Abstract
The earliest and most prevalent sensory experience includes tactile, thermal, and olfactory stimulation delivered to the young via contact with the mother, and in some mammals, the father. Prairie voles (Microtus ochrogaster), like humans, are biparental and serve as a model for understanding the impact of parent/offspring interactions on the developing brain. Prairie voles also exhibit natural variation in the level of tactile stimulation delivered by the parents to the offspring, and this has been well documented and quantified. Previous studies revealed that adult prairie vole offspring who received either high (HC) or low (LC) tactile contact from their parents have differences in the size of cortical fields and the connections of somatosensory cortex. In the current investigation we examined gene expression, intraneocortical connectivity, and cortical thickness in newborn voles to appreciate when differences in HC and LC offspring begin to emerge. We observed differences in developmentally-regulated genes, as well as variation in prelimbic and anterior cingulate cortical thickness at postnatal day 1 (P1) in HC and LC voles. Results from this study suggest that parenting styles, such as those involving high or low physical contact, impact the developing neocortex via very early sensory experience as well as differences in epigenetic modifications that may emerge in HC and LC voles.
Keywords: neocortical development, parental care, gene expression, cortical connections, RRID: NCBITaxon_79684, RRID: SCR_003070, RRID: SCR_002677
Graphical Abstract
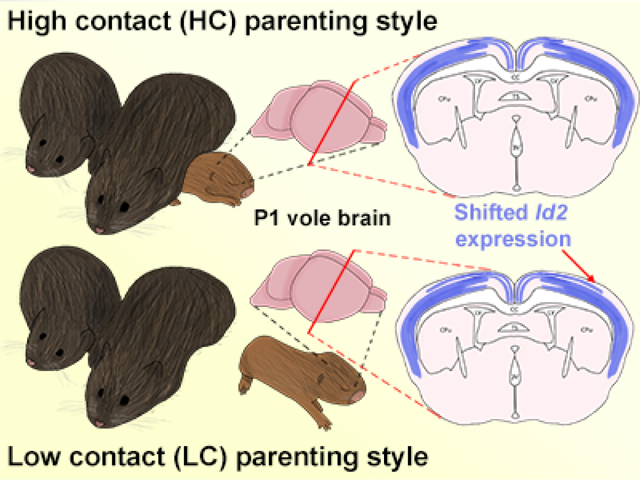
Prairie voles have a naturally occurring variation in parenting style. Dams and sires, left, can be classified as ‘High contact’ or ‘Low contact’ parents. The adult offspring of high and low contact parents show variation in cortical organization and connections. Here, we investigate whether these cortical variations are present at birth, possibly due to epigenetic modifications from altered rearing styles, or whether they are acquired through different contact-related sensory experiences in early life. By examining the gene expression patterns within the neocortex of newborn voles, we determined that some differences are present in early infancy, such as a lateral shift in Id2 gene expression in low contact offspring (right). These results suggest that differences in cortical connections are not present at birth and are likely modulated by differences in parental rearing style. However, differences in gene expression are present and may contribute to anatomical and functional differences observed in adult offspring.
1. Introduction:
During early life, critical developmental processes construct sensory and motor systems that will ultimately generate the animal’s behavioral repertoire throughout a lifetime. Although there is a great deal of genetic programming involved in the development of sensory and motor systems, early sensory experience, such as visual, auditory or somatic stimulation can significantly impact developmental outcomes. For many mammals, including humans, the most abundant and prominent multi-sensory inputs in early life arrive via physical contact with the mother. Seminal work from the 1950s by Harry Harlow highlighted the importance of these tactile inputs in developing monkeys. Following maternal separation and the introduction of a non-comforting “surrogate” mother made of wire, severe behavioral deficits resulted in the infants who were denied early-life physical contact with their natural mothers (Harlow and Zimmerman, 1959), indicating that early-life parental contact has long-lasting implications on offspring outcomes.
More recent studies that support the role of parental contact in neural development have made use of natural variations in parental care that exist within some populations of animals. One of the most well studied is the natural variation of maternal licking and grooming behavior (LG) in rats (Champagne et al., 2003). It has been demonstrated that the level of LG a rat offspring receives from its mother can impact multiple outcomes including the stress response and its corresponding neurological underpinnings (Liu et al., 1997; Francis et al., 1999; Champagne et al., 2003), spatial learning, NMDA receptor density, BDNF expression (Liu et al., 2000), and synaptic plasticity (van Hasselt et al., 2012; Nguyen et al., 2015). It has also been shown that nongenomic (or epigenetic) regulation plays a key role in establishing these differences reported in rat models (Francis et al., 1999; Bagot et al., 2012).
However, considering that rodents in the genus Rattus are uniparental (Saltzmann et al., 2017), rat maternal care studies fail to capture a key component of typical biparental human offspring care: care provided by the father. A more appropriate rodent model for human development is the prairie vole (Microtus ochrogaster), a biparental rodent that has been utilized to better understand how variations in parental care can impact developmental outcomes. Prairie voles are small rodents found primarily in central North America; they are noted for their monogamy and well-documented social behaviors (Getz et al., 1981; Williams et al., 1992; Bales and Carter, 2003). Important for the current study, prairie voles have also been shown to display natural variation in the amount of parental care they bestow upon offspring (Perkeybile et al., 2013; Perkeybile and Bales, 2015a; 2015b), where pair bonded male-female dyads can be characterized as either high contact (HC) or low contact (LC). Thus, prairie voles provide a natural model for studying the impact of early parental contact, and varying degrees of somatosensory and olfactory input, on offspring development.
A primary feature of the mammalian neocortex is the complexity of its organization. The cortex is comprised of areas that are both anatomically and functionally distinct, with an elaborate pattern of connectivity. This complex system generates sophisticated behavioral phenotypes. For example, primary somatosensory (S1) and motor (M1) cortex are areas responsible for processing sensory inputs and generating motor outputs that are critical for proper early development. Another cortical area, the anterior cingulate cortex (ACC), is involved in regulating social behavior including social cognition, social evaluation and interaction that is also developmentally relevant (Rudebeck et al., 2006; 2007; Scearse-Levie et al., 2008). These cortical areas develop through activity-independent mechanisms, as well as sensory-driven, activity-dependent mechanisms in a process termed arealization (O’Leary et al., 2007; Dye et al., 2011a; 2011b; Homman-Ludiye and Bourne, 2014). Early tactile and olfactory experience is often delivered to the offspring through interactions with the parents, and this early sensory input plays a key role in the establishment of distinct cortical areas. The Krubitzer laboratory has shown that differences in parental caregiving styles are associated with differences in the size of cortical fields (Seelke et al., 2016b), and cortico-cortical connections of these areas (Seelke et al., 2016a) in adult offspring of HC and LC voles. Previous studies using this model have also shown that the amount of parental care received by prairie vole offspring influences the development of social behaviors (Perkeybile et al., 2013) and stress reactivity (Perkeybile and Bales, 2015a; 2015b). While these data suggest that early differences in sensory experience impact the organization and connectivity of the neocortex and subsequent behavior, when these differences emerge, and the mechanisms by which these differences arise are still unclear (Bales et al., 2018).
It has been suggested that experience-related differences in brain and behavioral development in LC and HC voles arise from differences in early sensory inputs, which engage epigenetic mechanisms that activate or repress expression of genes crucial for cortical development (Seelke et al., 2015b; Perkeybile et al., 2019). This is supported by cross-fostering studies in rats (Francis et al., 1999) and prairie voles (Perkeybile et al., 2013) which show that maternal behavior, rather than genotype, plays a more prominent role in certain offspring behavioral outcomes. Despite a wealth of behavioral data in these species, studies on developing neocortex in different parental care models are lacking. In order to better understand the impact of sensory experience-related behavioral outcomes, it is critical to explore molecular and neuroanatomical changes in the developing brain that may underlie these phenotypes.
In this study, we examine neocortical gene expression patterns, intraneocortical connectivity, and other aspects of neuroanatomical development in newborn prairie voles (postnatal day (P)1) born to either HC or LC parents. We chose to examine connections in vole neocortex very early in life, prior to the time when external experiences could play a role in circuit modulation and neocortical development. This provides a baseline from which to compare neuroanatomical development at later developmental timepoints to determine at what age individual differences within a population begin to emerge. We compared the expression patterns of RZRβ and Id2 within the neocortex in HC and LC vole offspring. These two genes are involved in postmitotic patterning of cortical area connections and are proposed to be critical for the process of arealization in rodents (Rubenstein et al., 1999; Huffman et al., 2004; Dye et al., 2011a; 2011b; Jaboudon et al., 2012; Park et al., 2013). To our knowledge, no gene expression analyses has been done for RZRβ and Id2 within Microtus ochrogaster. We also examined patterns of intraneocortical connections (INCs) of S1 and anterior cingulate cortex (ACC) in both groups of P1 voles using lipophilic dyes in post-mortem tissue. Finally, we assessed architectonic features of five distinct cortical areas using Nissl stained cryosectioned tissue: frontal, prelimbic, anterior cingulate, primary somatosensory and primary visual cortex. Our results illuminate the underlying molecular mechanisms that may be involved in generating differences in cortical organization and connections in LC and HC voles, and at what point in development anatomical differences in cortical connectivity between these groups arise.
2. Materials and Methods:
A series of experiments were conducted in newborn vole offspring, from parents determined to have either high or low contact rearing styles. The experimental timeline, outlining the different methods used, is shown in Figure 1.
Figure 1. Breeding and experimental timeline.
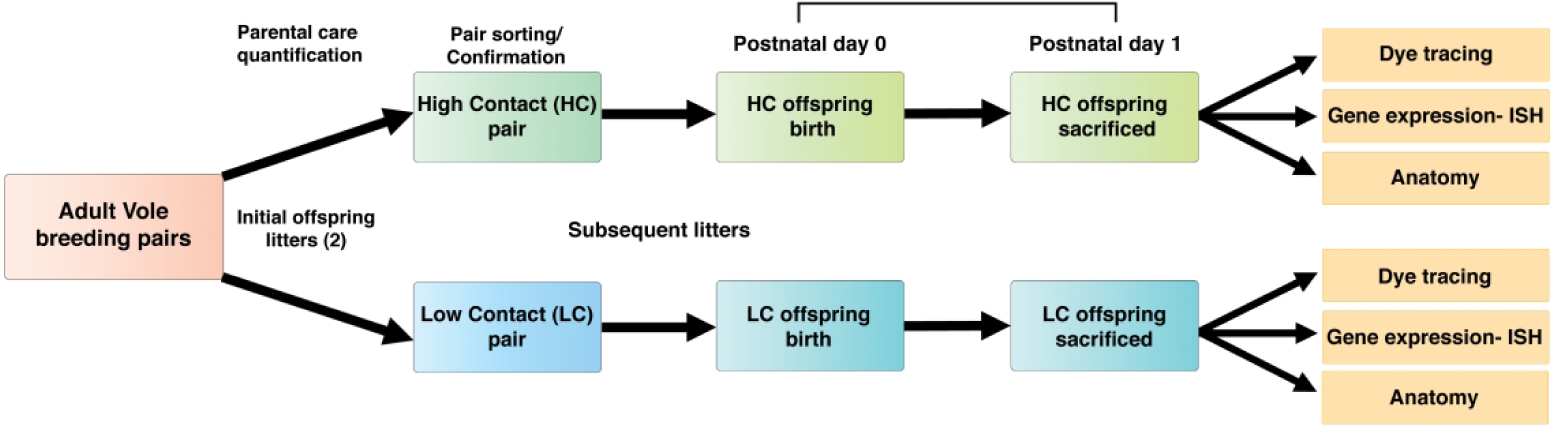
Breeding pairs of voles were scored for amounts of parental care they provided to their offspring during an initial offspring generation. Once sorted and categorized by the parental care amounts, top quartile and bottom quartile pairs were assigned as either high contact (HC) or low contact (LC) pairs, respectively. These same pairs generated a second litter which were subsequently referred to as HC or LC offspring. One day following parturition, pups were sacrificed, brain tissue was removed, and 3 experimental approaches were applied to offspring from each group as outlined in rightmost yellow boxes.
2.1. Subjects
Laboratory-bred prairie voles (Microtus ochrogaster, RRID: NCBITaxon_79684), which descended from a stock of wild voles originally caught near Champaign, Illinois, were used as subjects in this study. In total, 41 animals were utilized (23 females and 18 males; see Table 1). Voles were born and raised in the University of California, Davis, Psychology Vivarium, and were maintained on a 14:10 light/dark cycle starting at 6 am. Vole breeder pairs and offspring were housed in laboratory cages (44 × 22 × 16 cm) and were provided chow (high fiber Purina rabbit chow) and water ad libitum. Cotton nestlets were also provided for enrichment and nesting material. Data analysis for all cases was performed blind to the contact type. All experiments performed were approved by the UC Davis Institutional Animal Care and Use Committee and conform to NIH guidelines.
Table 1.
List of total litters and replicates (n) used per experimental endpoint.
| High Contact (HC) | Low Contact (LC) | |
|---|---|---|
| Total litters used | 8 | 8 |
| INC tracing: ACC and S1 | 5 (2 males, 3 females) | 3 (1 male, 2 females) |
| Gene expression: RZRβ | 5 (2 males, 3 females) | 7 (4 males, 3 females) |
| Gene expression: Id2 | 5 (2 males, 3 females) | 5 (3 males, 2 females) |
| Anatomy: cortical thickness | 5 (2 males, 3 females) | 6 (2 males, 4 females) |
Abbreviations: ACC, anterior cingulate cortex; HC, High contact; INC, intraneocortical connection; LC, low contact.
2.2. Parental Behavioral Assessment
This study made use of the naturally-occurring variation in the amount of parental care a prairie vole breeding pair provides its offspring, which has been quantified previously (Perkeybile et al., 2013; Perkeybile and Bales 2015a; 2015b; Seelke et al., 2016a; 2016b). During the early postpartum period, the home cage was observed, and the amount of pup-directed behavior generated by the parents was quantified (Fig. 1). Details of parental scoring and ranking are described elsewhere (Perkeybile et al., 2013). Briefly, four twenty-minute sessions, including two in the morning and two in the afternoon, occurred across P1–4. Behaviors were recorded live using Behavior Tracker software (behaviortracker.com). Both mother and father were examined for the following behaviors: huddling, pseudo-huddling, non-huddling contact, licking/grooming, anogenital licking/grooming, retrievals, hunching, nest building, and autogrooming. Additionally, the mother was scored for lateral, active, and neutral nursing. Each pup-directed contact behavior was scored as a continuous variable, with the precise parameter measured being seconds spent engaging within the specific behavior.
Following parental care quantification from 2 initial litters, the total amount of pup-directed contact time from each breeding pair was used to rank the pair in relation to the rest of the colony. The breeding pairs that exhibited contact scores within the top 25% of the colony distribution were designated as High Contact (HC) breeders, and those that exhibited scores within the bottom 25% were designated as Low Contact (LC) breeders. Breeding pairs within the middle two quartiles were not used within this study. Importantly, validity of the assignment appears to be very stable, as 92% of assigned previous breeding pairs retained their assignments in subsequent litters (Perkeybile et al., 2013). Once confirmation of breeding pair status was achieved, 8 LC and 8 HC breeding pairs were used to generate 8 subsequent HC and LC litters, and from these litters the P1 offspring used in this study were obtained.
2.3. Processing of P1 offspring brain tissue
All tissue for the following experiments was generated in the Department of Psychology at the University of California, Davis, and all post-mortem experiments were performed at the University of California, Riverside. At P1, HC and LC pups were euthanized by an overdose of sodium pentobarbital (250 mg/kg, IP), and were transcardially perfused with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde (PFA) in 0.1M phosphate buffer. Following perfusion, whole brains were rapidly dissected and extracted, postfixed in 4% PFA, and shipped overnight to UCR. For histology and dye tracing experiments, brains were placed in 4% PFA for long-term storage at 4 degrees Celsius. For in situ hybridization (ISH) experiments, brains were dehydrated using ascending concentrations of methanol, and were stored in 100% methanol at −20 degrees Celsius.
2.4. Dye tracing
In order to assess intraneocortical connection (INC) development at P1 in both LC and HC voles, single crystals of 1,1’-Dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine (DiI; Invitrogen) and 4-(4-(dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA; Invitrogen) were placed into the neocortex of single postmortem hemispheres (HC: n= 5; LC: n= 3; Table 1). Single dye crystals of each variety were placed in either putative anterior cingulate cortex (ACC) or putative primary somatosensory cortex (S1) in each hemisphere. The ACC is situated between motor and prelimbic cortex in rodents and the hemisected brain provides direct access to the ACC on the medial side. Somatosensory cortex is located between motor and visual cortex and the hemisected brain provides direct access to S1 on the lateral side. In order to reduce cross-case variability and to ensure proper placement of dyes, we used the Atlas of the Developing Mouse Brain (Paxinos, 2007) as a guide to locate ACC and S1. For the most part, major subdivisions of the rodent brain are conserved across species (Krubitzer and Seelke, 2012); because there is no developmental atlas for prairie voles, we used a well-established mouse atlas to guide our dye placements. In all cases, crystals were inserted perpendicular to the cortical layers to a point where the tip of the crystal was placed just below the cortical surface; the deepest tip of each dye crystal was inserted approximately 300 μm below the cortical surface. Detailed methodology on dye crystal placement has been described previously (Dye et al., 2011a; 2011b; El Shawa et al., 2013; Abbott et al., 2018). Following dye placement, hemispheres were stored in 4% PFA in the dark at room temperature for 4 – 6 weeks to allow for the transport of tracer. To ensure proper transport of dye, retrogradely labelled cells in the thalamus, originating from dye placements in each hemisphere, were verified as present in each case by visually examining the thalamus on the exposed medial surface via a fluorescent microscope. Once verified, the tissue was prepared for sectioning and embedded in 4% low-melting point agarose. Using a vibratome (Leica), hemispheres were sectioned coronally at 100 μm increments. Sections were collected in 1X PBS, counterstained with 4’, 6-diamidine-2- phenylindole dihydrochloride crystallized (DAPI; Roche), mounted onto glass slides and coverslipped with Flouromount (Sigma-Aldrich) medium. The positioning of dye placements was verified using thalamic back-labelling (Fig. 2). To verify S1 dye placements, we identified retrogradely labelled neurons in the ventral posterior nucleus of the thalamus (VP) originating from the S1 dye placement location (DPL). For ACC DPLs, we examined sections stained for Nissl and were able to determine the borders of the region by using nearby landmarks such as lateral and medial septal nuclei, the dorsal tenia tecta and prelimbic cortex as well as the prominent layer V in motor cortex at the dorsal border of ACC. Although we confirmed retrograde labelling in the medial dorsal nucleus (MD) of the thalamus resulting from ACC DPLs, confirmation of retrogradely labeled cells for ACC injections is less reliable due to the widespread nature of connections between the ACC and thalamus (Finch et al., 1984; Delevich et al., 2015).
Figure 2. Dye placement and thalamic verification.
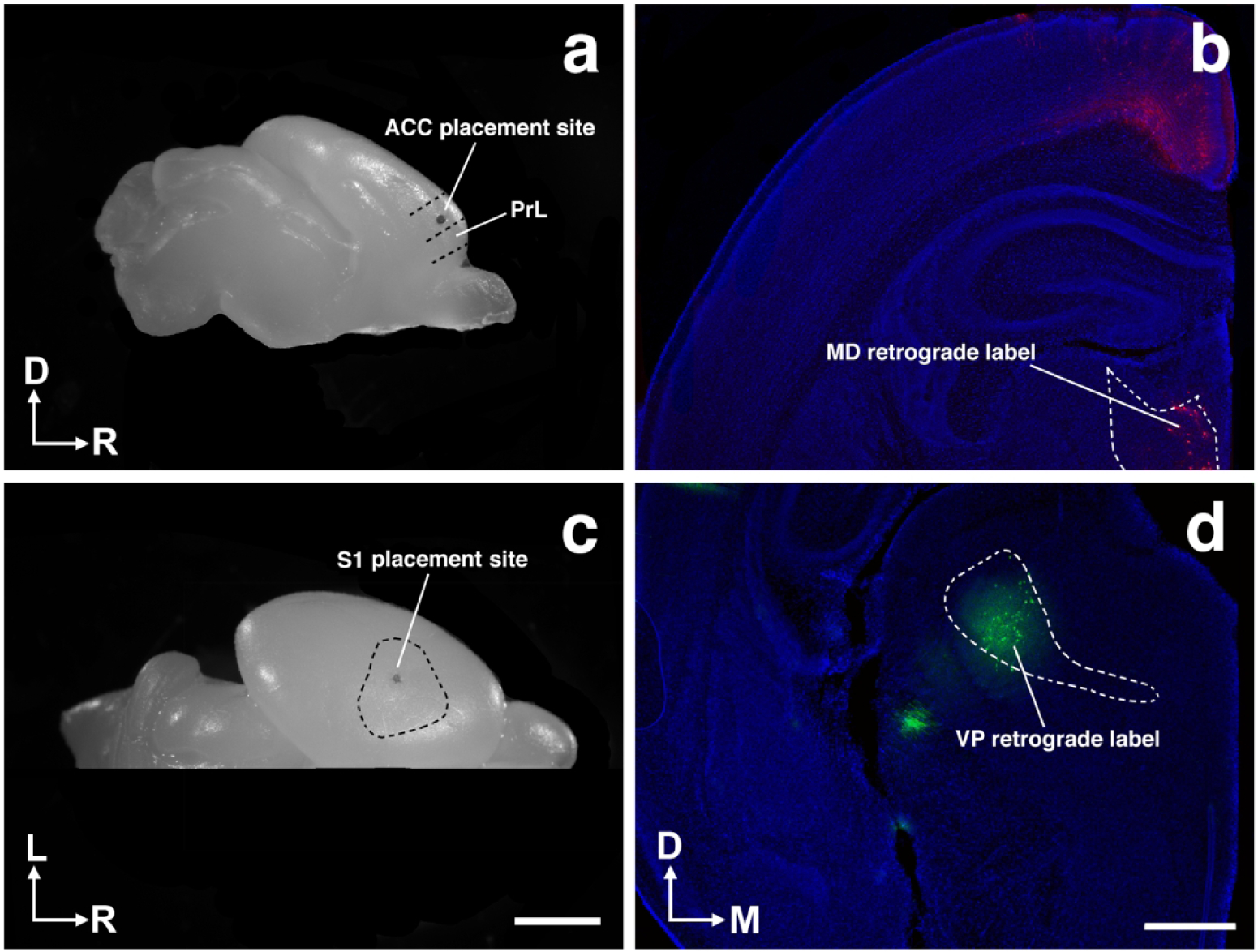
Representative whole hemisphere views of brains with dye placements in putative ACC (a) and S1 (c). b, Coronal section of P1 vole hemisphere demonstrating retrograde labeling in the mediodorsal nucleus of the thalamus (MD) stemming from ACC dye placement as seen in a. d, Coronal section of P1 vole hemisphere demonstrating retrograde labeling in the ventral posterior nucleus of the thalamus (VP) stemming from S1 dye placement as seen in (c). Image oriented dorsal (D) up, rostral (R) right in (a); lateral (L) up, rostral (R) right in (c); dorsal (D) up, medial (M) right in (b,d). Scale bars = 2 mm (a,c) and 500 μm (b,d).
All HC and LC sections were digitally imaged with three different filters using a Zeiss Axio Imager Upright Microscope equipped with fluorescence and captured using a digital high-resolution Zeiss Axio camera (HRm) using Axiovision software (version 4.7, RRID: SCR_002677). Three filters used were as follows: blue for DAPI counterstain, red for DiI, and green for DiA (excitation wavelengths: blue, DAPI 359 nm; red, Cy 3 550 nm; green, GFP 470 nm; emission wavelengths: blue, DAPI 461 nm; red, Cy 3 570 nm; green, GFP 509 nm). The three images for each section were merged and saved in high-resolution TIF format.
2.5. Dye tracing analyses
In order to assess the development of INCs, the dye placement locations (DPLs) were verified for each case by analyzing thalamocortical labeling in sections of both HC and LC hemispheres (Fig. 2) as well as cortical Nissl staining. Only cases in which DPLs were verified to be within the areas of interest were included and compared across contact types. Unedited images of coronal sections from HC and LC brains were anatomically aligned using DAPI stained landmarks and annotated examples are presented in a side-by-side, rostral to caudal series for comparison. In order to illustrate the overall pattern of INCs across the extent of the neocortex, we used coronal sections to create 2D, “flattened” reconstructions of a lateral view of the neocortex, on which the extent of the DPL and resulting retrogradely labelled cells were superimposed. To do this, we first draw the outlines of the coronal section, as well as any anatomical landmarks present at that level, along with the labelled cells, on each and every section. These sections are 100 μm thick. The drawn overlays for each section are stacked, aligned and co-registered and manually flattened using precise measurement and local anatomical landmarks using Adobe Illustrator. A standard cortical outline with dorsal, ventral, rostral, and caudal limits was applied to the transformation of serial coronal sections into a reconstructed flattened image. In this way, we can transform data from coronally cut tissue sections into flattened reconstructions of the neocortex. This methodology has been previously used in both embryonic and adult mice and can accurately demonstrate the boundaries of cortical sensory and motor regions within young mice (Huffman et al., 2004; Dye et al., 2011a; 2011b; El Shawa et al., 2013; Abbott et al., 2018).
Dye tracing data were also quantified in two ways to determine both the reproducibility of dye uptake at the DPL and the overall projection zones of neurons within individual hemispheres. First, the dye uptake extent (the area around the dye crystal insertion site where the dye was absorbed) was measured across the sections where present. The DPL uptake (in mm) was calculated as a percentage of total cortical length for each case. This method controlled for differences in cortical length and ensured the size of dye crystals and uptake zone was consistent across cases. This is critical for proper comparison of intraneocortical connectivity between HC and LC voles. In addition, the extent of neurons projecting to ACC and S1, in percentage of cortical length, was measured using the locations of the most caudal and most rostral retrogradely labeled cells for each DPL in each case. This way we could determine the expanse of the INCs for each dye placement. Differences in calculated projection zones from ACC and S1 dye placements were compared between groups using unpaired t tests.
2.6. In situ hybridization techniques and analyses
Gene expression patterns within the cortex of P1 voles were assessed using standard protocols for non-radioactive free-floating RNA in situ hybridization (ISH, Huffman et al., 2004; Dye et al., 2011a; 2011b; El Shawa et al., 2013; Abbott et al., 2018). Probes for RZRβ and Id2 (courtesy of John Rubenstein, UCSF) were used to identify patterns of neocortical expression in P1 HC and LC brains. Briefly, hemispheres designated for ISH were embedded in gelatin-albumin and sectioned coronally at 100 μm increments using a vibratome. After hybridization, sections were permeabilized in 50% glycerol, mounted onto glass slides, and coverslipped. All hybridized sections were digitally imaged using a Zeiss SteREO Discovery V.12 dissecting microscope and captured using a digital high-resolution Zeiss Axio camera (HRm) using Axiovision software (version 4.7). Anatomically matched-level HC and LC sections were presented together to directly compare RZRβ (HC: n= 5; LC: n= 7; Table 1) and Id2 (HC: n= 5; LC: n= 5; Table 1) expression patterns.
Id2 gene expression within a specific, highlighted region of interest (ROI) of neocortex of P1 HC and LC voles was further analyzed. Transcript densities were measured in HC and LC brains using a static ROI placed onto raw ISH data sections using ImageJ software (NIH, RRID: SCR_003070). Briefly, raw image densities were converted to binary values and adjusted to a standard threshold. This resulted in transformed images where transcript present in raw data was converted to black pixels and the absence of transcript was converted to white pixels. The area of black pixels within the static ROI was measured in each case using ImageJ, and the resulting transcript densities were presented as a percentage of the total pixels present within the defined ROI area. These calculated transcript densities were compared between groups using unpaired t tests. Details of this technique have been described previously (El Shawa et al., 2013; Abbott et al, 2018).
2.7. Histology (anatomy) measures
To assess the impact of parental care on gross neocortical development, we used Nissl stained tissue from HC and LC P1 brain hemispheres (HC: n= 5; LC: n= 6; Table 1). Briefly, brains kept in 4% PFA were washed with 1X PBS then placed into 30% sucrose/PBS for cryoprotection for 3 days. Once cryoprotected, hemispheres were frozen to a histology chuck positioned to cut in the coronal plane using Optimum Cutting Temperature (OCT) medium (Fisher). Hemispheres were then sectioned at 30 μm using a Leica Cryostat at −22 degrees Celsius chamber temperature. Serial sections were collected onto gelatin-coated slides and allowed to dry for 1–3 days. Once dry, slides were stained for Nissl substance and were cover slipped using Permount (Fisher) and digitally imaged using a Zeiss SteREO Discovery V.12 dissecting microscope and captured using a digital high-resolution Zeiss Axio camera (HRm) using Axiovision software (version 4.7).
Once imaged, regions of interest (ROIs) in matched individual tissue sections were measured across all cases using an electronic micrometer in ImageJ (NIH) by a trained researcher blind to the contact type. Sections taken from different rostral-caudal levels were matched across cases using established anatomical landmarks such as the corpus callosum, anterior commissure, size and subdivision of the hippocampus, and lateral ventricles. Cortical thickness measures in sections stained for Nissl were taken along a line perpendicular to the cortical sheet extending between the most superficial portion of layer I to the deepest portion of layer VI, which was judged based on the visible division between Nissl-stained cell bodies and the underlying white matter. These included frontal cortex (FC), prelimbic cortex (PrL), anterior cingulate cortex (ACC), primary somatosensory cortex (S1), and primary visual cortex (V1). ROIs were identified with the Atlas of Developing Mouse Brain (Paxinos et al., 2007) using specific cortical and subcortical landmarks such as the corpus callosum, the anterior commissure, the fimbria of hippocampus, and the superior colliculus. Cortical thickness measurements (in μm) were analyzed between groups via standard t test. In addition, a second trainer observer performed the same measurements in 40% of cases spanning all cortical areas measured to exclude systematic single experimenter error or bias. Based on these second set of measurements, the mean inter-observer difference was 0.0017 mm, and the intraclass correlation (ICC) was calculated to be 0.9665, indicative of a high degree of agreement among observers.
2.8. Statistical analyses
All statistical analyses were conducted using Prism 6 (Graphpad). For between group comparisons in DPL spread, projection zones, transcript densities, and cortical thickness measurements, standard unpaired t test analyses were used. In all experimental measures, data was assessed for in-group sex differences (data not shown). Due to lack of significant differences achieved by these analyses, all male and female subjects were combined for presented data. For all tests, statistical significance was set as p < 0.05. Data are presented as means ± SEM.
3. Results:
3.1. Dye labeling experiments
In all dye labeling experiments (HC: n=5; LC: n=3), small, single crystals of DiI were placed into putative anterior cingulate cortex (ACC) and similar size crystals of DiA were placed into putative primary somatosensory cortex (S1) within the same hemispheres. These particular areas were chosen based upon their involvement with behaviors that are highly sensitive to early sensory experience.
First, we present raw data from single cases from each contact type (LC and HC) as a series of coronal hemisections organized from rostral to caudal (Fig. 3). As mentioned above, HC (Fig. 3a1–6) and LC (Fig. 3b1–6) P1 hemispheres had DiI crystals placed into putative ACC (*ACC, Fig. 3a2, 3b2) and DiA crystals placed into putative S1 (*S, Fig. 3a4, 3b4) with DPLs denoted by asterisks. As shown by these two representative cases, the distribution patterns of retrogradely labelled cells was largely similar between HC and LC for both S1 and ACC dye placements. Putative S1 dye placements resulted in labeled cells largely within the area surrounding the DPL, likely within the developing boundaries of primary and secondary somatosensory cortex, as well as more rostral regions in the location of motor cortex in both HC and LC voles. Putative ACC dye placements resulted in labeled cells within cingulate cortex, in the frontal pole of neocortex, and in prelimbic cortex in both HC and LC animals. These overall patterns are more clearly visualized using lateral-view, “flattened” cortical reconstructions (Fig. 4). As confirmed from raw data analyses, S1 injections (green blobs) resulted in largely similar patterns of labeled INCs (green dots) in both HC (Fig. 4a1–3) and LC voles (Fig. 4b1–3). Putative ACC injections (red blobs) resulted in labeled INCs in similar locations in HC voles compared to the LC group (red dots, Fig. 4a1–3, b1–3).
Figure 3. Somatosensory and anterior cingulate intraneocortical connections (INC) in postnatal day 1 (P1) pups.
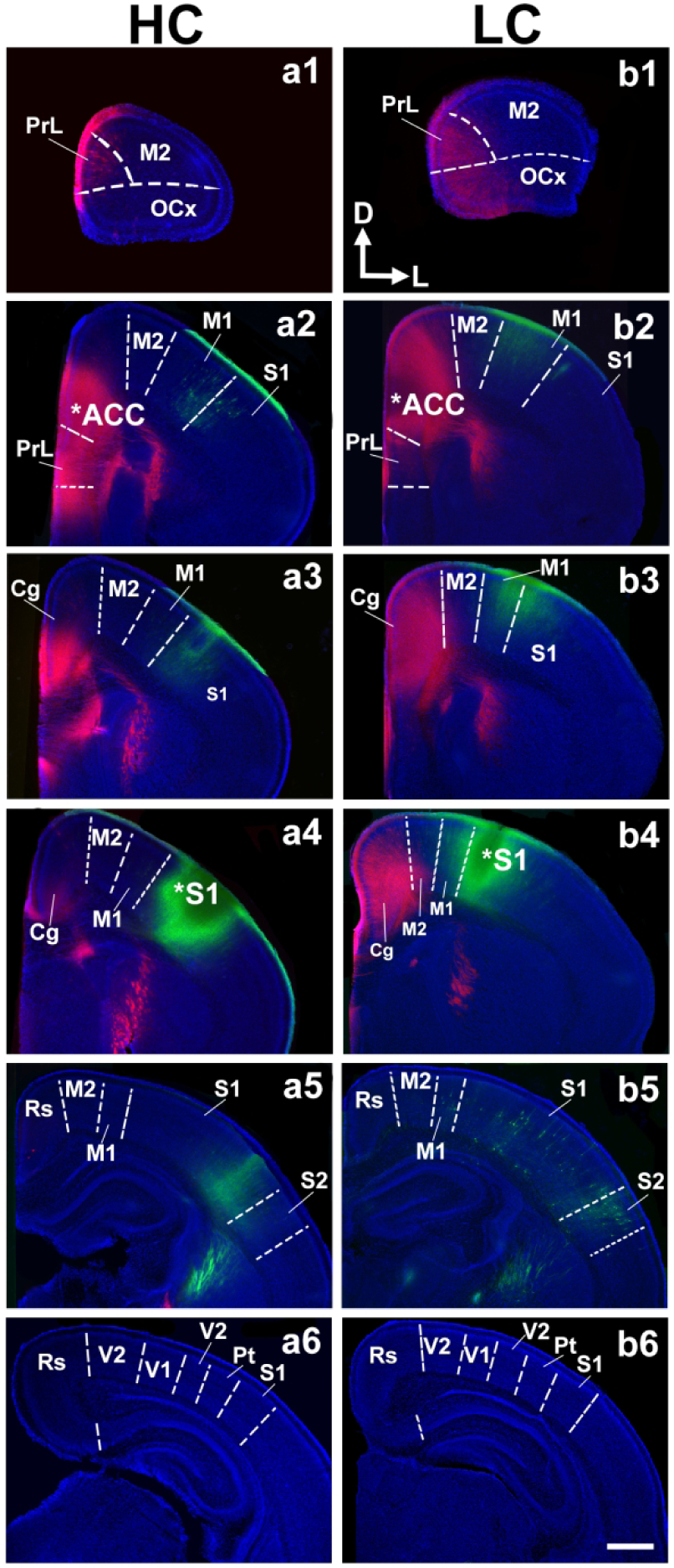
Vibratome-cut 100 μM coronal sections from P1 hemispheres are arranged in a rostral (top) to caudal (bottom) series following crystal placement of DiI (red) or DiA (green) in putative anterior cingulate cortex (ACC; a2, b2, stars) and somatosensory (S1; a4, b4, stars) cortex of high contact (HC; a1–6), and low-contact (LC; b1–6) offspring brains. Sections were counterstained with DAPI (blue). Analysis of ACC and S1 connections revealed no difference between LC and HC offspring. Sections are oriented dorsal (D) up and lateral (L) to the right. Scale bar = 500 μm.
Figure 4. Flattened reconstructions of LC and HC brains at P1.
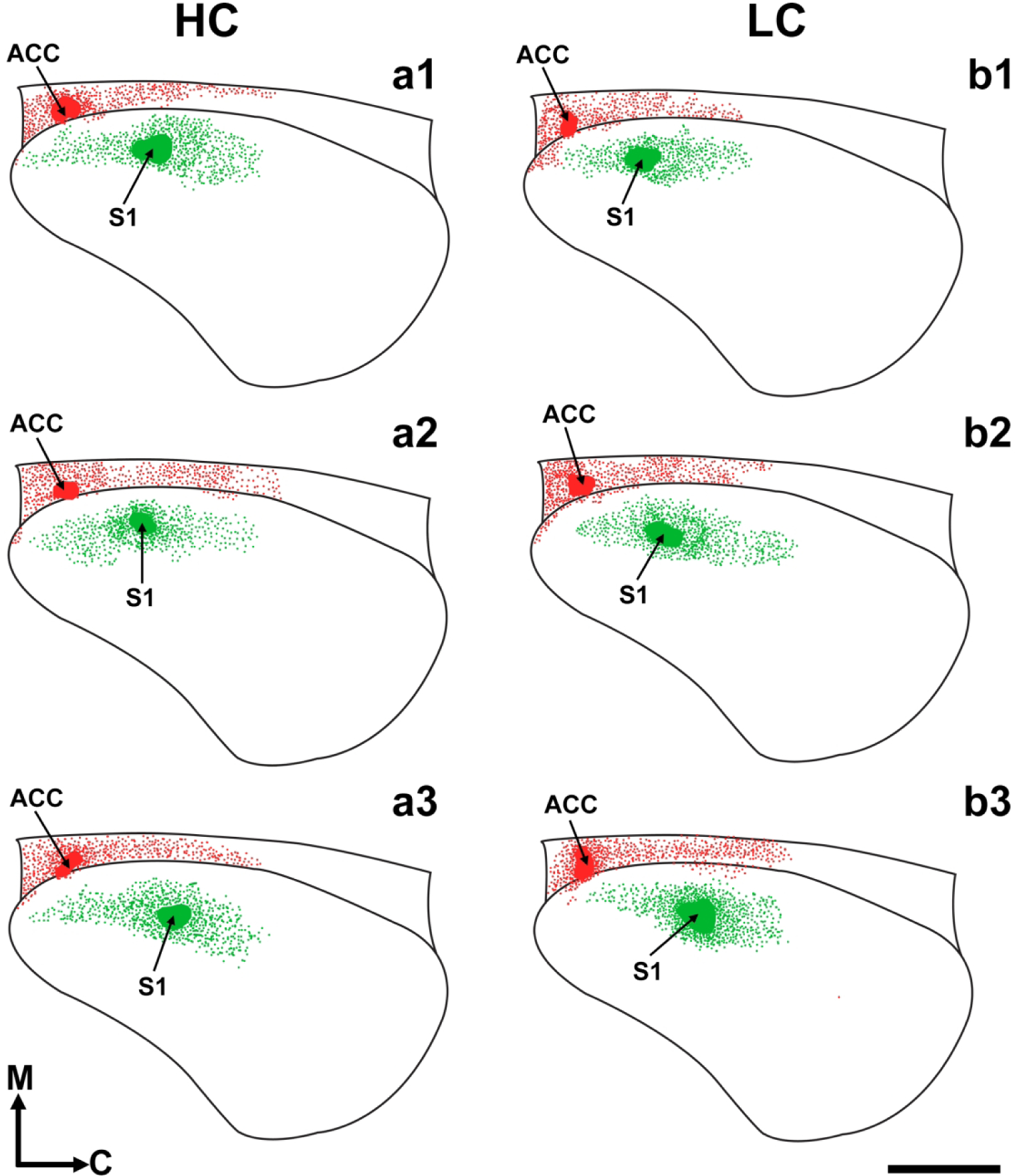
Drawn, ‘flattened’ neocortex images, reconstructed from coronal sections of three different HC (a1–3) and LC (b1–3) brains illustrating the retrograde connections of ACC (red) and S1 (green) at postnatal day 1. A standard cortical outline with dorsal, ventral, rostral, and caudal limits was applied to all cases for the transformation of serial coronal sections into reconstructed flattened cortical image. Black lines: Cortical outline; arrow/red patches: DiI anterior cingulate dye placement locations (DPLs); arrow/green patches: DiA somatosensory DPLs; green/red dots: Retrogradely labeled cell bodies. Reconstructions are oriented medial (M) up and caudal (C) to the right. Scale bar =1,000 μm.
In order to confirm the qualitative analyses described above, we quantified both DPL uptake and the associated projection zones from putative ACC and S1 dye placements, in both groups, as a function of percent total cortical length. Results of these measurements confirmed that there were no significant differences in DPL uptake between HC and LC voles in ACC DPLs (Fig. 5a; HC: 13.57 ± 0.5507% total cortical length; LC: 13.71 ± 1.460% total cortical length) or in S1 DPLs (Fig. 5c; HC: 8.095 ± 1.332% total cortical length; LC: 11.18 ± 1.979% total cortical length). Also, in accordance with the qualitative data analyses, S1 projection zones were not significantly different between HC (58.71 ± 4.802% total cortical length) and LC (47.55 ± 2.186% total cortical length) voles at P1 (Fig. 5d). Lastly, no significant differences were found when comparing ACC projection zones between HC and LC brains using t-test analyses (Fig. 5b; HC: 75.14 ± 1.8155 total cortical length; LC: 64.43 ± 4.771% total cortical length, p=0.0646). Overall, these results suggest that on the day of birth there are no statistically significant differences in the patterns of intracortical connections of S1 or ACC in low versus high contact voles.
Figure 5. Quantitative analysis of dye labeling.
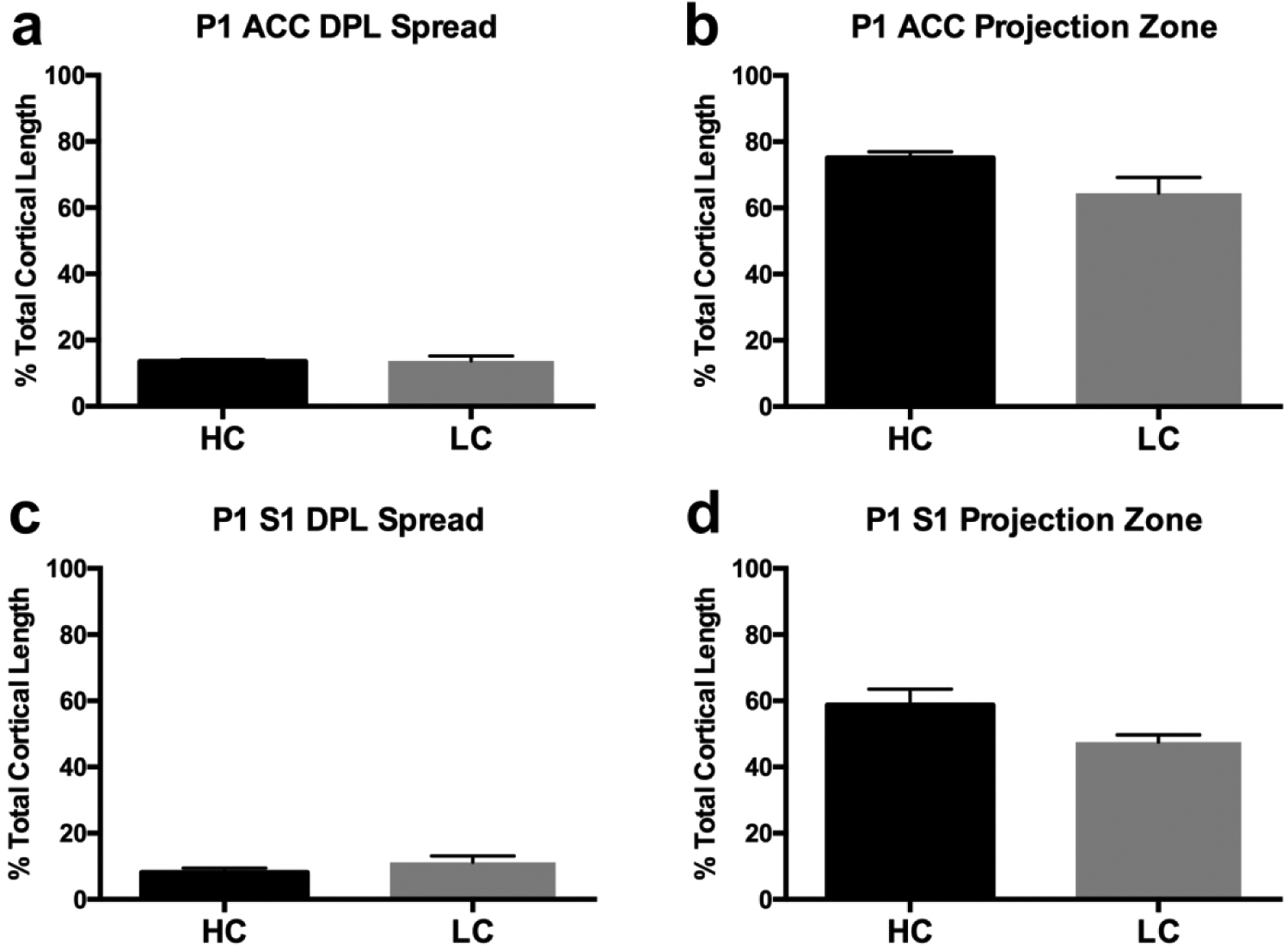
a, Putative anterior cingulate cortex (ACC) dye placement location (DPL) spread as a function of total cortical length. No differences are present among groups. b, ACC projection zones as a function of total cortical length. No differences are present among groups. c, Putative primary somatosensory cortex (S1) dye placement location (DPL) spread as a function of total cortical length. No differences are present among groups d, S1 projection zones as a function of total cortical length. No significant differences are present between HC and LC voles. Bars represent group means ± SEM.
3.2. Gene expression: ISH
Here, we examine the expression of RZRβ and Id2 in P1 HC and LC vole neocortex using in situ hybridization. Coronal sections of hemispheres hybridized to either RZRβ or Id2 are presented within Figure 6. RZRβ (also known as RORB) expression within the P1 prairie vole brain appears to mirror that of developing mouse and rat brain (Schaeren-Wiemers et al., 1997; Dye et al., 2011a), where expression is largely limited to cortical layer IV (Fig. 6a1–4, b1–4), and overall expression is patterned in a high-rostral to low-caudal gradient. Similar to other rodents (Woolsey and Van Der Loos, 1970; Woolsey et al., 1975), prairie voles have a characteristic barrel cortex in S1 where clusters of cells respond to stimulation of the whiskers, as previously described (Campi et al., 2007). We observed robust expression of RZRβ within the developing barrel cortex (arrows, Fig. 6a2–3, b2–3) of P1 voles, regardless of parental care style. Using carefully matched-levels, section analyses between HC (n=5) and LC (n=7) brains demonstrated no discernible differences between HC and LC voles in RZRβ expression patterns, suggesting that limited early sensory experience variation or potential inheritance does not affect RZRβ expression at P1.
Figure 6. Analysis of neocortical expression of RZRβ and Id2.
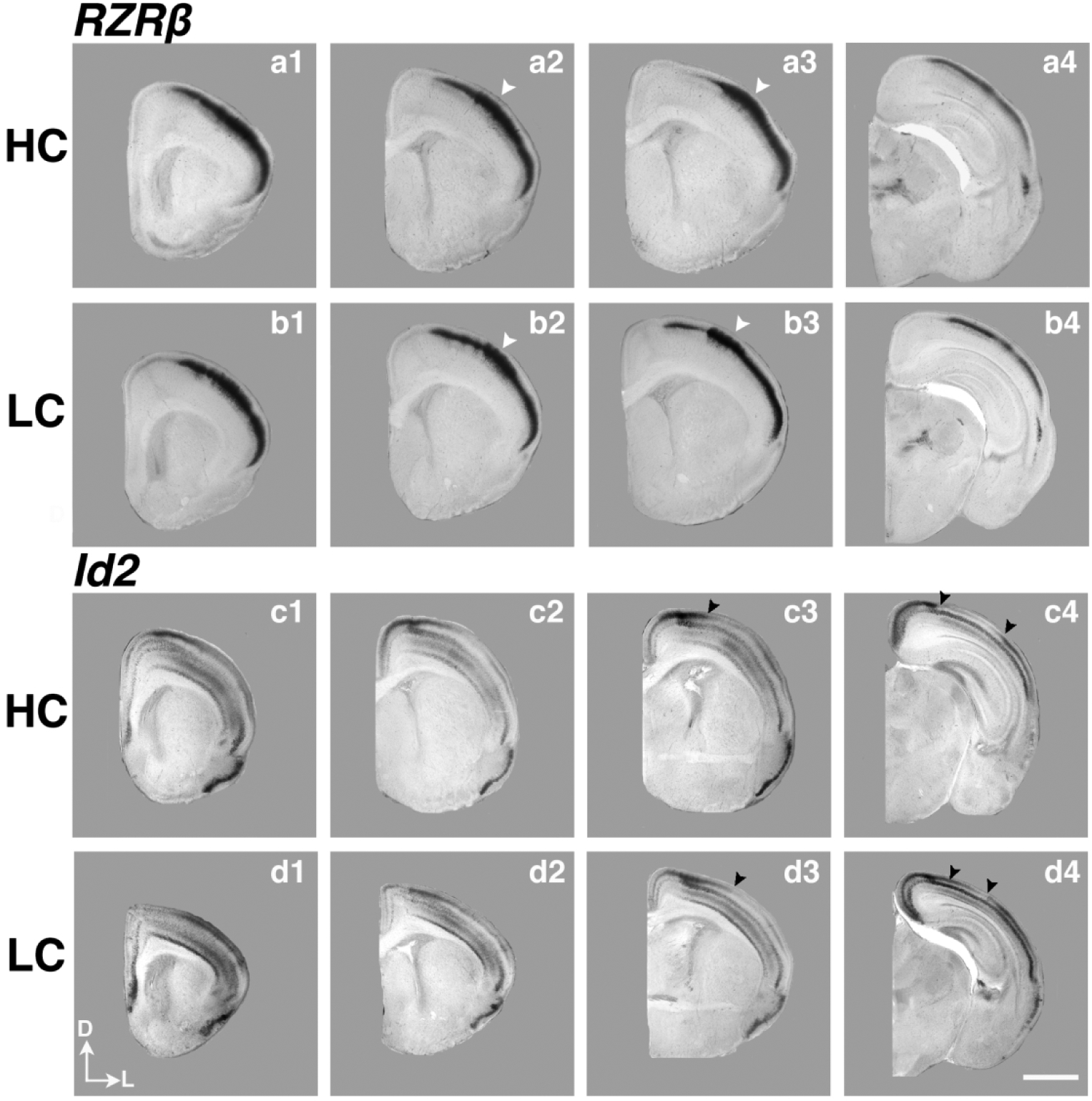
High magnification P1 coronal sections of high-contact (a1–4) and low-contact (b1–4) offspring in situ hybridized to RZRβ, as well as sections of high-contact (c1–4), low-contact (d1–4) offspring in situ hybridized to Inhibitor of DNA binding 2 (Id2). White arrowheads (a2–3, b2–3) indicate strong RZRβ expression in the developing barrel cortex in S1, similar to expression patterns in mouse development. Black arrowheads in c3–4 and d3–4 indicate the border of superficial Id2 expression, which is shifted laterally in LC pups compared to HC pups. Images oriented dorsal (D) up, lateral (L) right. Scale bar = 1,000 μm.
Id2 (Inhibitor of DNA binding-2) expression (Fig. 6c1–4, d1–4) within developing prairie vole neocortex also closely matches that of developing murine neocortex (Rubenstein et al., 1999; Dye et al., 2011a). At P1, Id2 expression patterns are present, with the majority of expression generally restricted to cortical layers II/III, as well as layers V and VI, and distinct absence of expression in Layer IV. Also apparent within voles at P1, there is an abrupt lack of expression within layers II/III near primary sensory areas, such as caudal S1 (black arrows, Fig. 6c3, d3) and V1 (black arrows, Fig. 6c4, d4). In contrast to RZRβ, differences were observed in Id2 expression at the level of caudal parietal cortex, in the putative S1, between LC and HC voles. Specifically, there was an abrupt cessation of expression in layers II/III in HC voles (arrow, Fig. 6c3; Fig. 7a). However, in LC animals, this border of expression is shifted laterally (arrow, Fig. 6d3; Fig. 7b). In addition, this lateral shift remains consistent in LC voles in caudal sections of the cortex (compare left arrow position in Fig. 6c4 vs. left arrow in Fig. 6d4). In order to quantify this observed difference, we used binary-converted images of raw data to measure the density of Id2 transcript present within a static region of interest (ROI). The ROI measured is denoted within the line drawing shown in Fig. 7c and corresponded to an area restricted to cortical layers II/III. Statistical analyses revealed a significant increase in transcript within the ROI in LC voles compared to the HC group (HC: n= 5, 19.80 ± 4.730% area fraction; LC: n=5, 61.97 ± 3.139% area fraction, ***p= 0.008).
Figure 7. Semi-quantitative analysis of Id2 transcript density within somatosensory cortex ROI.
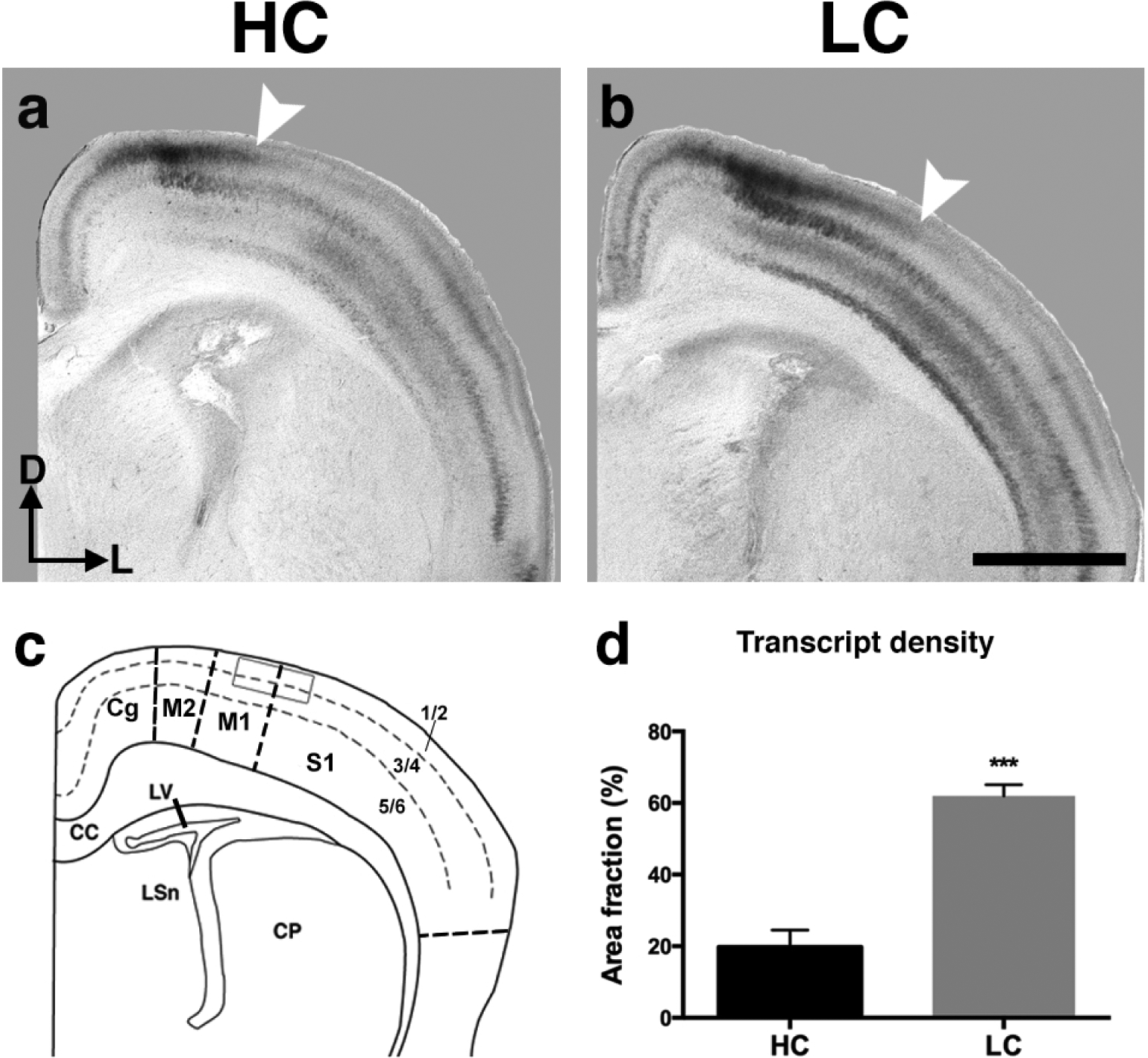
a, b, Representative coronal sections of HC (a) and LC (b) P1 vole hemispheres hybridized to Id2. Note the lateral shift in layer II/III Id2 expression in LC voles (arrow, b) compared to HC voles (arrow, a). c, Line drawing of the anatomical level (using Paxinos et al., 2007 as a guide) in which a static electronically-drawn region of interest (ROI) was placed on sections of binary-converted ISH experiments to quantify levels of mRNA expression. d, LC brains display an increase in Id2 transcript densities compared to HC in the ROI defined in (c) (***p=0.008, Student’s t). Bars represent group means ± SEM. ISH images and line drawing oriented dorsal (D) up, lateral (L) right. Scale bar = 1,000 μm.
Next, we analyzed the combined expression patterns of RZRβ and Id2, as overlapping and abutting expression of several genes has been implicated in patterning of developing neocortex, acting as cooperative and opposing molecular forces (Dye et al., 2011a; 2011b). In Figure 8, we present the RZRβ and Id2 expression and their merged patterns in coronal hemisections near the somatosensory/motor cortex border. Interestingly, the clear absence of expression of Id2 within layer IV in both HC and LC voles corresponds almost directly to the strong layer IV RZRβ expression, as seen in the merged images. This remarkably precise synergy in expression patterns is present in both HC (Fig. 8a1–3) and LC (Fig. 8b1–3) voles at P1, and we did not observe any phenotypic difference between the groups. Overall, these results suggest that prairie vole early forebrain development and genetic patterning is similar to what has been described in several other mammalian species, and that the general expression patterns are similar in both LC and HC animals. However, as noted above, there is a significance difference in the expression pattern of ID2 in parietal cortex in LC voles compared to HC voles.
Figure 8. Analysis of RZRβ and Id2 expression at the sensory-motor border.
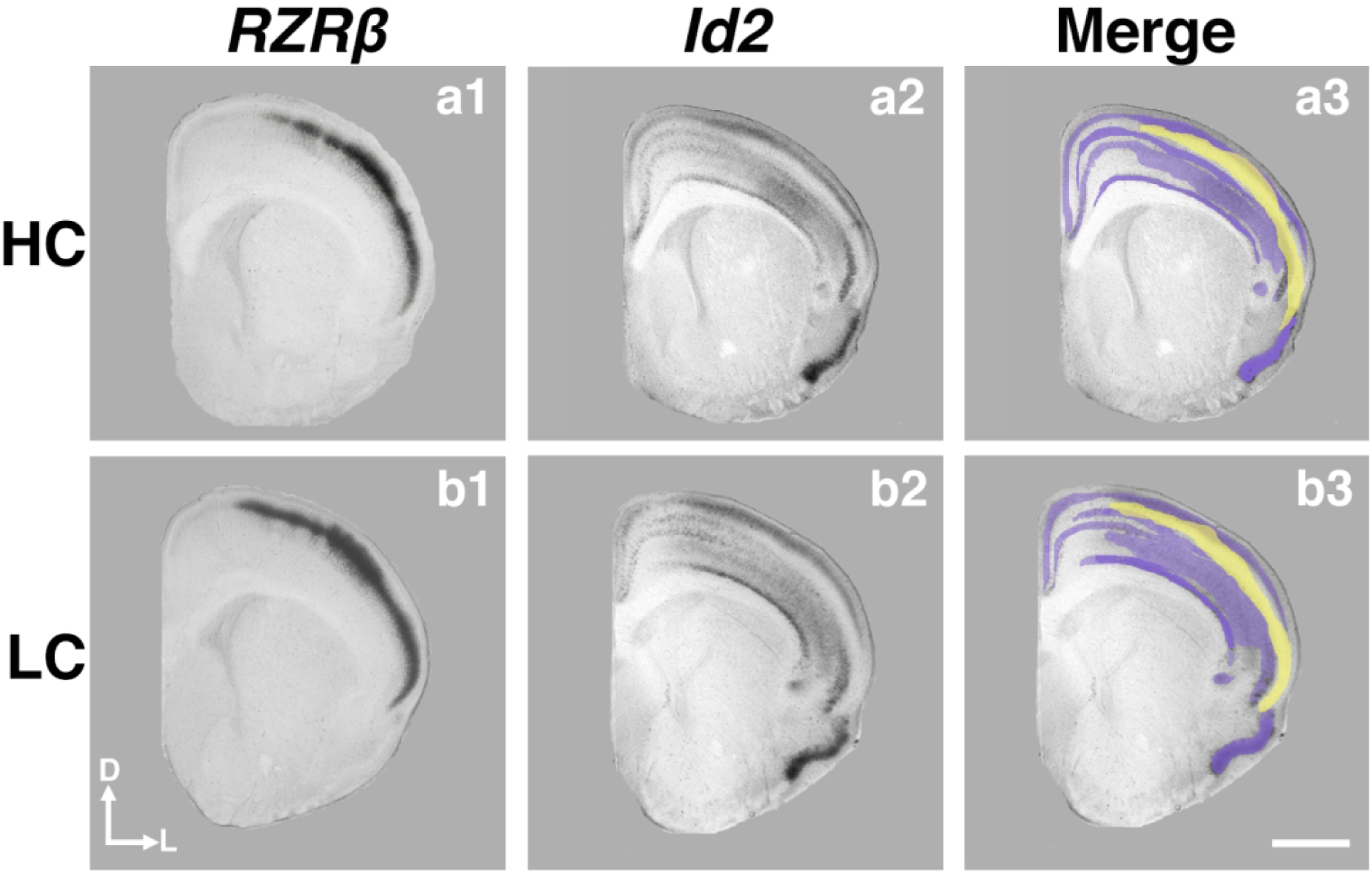
P1 coronal sections of HC (a1–3) and LC (b1–3) offspring hybridized to RZRβ (a1, b1) and Id2 (a2, b2) at the level of the somatosensory-motor cortex. Merging the expression patterns together (a3, b3) reveals the primarily complementary patterning of RZRβ and Id2 expression at this level, as well as a thin overlapping region in both LC and HC offspring. Purple, Id2 expression; yellow, RZRβ expression. Images oriented dorsal (D) up, lateral (L) right. Scale bar = 1,000 μm.
3.3. Cortical thickness in P1 HC and LC voles
Cortical thickness is a dynamic characteristic of the developing forebrain (Shaw et al., 2008), and has major implications for future neuronal processing and subsequent behavior (Karama et al., 2011; Burgaleta et al., 2014). We compared cortical thickness in coronal sections between HC (n=5) and LC (n=6) voles at P1 within 5 distinct, putative areas: frontal cortex (Fig. 9a), prelimbic cortex (Fig. 9b), anterior cingulate cortex (Fig. 9c), primary somatosensory cortex (Fig. 9d), and primary visual cortex (Fig. 9e).
Figure 9. Cortical thickness at P1.
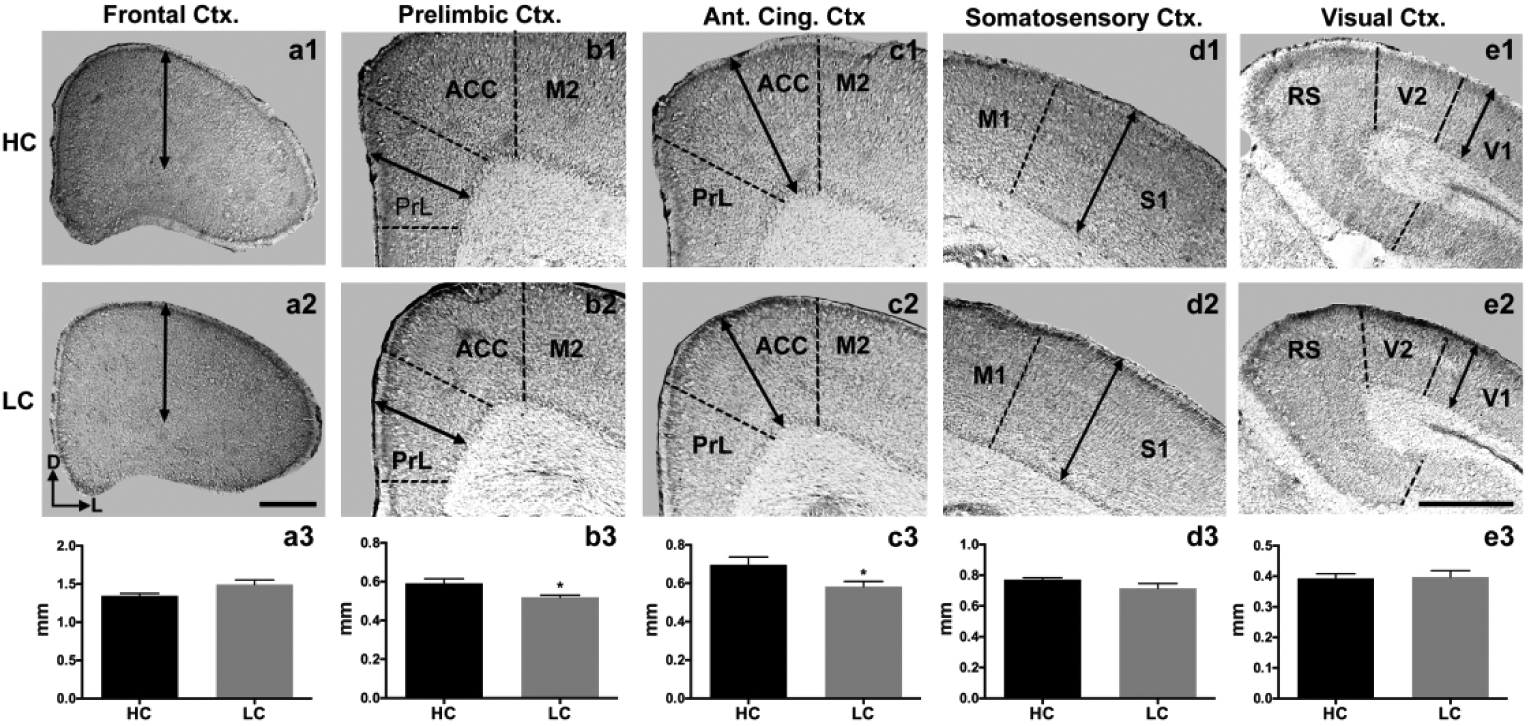
Coronal 40 μm sections in HC (top row, a1–e1) and LC P1 voles (middle row, a2–e2). Arrows indicate measurements of cortical thickness. No differences were observed in putative frontal cortex (a3), somatosensory (d3), and visual cortices (e3). However, a significant reduction was detected in putative prelimbic (b3; *p < 0.05) and anterior cingulate cortex (c3; *p < 0.05) in the LC group compared to HC voles. Bars represent group means ± SEM. Images oriented dorsal (D) up, lateral (L) left. Scale bar in (a2) applies to (a1–2). Scale bar in (e2) applies to all images in (b–e). Both scale bars = 500 μm.
At P1, there were no significant differences in cortical thickness between HC (1.346 ± 0.02901 mm) and LC (1.492 ± 0.06012 mm) voles within frontal cortex.(Fig. 9a3). Likewise, there were no significant differences between HC (0.7716 ± 0.01216 mm) and LC (0.7150 ± 0.03130 mm) voles within somatosensory cortex (Fig. 9d3) and visual cortex; HC (0.3932 ± 0.01534 mm) and LC (0.3973 ± 0.02072 mm) (Fig. 9e3). However, we did observe a significant decrease in prelimbic cortex thickness in LC (0.5196 ± 0.01102 mm) voles compared to HC (0.5908 ± 0.02422 mm) (Fig. 9b3; *p= 0.0281), as well as in the ACC (LC: 0.5826 ± 0.02718 mm; HC 0.6963 ± 0.04054 mm) (Fig. 9c3; *p= 0.0482).
4. Discussion:
The goal of this study was to investigate if differences in the cortical connections observed in HC and LC adult voles are present at birth, and to determine if differences in the expression patterns of genes involved in cortical development and connectivity exist at this early developmental stage in these two groups. It is well established that variations in sensory experience generated by experimental manipulations can produce long-lasting changes in cortical organization (Chang and Merzenich, 2003; Shepherd et al., 2003; Tagawa et al., 2005). However, it is not well understood how individual differences naturally emerge within a population, or the extent to which these differences are driven by direct alterations to the genomic sequence or via extrinsic, activity-dependent mechanisms.
In this study, we took advantage of systematic, naturally occurring variation in early sensory experiences due to differential parental contact in prairie voles. Behavioral variation in tactile contact of parents has been previously correlated with quantifiable differences in S1 cortical areal boundaries and patterns of connectivity in adult voles (Seelke et al., 2016a; 2016b). Furthermore, previous work has shown that voles who received differential parental care exhibited differences in social behavior (Perkeybile et al., 2013), which may be regulated by the anterior cingulate cortex (Apps et al., 2016). Using the vole as our animal model we examined intraneocortical connections of S1 and ACC, patterns of gene expression, and cortical thickness in LC and HC vole newborn offspring in order to determine if individual differences in cortical connections of LC and HC voles are present at birth, and to elucidate the potential mechanisms that may promote these differences observed in adult offspring.
4.1. Cortical Connections
Studies in adult LC and HC voles demonstrated quantifiable differences in the intra- and inter-hemispheric connections of the perioral face region of S1. Specifically, HC voles had a greater density of intrinsic connections within S1, and a more restricted pattern of ipsilateral connections was present when compared to LC voles. LC voles had denser and more widespread projections from parietal and frontal cortex, and more broadly distributed callosal projections compared to HC voles (Seelke et al., 2016a). In the current study, we found no statistically-significant differences between HC and LC offspring at P1 in either S1 or ACC connectivity, despite the differences previously reported in S1 in adult offspring (Seelke et al., 2016a). These findings suggest that the development of individual differences in cortical connections in HC versus LC voles must occur after P1, and, these difference in adults may reflect the consequences of differential tactile input bestowed upon them via parental care, which in turn may trigger activity dependent mechanisms involved in the establishment and modification of cortical connections. Although a comprehensive study of developing cortical connections within the prairie vole is not available, rodents (e.g. mouse) typically undergo extensive development of cortical connections in early life involving rudimentary patterns emerging in late prenatal stages, and beginning to take adult-like form within the first 2 weeks of postnatal life (Dye et al., 2011a; 2011b). As a member of the Order Rodentia, prairie voles likely undergo a similar trajectory of cortical development. Further work examining later postnatal juvenile ages will provide key information on when differences in cortical organization and connectivity emerge in high and low contact offspring.
In the current study we also examined the connections of ACC, an area which has been implicated in several complex behaviors in rodents including attention, motivation, reward processing and social behaviors (reviewed in Apps et al., 2016). Despite the role of ACC in a number of important behaviors, there are few studies that examine the development of this field and its patterns of connections in any rodent (Rash and Richards, 2001; Piper et al., 2009; Lim et al., 2015; Hossain et al., 2019), and these studies largely focus on callosal connectivity development in this area. Due to the difference in social behaviors observed in adolescent HC and LC voles (Perkeybile et al., 2013), we hypothesized that ACC may also represent a cortical area whose organization is susceptible to early parental care variation. However, our results demonstrate that differences do not exist between HC and LC offspring in ACC connectivity at P1. If HC and LC voles do display differences in patterns of ACC connections, they may emerge in later life.
4.2. Gene expression
Cortical development relies heavily on spatial and temporal-specific regulation of gene expression patterns, including that of several transcription factors. Here, we assessed patterns of two specific genes in P1 offspring, RZRβ and Id2, which represent a subset of genes involved in development of cortical connections as well as in patterning functionally and architectonically distinct cortical areas. RZRβ and Id2 are both strongly expressed in late prenatal and early postnatal cortical neurons in area- and layer-specific ways (Rubenstein et al., 1999). The expression patterns of these two genes have been shown to abut each other at the boundaries of developing sensorimotor areas (Huffman et al., 2004). These patterns at area boundaries, which are mostly complementary, have been hypothesized to provide a guiding influence on INC development (Dye et al., 2011a; 2011b). As we see in Figure 8, this also holds true for prairie voles. These early neocortical gene expression patterns have been directly implicated in the guidance and patterning of INC development (Huffman et al., 2004), making them ideal candidates for an initial characterization of gene expression in our vole model of differential parental care.
Although we report no significant differences in RZRβ expression, we do observe a significant difference in Id2 expression between HC and LC voles at P1. Specifically, we observed a lateral shift in layer II/III expression in LC offspring compared to HC offspring. Interestingly, this shift in expression coincides anatomically to the M1/S1 border, with LC voles displaying an expression pattern different from that seen in S1 of HC voles and other rodents such as mice (Dye et al., 2011a). Functionally, this differential gene expression could form part of the molecular substrate responsible for the alterations in cortical connections and cortical area size differences observed for S1 and M1 in adult voles (Seelke et al., 2016a).
Id2, a transcription factor in the inhibitor of differentiation/DNA binding protein family (Benezra et al., 1990), impacts a large number of genetic networks involved in brain development, including neurogenesis (Toma et al., 2000) and apoptosis (Gleichmann et al., 2002). Notably, in postmitotic neurons, Id2 is involved in axonal/neurite outgrowth (Lasorella et al., 2006; Ko et al., 2016; Huang et al., 2019), providing a potential mechanistic framework for the differences in cortical connectivity and overall area boundaries observed between HC and LC adult voles. Taken together, our results suggest that differential parental care (and thus, sensory inputs) may epigenetically alter expression patterns of early postnatal cortical genes and, in turn, drive the differences in both connectivity patterns and overall area boundaries observed in later life.
4.3. Cortical thickness
During early development, there is dynamic regulation of cortical thickness (Shaw et al., 2008; Tamnes et al., 2010). Here, we used cortical thickness measures to assess changes in cortical development possibly related to parenting style and physical contact. Specifically, we measured cortical thickness from five distinct, putative regions, including frontal cortex, prelimbic (PrL) cortex, anterior cingulate cortex (ACC), primary somatosensory cortex (S1), and primary visual cortex (V1) to assess if gross developmental trajectories were impacted by differential parental care in P1 voles. Following quantitative analyses, significant differences in PrL and ACC cortical thicknesses were observed between HC and LC voles, as LC voles had significantly thinner PrL and ACC regions.
PrL cortex, as a part of the medial prefrontal cortex and a part of the limbic system in mammals, has been implicated in several complex behaviors, including fear conditioning (Gilmartin and Helmstetter, 2010), attentional set shifting (Birrell and Brown, 2000), and regulation of the stress response (McKlveen et al., 2013). Additionally, the ACC is known to be involved in socio-emotional processing, and like PrL, is an important component of the limbic system (Rolls, 2019). Interestingly, HC and LC vole offspring have been shown to display differential stress responses in both non-social (Perkeybile and Bales, 2015b) and social contexts (Perkeybile and Bales, 2015a), suggesting that the differences in gross developmental trajectory of PrL and ACC at P1 may contribute to these later-life behaviors. Overall, these results suggest that development of prelimbic and anterior cingulate cortex may be altered by temporally-limited, very early sensory experiences (i.e. parental care variations), and/or by epigenetically-mediated changes that are distinct between the two contact types of voles (LC and HC). However, sensory areas, such as S1 and V1, may not be susceptible to alterations until later ages, after sensory organs are fully developed and sensory experience is more abundant and diverse.
4.4. Conclusions
We report that, at birth, vole offspring born to either HC or LC parental pairs exhibit significant shifts in Id2 gene expression near the M1/S1 boundary, as well as differences in prelimbic and anterior cingulate cortical thickness. In contrast, no significant differences were observed between HC and LC P1 offspring in ACC or S1 INC connectivity, suggesting that the differences in connections of S1 in adult LC and HC voles (Seelke et al., 2016a) arise after P1. Overall, these results support the hypothesis that individual differences in cortical connectivity and organization emerge in later life, potentially through epigenetic mechanisms that are driven by differential tactile experiences. In future studies, we will further investigate these processes by cross-fostering LC and HC and examining connections and patterns of gene expression at subsequent ages to disentangle how these naturally-occurring individual differences in brain and behavior emerge within a population.
Acknowledgements:
The authors would like to thank Dr. Adele Seelke for her extensive efforts in breeding and processing of brain tissue and members of the Krubitzer laboratory for their helpful comments on the manuscript.
In remembrance of Dr. Jack Pettigrew: If you are lucky enough, sometime in your life you will work with someone like Jack Pettigrew. Jack was the real deal; a true naturalist, biologist and scientist. I will always remember the nights that Jack, Paul and I went on our platypus hunts: “In Search of the Protypical Plan”, and Jack’s stories about everything. I’m gonna miss that guy! - Leah A. Krubitzer
Funding information: James S. McDonnell Foundation, grant/award number 220020516 to L.A.K and K.J.H.; National Institute of Health (NIH), grant/award number: RO1HD084362-01A1 to L.A.K. and K.J.H.
Footnotes
Data availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- Abbott CW, Rohac DJ, Bottom RT, Patadia S, & Huffman KJ (2018). Prenatal ethanol exposure and neocortical development: A transgenerational model of FASD. Cerebral Cortex, 28(8), 2908–2921. 10.1093/cercor/bhx168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Rushworth MFS, & Chang SWC (2016). The anterior cingulate gyrus and social Cognition: Tracking the motivation of others. Neuron, 90(4), 692–707. 10.1016/j.neuron.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Zhang T-Y, Wen X, Nguyen TTT, Nguyen H-B, Diorio J, … Meaney MJ (2012). Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proceedings of the National Academy of Sciences, 109(Supplement_2), 17200–17207. 10.1073/pnas.1204599109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Witczak LR, Simmons TC, Savidge LE, Rothwell ES, Rogers FD, … Arias del Razo R (2018). Social touch during development: Long-term effects on brain and behavior. Neuroscience & Biobehavioral Reviews, 95, 202–219. 10.1016/j.neubiorev.2018.09.019 [DOI] [PubMed] [Google Scholar]
- Bales KL, & Carter CS (2003). Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Hormones and Behavior, 44(3), 178–184. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14609540 [DOI] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, & Weintraub H (1990). The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell, 61(1), 49–59. 10.1016/0092-8674(90)90214-Y [DOI] [PubMed] [Google Scholar]
- Birrell JM, & Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(11), 4320–4324. 10.1523/JNEUROSCI.20-11-04320.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaleta M, Johnson W, Waber DP, Colom R, & Karama S (2014). Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. NeuroImage, 84, 810–819. 10.1016/j.neuroimage.2013.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Karlen SJ, Bales KL, & Krubitzer L (2007). Organization of sensory neocortex in prairie voles (Microtus ochrogaster). The Journal of Comparative Neurology, 502(3), 414–426. 10.1002/cne.21314 [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, & Meaney MJ (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. 10.1016/S0031-9384(03)00149-5 [DOI] [PubMed]
- Chang EF, & Merzenich MM (2003). Environmental noise retards auditory cortical development. Science, 300(5618), 498–502. 10.1126/science.1082163 [DOI] [PubMed] [Google Scholar]
- Delevich K, Tucciarone J, Huang ZJ, & Li B (2015). The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 35(14), 5743–5753. 10.1523/JNEUROSCI.4565-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye CA, El Shawa H, & Huffman KJ (2011a). A lifespan analysis of intraneocortical connections and gene expression in the mouse I. Cerebral Cortex, 21(6), 1311–1331. 10.1093/cercor/bhq213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye CA, El Shawa H, & Huffman KJ (2011b). A lifespan analysis of intraneocortical connections and gene expression in the mouse II. Cerebral Cortex, 21(6), 1331–1350. 10.1093/cercor/bhq213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Shawa H, Abbott CW, & Huffman KJ (2013). Prenatal ethanol exposure disrupts intraneocortical circuitry, cortical gene expression, and behavior in a mouse model of FASD. Journal of Neuroscience, 33(48), 18893–18905. 10.1523/JNEUROSCI.3721-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Derian EL, & Babb TL (1984). Afferent fibers to rat cingulate cortex. Experimental Neurology, 83(3), 468–485. 10.1016/0014-4886(84)90116-X [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, & Meaney MJ (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science (New York, N.Y.), 286(5442), 1155–1158. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10550053 [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, & Gavish L (1981). The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology, 8(3), 189–194. 10.1007/BF00299829 [DOI] [Google Scholar]
- Gilmartin MR, & Helmstetter FJ (2010). Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning & Memory, 17(6), 289–296. 10.1101/lm.1597410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann M, Buchheim G, El-Bizri H, Yokota Y, Klockgether T, Kugler S, … Schulz JB (2002). Identification of inhibitor-of-differentiation 2 (Id2) as a modulator of neuronal apoptosis. Journal of Neurochemistry, 80(5), 755–762. 10.1046/j.0022-3042.2002.00760.x [DOI] [PubMed] [Google Scholar]
- Harlow HF, & Zimmerman RR (1959). Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science (New York, N.Y.), 130(3373), 421–432. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13675765 [DOI] [PubMed] [Google Scholar]
- Homman-Ludiye J, & Bourne JA (2014). Mapping arealisation of the visual cortex of non-primate species: lessons for development and evolution. Frontiers in Neural Circuits, 8, 79. 10.3389/fncir.2014.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Tsuzuki T, Sakakibara K, Imaizumi F, Ikegaya A, Inagaki M, … Yukawa K (2019). PlexinA1 is crucial for the midline crossing of callosal axons during corpus callosum development in BALB/cAJ mice. PLOS ONE, 14(8), e0221440. 10.1371/journal.pone.0221440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Liu J, Jin J, Chen Q, Shields LBE, Zhang Y-P, … Yu P (2019). Inhibitor of DNA binding 2 promotes axonal growth through upregulation of Neurogenin2. Experimental Neurology, 320, 112966. 10.1016/J.EXPNEUROL.2019.112966 [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Garel S, & Rubenstein JLR (2004). Fgf8 regulates the development of intra-neocortical projections. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 24(41), 8917–8923. https://doi.org/20026779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D, J. Shnider S, J. Tischfield D, J. Galazo M, & Macklis JD (2012). RORβ induces barrel-like neuronal clusters in the developing neocortex. Cerebral Cortex, 22(5), 996–1006. 10.1093/cercor/bhr182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, … Brain Development Cooperative Group. (2011). Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. NeuroImage, 55(4), 1443–1453. 10.1016/j.neuroimage.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HR, Kwon I-S, Hwang I, Jin E-J, Shin J-H, Brennan-Minnella AM, … Ahn J-Y (2016). Akt1-Inhibitor of DNA binding2 is essential for growth cone formation and axon growth and promotes central nervous system axon regeneration. ELife, 5. 10.7554/eLife.20799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, & Seelke AMH (2012). Cortical evolution in mammals: the bane and beauty of phenotypic variability. Proceedings of the National Academy of Sciences of the United States of America, 109 Suppl 1(Supplement 1), 10647–10654. 10.1073/pnas.1201891109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorella A, Stegmüller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, … Iavarone A (2006). Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature, 442(7101), 471–474. 10.1038/nature04895 [DOI] [PubMed] [Google Scholar]
- Lim JWC, Donahoo A-LS, Bunt J, Edwards TJ, Fenlon LR, Liu Y, … Richards LJ (2015). EMX1 regulates NRP1-mediated wiring of the mouse anterior cingulate cortex. Development, 142(21), 3746–3757. 10.1242/DEV.119909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, … Meaney MJ (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science (New York, N.Y.), 277(5332), 1659–1662. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9287218 [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, & Meaney MJ (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience, 3(8), 799–806. 10.1038/77702 [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, & Herman JP (2013). Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biological Psychiatry, 74(9), 672–679. 10.1016/j.biopsych.2013.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H-B, Bagot RC, Diorio J, Wong TP, & Meaney MJ (2015). Maternal care differentially affects neuronal excitability and synaptic plasticity in the dorsal and ventral hippocampus. Neuropsychopharmacology, 40(7), 1590–1599. 10.1038/npp.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DDM, Chou SJ, & Sahara S (2007). Area patterning of the mammalian cortex. Neuron, 56(2), 252–269. 10.1016/j.neuron.2007.10.010 [DOI] [PubMed] [Google Scholar]
- Park HJ, Hong M, Bronson RT, Israel MA, Frankel WN, & Yun K (2013). Elevated Id2 expression results in precocious neural stem cell depletion and abnormal brain development. Stem Cells, 31(5), 1010–1021. 10.1002/stem.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, & Wang H (2007). Atlas of the developing mouse brain. 1st edition. Academic Press; Burlington, MA. [Google Scholar]
- Perkeybile AM, & Bales KL (2015a). Early rearing experience is related to altered aggression and vasopressin production following chronic social isolation in the prairie vole. Behavioural Brain Research, 283, 37–46. 10.1016/J.BBR.2015.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, & Bales KL (2015b). Early rearing experience is associated with vasopressin immunoreactivity but not reactivity to an acute non-social stressor in the prairie vole. Physiology & Behavior, 147, 149–156. 10.1016/J.PHYSBEH.2015.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, & Bales KL (2013). Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Frontiers in Behavioral Neuroscience, 7, 21. 10.3389/fnbeh.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, … Connelly JJ (2019). Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology, 99, 128–136. 10.1016/j.psyneuen.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Plachez C, Zalucki O, Fothergill T, Goudreau G, Erzurumlu R, … Richards LJ (2009). Neuropilin 1-Sema Signaling Regulates Crossing of Cingulate Pioneering Axons during Development of the Corpus Callosum. Cerebral Cortex, 19(suppl_1), i11–i21. 10.1093/cercor/bhp027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, & Richards LJ (2001). A role for cingulate pioneering axons in the development of the corpus callosum. The Journal of Comparative Neurology, 434(2), 147–157. 10.1002/cne.1170 [DOI] [PubMed] [Google Scholar]
- Rolls ET (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure and Function. 10.1007/s00429-019-01945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Anderson S, Shi L, Miyashita-Lin E, Bulfone A, & Hevner R (1999). Genetic control of cortical regionalization and connectivity. Cerebral Cortex (New York, N.Y. : 1991), 9(6), 524–532. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10498270 [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, & Rushworth MFS (2006). Separate neural pathways process different decision costs. Nature Neuroscience, 9(9), 1161–1168. 10.1038/nn1756 [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Millette BHP, Shirley E, Rushworth MFS, & Bannerman DM (2007). Distinct contributions of frontal areas to emotion and social behaviour in the rat. European Journal of Neuroscience, 26(8), 2315–2326. 10.1111/j.1460-9568.2007.05844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Harris BN, De Jong TR, Perea-Rodriguez JP, Horrell ND, Zhao M, & Andrew JR (2017). Paternal care in biparental rodents: Intra- and inter-individual variation. Integrative and Comparative Biology, 57(3), 589–602. 10.1093/icb/icx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, … Mucke L (2008). Abnormal social behaviors in mice lacking Fgf17. Genes, Brain and Behavior, 7(3), 344–354. 10.1111/j.1601-183X.2007.00357.x [DOI] [PubMed] [Google Scholar]
- Schaeren-Wierners N, André E, Kapfhammer JP, & Becker-André M (1997). The Expression pattern of the orphan nuclear receptor RORβ in the Developing and Adult Rat Nervous System Suggests a Role in the Processing of Sensory Information and in Circadian Rhythm. European Journal of Neuroscience, 9(12), 2687–2701. 10.1111/j.1460-9568.1997.tb01698.x [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Perkeybile AM, Grunewald R, Bales KL, & Krubitzer LA (2016a). Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster). Journal of Comparative Neurology, 524(3), 564–577. 10.1002/cne.23837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Yuan S-M, Perkeybile AM, Krubitzer LA, & Bales KL (2016b). Early experiences can alter the size of cortical fields in prairie voles (Microtus ochrogaster). Environmental Epigenetics, 2(3), dvw019. 10.1093/eep/dvw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, … Wise SP (2008). Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience, 28(14), 3586–3594. 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Pologruto TA, & Svoboda K (2003). Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron, 38(2), 277–289. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12718861 [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, & Shatz CJ (2005). Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nature Neuroscience, 8(3), 380–388. 10.1038/nn1410 [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, & Walhovd KB (2010). Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex, 20(3), 534–548. 10.1093/cercor/bhp118 [DOI] [PubMed] [Google Scholar]
- Toma JG, El-Bizri H, Barnabe-Heider F, Aloyz R, & Miller FD (2000). Evidence that helix-loop-helix proteins collaborate with retinoblastoma tumor suppressor protein to regulate cortical neurogenesis. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(20), 7648–7656. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11027225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt FN, Cornelisse S, Yuan Zhang T, Meaney MJ, Velzing EH, Krugers HJ, & Joëls M (2012). Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus, 22(2), 255–266. 10.1002/hipo.20892 [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, & Carter CS (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behavior, 26(3), 339–349. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1398553 [DOI] [PubMed] [Google Scholar]
- Woolsey TA, & Van der Loos H (1970). The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Research, 17(2), 205–242. 10.1016/0006-8993(70)90079-x [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Welker C, & Schwartz RH (1975). Comparative anatomical studies of the Sml face cortex with special reference to the occurrence of “barrels” in layer IV. The Journal of Comparative Neurology, 164(1), 79–94. 10.1002/cne.901640107 [DOI] [PubMed] [Google Scholar]


