Abstract
Objectives
Higher prevalence of progressive stages of nonalcoholic fatty liver disease (NAFLD) and hyperglucagonemia were observed in type 2 diabetes. We aim to investigate whether islet alpha cell dysfunction (evaluated by glucagon) associates with NAFLD progression in type 2 diabetic adults.
Methods
A total of 4937 diabetic participants were enrolled from seven communities in Shanghai, China. Probable nonalcoholic steatohepatitis (NASH) was defined by the presence of NAFLD and metabolic syndrome. Probable NAFLD fibrosis score was used to identify patients with different risk stratification of bridging fibrosis (stage 3) or cirrhosis (stage 4).
Results
After adjustment for age, sex, duration of diabetes, current smoking, waist circumference, C-peptide, HbA1c, dyslipidemia, hypertension and use of incretins and SGLT2 inhibitor, glucagon quartiles were negatively associated with probable NASH (Q4 vs. Q1 OR 0.71, 95% confidence interval, 0.53–0.96, P for trend=0.010), though they were not associated with simple NAFLD (P for trend=0.176). Furthermore, glucagon was not significantly associated with fibrotic progression of liver steatosis in diabetic patients with NAFLD (P for trend=0.889).
Conclusions
Significant associations were observed among glucagon and inflammatory progression of NAFLD, but not with fibrotic progression. Further understanding the association between islet alpha cell and liver may lead to development of treatment strategies for NAFLD patients with type 2 diabetes.
Keywords: advanced fibrosis, glucagon, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, type 2 diabetes mellitus
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in the world [1,2]. The prevalence of NAFLD is higher in patients with type 2 diabetes mellitus (T2DM) [3]. Moreover, patients with both T2DM and NAFLD are prone to develop severe histological forms of NAFLD, including NASH, advanced fibrosis and cirrhosis [4]. NASH has now become the second leading cause for liver transplantation in the USA [5] and fibrosis is closely associated with long-term mortality [6]. Therefore, great attention should be paid to the progression of NAFLD, especially in diabetic patients.
Glucagon is a peptide hormone secreted by islet α cells, contributing to the maintenance of euglycemia in human. Elevated plasma glucagon concentration is a biochemical hallmark of diabetes [7]. Glucagon can increase hepatic glucose production during fasting state [8]. Because glucagon secretion is highly affected by insulin, it may make sense to consider the glucagon-to-insulin ratio instead of assessing absolute values [9]. Moreover, a recent study with a small sample size found the association between glucagon-to-insulin ratio and the presence of NAFLD.
Thus, we suppose glucagon may be associated with NAFLD progression including inflammation and fibrosis. Liver biopsies could not be applied in epidemiology study, so we used metabolic syndrome, one of the strong noninvasive NASH predictors [10], to assess probable NASH; and we used NAFLD fibrosis score (NFS), a noninvasive system that identifies liver fibrosis in patients with NAFLD [11], to assess probable NAFLD fibrosis. NFS is a noninvasive scoring system that accurately separates NAFLD patients with and without advanced fibrosis [11].
Therefore, in this large community-based study, we aimed to investigate whether glucagon associates with inflammatory and fibrotic progression of NAFLD evaluated by above noninvasive measurements in Chinese type 2 diabetic adults.
Materials and methods
Study design and participants
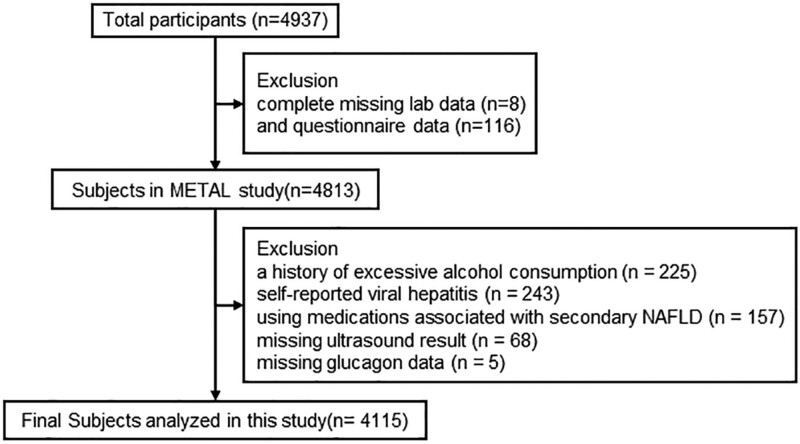
We designed a population-based study named METAL study in 2018 (Environmental Pollutant Exposure and Metabolic Diseases in Shanghai, www.chictr.org.cn, ChiCTR1800017573). Participants of this study were enrolled from seven communities in Huangpu and Pudong District, Shanghai, China. We randomly selected patients with diabetes from the community healthcare center registration platform. Chinese citizens adults who had lived in their current area for ≥6 months were included. Totally, 4937 subjects with diabetes received the examination. Participants who were missing laboratory results (n = 8), questionnaire data (n = 116), missing glucagon data (n = 5), had a history of excessive consumption (men ≥140 g/week, women ≥70 g/week) of pure alcohol (Chinese Society of Hepatology 2010) (n = 225), was using medications associated with secondary NAFLD (corticosteroids, estrogens, amiodarone, methotrexate) (n = 157), had self-reported viral hepatitis (including hepatitis B and hepatitis C virus) (n = 243), and did not have ultrasound result (n = 68) were excluded. Finally, 4115 diabetic participants were involved in the final analyses (Fig. 1).
Fig. 1.
Flowchart of participants’ inclusion and exclusion.
The study protocol was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee. Informed consent was obtained from all participants included in the study.
Measurements
We followed the methods of Wang et al. [12]. The questionnaire included sociodemographic characteristics, family history, medical history and lifestyle factors. The trained and experienced personnel were the same group as those in SPECT-China study [13]. The interviews and clinical examinations were conducted according to a standard protocol. Current smoking was defined as having smoked at least 100 cigarettes in one’s lifetime and currently smoking cigarettes [14]. Waist circumference was measured in the horizontal plane midway between the lowest ribs and the iliac crest, as suggested by the WHO and the International Diabetes Federation.
Blood samples were obtained between 6:00 a.m. and 9:00 a.m. after overnight fasting for at least 8 h. Blood was refrigerated immediately after phlebotomy, and it was centrifugated in 2 h and the serum was aliquoted and frozen in a central laboratory. Serum glucagon was measured by radioimmunoassay (SN-6105, Hesuorihuan, Shanghai, China). Glycated hemoglobin (HbA1c) was measured by HPLC (MQ-2000PT, Medconn, Shanghai, China). Fasting plasma glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lipid profile were performed with a Beckman Coulter AU 680 (Brea, USA).
Hypertension was assessed by systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg, or self-reported previous diagnosis of hypertension by physicians. Dyslipidemia was defined as total cholesterol ≥6.22 mmol/L (240 mg/dL), triglycerides ≥2.26 mmol/L (200 mg/dL), low-density lipoprotein≥4.14 mmol/L (160 mg/dL), high-density lipoprotein <1.04 mmol/L (40 mg/dL) or self-reported previous diagnosis of hyperlipidemia by physicians, according to the modified National Cholesterol Education Program-Adult Treatment Panel III.
Outcome definition
Liver fat accumulation (steatosis) was detected by ultrasound (Mindray M7, MINDRAY, Shenzhen, China) [15,16]. According to Saadeh et al.’s criteria, presentation of steatosis included increased liver echogenicity, stronger echoes in the hepatic parenchyma as compared to the renal parenchyma, vessel blurring, and narrowing of the lumen of the hepatic veins [17].
The presence of metabolic syndrome is a strong predictor for nonalcoholic steatohepatitis (NASH) in patients with NAFLD according to the guidelines by the American Association for the study of liver disease and the Chinese Society of Hepatology [18]. Thus, we categorized the subjects with metabolic syndrome and NAFLD into subjects with probable NASH and the left as simple NAFLD. Metabolic syndrome was diagnosed on the basis of the International Diabetes Federation criteria (2005) [19].
NFS was used to identify NAFLD patients with different risk stratification of having advanced fibrosis [18]. The formula to calculate NFS is −1.675 + 0.037*age (years) + 0.094*BMI (kg/m2) + 1.13* impaired fasting glucose /diabetes (yes =1, no =0) + 0.99*AST/ALT ratio − 0.013*platelet (*109/l) − 0.66*albumin (g/dL) [11]. A score <−1.455 was defined as probable absence of advanced fibrosis (90% sensitivity and 60% specificity to exclude advanced fibrosis); a score >0.676 was defined as probable presence of advanced fibrosis (67% sensitivity and 97% specificity to identify the presence of advanced fibrosis); and a score between −1.455 and 0.676 have indeterminate results [11].
Statistical analysis
Data analyses were performed using IBM SPSS Statistics, Version 21 (IBM Corporation, Armonk, New York, USA). Continuous variables were shown as mean±SD and categorical variables as percentages (%). P value <0.05 indicated significance (two-sided). The concentration of glucagon was logarithmically transformed to achieve a normal distribution.
Glucagon was divided into quartiles. The first quartile of glucagon (Q1) representing the lowest one and the fourth quartile (Q4) the highest. Multinomial logistic regression was used to measure the association of glucagon (independent variable) and progression category of NAFLD (dependent variable). Odds ratios (OR) with 95% confidence interval (CI) were calculated. The model was adjusted for age, sex, duration of diabetes, current smoking, waist circumference, C-peptide, HbA1c, dyslipidemia, hypertension and use of incretins and SGLT2 inhibitors.
Results
Characteristics of the diabetic participants by NAFLD progression
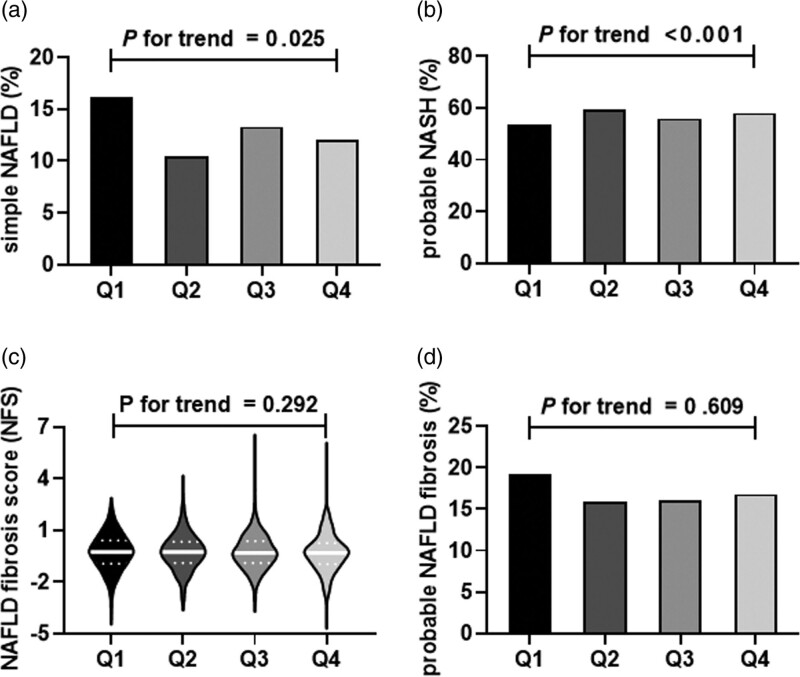
General clinical characteristics of the 4115 diabetic participants of this study by glucagon quartiles are shown in Table 1. The quartile ranges of glucagon were Q1 (≤115.31 pg/ml), Q2 (115.32–154.12 pg/mL), Q3 (154.13–201.99 pg/mL), Q4 (>202 pg/mL). Through the elevation of glucagon level, subjects were more prone to be women, had higher platelet, total cholesterol, HDL-C and LDL-C (Table 1). Meanwhile, the prevalence of probable NASH showed a significantly increasing trend, but no significant trend was observed in NFS or prevalence of probable advanced fibrosis (Fig. 2).
Table 1.
Characteristics of study participants by NAFLD category
| Glucagon (pg/mL) | |||||
|---|---|---|---|---|---|
| Characteristic | Q1 (≤115.31) | Q2 (115.32–154.12) | Q3 (154.13–201.99) | Q4 (>202) | P for trend |
| N | 1029 | 1029 | 1029 | 1028 | |
| Age, year | 67.29 ± 8.96 | 67.61 ± 8.88 | 67.84 ± 8.21 | 66.76 ± 8.82 | 0.266 |
| Men, % | 28.01 | 26.12 | 23.83 | 22.04 | <0.001 |
| Duration of diabetes, year | 10.68 ± 8.53 | 9.83 ± 7.6 | 10.34 ± 7.97 | 9.97 ± 7.92 | 0.164 |
| Current smoking, % | 17.01 | 16.03 | 14.29 | 15.18 | 0.946 |
| Platelet count, *109/L | 210.11 ± 58.18 | 214.39 ± 57.75 | 212.39 ± 57.59 | 216.37 ± 57.82 | 0.038 |
| Albumin, g/dL | 44.48 ± 2.74 | 44.46 ± 2.63 | 44.43 ± 2.71 | 44.48 ± 2.68 | 0.930 |
| AST/ALT | 1.17 ± 0.36 | 1.16 ± 0.39 | 1.16 ± 0.42 | 1.17 ± 0.43 | 0.921 |
| BMI, kg/m2 | 24.71 ± 3.49 | 25.16 ± 3.73 | 24.99 ± 3.54 | 25.03 ± 3.77 | 0.119 |
| Waist circumference, cm | 89.55 ± 9.84 | 90.58 ± 9.94 | 90.2 ± 9.66 | 90.25 ± 9.59 | 0.210 |
| FPG, mmol/L | 7.83 ± 2.36 | 7.74 ± 2.34 | 7.72 ± 2.41 | 7.77 ± 2.59 | 0.571 |
| HbA1c, % | 7.51 ± 1.34 | 7.49 ± 1.4 | 7.48 ± 1.38 | 7.52 ± 1.42 | 0.899 |
| HbA1c, mmol/mol | 58.58 ± −8.85 | 58.36 ± −8.2 | 58.25 ± −8.42 | 58.69 ± −7.98 | |
| SBP, mmHg | 143.57 ± 19.6 | 146.33 ± 19.83 | 144.49 ± 19.68 | 145.14 ± 19.78 | 0.307 |
| DBP, mmHg | 78.24 ± 10.4 | 78.94 ± 11.18 | 78.54 ± 10.9 | 78.57 ± 10.47 | 0.703 |
| Total cholesterol, mmol/L | 5.03 ± 1.19 | 5 ± 1.19 | 5.17 ± 1.2 | 5.25 ± 1.17 | <0.001 |
| Triglycerides, mmol/L | 1.83 ± 1.42 | 1.88 ± 1.39 | 1.9 ± 1.57 | 1.93 ± 1.45 | 0.119 |
| HDL-C, mmol/L | 1.18 ± 0.29 | 1.19 ± 0.28 | 1.23 ± 0.3 | 1.23 ± 0.3 | <0.001 |
| LDL-C, mmol/L | 3.14 ± 0.85 | 3.08 ± 0.84 | 3.18 ± 0.86 | 3.24 ± 0.84 | <0.001 |
| Hypertension, % | 76.48 | 83.67 | 76.38 | 78.5 | 0.829 |
| Dyslipidemia, % | 62.49 | 59.86 | 60.84 | 65.18 | 0.182 |
The data are summarized as the mean ± SD for continuous variables or as a percentage for categorical variables. P for trend was calculated by regression analysis.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP; diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; SBP, systolic blood pressure.
Fig. 2.
Distribution of NAFLD inflammatory and fibrotic progression in different glucagon quartiles among Chinese diabetic patients.
Association of glucagon with NAFLD inflammatory progression in diabetic patients
Table 2 shows that glucagon was negatively associated with inflammatory progression of liver steatosis in diabetic patients. Although glucagon quartiles were not associated with simple NAFLD (P for trend = 0.176), they were negatively associated with probable NASH after adjustment for age, sex, duration of diabetes, current smoking, waist circumference, C-peptide, HbA1c, dyslipidemia, hypertension and use of incretins and SGLT2 inhibitors (Q4 vs. Q1 OR 0.71, 95% CI, 0.53–0.96, P for trend=0.010). Moreover, in ordinal regression, glucagon was also negatively associated with the inflammatory progression of NAFLD, with OR of 1SD increment of ln(glucagon) 0.77 (95% CI 0.64–0.91, P for trend=0.008).
Table 2.
Relations of glucagon with NAFLD inflammatory progression in diabetic patients
| Glucagon, pg/mL | 1SD increment of ln(glucagon) | |||||
|---|---|---|---|---|---|---|
| Quartile 1 (≤115.31) | Quartile 2 (115.32–154.12) | Quartile 3 (154.13–201.99) | Quartile 4 (>202) | P for trend | ||
| Simple NAFLD | Reference | 0.7 (0.49,1.00) | 0.87 (0.62,1.22) | 0.73 (0.51,1.03) | 0.176 | 0.75 (0.58,0.97) |
| Probable NASH | Reference | 0.91 (0.68,1.22) | 0.75 (0.56,1.00) | 0.71 (0.53,0.96)a | 0.010 | 0.72 (0.58,0.90)a |
| Inflammatory progression of NAFLD | Reference | 0.91 (0.72,1.15) | 0.78 (0.62,0.99)a | 0.75 (0.59,0.95)a | 0.008 | 0.77 (0.64,0.91)a |
Data are shown as regression odds ratios (95% CI). Multinomial and ordinal logistic regression analyses were used. Inflammatory progression of NAFLD: from non-NAFLD, simple NAFLD to probable NASH. The model was adjusted for age, sex, duration of diabetes, current smoking, waist circumference, C-peptide, HbA1c, dyslipidemia, hypertension and use of incretins and SGLT2 inhibitors.
P < 0.05.
Association of glucagon with advanced fibrosis in diabetic patients with NAFLD
Table 3 shows that glucagon was not significantly associated with fibrotic progression of liver steatosis in diabetic patients with NAFLD. Glucagon quartiles were not associated with indeterminate group (P for trend=0.298), nor were they were related to presence of advanced fibrosis (P for trend=0.751). The fibrotic progression of NAFLD was not associated with glucagon (P for trend=0.889).
Table 3.
Relations of glucagon with NAFLD fibrotic progression in diabetic patients
| Glucagon, pg/mL | 1SD increment of ln(glucagon) | |||||
|---|---|---|---|---|---|---|
| Quartile 1 (≤115.31) | Quartile 2 (115.32–154.12) | Quartile 3 (154.13–201.99) | Quartile 4 (>202) | P for trend | ||
| Indeterminate group | Reference | 1.04 (0.69,1.57) | 1.50 (0.97,2.31) | 1.13 (0.76,1.70) | 0.298 | 1.15 (0.87,1.51) |
| Presence of significant fibrosis | Reference | 0.78 (0.46,1.33) | 1.19 (0.69,2.06) | 0.97 (0.57,1.64) | 0.751 | 1.04 (0.72,1.50) |
| Fibrotic progression of NAFLD | Reference | 0.87 (0.65,1.15) | 1.03 (0.78,1.37) | 0.96 (0.73,1.27) | 0.889 | 1.00 (0.82,1.22) |
Data are shown as regression odds ratios (95% CI). Multinomial and ordinal logistic regression analyses were used. Fibrotic progression of NAFLD: from absence of significant fibrosis, indeterminate results (NFS between −1.455 and 0.676) to presence of significant fibrosis (NFS >0.676). The model was adjusted for age, sex, duration of diabetes, current smoking, waist circumference, C-peptide, HbA1c, dyslipidemia, hypertension and use of incretins and SGLT2 inhibitors.
Discussion
In this study including 4115 Chinese diabetic adults, we reported that glucagon quartiles were negatively associated with probable NASH, independent of age, sex, duration of diabetes, current smoking, waist circumference, C-peptide, HbA1c, dyslipidemia, hypertension and use of incretins and SGLT2 inhibitors. Furthermore, glucagon was also negatively associated with the inflammatory progression of NAFLD from non-NAFLD, simple NAFLD to probable NASH. Whereas, glucagon was not significantly associated with fibrotic progression of liver steatosis in diabetic patients with NAFLD. Our results suggested that islet alpha cell function might influence the inflammatory progression of NAFLD in diabetic adults.
Several metabolic parameters in this study population were comparable among the subgroups divided by the quartiles of glucagon. The influence on glucagon from both T2DM and NAFLD could be the reason. Islet injury with alpha cell loss could reduce circulating glucagon, and in some, but not all, individuals with T2DM increased concentrations of glucagon can be observed [20]. For fatty liver disease, the typical hormonal environment included hyperglucagonemia [21]. Hence, in patients with both T2DM and NAFLD, the glucagon level was not necessarily elevated. The comparable metabolic characteristics in the subgroups of glucagon quartiles could eliminate the effects of duration of diabetes, obesity (BMI and waist circumference), blood glucose (FPG and HbA1c), hypertension and dyslipidemia on the NAFLD progression.
Glucagon was inversely related to NAFLD progression both in this study and in other researches. The association between glucagon and NASH could be explained by the beneficial role of glucagon in NAFLD in both animal and human studies. Exogenous glucagon administration could reduce fatty liver in human and animal studies [22]. Glucagon had hypolipidemic effects on hepatocytes and promotes mobilization of hepatic fat [23]. In addition, attenuation of glucagon receptor signaling is proposed to be associated with an increased risk of fatty liver [24]. Furthermore, glucagon/T3 substantially improved hepatic fat content and improve NASH in preclinical disease models [25]. Therefore, it could be inferred that glucagon might play a protective role in the inflammatory progress of NAFLD in type 2 diabetic patients.
There are several potential mechanisms underlying the function of glucagon on the progression of NAFLD. Glucagon increases and insulin inhibits follistatin secretion both in vivo and in vitro, mediated via the secondary messenger cAMP in the hepatocyte. Circulating follistatin plays a negative role in regulating hepatic gluconeogenesis through β-cell. Follistatin reduces hepatic lipid uptake and synthesis suggesting that circulating follistatin could counteract the development of hepatic steatosis in NAFLD and NASH [26,27]. Moreover, PNPLA3, an early signature of carbohydrate-induced lipogenesis, was unchanged by addition of glucagon in HepG2 cells [28]. Besides, glucagon could induce acetylation of different energy-sensing factors, which was involved in the advancement of NAFLD to liver cancer [29].
The liver-alpha cell axis in T2DM patients has now been receiving great clinical interests [30]. Physiologically, glucagon controls amino acid metabolism in the liver, and the plasma concentration of amino acids regulates alpha cell secretion [30]. In steatosis liver, expression of several genes involved in amino acid uptake and degradation, and the glucagon receptor gene, were downregulated [31]. The concentration and function of glucagon were both altered and might lead to compensatory hyperglucagonemia, which was similar to the development process of insulin resistance. Therefore, the pathophysiological phenomenon that developed in parallel with hepatic steatosis could result in glucagon resistance. However, the role of glucagon in NAFLD inflammatory progression has not been fully elucidated. Given the close relationship between liver and alpha cells, further study about glucagon, NASH and the feedback loop involving amino acid in T2DM could contribute to the biology of glucagon in metabolic diseases.
In contrast to our previous findings that C-peptide was positively associated with inflammatory progression and negatively associated with fibrosis progression [12], in this study, we found that glucagon was negatively associated with inflammatory progression of fatty liver but did not show significant relation with fibrotic progression of NAFLD. The diverse association between C-peptide and NAFLD progression might be a result of different functions of pancreas alpha and beta cells. The pancreas alpha and beta cells control glucose metabolism by modulating the relative concentrations of glucagon and insulin. Unger suggested the ‘bihormonal-abnormality’ hypothesis in regards to the development of diabetes, stating that both relative and absolute hyperglucagonemia and insulin deficiency may be present in diabetic subjects [32]. As the overall islet cell dysfunction becomes worse as type 2 diabetes progresses, C-peptide to glucagon ratio exhibited a decreasing tendency (and glucagon-to-insulin ratio exhibited an increasing tendency) as the duration of diabetes became longer [33]. Moreover, the ‘bihormonal-abnormality’ (glucagon-to-insulin ratio) was suggested to be negatively correlated with ALT, suggesting the close relationship between the balance between β and α islet cells with liver diseases [33]. These results suggest that islet alpha- and beta-cell dysfunction might be closely related to the metabolic and inflammatory changes in NAFLD. Mechanically, the balance between β and α islet cells could regulate FGF-21 which were served as important endocrine metabolic regulators [34].
There are several limitations to this study. First, as a cross-sectional study, this study cannot identify a causal relationship between glucagon and NAFLD inflammatory progression. Second, NFS was used to assess probable advanced fibrosis in patients with NAFLD and type 2 diabetes. Liver histology is the gold standard to assess liver fibrotic stages, but this invasive examination could not be performed in large-scale epidemiology studies. NFS could accurately predict the presence or absence of advanced fibrosis in NAFLD, with high sensitivity and specificity, and was recommended to be used in clinical decisions to identify those who are suspicious for NAFLD and NASH in patients with type 2 diabetes [18]. Lastly, despite a large sample size, the study population was from a single center, so there could be selection bias.
In conclusion, we provide evidence for significant associations between glucagon and inflammatory progression of NAFLD, but not fibrotic progression of NAFLD. These results indicate that alpha cell function may have a role in the NAFLD inflammatory progression. Further unraveling the mechanism linking glucagon and NASH might provide new insight into the potential new drug targets.
Acknowledgements
The authors thank all team members and participants in the METAL study.
This study was supported by National Natural Science Foundation of China (91857117); Science and Technology Commission of Shanghai Municipality (18410722300, 19140902400, 20015800400, 20ZR1432500); the Major Science and Technology Innovation Program of Shanghai Municipal Education Commission (2018YFC1705103); Shanghai Municipal Human Resources and Social Security Bureau (2020074); Clinical Research Plan of SHDC (SHDC2020CR4006); Commission of Health and Family Planning of Pudong District (PWZxq2017-17, PW2015D-5). The funders played no role in the design or conduct of the study, collection, management, analysis or interpretation of data or in the preparation, review, or approval of the article.
Y.L. and N.W. designed the study; Y.W., H.W., Z.L., W.Z., F.X., Y.C., X.C., C.W. and C.C. conducted the research; H.W., Y.W., Z.L. and N.W. analyzed the data and wrote the manuscript. The final manuscript was read and approved by all authors.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Yuying Wang, Zhiqi Lin and Heng Wan contributed equally to the writing of this article.
Ningjian Wang is the co-corresponding author.
References
- 1.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 2.Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis 2017; 49:471–483. [DOI] [PubMed] [Google Scholar]
- 3.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62:S47–S64. [DOI] [PubMed] [Google Scholar]
- 4.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015; 100:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148:547–555. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149:389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomark Med 2016; 10:1141–1151. [DOI] [PubMed] [Google Scholar]
- 8.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 2011; 13(Suppl 1):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrannini E, DeFronzo RA, Sherwin RS. Transient hepatic response to glucagon in man: role of insulin and hyperglycemia. Am J Physiol 1982; 242:E73–E81. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 11.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Wang Y, Zhang W, Chen Y, Chen X, Wang C, et al. C-peptide is associated with NAFLD inflammatory and fibrotic progression in type 2 diabetes. Diabetes Metab Res Rev 2020; 36:e3210. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr 2017; 36:253–259. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. ; 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310:948–959. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Chen Y, Ning Z, Li Q, Han B, Zhu C, et al. Exposure to famine in early life and nonalcoholic fatty liver disease in adulthood. J Clin Endocrinol Metab 2016; 101:2218–2225. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Chen C, Zhao L, Chen Y, Han B, Xia F, et al. Vitamin D and nonalcoholic fatty liver disease: bi-directional mendelian randomization analysis. Ebiomedicine 2018; 28:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–357. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome–a new worldwide definition. Lancet 2005; 366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 20.Færch K, Vistisen D, Pacini G, Torekov SS, Johansen NB, Witte DR, et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 2016; 65:3473–3481. [DOI] [PubMed] [Google Scholar]
- 21.Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol 2017; 234:R1–R21. [DOI] [PubMed] [Google Scholar]
- 22.Bobe G, Ametaj BN, Young JW, Beitz DC. Potential treatment of fatty liver with 14-day subcutaneous injections of glucagon. J Dairy Sci 2003; 86:3138–3147. [DOI] [PubMed] [Google Scholar]
- 23.Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol 2010; 6:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 2003; 284:E671–E678. [DOI] [PubMed] [Google Scholar]
- 25.Finan B, Clemmensen C, Zhu Z, Stemmer K, Gauthier K, Müller L, et al. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell 2016; 167:843–857.e14. [DOI] [PubMed] [Google Scholar]
- 26.Nakatani M, Kokubo M, Ohsawa Y, Sunada Y, Tsuchida K. Follistatin-derived peptide expression in muscle decreases adipose tissue mass and prevents hepatic steatosis. Am J Physiol Endocrinol Metab 2011; 300:E543–E553. [DOI] [PubMed] [Google Scholar]
- 27.Ungerleider NA, Bonomi LM, Brown ML, Schneyer AL. Increased activin bioavailability enhances hepatic insulin sensitivity while inducing hepatic steatosis in male mice. Endocrinology 2013; 154:2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao L, Ito K, Huang KH, Sae-tan S, Lambert JD, Ross AC. Shifts in dietary carbohydrate-lipid exposure regulate expression of the non-alcoholic fatty liver disease-associated gene PNPLA3/adiponutrin in mouse liver and HepG2 human liver cells. Metabolism 2014; 63:1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Yao W, Xia J, Wang T, Huang F. Glucagon-induced acetylation of energy-sensing factors in control of hepatic metabolism. Int J Mol Sci 2019; 20:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The liver-α-cell axis and type 2 diabetes. Endocr Rev 2019; 40:1353–1366. [DOI] [PubMed] [Google Scholar]
- 31.Eriksen PL, Vilstrup H, Rigbolt K, Suppli MP, Sørensen M, Heebøll S, et al. Non-alcoholic fatty liver disease alters expression of genes governing hepatic nitrogen conversion. Liver Int 2019; 39:2094–2101. [DOI] [PubMed] [Google Scholar]
- 32.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism 1978; 27:1691–1709. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Kim M, Park JS, Lee S, You J, Ahn CW, et al. Higher glucagon-to-insulin ratio is associated with elevated glycated hemoglobin levels in type 2 diabetes patients. Korean J Intern Med 2017; 34:1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 2015; 4:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]