Abstract
Objective
Inflammatory bowel disease (IBD) is a chronic intestinal disease. This study was attempted to investigate the effects of long noncoding RNA KIF9-AS1 (KIF9-AS1) on the development of IBD and its underlying mechanism of action.
Methods
Quantitative real time PCR (qRT-PCR) was implemented to examine the expression of KIF9-AS1 and microRNA-148a-3p (miR-148a-3p). The IBD mouse model was induced by dextran sulfate sodium (DSS). The body weight, disease activity index (DAI) score, colon length and histological injury were used to evaluate the colon injury. The levels of proinflammatory cytokines were measured by ELISA. In vitro, IBD was simulated by DSS treatment in colonic cells. Then the apoptosis of colonic cells was detected by flow cytometry assay. Furthermore, a dual-luciferase reporter assay was used to demonstrate the interactions among KIF9-AS1, miR-148a-3p and suppressor of cytokine signaling (SOCS3).
Results
KIF9-AS1 expression was upregulated in IBD patients, DSS-induced IBD mice and DSS-induced colonic cells, whereas miR-148a-3p expression was downregulated. KIF9-AS1 silencing attenuated the apoptosis of DSS-induced colonic cells in vitro and alleviated colon injury and inflammation in DSS-induced IBD mice in vivo. Additionally, the mechanical experiment confirmed that KIF9-AS1 and SOCS3 were both targeted by miR-148a-3p with the complementary binding sites at 3′UTR. Moreover, miR-148a-3p inhibition or SOCS3 overexpression reversed the suppressive effect of KIF9-AS1 silencing on the apoptosis of DSS-induced colonic cells.
Conclusion
KIF9-AS1 silencing hampered the colon injury and inflammation in DSS-induced IBD mice in vivo, and restrained the apoptosis of DSS-induced colonic cells by regulating the miR-148a-3p/SOCS3 axis in vitro, providing a new therapeutic target for IBD.
Keywords: apoptosis, inflammatory bowel disease, long noncoding RNA KIF9-AS1, microRNA-148a-3p, suppressor of cytokine signaling
Introduction
Inflammatory bowel disease (IBD) is a chronic intestinal disease which mainly includes Crohn’s disease and ulcerative colitis [1]. The etiology of IBD has involved genetic susceptibility, infectious agents and environmental factors, leading to aberrant immune responses to intestinal microbiota [2]. IBD is closely associated with fever and fatigue, loss of appetite, abdominal pain, diarrhea with bleeding and unintended weight loss [3]. The prevalence of IBD is high, and the costs of care for both crohn’s disease and ulcerative colitis have upregulated rapidly [4]. Thus, it is imperative to explore the mechanisms and targets associated with the treatment of IBD.
Long noncoding RNAs (lncRNAs) have been proved to modulate cell function and biological process in intestinal diseases such as irritable bowel syndrome [5], hirschsprung’s disease [6] and IBD [7]. Notably, numerous lncRNAs take part in the development of IBD. LncRNA colon cancer-associated transcript 1 (CCAT1) inhibits miR-185-3p expression to promote IBD malignancy [8]. LncRNA H19 overexpression has a destructive effect on intestinal epithelial barrier function in ulcerative colitis patients via regulating vitamin D receptor signaling [9]. Downregulation of lncRNA NEAT1 attenuates the inflammatory response in IBD [10]. Interestingly, lncRNA KIF9-AS1 (KIF9-AS1) is one of the 10 most high-expressed lncRNAs in Crohn’s disease pinch biopsies [11]. Wang et al., [12] have discovered that KIF9-AS1 can serve as a potential diagnostic biomarker for IBD. However, the underlying mechanism of KIF9-AS1 in the regulation of IBD is still unknown.
As biological molecules, microRNAs (miRNAs) participate in the progress of digestive system diseases including IBD [13,14]. In addition, multiple miRNAs have protective effects against IBD. For instance, miR-122 suppresses NOD2 expression to alleviate inflammatory response, lipopolysaccharide (LPS)-induced apoptosis and intestinal epithelial cell injury in IBD [15]. MiR-223 attenuates pathological intestinal inflammation via reducing NLPR3 activation in IBD [16]. Furthermore, miR-148a is regarded as a pivotal regulator in intestinal diseases. MiR-148a acts as a tumor suppressor by restraining Bcl-2 in colorectal cancer [17]. Interestingly, miR-148a is downregulated in IBD and protects mice against IBD and IBD-associated tumors [18]. However, the specific regulatory relationship between KIF9-AS1 and miR-148a-3p in IBD remains undefined.
Suppressor of cytokine signaling (SOCS3) belongs to the SOCS family and participates in acute and chronic inflammation [19]. Existing researches have reported that SOCS3 is involved in ulcerative colitis pathogenesis, and is high-expressed in biopsy specimens from patients with ulcerative colitis [20,21]. Moreover, SOCS3 plays a key role in the progress of IBD and IBD-related colorectal cancer by regulating the function of diverse cytokines [22]. Importantly, SOCS3 inhibition retards the development of Crohn’s disease by attenuating inflammatory response [23]. Nevertheless, the relationship between SOCS3 and KIF9-AS1 in IBD is still unclear.
Herein, we constructed dextran sulfate sodium (DSS)-induced IBD mice and DSS-induced colonic cell models. Then we evaluated the role of KIF9-AS1 in DSS-induced IBD mice. Furthermore, we explored whether KIF9-AS1 controlled the DSS-induced apoptosis of colonic cells via targeting the miR-148a-3p/SOCS3 axis. Our results may aid in the identification of potential therapeutic targets for IBD.
Materials and methods
Clinical specimens
Colonoscopic biopsies were obtained from inflamed mucosa of the colon of patients with Crohn’s disease (Crohn’s disease group, n = 25) and ulcerative colitis (ulcerative colitis group, n = 25) in our hospital between 2018 and 2019. In addition, 20 normal colon tissues from healthy donors were regarded as the normal group. The diagnosis of Crohn’s disease and ulcerative colitis met the Copenhagen criteria. The colon tissues were stored in liquid nitrogen until further analysis. This study was approved by the ethics committee of our hospital, and informed consent was obtained from each individual.
Animals
Thirty-two C57BL/6 mice (6–8 week-old) were purchased from Shanghai Laboratory Animal Research Center (Shanghai, China). Mice were fed standard chow and water and maintained under temperature-controlled conditions with an artificial 12-h light/dark cycle. The animal experiments were permitted by the ethics committee of our hospital.
The dextran sulfate sodium-induced inflammatory bowel disease model
Mice were randomly assigned to four groups (n = 8): DSS, sham, DSS + small interfering (si)-KIF9-AS1 and DSS + si-NC group. Briefly, the mice treated with 2% DSS for 10 days were regarded as the DSS group. The mice treated with the same volume of PBS for 10 days acted as the sham group. The mice were intraperitoneally injected with 100 μl si-KIF9-AS1 or si-negative control (NC) three times a week for 2 weeks, and they were treated with 2% DSS for 10 days from the second week of si-KIF9-AS1 (DSS + si-KIF9-AS1 group) or si-NC (DSS + si-NC group) injection. The mice were weighted daily, and the disease activity index (DAI) score, including occult blood and stool consistency, were measured every day until day 10. The DAI score was calculated by combining the bleeding score and stool score. After 10 days of DSS treatment, mice were fasting 12 h and subsequently anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg), and sacrificed by decapitation. The colon tissues were collected and the colon length was measured.
Haematoxylin-eosin staining
Colon tissues of mice were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, cut into 4-μm thick sections, dewaxed in xylene and rehydrated with ethanol. Sections were then stained with haematoxylin for 2 min and with eosin for 2 min. Using light microscopy, the histological injury was observed.
ELISA
The concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 in colon tissues of mice were measured using ELISA kits (Sigma, St. Louis, Missouri, USA) following the manufacturer’s recommendation.
Cell culture and dextran sulfate sodium-induced colonic cells
The human colonic epithelial cell lines HT-29 were purchased from the American Type Culture Collection (Manassas, Virginia, USA). HT-29 cells were cultured in DMEM (Invitrogen, Carlsbad, California, USA) with 10% fetal bovine serum (FBS, Invitrogen) at 37 °C containing 5% CO2. The HT-29 cells were stimulated with 2% DSS for 24 h were regarded as the DSS group. The HT-29 cells without treatment served as the control group.
Cell transfection
The si-KIF9-AS1, si-NC, miR-148a-3p mimics, miR-148a-3p inhibitor, miR-NC, pcDNA3.1 SOCS3 (pcDNA-SOCS3) and pcDNA-NC were synthesized by GenePharma (Shanghai, China). DSS-induced HT-29 cells grown to 85% confluence were transfected or cotransfected with these above agents using Lipofectamine 3000 (Invitrogen). The DSS-induced HT-29 cells in the Blank group did not receive any transfection.
Quantitative real time PCR
Total RNA was extracted from tissues and cells using the TRIzol reagent (Invitrogen). Then, cDNA samples were attained through reverse transcription using PrimeScript RT Reagent Kit (TaKaRa, Japan). Next, quantitative real time PCR (qRT-PCR) was conducted on 7500 real-time PCR System (Applied Biosystems, Waltham, Massachusetts, USA). Relative expression was calculated by the 2−ΔΔCt method. Glyceraldehyde-3-phosphate dehydrogenase, U6 and β-actin were used for the normalization of KIF9-AS1, miR-148a-3p and SOCS3, respectively. The primer sequences were shown in Table 1.
Table 1.
Primers sequences
| Name of primer | Sequences (5′–3′) |
|---|---|
| KIF9-AS1-F | AGTCCTTCCCATTCACAGGG |
| KIF9-AS1-R | GCCCTCTTCTTCCTCCACAT |
| GAPDH-F | TGTTCGTCATGGGTGTGAAC |
| GAPDH-R | ATGGCATGGACTGTGGTCAT |
| miR-148a-3p-F | AGCAGTTCAGTGCACTACAG |
| miR-148a-3p-R | GCAGGGTCCGAGGTATTC |
| U6-F | GCTTCGGCAGCACATATACTAAAAT |
| U6-R | CGCTTCACGAATTTGCGTGTCAT |
| SOCS3-F | ACCTTCAGCTCCAAAAGCGAGTAC |
| SOCS3-R | CGCTCCAGTAGAATCCGCTCTC |
| β-actin-F | ACACCTTCTACAATGAGCTG |
| β-actin-R | CTGCTTGCTGATCCACATCT |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Flow cytometry assay
DSS-induced HT-29 cells were trypsinized and washed with PBS twice. Then, cells were stained by using Annexin V-fluorescein isothiocyanate and propidium iodide (Invitrogen) for 15 min in a dark room. Afterward, the apoptotic cells were observed by MUSE flow cytometer (Beckman, Miami, Florida, USA).
Western blot
Total proteins were extracted from HT-29 cells and then transferred into SDS-PAGE. Separated protein was transferred onto polyvinylidene fluoride membranes, blocked with 5% skimmed milk, and incubated overnight at 4 °C with the following primary antibodies: anti-SOCS3 (1:1000, SAB4700831MSDS, Sigma), anticaspase-3 (1:1000, ABC495MSDS, Sigma) and anti-β-actin (1:5000, A5441MSDS, Sigma). Afterward, the membranes were subjected to horseradish peroxidase-labeled goat antimouse IgG (1:4000, 12-349MSDS, Sigma) secondary antibody at 25 °C for 1 h. The protein bands were visualized by exposure to enhanced chemiluminescence solution and quantified by ImageLab software (Bio-Rad, Hercules, California, USA).
Dual-luciferase reporter assay
The potential binding sites of KIF9-AS1 and miR-148a-3p or SOCS3 and miR-148a-3p were predicted by Starbase or TargetScan, respectively. KIF9-AS1 and SOCS3 with wild type (wt) or mutant type miR-148a-3p-binding sites were generated and fused to the psiCHECK-2 vectors (YouBio, Hunan, China). HT-29 cells were co-transfected with the above luciferase vectors and miR-NC or miR-148a-3p mimics using Lipofectamine 3000 (Invitrogen).
Statistical analysis
Data statistical analysis was performed using GraphPad Prism 7.0 (GraphPad, San Diego, California, USA). Data were presented as mean ± SD. The differences between two groups or among multiple groups were assessed by Student’s t-test or one-way analysis of variance followed by Tukey’s post hoc test. Differences were considered statistically significant at P < 0.05.
Results
KIF9-AS1 was upregulated while miR-148a-3p was downregulated in inflammatory bowel disease
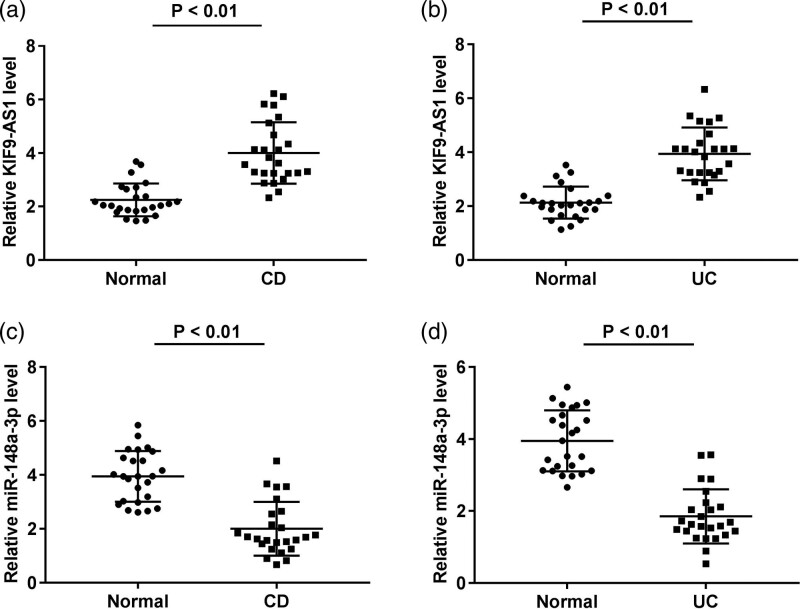
To demonstrate whether KIF9-AS1 is differently expressed in IBD patients (Crohn’s disease patients and ulcerative colitis patients), qRT-PCR was implemented. The KIF9-AS1 expression in both Crohn’s disease patients and ulcerative colitis patients was markedly increased by contrast to the normal healthy donors (P < 0.01, Fig. 1a and b). In addition, the miR-148a-3p expression in patients with Crohn’s disease and ulcerative colitis and normal healthy donors was evaluated. The miR-148a-3p expression in both the Crohn’s disease and ulcerative colitis group was lower than that in the normal group (P < 0.01, Fig. 1c and d).
Fig. 1.
KIF9-AS1 was upregulated while miR-148a-3p was downregulated in inflammatory bowel disease (IBD). (a) KIF9-AS1 expression in patients with Crohn’s disease (CD) and normal healthy donors were measured by quantitative real time PCR (qRT-PCR). P < 0.01 vs. normal; (b) qRT-PCR was performed to confirm the KIF9-AS1 expression in patients with ulcerative colitis (UC) and normal healthy donors. P < 0.01 vs. normal; (c) qRT-PCR was used to detect the expression of miR-148a-3p in crohn’s disease patients and normal healthy donors. P < 0.01 vs. normal; (d) the miR-148a-3p expression in UC patients and normal healthy donors was measured by qRT-PCR. P < 0.01 vs. normal.
The colon injury and inflammation were enhanced in dextran sulfate sodium-induced inflammatory bowel disease model
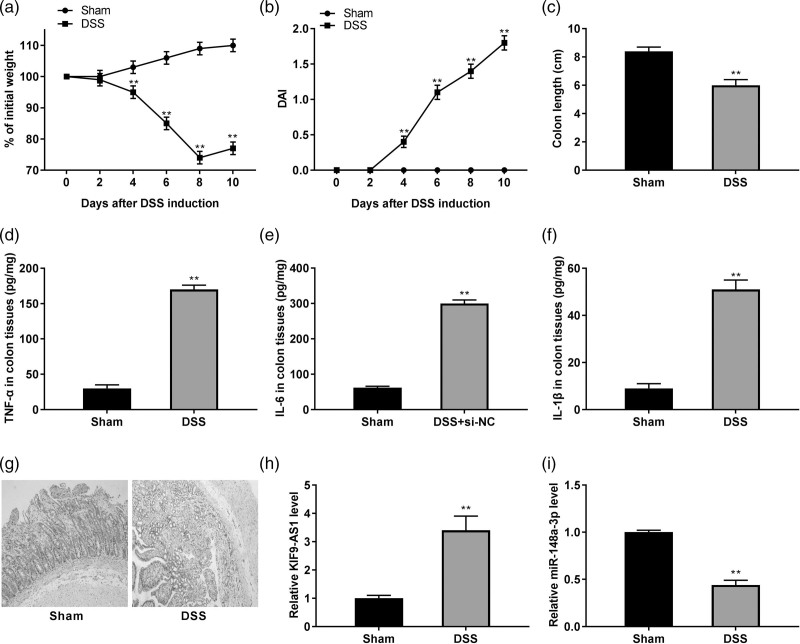
We established a DSS-induced IBD model in mice. As shown in Fig. 2a, the bodyweight of the DSS-induced mice was gradually declined during the 10 days (P < 0.01). Meanwhile, the DAI score in the DSS group displayed a gradual elevation compared to the sham group (P < 0.01, Fig. 2b). Additionally, the colon length of mice was considerably decreased after DSS treatment (P < 0.01, Fig. 2c). Furthermore, the concentrations of proinflammatory cytokines TNF-α, IL-6 and IL-1β were obviously increased in colon tissues of DSS-induced IBD mice (P < 0.01, Fig. 2d–f). Hematoxylin-eosin staining was used to assess histological injury of colon tissues. As shown in Fig. 2g, the folds disappeared with purulent and necrosis, and the intestinal wall was thickened, and recess and goblet cells were disappeared with the infiltrated mononuclear cells in the DSS group. Interestingly, the KIF9-AS1 expression was enhanced, whereas miR-148a-3p expression was inhibited in colon tissues of DSS-induced IBD mice (P < 0.01, Fig. 2h and i).
Fig. 2.
The colon injury and inflammation were enhanced in dextran sulfate sodium (DSS)-induced inflammatory bowel disease (IBD) model. (a) Bodyweight change in the sham group and the DSS group. **P < 0.01 vs. sham; (b) disease activity index (DAI) score of mice in the sham group and the DSS group. **P < 0.01 vs. sham; (c) colon length of mice in the sham group and the DSS group. **P < 0.01 vs. sham; (d–f) the concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β in colon tissues were determined by ELISA. **P < 0.01 vs. sham; (g) the histological injury of colon tissues was observed by hematoxylin-eosin (HE) staining; (h) quantitative real time PCR (qRT-PCR) was used to detect the expression of KIF9-AS1 in colon tissues. **P < 0.01 vs. sham; (i) the expression of miR-148a-3p in colon tissues was demonstrated using qRT-PCR. **P < 0.01 vs. sham.
Silencing of KIF9-AS1 reduced the dextran sulfate sodium-induced apoptosis of colonic cells
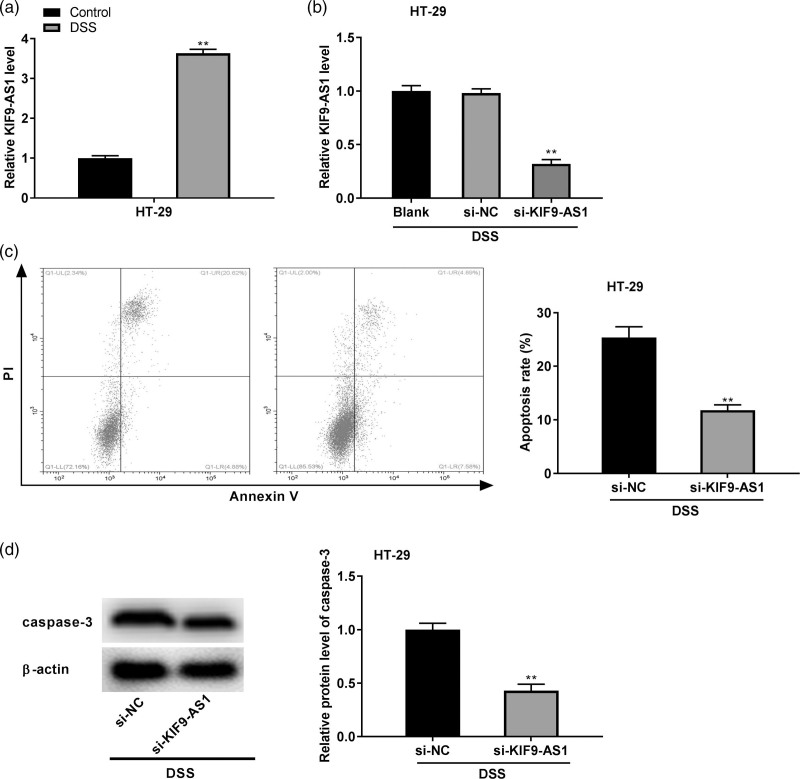
To construct the DSS-induced IBD model at the cellular level, HT-29 cells were stimulated with 2% DSS for 24 h. qRT-PCR discovered that KIF9-AS1 expression was upregulated in HT-29 cells after DSS treatment (P < 0.01, Fig. 3a). To further demonstrate the biological function of KIF9-AS1 in IBD in vitro, KIF9-AS1 was downregulated by the transfection of si-KIF9-AS1 (P < 0.01, Fig. 3b). Flow cytometry assay implied that KIF9-AS1 silencing reduced the apoptosis rate of DSS-induced HT-29 cells (P < 0.01, Fig. 3c). Additionally, western blot revealed that the expression of apoptosis-related protein caspase-3 in DSS-induced HT-29 cells was obviously inhibited by KIF9-AS1 silencing (P < 0.01, Fig. 3d).
Fig. 3.
Silencing of KIF9-AS1 reduced the dextran sulfate sodium (DSS)-induced apoptosis of colonic cells. (a) The expression of KIF9-AS1 in HT-29 cells was examined by quantitative real time PCR (qRT-PCR). **P < 0.01 vs. control; (b) qRT-PCR was used to evaluate the transfection efficiency of si-NC and si-KIF9-AS1 in HT-29 cells. **P < 0.01 vs. si-NC; (c) flow cytometry assay was used to determine the apoptosis rate of HT-29 cells after DSS treatment. **P < 0.01 vs. si-NC; (d) the expression of apoptosis-related protein caspase-3 in DSS-induced HT-29 cells was detected by western blot. **P < 0.01 vs. si-NC.
MiR-148a-3p suppressed the dextran sulfate sodium-induced apoptosis of colonic cells
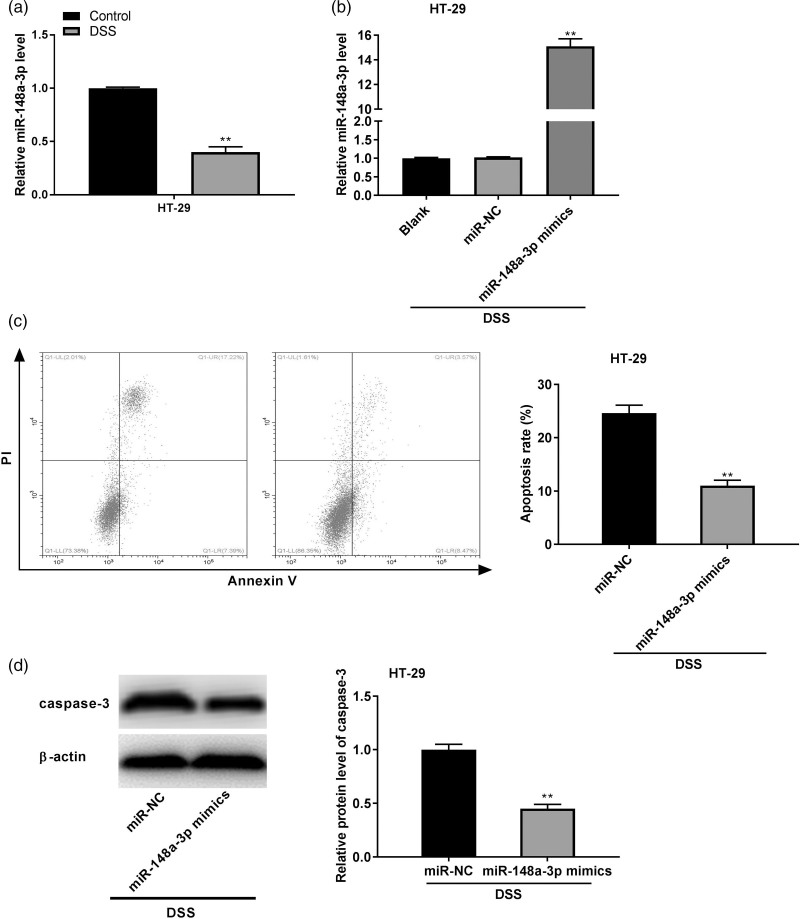
As shown in Fig. 4a, the miR-148a-3p expression was visibly downregulated in the DSS-induced HT-29 cells (P < 0.01). To verify the regulatory effect of miR-148a-3p on DSS-induced HT-29 cells, miR-148a-3p expression was enhanced by the transfection of miR-148a-3p mimics (P < 0.01, Fig. 4b). Moreover, miR-148a-3p overexpression could considerably attenuate the DSS-induced apoptosis of HT-29 cells (P < 0.01, Fig. 4c). The elevation of caspase-3 protein expression caused by DSS treatment in HT-29 cells was markedly down-regulated by miR-148a-3p overexpression (P < 0.01, Fig. 4d).
Fig. 4.
MiR-148a-3p suppressed the dextran sulfate sodium (DSS)-induced apoptosis of colonic cells. (a) quantitative real time PCR (qRT-PCR) was performed to measure the expression of miR-148a-3p in HT-29 cells. **P < 0.01 vs. control; (b) the transfection efficiency of miR-NC and miR-148a-3p in HT-29 cells was assessed by qRT-PCR. **P < 0.01 vs. miR-NC; (c) the apoptosis rate of HT-29 cells after DSS treatment was examined by flow cytometry assay. **P < 0.01 vs. miR-NC; (d) western blot was implemented to measure the expression of apoptosis-related protein caspase-3 in DSS-induced HT-29 cells. **P < 0.01 vs. miR-NC.
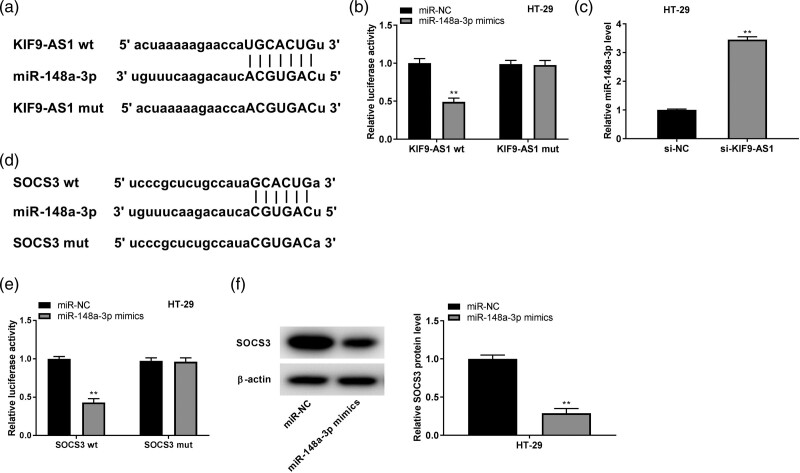
KIF9-AS1 directly interacted with miR-148a-3p, and miR-148a-3p directly interacted with suppressor of cytokine signaling
To demonstrate the deep mechanism by which KIF9-AS1 mediated the progression of IBD in vitro, we used Starbase to predict the miRNAs interacted with KIF9-AS1 and uncovered that miR-148a-3p binds to complementary sequences in KIF9-AS1 (Fig. 5a). The relative luciferase activity was dramatically declined in HT-29 cells cotransfected with miR-148a-3p mimics and KIF9-AS1 wt compared with HT-29 cells co-transfected with miR-NC and KIF9-AS1 wt (P < 0.01, Fig. 5b). Interestingly, si-KIF9-AS1 could visibly upregulate miR-148a-3p expression in HT-29 cells (P < 0.01, Fig. 5c). In addition, bioinformatics analysis by using Targetscan exhibited that miR-148a-3p binds to 3′UTR of SOCS3 mRNA (Fig. 5d). The luciferase activity of HT-29 cells treated with SOCS3 3′UTR-wt reporter was strikingly inhibited by miR-148a-3p mimics (P < 0.01, Fig. 5e). Besides, miR-148a-3p could obviously reduce the protein expression of SOCS3 in HT-29 cells (P < 0.01, Fig. 5f).
Fig. 5.
KIF9-AS1 directly interacted with miR-148a-3p, and miR-148a-3p directly interacted with suppressor of cytokine signaling (SOCS3). (a) The putative binding site of miR-148a-3p in KIF9-AS1 was predicted by starBase; (b) relative luciferase activity in HT-29 cells was measured by dual-luciferase reporter assay. **P < 0.01 vs. miR-NC; (c) the expression of miR-148a-3p in HT-29 cells was detected by quantitative real time PCR (qRT-PCR). **P < 0.01 vs. si-NC. (d) The binding site for miR-148a-3p on the 3′ UTR of SOCS3 was predicted by targetScan. (e) Dual-luciferase reporter assay was performed to detect the relative luciferase activity in HT-29 cells. **P < 0.01 vs. miR-NC; (e) The protein expression of SOCS3 in HT-29 cells was measured by western blot. **P < 0.01 vs. miR-NC.
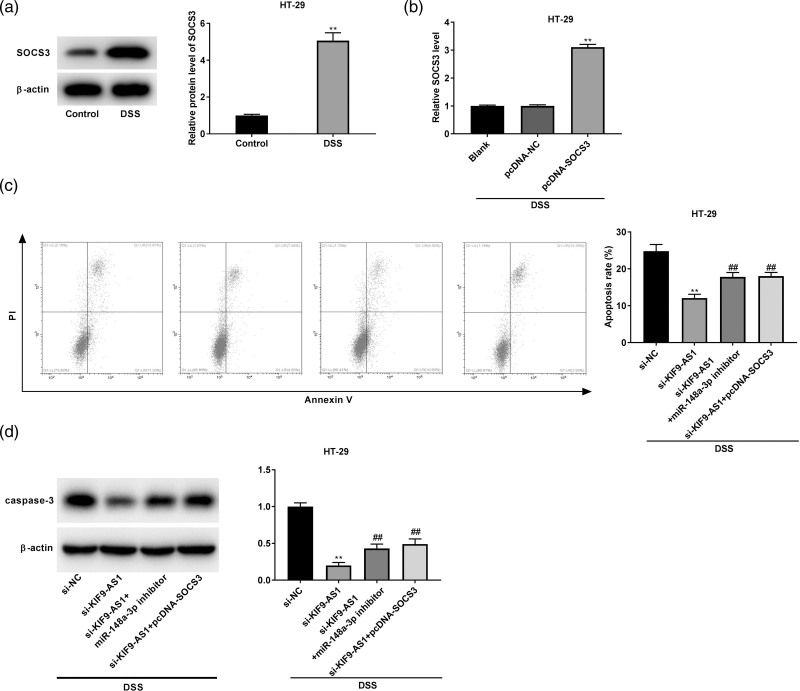
KIF9-AS1 promoted the dextran sulfate sodium-induced apoptosis of colonic cells by targeting miR-148a-3p/suppressor of cytokine signaling axis
As illustrated in Fig. 6a, the protein expression of SOCS3 in DSS-induced HT-29 cells was markedly enhanced (P < 0.01). SOCS3 expression was obviously upregulated by the transfection of pcDNA-SOCS3 (P < 0.01, Fig. 6b). To further confirm the molecular mechanism by which KIF9-AS1 overexpression accelerated the DSS-induced apoptosis of colonic cells, rescue experiments were performed. As shown in Fig. 6c and d, miR-148a-3p inhibition or SOCS3 overexpression could visibly reverse the inhibitory effects of KIF9-AS1 silencing on the apoptosis rate and caspase-3 protein expression of DSS-induced HT-29 cells (P < 0.01).
Fig. 6.
KIF9-AS1 promoted the dextran sulfate sodium (DSS)-induced apoptosis of colonic cells by targeting miR-148a-3p/suppressor of cytokine signaling (SOCS3) axis. (a) The protein expression of SOCS3 was markedly enhanced in DSS-induced HT-29 cells. **P < 0.01 vs. control; (b) the transfection efficiency of pcDNA-NC and pcDNA-SOCS3 was demonstrated using quantitative real time PCR (qRT-PCR) in DSS-induced HT-29 cells. **P < 0.01 vs. pcDNA-NC; (c and d) Inhibition of miR-148a-3p or overexpression of SOCS3 weakened the inhibitory effect of KIF9-AS1 silencing on the apoptosis rate and caspase-3 protein expression of DSS-induced HT-29 cells. **P < 0.01 vs. si-NC; ##P < 0.01 vs. si-KIF9-AS1.
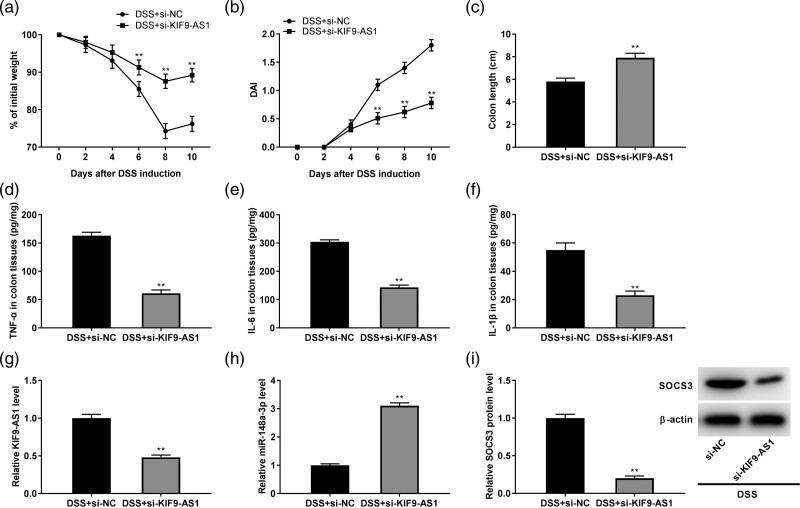
KIF9-AS1 silencing alleviated the colon injury and inflammation in dextran sulfate sodium-induced inflammatory bowel disease model
To further demonstrate whether KIF9-AS1 is associated with the progress of IBD in vivo, mice were intraperitoneally injected with si-NC and si-KIF9-AS1. As exhibited in Fig. 7a, KIF9-AS1 silencing could visibly increase the bodyweight of DSS-induced mice (P < 0.01). In contrary, the DAI score of DSS-induced mice was noticeably reduced by KIF9-AS1 knockdown (P < 0.01, Fig. 7b). In addition, si-KIF9-AS1 could strikingly increase the colon length of DSS-induced mice (P < 0.01, Fig. 7c). Furthermore, silencing of KIF9-AS1 could obviously downregulate the concentrations of TNF-α, IL-6 and IL-1β in colon tissues of DSS-induced IBD mice (P < 0.01, Fig. 7d–f). Notably, KIF9-AS1 silencing obviously downregulated the KIF9-AS1 expression while upregulated the miR-148a-3p expression in DSS-induced mice (P < 0.01, Fig. g and h). Moreover, the protein expression of SOCS3 in DSS-induced mice was considerably downregulated by KIF9-AS1 silencing (P < 0.01, Fig. 7i).
Fig. 7.
KIF9-AS1 silencing alleviated the colon injury and inflammation in dextran sulfate sodium (DSS)-induced inflammatory bowel disease (IBD) model. (a) Si-KIF9-AS1 elevated the bodyweight in DSS-induced mice. **P < 0.01 vs. DSS + si-NC; (b) disease activity index (DAI) score of mice was declined by silencing of KIF9-AS1. **P < 0.01 vs. DSS + si-NC; (c) KIF9-AS1 silencing increased the colon length of DSS-induced mice. **P < 0.01 vs. sham; (d–f) the concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-1β in colon tissues of DSS-induced mice were downregulated by KIF9-AS1 knockdown. **P < 0.01 vs. DSS + si-NC; (g) KIF9-AS1 silencing inhibited the expression of KIF9-AS1 in colon tissues of DSS-induced mice. **P < 0.01 vs. DSS + si-NC; (h) downregulation of KIF9-AS1 enhanced the expression of miR-148a-3p in colon tissues of DSS-induced mice. **P < 0.01 vs. DSS + si-NC; (I) KIF9-AS1 knockdown reduced the protein expression of suppressor of cytokine signaling (SOCS3) in colon tissues of DSS-induced mice. **P < 0.01 vs. DSS + si-NC.
Discussion
The IBD is a chronic and relapsing inflammatory disorder of the intestinal tract [24]. In this study, the bodyweight and colon length were decreased, whereas the DAI score, histological injury and concentrations of proinflammatory cytokines were increased in DSS-induced IBD mice. Previous studies have verified that the bodyweight and colon length were declined, while the DAI score, histological injury and inflammation were elevated in the DSS-induced IBD model [25–27]. All above data indicated that the DSS-induced IBD model in mice was constructed successfully. Enhanced expression of lncRNAs, such as lncRNA DQ786243 [28], lncRNA MALAT1 [29] and LINC00657 [30] has been uncovered in IBD. Here, KIF9-AS1 expression was elevated in IBD patients, DSS-induced IBD mice, and DSS-induced colonic cells. Above all, we suspect that KIF9-AS1 may be a regulator of IBD.
Imbalance in the intracellular events that regulate apoptosis may accelerate the pathogenesis of IBD [31]. Colonic lamina propria and epithelium from ulcerative colitis patients display higher rates of apoptosis than controls [32]. LncRNAs have been proved to take part in the regulation of intestinal epithelial cell apoptosis and inflammation in IBD [33]. LncRNA BC012900 overexpression promotes apoptosis and also elevates the caspase-3 activity of intestinal epithelial cells in ulcerative colitis [34]. LncRNA ANRIL facilitates the injury of the ulcerative colitis model by accelerating the apoptosis and suppressing the proliferation of LPS-treated fetal human cells in vitro [35]. In this study, KIF9-AS1 silencing reduced the apoptosis rate and protein level of caspase-3 in DSS-induced colonic cells. We hypothesize that KIF9-AS1 silencing may protect against IBD by attenuating the apoptosis of DSS-induced colonic cells in vitro. In support of this hypothesis, we investigated the biological effects of KIF9-AS1 in DSS-induced IBD mice and found that KIF9-AS1 silencing not only increased the body weight and colon length but also decreased the DAI score, histological injury and inflammation in DSS-induced IBD mice. The function of KIF9-AS1 was similar to lncRNA NEAT1. LncRNA NEAT1 knockdown suppresses the injury and inflammatory response of colon tissues, as well as elevates the bodyweight and colon length of DSS-induced IBD mice [10]. Taken together, we indicate that KIF9-AS1 silencing has a protective effect against IBD in both vitro and vivo.
Increasing evidence has exhibited that multiple miRNAs are downregulated and play a pivotal role in the regulation of IBD. MiR-125a is downregulated in IBD patients and restrains intestinal mucosal inflammation by modulating ETS-1 [36]. MiR-200b facilitates the proliferation of intestinal epithelial cells in IBD patients by decreasing SAMD2 [37]. MiR-320 contributes to the maintenance of intestinal homeostasis through controlling NOD2 expression in IBD patients [38]. Notably, miR-148a alleviates colon injury and inflammation and inhibits cell apoptosis in the DSS-induced IBD mice [18]. Here, we observed that miR-148a-3p was downregulated in IBD patients, DSS-induced IBD mice and DSS-induced colonic cells. MiR-148a-3p overexpression declined the apoptosis rate and protein level of caspase-3 in DSS-induced colonic cells. Our results suggest that miR-148a-3p protects against IBD through inhibiting apoptosis of DSS-induced colonic cells in vitro. Certain lncRNAs have been shown to interact with miRNAs, participating in the regulation of IBD. LncRNA CRNDE facilitates cell apoptosis by repressing miR-495 in IBD [39]. LncRNA CCAT1 accelerates IBD malignancy by destroying the intestinal barrier by inhibiting miR-185-3p [8]. LncRNA ANRIL silencing attenuates the development of ulcerative colitis by downregulating miR-323b-5p [35]. In this study, miR-148a-3p was regarded as a target of KIF9-AS1, and KIF9-AS1 silencing enhanced the miR-148a-3p expression. We assume that KIF9-AS1 influences IBD through regulating miR-148a-3p. Interestingly, the rescue experiment exhibited that miR-148a-3p knockdown strikingly reversed the effect that si-KIF9-AS1 exerted. Taken together, KIF9-AS1 may promote apoptosis of DSS-induced colonic cells by suppressing miR-148a-3p.
It has been documented that SOCS3 can promote the development of intestinal diseases. For instance, SOCS3 overexpression leads to the destruction of mucosal homeostasis in a model of chronic inflammation [40]. SOCS3 enhances the vulnerability of intestinal epithelial cells during remission and can predict mucosal relapse in ulcerative colitis patients without any signs of mucosal inflammation [41]. Here, SOCS3 protein expression was elevated in DSS-induced colonic cells and suggesting that SOCS3 may have a promoting effect on the progression of IBD. Some miRNAs exert their function through targeting SOCS3 in inflammatory diseases. LncRNA GAS5 accelerates M1 macrophage polarization in childhood pneumonia via modulating miR-455-5p/SOCS3 axis [42]. MiR-19b attenuates intestinal inflammation in Crohn’s disease via inhibiting intestinal SOCS3 [23]. Additionally, certain miRNAs can achieve antiapoptotic effect by targeting SOCS3 [43,44]. In this study, SOCS3 was a target of miR-148a-3p, and miR-148a-3p overexpression inhibited the SOCS3 protein expression. Considering the interaction of KIF9-AS1/miR-148a-3p, we hypothesize that KIF9-AS1 may mediate SOCS3 expression in IBD. Notably, the rescue experiment displayed that SOCS3 overexpression reversed the reduction effects on the apoptosis rate and protein level of caspase-3 caused by KIF9-AS1 silencing in DSS-induced colonic cells. To sum up, KIF9-AS1 promotes the apoptosis rate and protein level of caspase-3 in DSS-induced colonic cells through regulating miR-148a-3p/SOCS3 axis.
In summary, we discovered that KIF9-AS1 expression was increased in IBD. Additionally, KIF9-AS1 silencing exerted the protective role against IBD in mice. Moreover, KIF9-AS1 silencing restrained DSS-induced apoptosis of colonic cells via targeting miR-148a-3p/SOCS3 axis. Our research may give a new therapeutic target for IBD.
Acknowledgements
The study received funds from the National Natural Science Foundation of China (No. 81800489). Three engineering training funds in Shenzhen (No. SYLY201718). Technical Research and Development Project of Shenzhen (No. JCYJ20170307100538697). Technical Research and Development Project of Shenzhen (No. JCYJ20160429174927286). Technical Research and Development Project of Shenzhen (No. JCYJ20170307100911479).
This study was approved by the Ethics Committee of Shenzhen Municipal People’s Hospital. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. And all animal experiments were conducted in strict accordance with the guidelines for laboratory care and use, and approved by the ethics Committee of our hospital.
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr.Jun Yao, Dr. Ruoyu Gao and Dr. Minghan Luo contributed equally to the writing of this article.
References
- 1.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152:313–321.e2. [DOI] [PubMed] [Google Scholar]
- 2.Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020; 16:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norouzinia M, Chaleshi V, Alizadeh AHM, Zali MR. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench 2017; 10:155–167. [PMC free article] [PubMed] [Google Scholar]
- 4.Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K, et al. Corrigendum to the cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis 2020; 26:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang H, Zhang W, Zhang Y, Wang W, Nie L. LncRNA XIST modulates 5-hydroxytrytophan-induced visceral hypersensitivity by epigenetic silencing of the SERT gene in mice with diarrhea-predominant IBS. Cell Signal 2020; 73:109674. [DOI] [PubMed] [Google Scholar]
- 6.Cai P, Li H, Huo W, Zhu H, Xu C, Zang R, et al. Aberrant expression of LncRNA-MIR31HG regulates cell migration and proliferation by affecting miR-31 and miR-31* in Hirschsprung’s disease. J Cell Biochem 2018; 119:8195–8203. [DOI] [PubMed] [Google Scholar]
- 7.Lucafò M, Di Silvestre A, Romano M, Avian A, Antonelli R, Martelossi S, et al. Role of the long non-coding RNA growth arrest-specific 5 in glucocorticoid response in children with inflammatory bowel disease. Basic Clin Pharmacol Toxicol 2018; 122:87–93. [DOI] [PubMed] [Google Scholar]
- 8.Ma D, Cao Y, Wang Z, He J, Chen H, Xiong H, et al. CCAT1 lncRNA promotes inflammatory bowel disease malignancy by destroying intestinal barrier via downregulating miR-185-3p. Inflamm Bowel Dis 2019; 25:862–874. [DOI] [PubMed] [Google Scholar]
- 9.Chen SW, Wang PY, Liu YC, Sun L, Zhu J, Zuo S, et al. Effect of long noncoding RNA H19 overexpression on intestinal barrier function and its potential role in the pathogenesis of ulcerative colitis. Inflamm Bowel Dis 2016; 22:2582–2592. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Tang A, Wang X, Chen X, Zhao L, Xiao Z, Shen S. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int J Mol Med 2018; 42:2903–2913. [DOI] [PubMed] [Google Scholar]
- 11.Zacharopoulou E, Gazouli M, Tzouvala M, Vezakis A, Karamanolis G. The contribution of long non-coding RNAs in inflammatory bowel diseases. Dig Liver Dis 2017; 49:1067–1072. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Hou Y, Chen W, Wang J, Xie W, Zhang X, Zeng L. KIF9-AS1, LINC01272 and DIO3OS lncRNAs as novel biomarkers for inflammatory bowel disease. Mol Med Rep 2018; 17:2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2016; 65:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou J, Hu X, Chen B, Chen X, Zhao L, Chen Z, et al. miR-155 targets Est-1 and induces ulcerative colitis via the IL-23/17/6-mediated Th17 pathway. Pathol Res Pract 2017; 213:1289–1295. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Wang C, Liu Y, Tang L, Zheng M, Xu C, et al. miR-122 targets NOD2 to decrease intestinal epithelial cell injury in Crohn’s disease. Biochem Biophys Res Commun 2013; 438:133–139. [DOI] [PubMed] [Google Scholar]
- 16.Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med 2017; 214:1737–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Li Y, Huang Q, Ren X, Hu H, Sheng H, Lai M. MiR-148a promotes apoptosis by targeting Bcl-2 in colorectal cancer. Cell Death Differ 2011; 18:1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Gu L, Li Y, Lin X, Shen H, Cui K, et al. miR-148a inhibits colitis and colitis-associated tumorigenesis in mice. Cell Death Differ 2017; 24:2199–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White GE, Cotterill A, Addley MR, Soilleux EJ, Greaves DR. Suppressor of cytokine signalling protein SOCS3 expression is increased at sites of acute and chronic inflammation. J Mol Histol 2011; 42:137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 2010; 59:227–235. [DOI] [PubMed] [Google Scholar]
- 21.Miyanaka Y, Ueno Y, Tanaka S, Yoshioka K, Hatakeyama T, Shimamoto M, et al. Clinical significance of mucosal suppressors of cytokine signaling 3 expression in ulcerative colitis. World J Gastroenterol 2007; 13:2939–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, de Haar C, Peppelenbosch MP, van der Woude CJ. SOCS3 in immune regulation of inflammatory bowel disease and inflammatory bowel disease-related cancer. Cytokine Growth Factor Rev 2012; 23:127–138. [DOI] [PubMed] [Google Scholar]
- 23.Cheng X, Zhang X, Su J, Zhang Y, Zhou W, Zhou J, et al. miR-19b downregulates intestinal SOCS3 to reduce intestinal inflammation in Crohn’s disease. Sci Rep 2015; 5:10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006; 12 (Suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 25.Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 201260:3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sann H, Erichsen Jv, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sci 2013; 92:708–718. [DOI] [PubMed] [Google Scholar]
- 27.Munyaka PM, Rabbi MF, Khafipour E, Ghia JE. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J Basic Microbiol 2016; 56:986–998. [DOI] [PubMed] [Google Scholar]
- 28.Qiao YQ, Huang ML, Xu AT, Zhao D, Ran ZH, Shen J. LncRNA DQ786243 affects Treg related CREB and Foxp3 expression in Crohn’s disease. J Biomed Sci 2013; 20:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu M, Xie J. LncRNA MALAT1 promotes ulcerative colitis by upregulating lncRNA ANRIL. Dig Dis Sci 2020; 65:3191–3196. [DOI] [PubMed] [Google Scholar]
- 30.Shaker OG, Ali MA, Ahmed TI, Zaki OM, Ali DY, Hassan EA, et al. Association between LINC00657 and miR-106a serum expression levels and susceptibility to colorectal cancer, adenomatous polyposis, and ulcerative colitis in Egyptian population. IUBMB Life 2019; 71:1322–1335. [DOI] [PubMed] [Google Scholar]
- 31.Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut 2007; 56:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souza HS, Tortori CJ, Castelo-Branco MT, Carvalho AT, Margallo VS, Delgado CF, et al. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways. Int J Colorectal Dis 2005; 20:277–286. [DOI] [PubMed] [Google Scholar]
- 33.Yarani R, Mirza AH, Kaur S, Pociot F. The emerging role of lncRNAs in inflammatory bowel disease. Exp Mol Med 2018; 50:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu F, Huang Y, Dong F, Kwon JH. Ulcerative colitis-associated long noncoding RNA, BC012900, regulates intestinal epithelial cell apoptosis. Inflamm Bowel Dis 2016; 22:782–795. [DOI] [PubMed] [Google Scholar]
- 35.Qiao C, Yang L, Wan J, Liu X, Pang C, You W, Zhao G. Long noncoding RNA ANRIL contributes to the development of ulcerative colitis by miR-323b-5p/TLR4/MyD88/NF-κB pathway. Biochem Biophys Res Commun 2019; 508:217–224. [DOI] [PubMed] [Google Scholar]
- 36.Ge Y, Sun M, Wu W, Ma C, Zhang C, He C, et al. MicroRNA-125a suppresses intestinal mucosal inflammation through targeting ETS-1 in patients with inflammatory bowel diseases. J Autoimmun 2019; 101:109–120. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Xiao Y, Ge W, Zhou K, Wen J, Yan W, et al. miR-200b inhibits TGF-β1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis 2013; 4:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierdomenico M, Cesi V, Cucchiara S, Vitali R, Prete E, Costanzo M, et al. NOD2 is regulated by Mir-320 in physiological conditions but this control is altered in inflamed tissues of patients with inflammatory bowel disease. Inflamm Bowel Dis 2016; 22:315–326. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Li XF, Cheng LN, Li XL. Long non-coding RNA CRNDE promotes cell apoptosis by suppressing miR-495 in inflammatory bowel disease. Exp Cell Res 2019; 382:111484. [DOI] [PubMed] [Google Scholar]
- 40.Shaw EJ, Smith EE, Whittingham-Dowd J, Hodges MD, Else KJ, Rigby RJ. Intestinal epithelial suppressor of cytokine signaling 3 (SOCS3) impacts on mucosal homeostasis in a model of chronic inflammation. Immun Inflamm Dis 2017; 5:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Nuij VJ, Baars JE, Biermann K, Kuipers EJ, Peppelenbosch MP, et al. Increased suppressor of cytokine signaling-3 expression predicts mucosal relapse in ulcerative colitis. Inflamm Bowel Dis 2013; 19:132–140. [DOI] [PubMed] [Google Scholar]
- 42.Chi X, Ding B, Zhang L, Zhang J, Wang J, Zhang W. lncRNA GAS5 promotes M1 macrophage polarization via miR-455-5p/SOCS3 pathway in childhood pneumonia. J Cell Physiol 2019; 234:13242–13251. [DOI] [PubMed] [Google Scholar]
- 43.Zou YF, Liao WT, Fu ZJ, Zhao Q, Chen YX, Zhang W. MicroRNA-30c-5p ameliorates hypoxia-reoxygenation-induced tubular epithelial cell injury via HIF1α stabilization by targeting SOCS3. Oncotarget 2017; 8:92801–92814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen P, Miao Y, Yan P, Wang XJ, Jiang C, Lei Y. MiR-455-5p ameliorates HG-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J Cell Physiol 2019; 234:21915–21924. [DOI] [PubMed] [Google Scholar]