit should be assessed in each case before initiating VIT is consistent with our conclusions. They did not address adverse effects in detail. Our findings that VIT is an effective treatment for allergy to insect stings are consistent with British, European, and North American practice guidelines (Bonifazi 2005; Golden 2011; Krishna 2011). The statement that VIT is not indicated for children with a history of systemic reactions limited to cutaneous involvement is included in some reviews and guidelines (Golden 2011; Krishna 2011; Watanabe 2010), but not others (Bonifazi 2005). This statement is based on the findings of 1 observational study that 12 of 89 (13%) such children who declined VIT treatment had a systemic reaction to a field sting during long‐term follow‐up, but no cases of severe systemic reaction were noted (Golden 2004).
Our findings suggest that quality of life improvement may be a valid indication for VIT in some such cases (Bonifazi 2005), and that in all cases, an assessment must be made of likelihood of a future sting, of a future sting reaction, and of an allergic reaction to treatment. A recent Health Technology Assessment of one manufacturer's VIT products concluded that VIT may only be cost effective for preventing systemic reactions in those at risk of > five insect stings per year, which would exclude most people with a previous systemic reaction to a sting from treatment (Hockenhull 2012). However, the treatment is likely to be cost effective for improving quality of life in a wider group of people, and this needs further study. While this and other forms of allergen immunotherapy have generally been proven to be clinically effective, there is a need for more data describing effects on overall quality of life and health economic outcomes and long‐term outcomes beyond the treatment period (Calderon 2011).
Authors' conclusions
Implications for practice.
In those who have had a previous allergic reaction to an insect sting, venom immunotherapy (VIT) using extracted insect venom is effective for preventing a further allergic reaction to a future sting and improves people's quality of life.
There is a significant risk of systemic allergic reaction to VIT during treatment, but this differs across insect species and populations.
The decision whether to initiate VIT depends on an accurate diagnosis, followed by careful assessment of a person's risk of further insect sting reactions, their quality of life, and their risk of a systemic reaction to VIT.
Implications for research.
Further research is needed to identify the patient groups who benefit most from this treatment, and the optimal treatment duration.
Future trials in this area should include quality of life and health economic outcomes.
Future trials should investigate modes of VIT, which may carry a reduced risk of systemic allergic reaction, particularly sublingual treatment, and outcome assessors should be blinded to treatment allocation.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2014 | Review declared as stable | There were no ongoing studies or studies awaiting classification listed in the last published review, but a search of MEDLINE and Embase in October 2013 found 2 more studies. However, the review team confirmed that neither of these trials would meet the review's inclusion criteria, so the update was postponed. Another search in December 2014 did not identify any further trials, so we have marked this review as stable. Our Trials Search Co‐ordinator will run a new search towards the end of 2015 to re‐assess whether an update is needed. |
Notes
There were no ongoing studies or studies awaiting classification listed in the last published review, but a search of MEDLINE and Embase in October 2013 found 2 more studies. However, the review team confirmed that neither of these trials would meet the review's inclusion criteria, so the update was postponed. Another search in December 2014 did not identify any further trials, so we have marked this review as stable. Our Trials Search Co‐ordinator will run a new search towards the end of 2015 to re‐assess whether an update is needed.
Acknowledgements
We are grateful to Alexandra Watanabe, Fabio Morato Castro, and Luiz Fonseca who initiated the protocol for this systematic review but were unable to complete the work due to other commitments. We are grateful to the trial authors, Simon Brown, David Golden, Gianni Passalacqua, and Kevin Hunt, for providing original data, clarification about methodology and data from their trials, or both, and to David Golden for also providing unpublished details for one trial (Valentine 1990).
The Cochrane Skin Group editorial base wishes to thank Ching‐Chi Chi who was the Key Editor for this review; Matthew Grainge and Philippa Middleton who were the Statistical and Methods Editors, respectively; the clinical referees, Cristoforo Incorvaia and James Tracy; and the consumer referee, David Glaser.
Appendices
Appendix 1. CENTRAL search strategy (Cochrane Library)
#1 MeSH descriptor Hymenoptera explode all trees #2 MeSH descriptor Arthropod Venoms explode all trees #3 (bee or wasp or ant or hymenoptera or hornet or venom) #4 MeSH descriptor Bees explode all trees #5 MeSH descriptor Ants explode all trees #6 MeSH descriptor Wasps explode all trees #7 MeSH descriptor Ant Venoms explode all trees #8 MeSH descriptor Bee Venoms explode all trees #9 MeSH descriptor Wasp Venoms explode all trees #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 MeSH descriptor Insect Bites and Stings explode all trees #12 MeSH descriptor Hypersensitivity explode all trees #13 MeSH descriptor Anaphylaxis explode all trees #14 MeSH descriptor Shock explode all trees #15 MeSH descriptor Death explode all trees #16 MeSH descriptor Edema explode all trees #17 MeSH descriptor Urticaria explode all trees #18 MeSH descriptor Airway Obstruction explode all trees #19 venom allerg* #20 hypersensitivity #21 anaphyla* #22 allerg* #23 swell* #24 edema #25 systemic reaction #26 hives #27 shock #28 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29 MeSH descriptor Immunotherapy explode all trees #30 MeSH descriptor Epinephrine explode all trees #31 MeSH descriptor Desensitization, Immunologic explode all trees #32 MeSH descriptor Dose‐Response Relationship, Immunologic explode all trees #33 adrenaline #34 epipen #35 (venom immunotherapy) #36 immunomodulatory #37 immune therapy #38 immunologic response #39 hyposensitization #40 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39) #41 (#10 AND #28 AND #40)
Appendix 2. MEDLINE search strategy (OVID)
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (human and animals)).sh. 10. 8 not 9 11. bees.mp. or exp BEES/ 12. bee venom.mp. or exp Bee Venoms/ 13. (honey adj bee).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 14. honeybee.mp. 15. hymenoptera.mp. or exp HYMENOPTERA/ 16. apis mellifera.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 17. wasps.mp. or exp WASPS/ 18. wasp venom.mp. or exp Wasp Venoms/ 19. vespid.mp. 20. vespula.mp. 21. white hornet.mp. 22. yellow jacket.mp. 23. yellow hornet.mp. 24. polistes.mp. 25. ants.mp. or exp ANTS/ 26. ant venom.mp. or exp Ant Venoms/ 27. exp Arthropod Venoms/ 28. solenopsis invicta.mp. 29. myrmecia pilosula.mp. 30. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. exp "Insect Bites and Stings"/ 32. (venom adj allergy).mp. 33. exp HYPERSENSITIVITY, DELAYED/ or exp HYPERSENSITIVITY/ or hypersensitivity.mp. or exp HYPERSENSITIVITY, IMMEDIATE/ 34. exp Anaphylaxis/ or anaphyla$.mp. 35. (allergic or allergy).mp. 36. swelling.mp. 37. exp EDEMA/ or edema.mp. 38. systemic reaction.mp. 39. shock.mp. or exp SHOCK, TRAUMATIC/ or exp SHOCK/ 40. hives.mp. or exp Urticaria/ 41. laryngeal obstruction.mp. 42. exp Death/ or exp Death, Sudden/ or death.mp. 43. exp Angioedema/ or exp Airway Obstruction/ 44. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 45. exp IMMUNOTHERAPY, ACTIVE/ or immunotherapy.mp. or exp IMMUNOTHERAPY/ 46. adrenaline.mp. or exp Epinephrine/ 47. epipen.mp. 48. (venom adj immunotherapy).mp. 49. specific immunotherapy for hymenoptera venom.mp. 50. immunomodulatory.mp. 51. immune therapy.mp. 52. immunologic response.mp. 53. desensiti$.mp. or Desensitization, Immunologic/ 54. dose response relationship immunologic.mp. or exp Dose‐Response Relationship, Immunologic/ 55. hyposensitization.mp. 56. 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 57. 30 and 44 and 56 58. 10 and 57
Appendix 3. EMBASE search strategy (OVID)
1. bee.mp. or exp BEE/ 2. bee venom.mp. or exp bee venom/ 3. honey bee.mp. or exp honeybee/ 4. exp HYMENOPTERA VENOM/ or exp HYMENOPTERA/ or hymenoptera.mp. 5. apis mellifera.mp. 6. exp WASP/ or wasp.mp. 7. wasp venom.mp. or exp wasp venom/ 8. vespid.mp. 9. vespula.mp. 10. white hornet.mp. 11. yellow jacket.mp. 12. yellow hornet.mp. 13. polistes.mp. 14. exp ANT/ or ant.mp. 15. ant venom.mp. or exp ant venom/ 16. arthropod venom.mp. or exp arthropod venom/ 17. solenopsis invicta.mp. 18. myrmecia pilosula.mp. 19. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20. insect sting.mp. or exp insect sting/ 21. insect bite.mp. or exp insect bite/ 22. insect allergy.mp. or exp insect allergy/ 23. venom allergy.mp. 24. hypersensitivity.mp. or exp IMMEDIATE TYPE HYPERSENSITIVITY/ or exp DELAYED HYPERSENSITIVITY/ or exp HYPERSENSITIVITY REACTION/ or exp HYPERSENSITIVITY/ 25. anaphyla$.mp. 26. exp ANAPHYLAXIS/ 27. allergic.mp. 28. exp INSECT ALLERGY/ or exp ALLERGY/ or allergy.mp. 29. swelling.mp. or exp SWELLING/ 30. edema.mp. or exp EDEMA/ 31. systemic reaction.mp. or exp allergic reaction/ 32. exp SHOCK/ or exp TRAUMATIC SHOCK/ or shock.mp. 33. hives.mp. or exp urticaria/ 34. laryngeal obstruction.mp. or exp larynx stenosis/ 35. exp DEATH/ or exp SUDDEN DEATH/ or death.mp. 36. angioedema.mp. 37. airway obstruction.mp. or exp airway obstruction/ 38. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39. immunotherapy.mp. or exp IMMUNOTHERAPY/ 40. adrenaline.mp. or exp adrenalin/ 41. epipen.mp. 42. venom immunotherapy.mp. 43. specific immunotherapy for hymenoptera venom.mp. 44. exp immunomodulation/ or immunomodulatory.mp. 45. immune therapy.mp. or exp immunotherapy/ 46. immunologic response.mp. or exp immune response/ 47. exp DESENSITIZATION/ or desensitization.mp. 48. exp dose response/ or immunologic dose response relationship.mp. 49. hyposensitization.mp. 50. 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 51. random$.mp. 52. factorial$.mp. 53. (crossover$ or cross‐over$).mp. 54. placebo$.mp. or PLACEBO/ 55. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 56. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 57. (assign$ or allocat$).mp. 58. volunteer$.mp. or VOLUNTEER/ 59. Crossover Procedure/ 60. Double Blind Procedure/ 61. Randomized Controlled Trial/ 62. Single Blind Procedure/ 63. 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 64. 19 and 38 and 50 65. 63 and 64
Appendix 4. PsycINFO search strategy (OVID)
1. double‐blind.tw. 2. random$ assigned.tw. 3. control.tw. 4. 1 or 2 or 3 5. wasp.mp. or exp Wasps/ 6. ant.mp. or exp Ants/ 7. bee.mp. or exp Bees/ 8. hornet.mp. 9. venom.mp. 10. 5 or 6 or 7 or 8 or 9 11. bite.mp. 12. sting.mp. 13. hypersensitivity.mp. 14. allerg$.tw. 15. exp Anaphylactic Shock/ or anaphylaxis.mp. 16. exp Allergic Disorders/ 17. swelling.mp. 18. edema.mp. 19. shock.mp. or exp Shock/ 20. death.mp. 21. hives.mp. 22. systemic reaction.mp. 23. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24. exp immunotherapy/ 25. immune therapy.mp. 26. adrenaline.mp. or exp Epinephrine/ 27. desensiti$.tw. 28. hyposensitization.tw. 29. 24 or 25 or 26 or 27 or 28 30. 10 and 23 and 29 31. 4 and 30
Appendix 5. AMED search strategy (OVID)
1. exp Apis mellifica/ or bee.mp. 2. wasp.mp. 3. exp insects/ or ant.mp. 4. hornet.mp. or exp Venoms/ 5. 1 or 2 or 3 or 4 6. bite.mp. 7. sting.mp. or exp "Bites and stings"/ 8. allerg$.tw. 9. exp Shock/ or exp Anaphylaxis/ or shock.mp. 10. exp Death/ or death.mp. 11. 6 or 7 or 8 or 9 or 10 12. 5 and 11 13. immunotherapy.mp. or exp Immunotherapy/ 14. 5 and 13
Appendix 6. LILACS search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Words] and alergia or alergeno [Words] and picadura or insecto [Words]
Data and analyses
Comparison 1. Risk of systemic reaction ‐ Immunotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any systemic reaction to field or challenge sting | 6 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.28] |
| 2 Mild systemic reaction to field or challenge sting | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.85] |
| 3 Moderate systemic reaction to field or challenge sting | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.09] |
| 4 Severe systemic reaction to field or challenge sting | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.81] |
| 5 Systemic reaction to field or challenge sting requiring adrenaline treatment | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.12] |
Comparison 2. Risk of systemic reaction ‐ Analysed by number treated.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any systemic reaction to field or challenge sting | 7 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.31] |
Comparison 3. Risk of local reaction ‐ Immunotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Large local reaction to field or challenge sting | 4 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.24, 0.69] |
Comparison 4. Quality of life ‐ Immunotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vespid Quality of Life Questionnaire ‐ end of treatment | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 1.21 [0.75, 1.67] |
| 2 Vespid Quality of Life Questionnaire ‐ change during treatment | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.97, 1.63] |
| 3 Vespid Quality of Life Questionnaire ‐ response rate | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 7.11 [3.02, 16.71] |
Comparison 5. Adverse reaction to treatment ‐ Immunotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any local reaction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Any systemic reaction | 6 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [1.53, 43.46] |
| 3 Number of systemic reactions per participant | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Number of systemic reactions per visit | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Mild systemic reaction | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Moderate systemic reaction | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Severe systemic reaction | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Systemic reaction requiring adrenaline treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Planned subgroup analyses ‐ Immunotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic reaction to field or challenge sting by route of immunotherapy | 6 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.28] |
| 1.1 Subcutaneous immunotherapy | 5 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.03, 0.26] |
| 1.2 Sublingual immunotherapy | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.50] |
| 2 Systemic reaction to field or challenge sting by age | 5 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.35] |
| 2.1 Under 18 years | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.21] |
| 2.2 Aged 18 years or over | 4 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.48] |
| 3 Systemic reaction to field or challenge sting by duration of immunotherapy | 6 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.28] |
| 3.1 Under 1 year of treatment | 4 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.28] |
| 3.2 1 to 3 years of treatment | 1 | 5 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Over 3 years of treatment | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.21] |
| 4 Systemic reaction to field or challenge sting by insect species | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.48] |
| 4.1 Honey bee | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.50] |
| 4.2 Wasp | 1 | 5 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Ant | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.28] |
| 5 Systemic reaction to field or challenge sting by updosing schedule | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Rush or ultrarush | 1 | 5 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Weekly or 2‐weekly | 4 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.03, 0.26] |
| 6 Systemic adverse reaction to treatment by route of immunotherapy | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Subcutaneous immunotherapy | 5 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [1.53, 43.46] |
| 6.2 Sublingual immunotherapy | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Systemic adverse reaction to treatment by age | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Under 18 years | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Aged 18 years or over | 5 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Systemic adverse reaction to treatment by duration of immunotherapy | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Under 1 year of treatment | 4 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [1.53, 43.46] |
| 8.2 1 to 3 years of treatment | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Over 3 years of treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Systemic adverse reaction to treatment by insect species | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Honey bee | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Wasp | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Ant | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 11.89 [1.65, 85.80] |
| 10 Systemic adverse reaction to treatment by updosing schedule | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Rush or ultrarush | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Weekly or 2‐weekly | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [1.53, 43.46] |
Comparison 7. Unplanned subgroup analyses ‐ Immunotherapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic reaction to field or challenge sting by history of prior systemic reaction | 6 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.03, 0.28] |
| 1.1 No prior systemic reaction to insect sting | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.50] |
| 1.2 Prior systemic reaction to insect sting | 4 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.03, 0.26] |
| 2 Systemic adverse reaction to treatment by history of prior systemic reaction | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 No prior systemic reaction to insect sting | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Prior systemic reaction to insect sting | 4 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [1.53, 43.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 2003.
| Methods | This was a randomised, double‐blind, placebo‐controlled, parallel‐group trial. | |
| Participants | This trial was conducted in Australia. The participants were adults aged 18 to 63 years. The total number of participants was 68. The total number of participants in the treatment group was 35 (22 men). The total number of participants in the control group was 33 (18 men). The species of insect venom that the participants were allergic to was Myrmecia pilosula (M. pilosula) venom (ant venom). Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: NSL Health Ltd., Melbourne, Australia Duration: 4 to 5 months Updosing: Outpatient cluster hyposensitisation regimen (10 visits) Maintenance dose: 100 µg monthly
|
|
| Outcomes |
Outcomes of the trial 1. Systemic reaction to insect sting challenge 2. Fatal systemic reaction to insect sting challenge 3. Adverse events to immunotherapy |
|
| Notes | This study was funded by NSL Health Ltd. (VIT manufacturer), the Department of Health and Human Services Tasmania, and Royal Hobart Research Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number‐based sequential allocation was done by a third party. |
| Allocation concealment (selection bias) | Low risk | This was done by a third party. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with participants followed to designated follow‐up periods (0 out of 35 participants in the treatment group and 4 out of 33 participants in the control group). |
| Selective reporting (reporting bias) | Low risk | The specified outcomes in the methodology were reported in the results section. |
| Other bias | Low risk | ‐ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Packs were identical in appearance, and contents containing placebo or lyophilised venom were dispensed. Early unblinding meant that 12 of 35 VIT‐treated participants were unblinded at the time of the sting challenge; however, outcomes were similar in this group to the other 23 who had blinded sting challenges. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded. |
Golden 2009.
| Methods | This was a quasi‐randomised, open‐label, controlled, parallel‐group trial. | |
| Participants | This trial was conducted in the USA. The participants were adults aged 21 to 63 years. The total number of participants was 29. The total number of participants in the treatment group was 19. The total number of participants in the control group was 10. No distribution by gender was reported. The species of insect venoms that the participants were allergic to were honeybee (7), yellowjacket (2), polistes wasp (2), and mixed vespid (18). Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: ALK‐Abelló Duration: 12 weeks Updosing: Used a dose schedule to achieve the maintenance dose in 7 weekly visits Maintenance dose: 100 µg every 4 weeks
|
|
| Outcomes |
Outcomes of the trial 1. Systemic reaction to insect sting challenge 2. Fatal systemic reaction to insect sting challenge 3. Large local reactions to insect sting challenge (measured by participants at home using study measuring equipment) 4. Adverse events to immunotherapy |
|
| Notes | This study was funded by the National Institutes of Health and ALK‐Abelló (VIT manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was done based on sequence of enrolment, probably using alternation ‐ therefore, this was a quasi‐randomised study. |
| Allocation concealment (selection bias) | High risk | This was inadequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow up during the 12‐week period. |
| Selective reporting (reporting bias) | Low risk | The specified outcomes in the methodology were reported in the results section. |
| Other bias | Low risk | No other sources of bias were detected or suspected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
Hunt 1978.
| Methods | This was a randomised, single‐blind, placebo‐controlled, parallel‐group trial. | |
| Participants | This trial was conducted in the USA. The participants were adults and children aged 15 to 69 years. The total number of participants was 39. The total number of participants in the treatment group was 19. The total number of participants in the control group was 20. No distribution by gender was reported. The species of insect venoms that the participants were allergic to were honeybee, yellowjacket, yellow hornet, and white‐faced hornet. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: not stated Duration: 6 to 10 weeks Updosing: Cluster updosing (modified rush protocol) weekly Maintenance dose: 100 µg weekly
|
|
| Outcomes |
Outcomes of the trial 1. Systemic reaction to insect sting challenge 2. Fatal systemic reaction to insect sting challenge 3. Adverse events to immunotherapy |
|
| Notes | This study was funded by the US' National Institute of Allergy and Infectious Diseases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned a treatment regimen by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 of the 19 participants in the treatment group was lost to follow up (did not tolerate maintenance dose). None of the 20 participants in the control group were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | This was unclear. |
| Other bias | High risk | Only 12 of 20 participants in the placebo group underwent sting challenge due to cessation of placebo group challenges on safety grounds. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded, but the physicians were not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The clinician and outcome assessor were not blinded. |
Oude Elberink 2006.
| Methods | This was a randomised, open‐label, controlled, parallel‐group trial. | |
| Participants | This trial was conducted in the Netherlands. The participants were adults aged 18 to 65 years. The total number of participants was 91. The total number of participants in the treatment group was 47. The total number of participants in the control group was 44. No distribution by gender was reported. The species of insect venom that the participants were allergic to was yellowjacket. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: ALK‐Abelló Duration: 1 year Updosing: Modified semi‐rush schedule over approximately 6 weeks Maintenance dose: 100 µg every 6 weeks
|
|
| Outcomes |
Outcomes of the trial 1. Systemic reaction to accidental insect sting 2. Quality of life assessment using "Burden of Treatment" questionnaire at 1 year |
|
| Notes | This is part of the same study reported in the Oude Elberink 2001 and 2002 publications. This study was funded by ALK‐Abelló (VIT manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A minimisation computer program was used. Participants were stratified for age and time of reaction. |
| Allocation concealment (selection bias) | Low risk | This was done by a third party. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of 47 participants were lost to follow up in the treatment group; 3 of 44 participants were lost to follow up in the control group. |
| Selective reporting (reporting bias) | Low risk | The specified outcomes in the methodology were either reported in the results section or provided by the authors as the original dataset. |
| Other bias | Low risk | No other sources of bias were detected or suspected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
Oude Elberink 2009.
| Methods | This was a randomised, open‐label, controlled, parallel‐group trial. | |
| Participants | This trial was conducted in the Netherlands. The participants were adults aged 18 years and older. The total number of participants was 29. The total number of participants in the treatment group was 15 (9 men). The total number of participants in the control group was 14 (6 men). The species of insect venom that the participants were allergic to was yellowjacket. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: ALK‐Abelló Duration: 1 year Updosing: Modified semi‐rush schedule Maintenance dose:100 µg every 6 weeks
|
|
| Outcomes |
Outcomes of the trial 1. Quality of life assessment using the Vespid Quality of Life Questionnaire (VQLQ) at 1 year |
|
| Notes | This study was funded by ALK‐Abelló (VIT manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A minimisation computer program was used. Participants were stratified for age and time of reaction. |
| Allocation concealment (selection bias) | Low risk | This was done by a third party. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow up or excluded were accounted for along with participants followed to designated follow‐up periods. None of the 15 participants in the treatment group, and 1 of 14 in the control group were lost to follow up. |
| Selective reporting (reporting bias) | Low risk | The specified outcomes in the methodology were reported in the results section. |
| Other bias | Low risk | No other sources of bias were detected or suspected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
Severino 2008.
| Methods | This was a randomised, double‐blind, placebo‐controlled, parallel‐group trial. | |
| Participants | This trial was conducted in Italy. The participants were adults aged 18 years and older. The total number of participants was 30. The total number of participants in the treatment group was 15 (10 men). The total number of participants in the control group was 15 (12 men). The species of insect venom that the participants were allergic to was honeybee. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: Anallergo SpA, Florence, Italy Duration: 6 months Updosing: Build up regimen by gradually administering increasing amounts of venom, starting from 0.05 to 35 µg per day over 33 days Maintenance dose: 35 µg taken on alternate days with cumulative dose of 525 µg of venom monthly
|
|
| Outcomes |
Outcomes of the trial 1. Systemic reaction to insect sting challenge 2. Fatal systematic reaction to insect sting challenge 3. Adverse events to immunotherapy 4. Large local reactions to insect sting challenge |
|
| Notes | This study was funded by Anallergo SpA (VIT manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was made using a computer list. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low loss to follow up rate ‐ just 1 out of 15 participants in the treatment group and 3 out of 15 participants in the control group were not assessed for the primary outcome measure; all were due to refusal to undergo the sting challenge. |
| Selective reporting (reporting bias) | Low risk | The specified outcomes in the methodology were reported in the results section. |
| Other bias | Low risk | No other sources of bias were detected or suspected. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
Valentine 1990.
| Methods | This was a randomised, open‐label, controlled, parallel‐group trial. | |
| Participants | This trial was conducted in the USA. The participants were children aged 2 to 16 years. The total number of participants was 106. The total number of participants in the treatment group was 45 (70% men). The total number of participants in the control group was 61 (75% men). It was unclear how many participants were lost to follow up. The species of insect venoms that the participants were allergic to were honeybee, yellowjacket, yellow hornet, white‐faced hornet, and Polistes. Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
VIT Manufacturer: Pharmacia Duration: 4 years Updosing: weekly injections Maintenance dose: 100 µg monthly
|
|
| Outcomes |
Outcomes of the trial 1. Systemic reaction to accidental (field) insect sting 2. Large local reactions to accidental insect sting 3. Both recorded at periodic study visits using a structured questionnaire |
|
| Notes | This study included the participants reported in the study of Schuberth 1983. This study was funded by the National Institutes of Health and a Pfizer Biomedical research award. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was unclear how many participants were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | This was unclear. |
| Other bias | Low risk | No other sources of bias were detected or suspected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berchtold 1992 | There was no appropriate control group. |
| Bilo 2009 | There was no appropriate control group. |
| Bousquet 1987 | This was not a trial of venom immunotherapy. |
| Brockow 1997 | There was no appropriate control group. |
| Brown 2008 | There was no appropriate control group. |
| Divanovic 1997 | There was no appropriate control group. |
| Malling 1985 | This was not a randomised controlled trial. |
| Mosbech 1986 | There was no appropriate control group. |
| Muller 1985 | There was no appropriate control group. |
| Muller 1987 | There was no appropriate control group. |
| Muller 1992 | This was not a randomised controlled trial. |
| Muller 2001 | There was no appropriate control group. |
| Muller 2008 | There was no appropriate control group. |
| Ohman 1986 | This was not a randomised controlled trial. |
| Quercia 2001 | There was no appropriate control group. |
| Reimers 2000 | There was no appropriate control group. |
| Roumana 2009 | There was no appropriate control group. |
| Spertini 2000 | This was not a randomised controlled trial. |
| Thurnheer 1983 | There was no appropriate control group. |
| Wohrl 2007 | There was no appropriate control group. |
| Zubrinich 2010 | This was not a randomised controlled trial. |
Differences between protocol and review
Hanneke Oude Elberink joined as a co‐author. We changed the order of the secondary outcomes. In the Data Collection and Analysis section (Measures of treatment effect), we elaborated on how we analysed the risk of systemic reaction. We added a post‐hoc subgroup analysis according to the severity of the previous reaction to insect sting. We included quasi‐randomised trials, which had not been specifically considered for inclusion or exclusion at the protocol stage.
Contributions of authors
ME was the contact person with the editorial base at the protocol stage, and RB, at the review stage. RB co‐ordinated contributions from the co‐authors and wrote the final draft of the review. ME and RB screened papers against eligibility criteria. ME and RB obtained data on ongoing and unpublished studies. ME and RB appraised the quality of papers. ME and RB extracted data for the review and sought additional information about papers. ME and RB entered data into RevMan. JH and MC undertook the adverse events search and summarised the adverse events data. RB analysed and interpreted data, with advice from MB. ME and RB drafted the clinical sections of the background, with contributions from all authors. RB responded to the referees' comments. All authors reviewed and commented on the review. MD was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. RB is the guarantor of the update.
Sources of support
Internal sources
Imperial College London, UK.
National Institute for Health Research Biomedical Research Centre, UK.
External sources
No sources of support supplied
Declarations of interest
Robert Boyle has received research funding from Lincoln Medical Ltd and support for conference attendance from Meda Pharmaceuticals, both of which market adrenaline autoinjector devices, and his department has received research support from Allergy Therapeutics who market allergen immunotherapy products, including venom immunotherapy.
Hanneke Oude Elberink (J.N.G. Oude Elberink) undertook two of the trials included in this review. She has received research support from ALK‐Abello who market allergen immunotherapy, and HAL Allergy; she has received honorarium from MSD, Allergopharma, ALK‐Abello, and AstraZeneca; and she has received fees for consulting from ALK‐Abello and HAL Allergy.
Mariam Elremeli, Max Bulsara, Juliet Hockenhull, Gemma Cherry, and Michael Daniels have no interests to declare.
A clinical referee on the review, Dr Cristoforo Incorvaia, has received fees as a scientific consultant from the producer of allergen extracts, Stallergenes.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Brown 2003 {published and unpublished data}
- Brown SG, Wiese MD, Blackman KE, Heddle RJ. Ant venom immunotherapy: a double‐blind, placebo‐controlled, crossover trial. Lancet 2003;361(9362):1001‐6. [DOI] [PubMed] [Google Scholar]
Golden 2009 {published and unpublished data}
- Golden DB, Kelly D, Hamilton RG, Craig TJ. Venom immunotherapy reduces large local reactions to insect stings. Journal of Allergy and Clinical Immunology 2009;123(6):1371‐5. [DOI] [PubMed] [Google Scholar]
Hunt 1978 {published and unpublished data}
- Hunt KJ, Valentine MD, Sobotka AK, Benton A, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. New England Journal of Medicine 1978;299(4):157‐61. [DOI] [PubMed] [Google Scholar]
Oude Elberink 2006 {published and unpublished data}
- Oude Elberink JNG, Monchy JGR, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health‐related quality of life in patients with allergic reactions following yellow‐jacket stings ‐ extended observations. Journal of Allergy and Clinical Immunology 2001;107(2):S222‐3. [Google Scholar]
- Oude Elberink JNG, Monchy JGR, Heide S, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health‐related quality of life in patients allergic to yellow jacket venom. Journal of Allergy and Clinical Immunology 2002;110(1):174‐82. [DOI] [PubMed] [Google Scholar]
- Oude Elberink JNG, Heide S, Guyatt GH, Dubois AEJ. Analysis of the burden of treatment in patients receiving an EpiPen for yellow jacket anaphylaxis. Journal of Allergy & Clinical Immunology 2006;118(3):699‐704. [DOI] [PubMed] [Google Scholar]
Oude Elberink 2009 {published and unpublished data}
- Oude Elberink JNG, Heide S, Guyatt GH, Dubois AEJ. Immunotherapy improves health‐related quality of life of adult patients with dermal reactions following yellow jacket stings. Clinical and Experimental Allergy 2009;39(6):883‐9. [DOI] [PubMed] [Google Scholar]
Severino 2008 {published and unpublished data}
- Severino M, Cortellini, G, Bonadonna P, Francescato E, Panzini I, Macchia D, et al. Sublingual immunotherapy with honeybee venom reduces large local reactions: a randomised, double blind controlled study. EAACI Conference Abstract. 2008:A170. [DOI] [PubMed]
- Severino MG, Cortellini G, Bonadonna P, Francescato E, Panzini I, Macchia D, et al. Sublingual immunotherapy for large local reactions caused by honeybee sting: a double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2008;122(1):44‐8. [DOI] [PubMed] [Google Scholar]
Valentine 1990 {published data only (unpublished sought but not used)}
- Schuberth KC, Lichtenstein LM, Kagey‐Sobotka A, Szklo M, Kwiterovich KA, Valentine MD. Epidemiologic study of insect allergy in children. II. Effect of accidental stings in allergic children. Journal of Pediatrics 1983;102(3):361‐5. [DOI] [PubMed] [Google Scholar]
- Valentine MD, Schuberth KC, Kagey‐Sobotka A, Graft DF, Kwiterovich KA, Szklo M, et al. The value of immunotherapy with venom in children with allergy to insect stings. New England Journal of Medicine 1990;323(23):1601‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berchtold 1992 {published data only}
- Berchtold E, Maibach R, Müller U. Reduction of side effects from rush‐immunotherapy with honey bee venom by pretreatment with terfenadine. Clinical and Experimental Allergy 1992;22(1):59‐65. [DOI] [PubMed] [Google Scholar]
Bilo 2009 {published data only}
- Bilò MB, Severino M, Cilia M, Pio A, Casino G, Ferrarini E, et al. The VISYT trial: Venom Immunotherapy Safety and Tolerability with purified vs nonpurified extracts. Annals of Allergy Asthma and Immunology 2009;103(1):57‐61. [DOI] [PubMed] [Google Scholar]
Bousquet 1987 {published data only}
- Bousquet J, Fontez A, Aznar R, Robinet‐Levy M, Michel FB. Combination of passive and active immunization in honeybee venom immunotherapy. Journal of Allergy and Clinical Immunology 1987;79(6):947‐54. [DOI] [PubMed] [Google Scholar]
Brockow 1997 {published data only}
- Brockow K, Kiehn M, Riethmüller C, Vieluf D, Berger J, Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 1997;100(4):458‐63. [DOI] [PubMed] [Google Scholar]
Brown 2008 {published data only}
- Brown SG, Wiese MD, Chuter CL, Gunner J. Rapid (ultra‐rush) versus clustered (semi rush) initiation of insect venom immunotherapy: an open randomised controlled trial with patient choice arms. Internal Medicine Journal 2008;38(S6):A151. [Google Scholar]
Divanovic 1997 {published data only}
- Divanovic A, Melac M, Herman D. Cetirizine (20 mg/day) versus placebo in the prevention of reactions secondary to accelerated desensitization to hymenoptera venoms [Cetirizine (20 mg/j) versus placebo dans la prevention des reactions secondaires de la desensibilisation acceleree aux venins d'hymenopteres]. Revue Francaise D'Allergologie Et D'Immunologie Clinique 1997;37(6):741‐5. [Google Scholar]
Malling 1985 {published data only}
- Malling HJ, Djurup R, Søndergaard I, Weeke B. Clustered immunotherapy with yellow jacket venom. Evaluation of the influence of time interval on in vivo and in vitro parameters. Allergy 1985;40(5):373‐83. [DOI] [PubMed] [Google Scholar]
Mosbech 1986 {published data only}
- Mosbech H, Malling H J, Biering I, Böwadt H, Søborg M, Weeke B, et al. Immunotherapy with yellow jacket venom. A comparative study including three different extracts, one adsorbed to aluminium hydroxide and two unmodified. Allergy 1986;41(2):95‐103. [DOI] [PubMed] [Google Scholar]
Muller 1985 {published data only}
- Müller U, Lanner A, Schmid P, Bischof M, Dreborg S, Hoigné R. A double blind study on immunotherapy with chemically modified honey bee venom: monomethoxy polyethylene glycol‐coupled versus crude honey bee venom. International Archives of Allergy and Applied Immunology 1985;77(1‐2):201‐3. [DOI] [PubMed] [Google Scholar]
Muller 1987 {published data only}
- Müller U, Rabson AR, Bischof M, Lomnitzer R, Dreborg S, Lanner A. A double‐blind study comparing monomethoxy polyethylene glycol‐modified honeybee venom and unmodified honeybee venom for immunotherapy. Journal of Allergy and Clinical Immunology 1987;80(3 Pt 1):252‐61. [DOI] [PubMed] [Google Scholar]
Muller 1992 {published data only}
- Müller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. Journal of Allergy and Clinical Immunology 1992;89(2):529‐35. [DOI] [PubMed] [Google Scholar]
Muller 2001 {published data only}
- Müller U, Hari Y, Berchtold E. Premedication with antihistamines may enhance efficacy of specific‐allergen immunotherapy. Journal of Allergy and Clinical Immunology 2001;107(1):81‐6. [DOI] [PubMed] [Google Scholar]
Muller 2008 {published data only}
- Müller UR, Jutel M, Reimers A, Zumkehr J, Huber C, Kriegel C, et al. Clinical and immunologic effects of H1 antihistamine preventive medication during honeybee venom immunotherapy. Journal of Allergy and Clinical Immunology 2008;122(5):1001‐7. [DOI] [PubMed] [Google Scholar]
Ohman 1986 {published data only}
- Öhman S, Björkander J, Dreborg S, Lanner Å, Malling HJ, Weeke B. A preliminary study of immunotherapy with a monomethoxy polyethylene glycol modified honey bee venom preparation. Allergy 1986;41(2):81‐8. [DOI] [PubMed] [Google Scholar]
Quercia 2001 {published data only}
- Quercia O, Rafanelli S, Puccinelli P, Stefanini GF. The safety of cluster immunotherapy with aluminium hydroxide‐adsorbed honey bee venom extract. Journal of Investigative Allergology and Clinical Immunology 2001;11(1):27‐33. [PubMed] [Google Scholar]
Reimers 2000 {published data only}
- Reimers A, Hari Y, Müller U. Reduction of side‐effects from ultrarush immunotherapy with honeybee venom by pretreatment with fexofenadine: a double‐blind, placebo‐controlled trial. Allergy 2000;55(5):484‐8. [DOI] [PubMed] [Google Scholar]
Roumana 2009 {published data only}
- Roumana A, Pitsios C, Vartholomaios S, Kompoti E, Kontou‐Fili K. The safety of initiating Hymenoptera immunotherapy at 1 mug of venom extract. Journal of Allergy and Clinical Immunology 2009;124(2):379‐81. [DOI] [PubMed] [Google Scholar]
Spertini 2000 {published data only}
- Spertini F, Fellrath JM, Kettner A, Dufour N, Frigerio C, Schneeberger D, et al. Allergen‐derived long peptide immunotherapy in bee venom hypersensitive patients. Allergologie 2000;23(3):156. [Google Scholar]
Thurnheer 1983 {published data only}
- Thurnheer U, Müller U, Stoller R, Lanner A, Hoigné R. Venom immunotherapy in hymenoptera sting allergy. Comparison of rush and conventional hyposensitization and observations during long‐term treatment. Allergy 1983;38(7):465‐73. [DOI] [PubMed] [Google Scholar]
Wohrl 2007 {published data only}
- Wöhrl S, Gamper S, Hemmer W, Heinze G, Stingl G, Kinaciyan T. Premedication with montelukast reduces local reactions of allergen immunotherapy. International Archives of Allergy and Immunology 2007;144(2):137‐42. [DOI] [PubMed] [Google Scholar]
Zubrinich 2010 {published data only}
- Zubrinich C, Weber E, Stirling R, Puy R, O'Hehir R, Douglass J. Omalizumab for anaphylaxis during hymenopteravenom immunotherapy [Poster 60]. Australasian Society of Clinical Immunology and Allergy (ASCIA) 21st Annual Scientific Meeting, 1‐3 September 2010, Queensland. Internal Medicine Journal 2010;40(S4):20. [Google Scholar]
Additional references
Akdis 2007
- Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy. Journal of Allergy and Clinical Immunology 2007;119(4):780‐91. [DOI] [PubMed] [Google Scholar]
Antonicelli 2002
- Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Current Opinion in Allergy and Clinical Immunology 2002;2(4):341‐6. [DOI] [PubMed] [Google Scholar]
Bilo 2009
- Bilo MB, Bonifazi F. The natural history and epidemiology of insect venom allergy: clinical implications. Clinical and Experimental Allergy 2009;39(10):1467‐76. [DOI] [PubMed] [Google Scholar]
Bilo 2005
- Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude Elberink JNG, EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005;60(11):1339‐49. [DOI] [PubMed] [Google Scholar]
Bonadonna 2009
- Bonadonna P, Perbellini O, Passalacqua G, Caruso B, Colarossi S, Dal Fior D, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. Journal of Allergy and Clinical Immunology 2009;123(3):680‐6. [DOI] [PubMed] [Google Scholar]
Bonifazi 2005
- Bonifazi F, Jutel M, Bilo BM, Birnbaum J, Muller U, EACCI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of Hymenoptera venom allergy: guidelines for clinical practice. Allergy 2005;60(12):1459‐70. [DOI] [PubMed] [Google Scholar]
Brown 2004
- Brown SG. Clinical features and severity grading of anaphylaxis. Journal of Allergy & Clinical Immunology 2004;114(2):371‐6. [DOI] [PubMed] [Google Scholar]
Calderon 2011
- Calderon MA, Boyle RJ, Penagos M, Sheikh A. Immunotherapy: The Meta‐Analyses. What have we learned?. Immunology and Allergy Clinics of North America 2011;31(2):159‐73. [DOI] [PubMed] [Google Scholar]
Carballada 2003
- Carballada F, Martin S, Boquete M. High efficacy and absence of severe systemic reactions after venom immunotherapy. Journal of Investigative Allergology and Clinical Immunology 2003;13(1):43‐9. [PubMed] [Google Scholar]
Carballada 2009
- Carballada FJ, Crehuet AM, Manjon HA, Torre F, Boquete M. Hymenoptera venom allergy: characterisitics, tolerance and efficacy of immunotherapy in the paediatric population. Allergologia et Immunopathologia 2009;37(3):111‐5. [DOI] [PubMed] [Google Scholar]
Carballada 2010
- Carballada F, Boquete M, Nunez R, Lombardero M, Torre F. Follow‐up of venom immunotherapy based on conventional techniques and monitoring of immunoglobulin E to individual venom allergens. Journal of Investigative Allergology and Clinical Immunology 2010;20(6):506‐13. [PubMed] [Google Scholar]
Clark 2007
- Clark S, Camargo CA Jr. Epidemiology of anaphylaxis. Immunology and Allergy Clinics of North America 2007;27(2):145‐63. [DOI] [PubMed] [Google Scholar]
Ewan 2001
- Ewan PW. New insight into immunological mechanisms of venom immunotherapy. Current Opinion in Allergy and Clinical Immunology 2001;1(4):367‐74. [DOI] [PubMed] [Google Scholar]
Freeman 1992
- Freeman TM, Hylander R, Ortiz A, Martin ME. Imported fire ant immunotherapy: effectiveness of whole body extracts. Journal of Allergy & Clinical Immunology 1992;90(2):210‐15. [DOI] [PubMed] [Google Scholar]
Fricker 1997
- Fricker M, Helbling A, Schwartz L, Muller U. Hymenoptera sting anaphylaxis and urticaria pigmentosa: clinical findings and results of venom immunotherapy in ten patients. Journal of Allergy and Clinical Immunology 1997;100(1):11‐15. [DOI] [PubMed] [Google Scholar]
Golden 2004
- Golden DB, Kagey‐Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. New England Journal of Medicine 2004;351(7):668‐74. [DOI] [PubMed] [Google Scholar]
Golden 2005
- Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. Journal of Allergy & Clinical Immunology 2005;115(3):439‐47. [DOI] [PubMed] [Google Scholar]
Golden 2011
- Golden DB, Moffit J, Nicklas RA, Freeman T, Graft DF, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update 2011. Journal of Allergy and Clinical Immunology 2011;127(4):852‐4. [DOI] [PubMed] [Google Scholar]
Haeberli 2003
- Haeberli G, Bronnimann M, Hunziker T, Muller U. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clinical and Experimental Allergy 2003;33(9):1216‐20. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hockenhull 2012
- Hockenhull JC, Elremeli M, Cherry MG, Mahon J, Lai M, Darroch J, et al. The clinical and cost effectiveness of pharmalgen for the treatment of bee and wasp venom allergy. Health Technology Assessment (in preparation). [DOI] [PMC free article] [PubMed]
Kalogeromitros 2010
- Kalogeromitros D, Makris M, Koti I, Chliva C, Mellios A, Avgerinou G. A simple 3‐day 'rush' venom immunotherapy protocol: documentation of safety. Allergologia et Immunopathologia 2010;38(2):69‐73. [DOI] [PubMed] [Google Scholar]
Krishna 2011
- Krishna MT, Ewan PW, Diwakar L, Durham SR, Frew AJ, Leech SC, et al. Diagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines. Clinical & Experimental Allergy 2011;41(9):1201‐20. [DOI] [PubMed] [Google Scholar]
Liew 2009
- Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. Journal of Allergy and Clinical Immunology 2009;123(2):434‐42. [DOI] [PubMed] [Google Scholar]
Moffit 2004
- Moffitt JE, Golden DB, Reisman RE, Lee R, Nicklas R, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update. Journal of Allergy and Clinical Immunology 2004;114(4):869‐86. [DOI] [PubMed] [Google Scholar]
Mosbech 2000
- Mosbech H, Muller U. Side‐effects of insect venom immunotherapy: results from an EAACI multicenter study. Allergy 2000;55(11):1005‐10. [DOI] [PubMed] [Google Scholar]
Mueller 1966
- Mueller, HL. Diagnosis and treatment of insect sensitivity. Journal of Asthma Research 1966;3(4):331‐3. [DOI] [PubMed] [Google Scholar]
Muller 1989
- Muller U, Helbling A, Bischof M. Predictive value of venom‐specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy 1989;44(6):412‐8. [DOI] [PubMed] [Google Scholar]
Muller 2005
- Muller UR. Bee venom allergy in beekeepers and their family members. Current Opinion in Allergy and Clinical Immunology 2005;5(4):343‐7. [DOI] [PubMed] [Google Scholar]
Oude Elberink 2002
- Oude Elberink JNG, Monchy JG, Golden DB, Brouwer JL, Guyatt GH, Dubois AEJ. Development and validation of a health‐related quality‐of‐life questionnaire in patients with yellow jacket allergy. Journal of Allergy and Clinical Immunology 2002;109(1):162‐70. [DOI] [PubMed] [Google Scholar]
Oude Elberink 2003
- Oude Elberink JNG, Dubois AEJ. Quality of life in insect venom allergic patients. Current Opinion in Allergy and Clinical Immunology 2003;3(4):287‐93. [DOI] [PubMed] [Google Scholar]
Pumphrey 2000
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clinical and Experimental Allergy 2000;30(8):1144‐50. [DOI] [PubMed] [Google Scholar]
Radulovic 2011
- Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy. Allergy 2011;66(6):740‐52. [DOI] [PubMed] [Google Scholar]
Ramirez 1981
- Ramirez DA, Londono S, Evans IRD. Adverse reactions to venom immunotherapy. Annals of Allergy 1981;47(6):435‐9. [PubMed] [Google Scholar]
Rueff 2009
- Rueff F, Przybilla B, Bilo MB, Muller U, Scheipl F, Aberer W, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase‐a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. Journal of Allergy and Clinical Immunology 2009;124(5):1047‐54. [DOI] [PubMed] [Google Scholar]
Sanchez‐Machin 2010
- Sanchez‐Machin I, Moreno C, Gonzalez R, Iglesias‐Souto J, Perez E, Matheu, V. Safety of a 2‐visit cluster schedule of venom immunotherapy in outpatients at risk of life‐threatening anaphylaxis. Journal of Investigative Allergology and Clinical Immunology 2010;20(1):91‐2. [PubMed] [Google Scholar]
Schiavino 2004
- Schiavino D, Nucera E, Pollastrini E, Pasquale T, Buonomo A, Bartolozzi F, et al. Specific ultrarush desensitisation in hymenoptera venom‐allergic patients. Annals of Allergy Asthma and Immunology 2004;92(4):409‐13. [DOI] [PubMed] [Google Scholar]
Senti 2006
- Senti G, Johansen P, Oliver R, Prinz Vavricka BM, Graf N, Wuthrich B, et al. A cutaneous allergen neutralisation test that correlates with the duration of venom immunotherapy. Internal Archives of Allergy and Immunology 2006;141(4):377‐83. [DOI] [PubMed] [Google Scholar]
Watanabe 2010
- Watanabe AS, Fonseca LA, Galvao CE, Kalil J, Castro FF. Specific immunotherapy using Hymenoptera venom: systematic review [Imunoterapia especifica com venenos de Hymenoptera: Revisao sistematica]. Sao Paolo Medical Journal. Revista Paulista de Medicina 2010;128(1):30‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1994
- Wilson AB, Deighton J, Lachmann PJ, Ewan PW. A comparative study of IgG subclass antibodies in patients allergic to wasp or bee venom. Allergy 1994;49(4):272‐80. [DOI] [PubMed] [Google Scholar]


