Abstract
The Mycobacterium tuberculosis strains H37Rv and H37Ra are the most commonly used controls for M. tuberculosis identification in the clinical and research laboratory setting. To reduce the likelihood of misidentification and possible cross-contamination with this laboratory neotype, it is important to be able to distinguish H37 from clinical isolates. To provide a reference for identifying H37, we used multiple molecular techniques to characterize H37 strains, including 18 of the most frequently used variants available through the American Type Culture Collection. Isolates were genotyped using gene probes to IS6110 and IS1085. In addition, we performed polymorphic GC-rich sequence typing (PGRS), spoligotyping, determination of variable number of tandem repeats (VNTR), and PCR amplification of the mtp40, msx4, and mpp8 polymorphic regions. Southern hybridization with IS6110 provided the most discrimination, differentiating the 18 H37 isolates into 10 discrete patterns made up of 9 H37Rv variants and 1 H37Ra variant. PGRS, IS1085, mpp8, and spoligotyping were not able to distinguish any H37 variants, while VNTR and msx4 discriminated two. Only IS6110 and spoligotyping could distinguish the H37 strain from clinical isolates. In summary, spoligotyping and IS6110 provide a rapid and accurate way to identify H37 contamination, though IS6110 can, in addition, classify many of the H37 variants that would otherwise require phenotypic segregation.
The genotyping of Mycobacterium tuberculosis, primarily for outbreak identification, has become a model for the application of strain typing in the field of molecular epidemiology. In the clinical mycobacteriology laboratory, strain typing has been essential in the identification of laboratory cross-contamination (1, 2, 5, 16, 25), an almost impossible task prior to the inception of molecular techniques. The source of laboratory cross-contamination can be a clinical sample (3, 15, 16) or often the M. tuberculosis control strain maintained by the clinical mycobacteriology laboratory (12, 13). In this regard, the virulent and attenuated H37 variants are the most commonly used control isolates and thus are a major source of false-positive results in M. tuberculosis identification as well as cross-contamination (13). The purpose of this study is to provide the mycobacteriologist with a molecular guide for discriminating H37 from clinical isolates in the genotyping laboratory.
The strain H37 was originally isolated in 1905 and gained attention for its noted virulence in the guinea pig model, a distinctive characteristic used in the classification of “human tuberculosis” in the early 1900s. In 1934, H37 was dissociated into “virulent” (Rv) and “avirulent” (Ra) strains (18, 24). The original 1905 H37 isolate was then discontinued, and the H37Rv and H37Ra isolates have been maintained at the Trudeau Institute ever since. Several drug-resistant derivatives have been generated during the years, accounting in part for the 18 H37 variants available through the Trudeau Mycobacterial Collection (TMC) and the American Type Culture Collection (ATCC). Hence, there are 15 H37Rv and 3 H37Ra progenies maintained at the Trudeau Institute and the ATCC.
Although H37 variants are widely used as reference strains in mycobacteriology and molecular biology laboratories, their IS6110 patterns are often mistaken for clinical isolates displaying similar fingerprint patterns (unpublished data). In this respect, it is essential for both patient care and tuberculosis control to be able to properly recognize and genotype all possible H37 variants. To do so, we have employed several of the most common M. tuberculosis typing techniques in order to characterize the 18 H37 variants available through the ATCC. The results indicate that both spoligotyping and IS6110 provide a rapid means of distinguishing H37 strains from clinical isolates. In addition, IS6110 DNA fingerprinting analysis further discriminates the collection into 10 distinct H37 variants.
MATERIALS AND METHODS
M. tuberculosis reference strains.
The 18 different catalogued H37 variants were purchased from the ATCC. The strains were deposited at the ATCC as follows: 25177 (C. L. Larson, University of Montana, lot 1-23-69), 35618 (A. G. Karlson, Mayo Clinic, lot 1-21-70), and 27294 (G. P. Kubica, Trudeau Laboratories, lot 1-27-72). All other strains (35820, 35821, 35822, 35823, 35824, 35825, 35826, 35827, 35828, 35829, 35830, 35835, 35836, 35837 and 35838) were deposited by the Trudeau Institute in lot 2-01-85 (data were kindly provided by the ATCC). In addition, the primary collection of H37 variants was also received, as a kind gift of R. North, from the TMC, Trudeau Institute, Saranac Lake, N.Y. TMC and ATCC reference numbers and susceptibility data are shown in Table 1.
TABLE 1.
H37Rv and H37Ra collectiona
| ATCC no. | TMC no. | Genotype | Phenotype | Strain origin | Source or reference(s) (yr) |
|---|---|---|---|---|---|
| Rv | |||||
| 25618 | H37Rv | Rv9 | Pans | A. G. Karlson, Mayo Clinic | ATCC (1992) |
| 27294 | 102(Rv) | Rv7 | Pans | Dissociated in 1934 | 18, 24 |
| 35820 | 301(Rv) | Rv1 | Strr | Mutant of TMC102 | 21 |
| 35821 | 302(Rv) | Rv2 | Pasr | Mutant of TMC102 | W. Steenken (1948) |
| 35822 | 303(Rv) | Rv8 | Inhr | Mutant of TMC102 | 29 |
| 35823 | 304(Rv) | Rv3 | StrrInhr | Mutant of TMC102 | TMC (1954) |
| 35824 | 305(Rv) | Rv5 | Pasr Strr | Mutant of TMC102 | 20 |
| 35825 | 306(Rv) | Rv6 | Pasr Strr Inhr | Mutant of TMC102 | TMC (1961) |
| 35826 | 307(Rv) | Rv8 | Cycr | Mutant of TMC102 | 19 |
| 35827 | 309(Rv) | Rv8 | Kanr | Mutant of TMC102 | 22 |
| 35828 | 311(Rv) | Rv8 | Pzar | Mutant of TMC102 | 29 |
| 35829 | 313(Rv) | Rv4 | Tacr | Mutant of TMC102 | 29 |
| 35830 | 314(Rv) | Rv8 | Ethr | Mutant of TMC102 | 17 |
| 35837 | 330(Rv) | Rv7 | Embr | Mutant of TMC102 | TMC (1970) |
| 35838 | 331(Rv) | Rv7 | Rifr | Mutant of TMC102 | TMC (1970) |
| Ra | |||||
| 35835 | 326(Ra) | Ra1 | Inhr | Mutant of TMC201 | TMC (1961) |
| 35836 | 327(Ra) | Ra1 | Strr | Mutant of TMC201 | TMC (1949) |
| 25177 | 201(Ra) | Ra1 | Pans | Dissociated in 1934 | 18, 24 |
M. tuberculosis clinical isolates.
A search of the IS6110 fingerprint database maintained at the Public Health Research Institute Tuberculosis Center (TB Center) (n = 11,000) identified 131 H37Rv and H37Ra isolates which matched at least one of the nine H37Rv and one H37Ra patterns reported in this study. The fingerprint search was conducted using each of the 10 possible H37Rv and H37Ra patterns as a prototype. The TB Center database includes approximately 8,600 isolates from New York City and New Jersey, while the remaining samples are from seven additional states in the United States and from the former USSR, Singapore, South Africa, Romania, Egypt, Israel, Venezuela, Honduras, Mexico, India, Chile, the Czech Republic, and Kenya.
Genotyping by IS6110.
Chromosomal DNA extraction and strain typing by IS6110 was performed according to the standard method using the right-side hybridization probe (IS6110-3′-probe) (27). The same membrane was rehybridized with the left-side IS6110 probe (IS6110-5′-probe), the direct repeat (DR) probe, and the insertion sequence IS1085.
Genotyping by using PGRS probe.
Chromosomal DNA was restricted with AluI and hybridized with the polymorphic GC-rich repetitive sequence (PGRS) probe (GenBank accession no. M95490) (14). All other electrophoretic and hybridization conditions were the same as for IS6110 genotyping (26).
Spoligotyping.
The DR of the extracted M. tuberculosis DNA was amplified by PCR and analyzed according to the spoligotyping protocol as described by Kamerbeek and colleagues (8). The AluI-digested DNA membranes generated for PGRS typing were also probed with the DR probe to confirm spoligotyping results (27).
Determination of VNTR.
The variable number of tandem repeat (VNTR) loci ETR-A to ETR-E were determined as described by Frothingham and Meeker-O'Connell (7). Briefly, the five selected loci were amplified by PCR and analyzed on a 2% agarose gel.
PCR amplification of the polymorphic fragments msx4, mpp8, and mtp40.
Amplicons to segments msx4, mpp8, and mtp40 were generated and compared from all 18 H37 variants. The polymorphic segments msx4 and mpp8 containing two DR sequences were PCR amplified with primer pairs SX1-SX2 and PP3-PP4, respectively, as described by Namwat et al. (11). PCR amplification of the mtp40 fragment was accomplished using primers PT1 and PT2 (6).
Computer analysis of fingerprint patterns.
The IS6110 hybridization patterns were electronically digitized and compared with a pattern-matching computer program on a Sun Sparc5 workstation using a Bioimage Whole Band Analyzer (software version 3.4; Genomic Solutions, Ann Arbor, Mich.). The Jaccard matching method and unweighted-pair-group method using arithmetic averages (UPGMA)-average linkage clustering was used to identify related patterns, in accord with the protocol of the Centers for Disease Control and Prevention, The National Tuberculosis Genotyping and Surveillance Network.
RESULTS
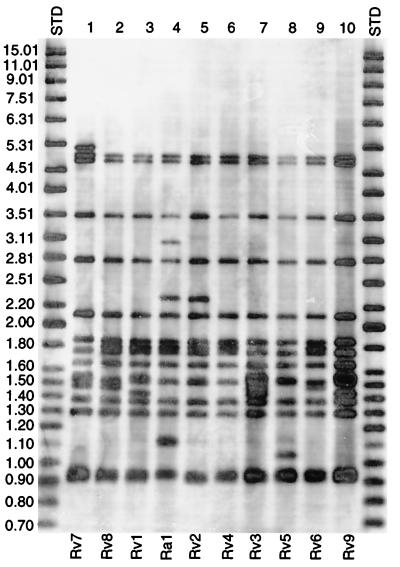
Eighteen H37 variants available through the ATCC and their respective parent strains from the Trudeau Institute were typed by the now standard IS6110 Southern blot hybridization analysis (26). A total of 10 distinct fingerprint patterns were identified (Fig. 1). The nine patterns associated with the H37Rv strains were assigned the genotypes Rv1 through Rv9 and all three H37Ra strains (35835, 35836, and 25177) shared the same Ra1 fingerprint pattern (Table 1). The Rv variants were Rv1 (strain 35820), Rv2 (strain 35821), Rv3 (strain 35823), Rv4 (strain 35829), Rv5 (strain 35824), Rv6 (strain 35825), Rv7 (strains 35837, 35838, and 27294), Rv8 (strains 35822, 35826, 35827, 35828, and 35830), and Rv9 (strain 35618). The number of IS6110 hybridizing bands ranged from 14 (Rv4) to 16 (Rv7) bands for H37Rv and was 16 bands for H37Ra (Ra1; Fig. 1).
FIG. 1.
IS6110 hybridization patterns of H37. Ten different IS6110 patterns were identified from the 18 H37 variants available through the ATCC and the TMC.
The spoligopattern of H37 variants was unique (spoligotype S00001; Fig. 2). The S00001 spoligopattern has only been observed in isolates of H37 variants and clinical samples determined to have been cross-contaminated by H37 strains. VNTR patterns were determined by analysis of the products of the PCR amplification of the five chromosomal loci ETR-A to ETR-E. All 18 ATCC isolates displayed the same VNTR pattern (33433), except for Rv5. PGRS-typing, spoligotyping, and VNTR analysis did not differentiate the 18 H37 variants within the TMC and ATCC isolates (see Fig. 4).
FIG. 2.
Spoligotype pattern of H37 variants. All 18 H37 ATCC and TMC samples, as well as 5 H37 laboratory variants, were found to have the same pattern. This pattern was not seen in a spoligotype database of 2,400 samples other than for H37 control samples.
FIG. 4.
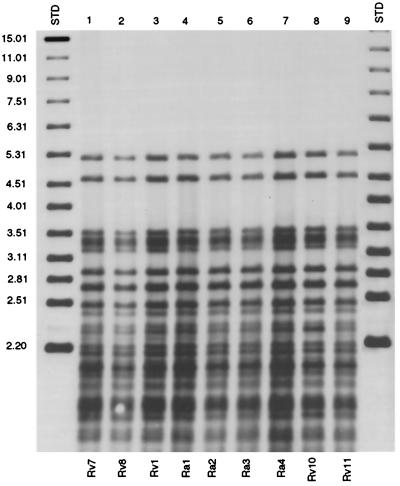
Southern hybridization of AluII-digested chromosomal DNA with the PGRS probe for strains Rv7, Ra1, and five H37 polymorphic variants. PGRS grouped all H37 variants but could not differentiate between them.
PCR amplification of three other polymorphic regions, the two repetitive sequences msx4, mpp8, and mtp40, and hybridization with IS1085, could not be used to distinguish H37 variants from other genetically unrelated M. tuberculosis strains. The msx4 PCR products of all H37 variants were shown to be 437 bp in length, with the exception of strain ATCC 35825, which generated a 720-bp amplicon (data not shown).
Genotyping clinical isolates.
Searching the IS6110 DNA fingerprint pattern database at the TB Center (n = ∼11,000) using Rv1 to Rv9 and Ra1 as prototypes, identified 131 IS6110 patterns that matched exactly at least 1 of the 18 H37 variants. A total of 102 samples matched H37Ra type Ra1, 27 matched H37Rv type Rv7, and 1 each matched Rv1 and Rv8. Of these, 45 samples identified as H37Ra were previously reported as known cases of cross-contamination following an investigation by the New York City Department of Health (13). Three H37Ra samples were from outside the United States. The New York State Department of Health confirmed an additional 14 H37Ra strains to be cases of laboratory cross-contamination (12). Of the remaining 69 cases, 37 were confirmed to be contamination by the source laboratories, and the remaining are being investigated by the responsible entities.
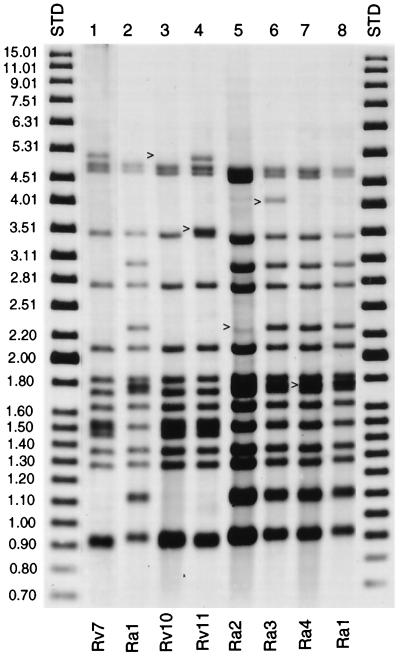
Five additional samples had a similar but not exact match with one of the H37Rv or H37Ra variants. The five samples differed from Rv1 to Rv9 and Ra1 by one or two hybridizing bands according to IS6110 analysis (Fig. 3). These samples were designated Rv10 and Rv11 and Ra2 through Ra4 (Fig. 3). Further analysis of all five isolates by the IS1085, VNTR, PGRS, and spoligotype methods failed to distinguish these samples from the reference TMC-ATCC prototypes (Fig. 4). The PGRS profile of H37 Rv1, Rv7, Rv8, Rv10 and Rv11, and Ra1 and Ra4 can be seen in Fig. 4.
FIG. 3.
IS6110 hybridization patterns of Rv7, Ra1, and five H37 polymorphic variants. As indicated, Rv10 and Rv11 (lanes 4 and 5) differ from Rv7 (lane 2) by the loss and addition of an IS6110 insertion, respectively (see arrows). Ra2 (lane 6) has lost an IS6110 insertion, while Ra3 and Ra4 (lanes 7 and 8) have gained an insertion compared to the reference strain Ra1 (lane 3 and 9).
Two of the strains, designated Rv10 and Ra2 (Fig. 3), were received as part of a large cluster of H37Rv and H37Ra cross-contamination investigations (13). Furthermore, strains Rv10 and Ra2 were very closely related to the standard laboratory reference strains Rv1 and Ra1, respectively. Rv10 and Ra2 differ from Rv1 and Ra1 by the deletion of one IS6110 insertion (Fig. 3). Another variant (Rv11) was routinely used as a reference strain by a clinical laboratory and was supplied for fingerprint analysis as part of a control experiment. Cross-contamination by the remaining two H37 isolates (Ra3 and Ra4) was confirmed by reviewing laboratory and clinical data pertaining to these two cases. Thus, it can be inferred that Rv10, Rv11, Ra2, Ra3, and Ra4 are variants of an H37 reference strain which have further evolved in the lab.
DISCUSSION
In the speciation and susceptibility testing of M. tuberculosis in the clinical laboratory, suspicion of contamination is heightened by the finding of an unusually high number of positive cultures per time period and by inconsistencies between the patient's clinical presentation and his or her laboratory results (10). Suggested cases of contamination are forwarded to a limited number of genotyping laboratories to perform molecular analysis to confirm or reject the possibility of contamination, primarily on a case-by-case basis (1, 2, 5, 16, 25). Often, genotyping and mycobacteriology laboratories are unaware of which H37 variant they are employing as controls, confounding the identification of contaminants. In this study, we have provided a collection of patterns for H37 and its variants for use as a reference by genotyping laboratories.
Genotyping analysis of H37 variants.
All 18 ATCC H37 variants had the same spoligotype pattern, designated S00001. Although spoligotype pattern S00001 did not discriminate one ATCC H37 variant from another, it proved definitive in distinguishing the ATCC H37 collection from all other clinical isolates analyzed by this technique (n ≈ 2,400).
Based on the similarity of the IS6110 DNA fingerprint patterns, all H37 variants were grouped as related. IS6110 discriminated the H37 variants, identifying 9 distinct yet similar patterns (Rv1 to Rv9) of 15 possible phenotypically (drug resistance profile) diverse H37Rv isolates and a single pattern for all 3 H37Ra (Ra1) isolates. The differences in IS6110 fingerprint patterns between H37Rv and H37Ra have been previously examined by restriction analysis using four different endonucleases (9), and the molecular bases for the alternate IS6110 patterning have been investigated by comparative sequence analysis using the bacterial-artificial-chromosome method (4). Unfortunately, since the vast majority of studies involve H37 isolates, the above-mentioned investigations failed to properly identify the H37Rv and H37Ra used (4, 9).
The H37 IS6110 patterns shown in this study may be used as a reference for genotyping analysis; however, it is conceivable that cultures that have been maintained over the years in different laboratories or else different culture lots available from the ATCC might have evolved additional related IS6110 patterns.
The accuracy of spoligotyping and IS6110 fingerprint analysis in the identification of H37 variants was evaluated by comparison with other well-established molecular techniques. As with spoligotyping, PGRS and VNTR analyses were found to be nondiscriminating within the 18 ATCC H37 variants. Thus, the spoligotyping, PGRS, and VNTR methods can group the ATCC H37 collection as shown but cannot distinguish any variants. In addition, when H37 PGRS and VNTR patterns were compared to our database (PGRS, n > 600; VNTR, n > 400), a close similarity was noted with a large cluster of clinical isolates (unpublished data), rendering detection of the H37 variants by one of these two techniques unreliable. In agreement with VNTR analysis of an unspecified H37Rv and H37Ra in another work (7), we found that all 18 TMC-ATCC H37 variants (except for Rv5) share the same VNTR pattern for loci ETR-A to ETR-E, i.e., strain 33433. Polymorphisms encountered in amplicons, msx4, mpp8, and mtp40 could not be used to discriminate the H37 variants from other clinical isolates, in agreement with other studies (11, 28).
Identifying H37 cross-contamination among clinical isolates.
The ability to discriminate between the H37 variants and real clinical samples has important public health implications. In this study, the IS6110 fingerprint patterns of 131 clinical isolates, most of which were confirmed contaminants, matched that of one of the reference H37 strains. Random spoligotyping, PGRS, and VNTR analyses confirmed the relatedness of these 131 isolates to the reference ATCC H37 variants.
In addition, two H37Rv and three H37Ra IS6110 patterns (Rv10 and Rv11 and Ra2 to Ra4), which are distinct from but related to the ATCC collection, were identified among clinical specimens from our database of 11,000 fingerprints. These five isolates, also confirmed cases of laboratory contamination, were found to have the same spoligotype, PGRS, and VNTR as the TMC-ATCC isolates, and we infer that the isolates have evolved in the clinical laboratory from one of the reference strains.
However, investigators should be aware that, given the origins of H37, it is possible that “true” clinical isolates exist with an identical spoligotype and similar IS6110 pattern as the reference strain H37. Thus, while genotyping may be used to initiate or direct investigation, clinical decisions regarding contamination should be based on a combination of molecular and medical information.
Taken together, analysis of our clinical M. tuberculosis collection as well as ATCC isolates indicates that a combination of spoligotyping and IS6110 fingerprinting has proven to be a reliable tool in the proper identification of H37 cross-contamination. Unlike PGRS analysis, the H37 spoligopatterns (S00001) were unambiguous, making interpretation consistent. IS6110 fingerprinting should be used to confirm the proper identification of an H37 isolate.
ACKNOWLEDGMENTS
This research was supported in part by the Centers for Disease Control and Prevention, National Tuberculosis Genotyping and Surveillance Network cooperative agreement.
We are grateful to R. North for providing us with the H37 variants from the TMC. We thank W. Eisner and H. Marasco for assistance in preparing the manuscript.
Footnotes
Publication 72 from the Public Health Research Institute Tuberculosis Center.
REFERENCES
- 1.Bauer J, Thomsen V O, Poulsen S, Andersen A B. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J Clin Microbiol. 1997;35:988–991. doi: 10.1128/jcm.35.4.988-991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya M, Dietrich S, Mosher L, Siddiqui F, Reisberg B E, Paul W S, Warren J R. Cross-contamination of specimens with Mycobacterium tuberculosis: clinical significance, causes, and prevention. Am J Clin Pathol. 1998;109:324–330. doi: 10.1093/ajcp/109.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Braden C R, Templeton G L, Stead W W, Bates J H, Cave M D, Valway S E. Retrospective detection of laboratory cross-contamination of Mycobacterium tuberculosis cultures with use of DNA fingerprint analysis. Clin Infect Dis. 1997;24:35–40. doi: 10.1093/clinids/24.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Brosch R, Philipp W J, Stavropoulos E, Colston M J, Cole S T, Gordon S V. Genomic analysis reveals variation between Mycobacterium tuberculosis H37Rv and the attenuated M. tuberculosis H37Ra strain. Infect Immun. 1999;67:5768–5774. doi: 10.1128/iai.67.11.5768-5774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De C R M, Soini H, Roscanni G C, Jaques M, Villares M C, Musser J M. Extensive cross-contamination of specimens with Mycobacterium tuberculosis in a reference laboratory. J Clin Microbiol. 1999;37:916–919. doi: 10.1128/jcm.37.4.916-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Portillo P, Murillo L A, Patarroyo M E. Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol. 1991;29:2163–2168. doi: 10.1128/jcm.29.10.2163-2168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frothingham R, Meeker-O'Connell W A. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144(Pt. 5):1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- 8.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lari N, Rindi L, Lami C, Garzelli C. IS6110-based restriction fragment length polymorphism (RFLP) analysis of Mycobacterium tuberculosis H37Rv and H37Ra. Microb Pathog. 1999;26:281–286. doi: 10.1006/mpat.1998.0270. [DOI] [PubMed] [Google Scholar]
- 10.MacGregor R R, Clark L W, Bass F. The significance of isolating low numbers of Mycobacterium tuberculosis in culture of sputum specimens. Chest. 1975;68:518–523. doi: 10.1378/chest.68.4.518. [DOI] [PubMed] [Google Scholar]
- 11.Namwat W, Luangsuk P, Palittapongarnpim P. The genetic diversity of Mycobacterium tuberculosis strains in Thailand studied by amplification of DNA segments containing direct repetitive sequences. Int J Tuberc Lung Dis. 1998;2:153–159. [PubMed] [Google Scholar]
- 12.Nivin, B., J. Driscoll, T. Glaser, P. Bifani, and S. Munsiff. Use of spoligotype analysis to detect laboratory cross-contamination. Infect. Control Hosp. Epidemiol., in press. [DOI] [PubMed]
- 13.Nivin B, Fujiwara P I, Hannifin J, Kreiswirth B N. Cross-contamination with Mycobacterium tuberculosis: an epidemiological and laboratory investigation. Infect Control Hosp Epidemiol. 1998;19:500–503. doi: 10.1086/647856. [DOI] [PubMed] [Google Scholar]
- 14.Palittapongarnpim P, Luangsook P, Tansuphaswadikul S, Chuchottaworn C, Prachaktam R, Sathapatayavongs B. Restriction fragment length polymorphism study of Mycobacterium tuberculosis in Thailand using IS6110 as probe. Int J Tuberc Lung Dis. 1997;1:370–376. [PubMed] [Google Scholar]
- 15.Segal-Maurer S, Kreiswirth B N, Burns J M, Lavie S, Lim M, Urban C, Rahal J J., Jr Mycobacterium tuberculosis specimen contamination revisited: the role of laboratory environmental control in a pseudo-outbreak. Infect Control Hosp Epidemiol. 1998;19:101–105. doi: 10.1086/647774. [DOI] [PubMed] [Google Scholar]
- 16.Small P M, McClenny N B, Singh S P, Schoolnik G K, Tompkins L S, Mickelsen P A. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol. 1993;31:1677–1682. doi: 10.1128/jcm.31.7.1677-1682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenken W, Montalbine V. The antituberculous activity of thiomide in vitro and in the experimental animal (mouse and guinea pig) Am Rev Respir Dis. 1959;81:761–763. doi: 10.1164/arrd.1960.81.5.761. [DOI] [PubMed] [Google Scholar]
- 18.Steenken W, Oatway W H, Petroff S A. Biological studies of the tubercle bacillus. J Exp Med. 1934;60:515–543. doi: 10.1084/jem.60.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steenken W, Wolinsky E. Cycloserine: antituberculous activity in vitro and in the experimental animal. Am Rev Tuberc. 1956;73:539–546. doi: 10.1164/artpd.1956.73.4.539. [DOI] [PubMed] [Google Scholar]
- 20.Steenken W, Wolinsky E. Effect of antimicrobial agents on the tubercle bacillus and on experimental tuberculosis. Am J Med. 1950;9:633–653. doi: 10.1016/0002-9343(50)90213-0. [DOI] [PubMed] [Google Scholar]
- 21.Steenken W, Wolinsky E. Streptomycin in experimental tuberculosis. Am Rev Tuberc. 1948;58:353–362. doi: 10.1164/art.1948.58.3.353. [DOI] [PubMed] [Google Scholar]
- 22.Steenken W, Wolinsky E, Smith M M, Montalbine V. Further observations on pyrazinamide alone and in combination with other drugs in experimental tuberculosis. Am Rev Tuberc. 1957;76:643–659. doi: 10.1164/artpd.1957.76.4.643. [DOI] [PubMed] [Google Scholar]
- 23.Steenken W, Wolinsky E, Thurston J R. The antituberculous activity of kanamycin in vitro and in the experimental animal (guinea pig) Am Rev Tuberc. 1959;79:66–71. doi: 10.1164/artpd.1959.79.1.66. [DOI] [PubMed] [Google Scholar]
- 24.Steenken W J, Gardner L U. History of H37 strain of tubercle bacillus. Am Rev Tuberc. 1946;54:62–66. doi: 10.1164/art.1946.54.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Van Duin J M, Pijnenburg J E, van Rijswoud C M, de Haas P E, Hendriks W D, van Soolingen D. Investigation of cross contamination in a Mycobacterium tuberculosis laboratory using IS6110 DNA fingerprinting. Int J Tuberc Lung Dis. 1998;2:425–429. [PubMed] [Google Scholar]
- 26.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Soolingen D, de Haas P E, Hermans P W, Groenen P M, van Embden J D. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993;31:1987–1995. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weil A, Plikaytis B B, Butler W R, Woodley C L, Shinnick T M. The mtp40 gene is not present in all strains of Mycobacterium tuberculosis. J Clin Microbiol. 1996;34:2309–2311. doi: 10.1128/jcm.34.9.2309-2311.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolinsky E, Steenken W. Antituberculous activity of hinconstarch, a synthetic polymer of isoniazid, amithiozone, and starch. Am Rev Tuberc. 1956;73:7–8. doi: 10.1164/artpd.1956.73.1.72. [DOI] [PubMed] [Google Scholar]