In a recent issue of the Journal, Thomas et al (1) reported that minority (excluding White and Asian) participants in the CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation) trial, randomized to receive catheter ablation therapy, experienced lower rates of primary and secondary endpoints than did those randomized to drug therapy. Notably, this difference was not observed among nonminority participants. These findings are significant because racial/ethnic minority individuals experience higher rates of atrial fibrillation (AF) complications than do their nonminority counterparts (2). We comment here on these findings describing outcome differences by minority status.
Diverse participation in AF clinical trials is limited (2). Black race and Hispanic ethnicity represent 13.4% and 18.5% of the U.S. population, respectively, but constituted only 8.9% of the CABANA trial (3). Underrepresentation of minority participants, consistent with other AF studies (Figure 1), has implications for achieving equitable AF management. Despite prior work demonstrating improved outcomes of catheter ablation therapy in AF, Black patients are significantly less likely to receive such therapy (4).
FIGURE 1. Racial/Ethnic Representation in Clinical Trials and Observational Studies of Atrial Fibrillation.
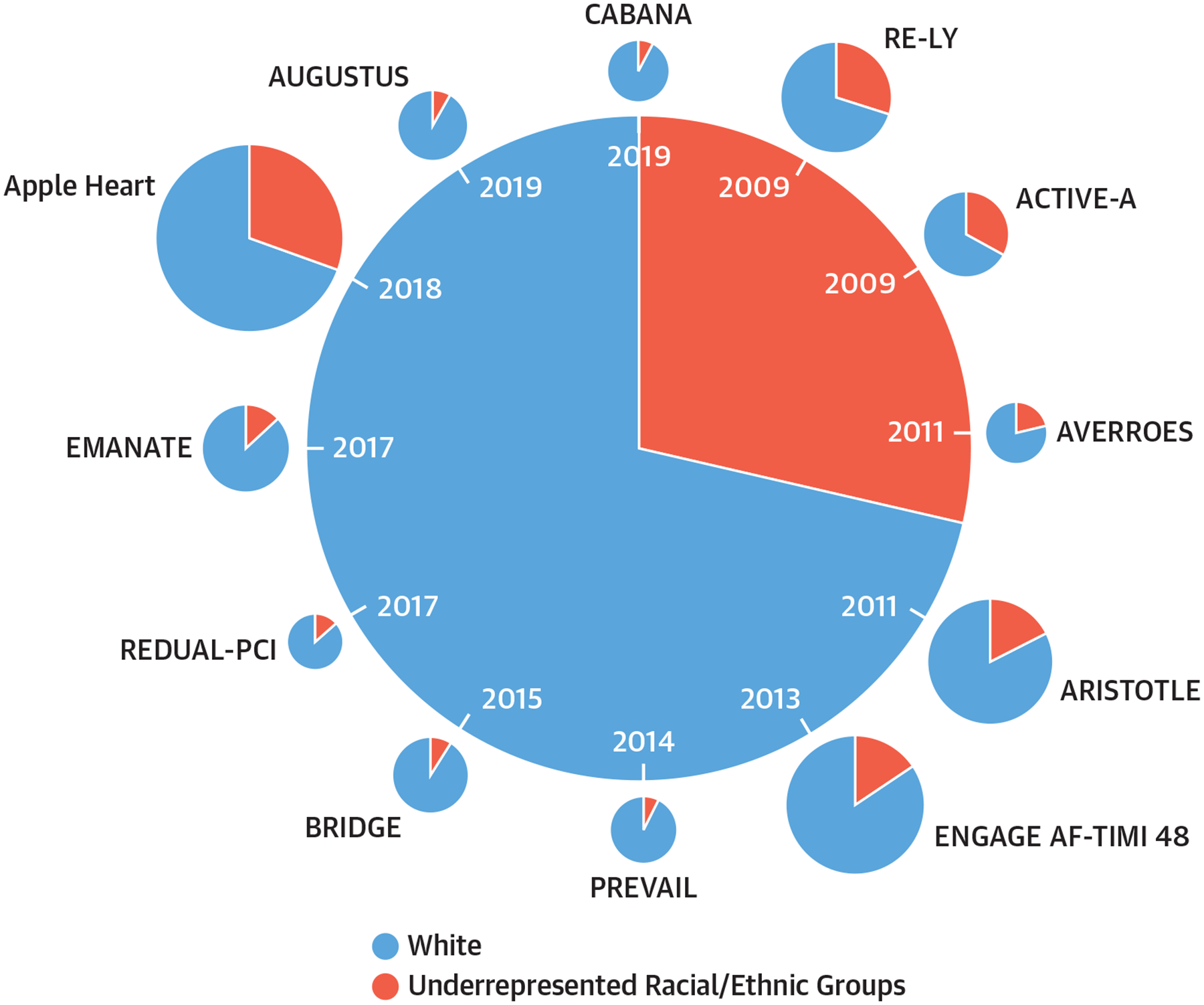
Proportion of White versus racial/ethnic minorities in clinical trials and observational studies of atrial fibrillation. ACTIVE-A = Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events; ARISTOTLE = Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AUGUSTUS = The Open-Label, 2 × 2 Factorial, Randomized, Controlled Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients with Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention Trial; AVERROES = Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment Trial; BRIDGE = Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery Trial; CABANA = Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial; EMANATE = Eliquis evaluated in acute cardioversion coMpared to usual treatmeNts for AnticoagulaTion in subjEcts with atrial fibrillation Trial; ENGAGE AF-TIMI 48 = Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48; PREVAIL = Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation; RE-DUAL PCI = Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention Trial; RE-LY = Randomized Evaluation of Long-Term Anticoagulation Therapy. Reprinted with permission from Essien et al (4).
Beyond trial representation, the finding of different outcomes by race/ethnicity and treatment type is notable. Race is a social construct, not a biological determinant, and as such, caution should be taken that it does not replace the social disadvantages commonly experienced by minority individuals. By condensing broad social heterogeneity into 1 term, “minority,” we have historically ignored interactions of social factors with race that contribute to health disparities. Collecting and adjusting for individuallevel and neighborhood-level social factors will guide the application of clinical trial findings.
Thomas et al (1) remind us of the multidimensional barriers to research participation by minority individuals. Often low minority research participation is explained by distrust, poor access, and provider bias. However, some health systems caring for higher proportions of minority patients may be less likely to receive funding to lead trials. Furthermore, certain communities may see biomedical research as a unilateral relationship, whereby researchers use data to advance their scientific careers with limited effect on community well-being (5).
Clinical trials must be more inclusive to address the perpetual disparities in AF care (4). Investigators should broadly incorporate a community-based participatory research model into their work to help design protocols that effectively recruit, retain, and engage historically excluded participants. Focusing on equity is key to ensuring high-quality care for all patients with AF.
Acknowledgments
This work was supported by grants NIH/NHLBI R01HL143010 and R33HL144669 to Dr Magnani and the Department of Veterans Affairs (CDA-20-049) to Dr Essien. Dr Tertulien has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Thomas KL, Al-Khalidi HR, Silverstein AP, et al. Ablation versus drug therapy for atrial fibrillation in racial and ethnic minorities. J Am Coll Cardiol. 2021;78:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magnani JW, Norby FL, Agarwal SK, et al. Racial differences in atrial fibrillation-related cardiovascular disease and mortality. JAMA Cardiol. 2016;1: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Census Bureau (2016). State and County QuickFacts. Accessed September 27, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 4.Essien UR, Kornej J, Johnson AE, Schulson LB, Benjamin EJ, Magnani JW. Social determinants of atrial fibrillation. Nat Rev Cardiol. 2021;18:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer LC, Pasha M, Seele P, et al. Overcoming historical barriers: enhancing positive perceptions of medical research among African Americans through a conference-based workshop. J Gen Intern Med. 2021;36:2547–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]


