Abstract
Our analysis of rotund (rn) null mutations in Drosophila melanogaster revealed that deletion of the rn locus affects both spermatid and retinal differentiation. In the male reproductive system, the absence of RnRacGAP induced small testes, empty seminal vesicles, short testicular cysts, reduced amounts of interspermatid membrane, the absence of individualization complexes, and incomplete mitochondrial condensation. Flagellar growth continued within the short rn null cysts to produce large bulbous terminations of intertwined mature flagella. Organization of the retina was also severely perturbed as evidenced by grossly misshapen ommatidia containing reduced numbers of photoreceptor and pigment cells. These morphological phenotypes were rescued by genomic rnRacGAP transgenes, demonstrating that RnRacGAP function is critical to spermatid and retinal differentiation. The testicular phenotypes were suppressed by heterozygous hypomorphic mutations in the Dras1 and drk genes, indicating cross talk between RacGAP-regulated signaling and that of the Ras pathway. The observed genetic interactions are consistent with a model in which Rac signaling is activated by Ras and negatively regulated by RnRacGAP during spermatid differentiation. RnRacGAP and Ras cross talk also operated during retinal differentiation; however, while the heterozygous hypomorphic drk mutation continued to act as a suppressor of the rn null mutation, the heterozygous hypomorphic Dras1 mutation induced novel retinal phenotypes.
Cellular responses to environmental cues are often manifested by changes in cell shape and cell surface properties which may themselves influence subsequent developmental and homeostatic decisions in the organism. Genetic and biochemical studies of the model RTK–Ras–mitogen-activated protein kinase signal transduction pathway have been particularly instructive in defining molecular mechanisms which govern certain of these responses, underlining the importance of interaction between signaling pathways and the resulting modulation of their kinetics (48). For example, membrane and cytoskeleton modifications induced after mitogenic stimulation are the products of the parallel and synergistic functions of the Ras and Rho signaling networks. Specifically, the Rho family proteins regulate the actin cytoskeleton through a GTPase cascade in which activation of Cdc42 activates Rac, which activates Rho, to induce membrane filopodia, lammellipodia, and stress fibers, respectively (36). Microinjection of fibroblasts with oncogenic Ras stimulated membrane ruffling which was inhibited by the expression of the dominant-negative form of Rac, thereby leading to a model in which Ras activates Rac to regulate membrane remodeling through changes in F-actin localization (45). The Ral subfamily of proteins has also been implicated in this cross talk through its modulation of the activity of the Cdc42-Rac-Rho pathway in response to Ras stimulation (29).
Ras and Rho family proteins, like all members of the Ras superfamily, cycle between active and inactive conformations, finely regulated by association with proteins modulating GTP or GDP nucleotide binding, respectively. The GTPase-activating proteins (GAPs) have been defined in biochemical and cell culture studies as negative regulators of these proteins (30), stimulating their intrinsic GTPase activity, thereby leading to GDP binding and downregulation (7). We previously isolated a GAP gene, rotundRacGAP (rnRacGAP), from Drosophila melanogaster (1). The protein product, RotundRacGAP (RnRacGAP), shows high sequence similarity in C-terminal regions (3) to the RacGAP protein Bcr (13), the N-chimaerins (24), and a remarkable overall similarity (60%) with MgcRacGAP, the product of the human gene male germ cell RacGAP (52). As expected for a protein regulating Rho family function, overexpression of rnRacGAP during development causes modification of the actin cytoskeleton (22); during embryogenesis, for example, ectopic expression of RnRacGAP in the cellularizing embryo caused polymerized actin, normally present in the subcortical layer, to redistribute throughout the apical cell cytoplasm. This change in the localization of F-actin was correlated with extensive modifications in cell shape and polarity, abnormally smooth cell membranes, and altered cell adhesivity.
rnRacGAP is found within the complex rotund (rn) locus. While no mutations exist which specifically affect rnRacGAP (2), several rn deletion mutations are available. The resulting rn null mutants are viable, show a short appendage phenotype and small, roughened eyes, and are completely male sterile (3). We determined the testes, and specifically the primary spermatocytes, to be the major site of rnRacGAP transcript expression (28). A number of RacGAPs from different species are expressed in the testes, suggesting that RacGAP proteins may have an evolutionarily conserved function during spermatogenesis (32, 52). While no biological function has yet been defined for these RacGAPs, genetic evidence has now accumulated for the function of diverse signaling networks during early Drosophila spermatogenesis, and it has been shown that signals from the germ line can modulate the regulation of proliferation in the somatic tissue and vice versa. For example, the diaphanous (dia) gene is expressed in the germ line and encodes a formin-related protein required for regulation of surrounding somatic cell proliferation and fate (20). In mouse, diaphanous-related formins bridge Rho-GTPase and Src tyrosine kinase during signaling and the regulation of actin dynamics (51). Conversely, the transforming growth factor β signaling pathway, a potential downstream target of Rac, appears to operate in the soma to regulate germ cell proliferation through the action of the genes punt and schnurri (35).
Much less is known about the signal cascades operating during spermatid formation, or spermiogenesis, when germ cells transform from typical round cells to the highly specialized spermatozoa. In this study, we used transgenic rescue experiments to demonstrate that RnRacGAP was necessary to recruit the membrane and direct its deposition during sperm elongation and individualization; in its absence, the interspermatid membrane was much reduced and testicular cysts were dramatically shortened. We also genetically assayed for cross talk between RacGAP-regulated signaling and the Ras pathway and showed that hypomorphic mutations in the Dras1 and drk genes suppressed the morphological defects in the testes of rn null mutants. This RacGAP-Ras cross talk also operated during eye differentiation, although the combination of the rn null mutation with the heterozygous hypomorphic Dras1 mutation induced novel retinal phenotypes.
MATERIALS AND METHODS
Fly strains.
Three null rn mutant strains were initially employed for both ultrastructural observations and fertility tests: rn20 pp/rn20 red e, rn17red e/rn20 red e, and rn22 red/rn20 red e (see reference 1 for a fuller description). These alleles contain large deficiencies covering the entire rn locus and neighboring genes (cytogenetic locus 84D3,4), and no differences were seen among them. We also examined Df(3R)dsxMas+R29, a deletion which covers both doublesex and rn loci, by the sperm release assay and found the seminal vesicles to be empty. Data in this paper are shown for the rn20 mutation, which was chosen as representative for extended analyses in transgenic rescue experiments. The original rn20 mutation is described in Agnel et al. (1): the proximal breakpoint lies between Antennapedia (27) and the glucose dehydrogenase gene (10); the distal breakpoint does not include the cluster of four male-sterile genes, Mst84Da, Mst84Db, Mst84Dc, and Mst84Dd, described previously (31). To control for possible effects of genetic background on the sterility of flies carrying the original rn20 red e chromosome, the mutant strain was outcrossed over seven generations to a P-insertion line, e(Pc)84DE, obtained from a w1118 isogenic strain carrying an insertion close to rn (16). This outcrossed strain was then tested in the fertility assay described below and found to remain 100% sterile.
The drk allele E(sev)EDA/CyO (19) was introduced into the rn null background by crossing the appropriate strains, and the alleles Ras1E1B and sose2H (46) were introduced into the rn20 pp third chromosome in separate conventional recombination experiments. The various mutant and transgene combinations were generated by crosses of the relevant strains. The rnRacGAP transgenes are genomic inserts situated on chromosomes 2 and 3 (transgenic lines 10 and 13, respectively) and contain a 5.0-kb HindIII-HindIII genomic fragment. This fragment includes the entire RnRacGAP sequence with its two associated introns (1.7 kb) together with approximately 1.5 kb each of upstream and downstream genomic sequence (28).
RNA preparation and RT-PCR amplification.
Fifty testes were dissected from the indicated strains in phosphate-buffered saline (PBS; 130 mM NaCl, 7 mM Na2HPO4 · 2H2O, 3 mM NaH2PO4 · 2H2O, pH 7.0). Total RNA was isolated by the RNA+ method (Quantum) and amplified by reverse transcription (RT)-PCR with the following primers: GAP1, 5′-CTTGCCGTGATCTTCGCTCC, and 1.7.1as, 5′-GGTGAGTACTGCTAAGGTTGAC (28). The reaction products were separated by agarose gel electrophoresis, transferred to a nylon membrane, and probed with a 32P-labeled fragment of rnRacGAP cDNA.
Fertility tests.
Male flies (5 to 15) were placed in tubes with an excess of virgin females of the genotype w1118; the white mutation does not affect fertility and allowed detection of any instances of prior insemination by white brothers from the same stock.
Electron microscopic observations.
Testes or heads (cut in half) of wild-type, mutant, and transgenic flies were dissected in PBS and immediately fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, for 2 h. After subsequent washing, they were postfixed in 1% OsO4 for 1 h and then dehydrated and embedded in Epon. Thin sections (2 μm) stained with toluidine blue were examined by phase-contrast and fluorescence optical microscopy; ultrathin sections (90 nm) stained with heavy metal were examined with a JEOL 1200ExII electron microscope.
Cyst dissection and fluorescence microscopy.
Testes were dissected on slides in Ringer's solution or PBS, the testicular envelope was removed with fine tweezers, and the slides were agitated to disperse the cysts. Samples were air dried and rinsed in 70% ethanol, and some were mounted directly in glycerol-Ringer's solution with 4′,6′-diamidino-2-phenylindole (DAPI); others were rehydrated for staining with rhodamine-coupled phalloidin. Samples were observed with a Zeiss Axioplan microscope equipped with epifluorescence illumination.
RESULTS
RnRacGAP dosage is critical to testicular cyst elongation and actin localization in individualization complexes (ICs).
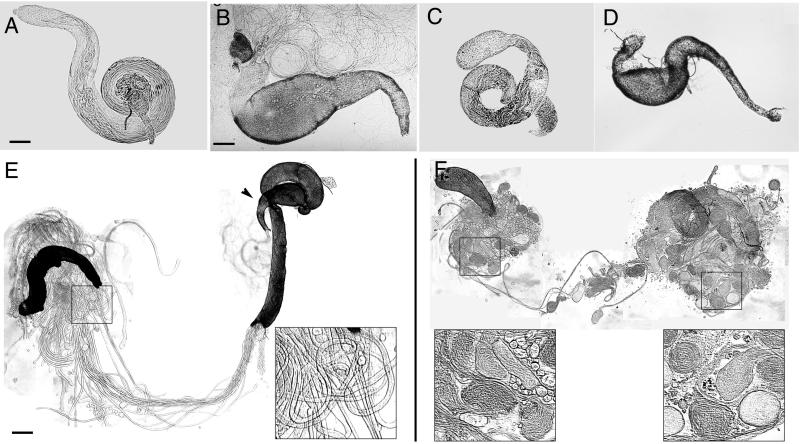
Dissection of the reproductive systems from rn null mutant males shows that both the testes and the seminal vesicle, the organ depot for stockage of mature sperm, are smaller than those of the wild type, and optical microscopy reveals specific internal differences. In wild-type testes, phase-contrast microscopy revealed striations in the helicoidal regions reflecting strict alignment of spermatid flagella (Fig. 1A); in rn null mutants (rn20/rn20), spermatid tails appeared clumped along much of their length (Fig. 1C). Detachment of wild-type vesicles from the testes released a cloud of motile sperm (Fig. 1B), while rn null mutant vesicles (Fig. 1D) were completely empty. Gentle teasing apart of the testes revealed only thin long cysts in the wild type (Fig. 1E), but large round tissue masses were present in the rn null mutants (Fig. 1F). Removal of the enveloping testicular membrane allowed visualization of the individual testicular cysts, each composed of a syncytial network of 64 developing spermatids surrounded by two somatic cells, the cyst cells. UV fluorescence microscopy of isolated cysts stained with DAPI showed no visible changes in the cyst head region, but more distally the lack of RnRacGAP signaling induced two linked phenotypes: extremely short cysts and aberrant terminations. Normally, wild-type cysts are uniform along their 1.8-mm length (Fig. 2A), up to and including the distal extremity (Fig. 2D). In contrast, null mutant cysts were extremely short with less than 10 to 15% exceeding even a third of the normal length (Fig. 2B), and these aberrant cysts terminated in tails several times the normal diameter. Considerable internal structure was present within these abnormal terminations, and phase-contrast microscopy revealed a “ball-of-yarn” configuration suggesting masses of intertwining spermatid flagella (Fig. 2E) which was confirmed by transmission electron microscopy of ultrathin sections (Fig. 2F) from regions within the terminal bulge (such as that situated within Fig. 2E, bracket F). Sections cut just proximal to the bulge (such as that situated within Fig. 2E, bracket G) revealed an area of transition in which variable numbers of flagellar structures were visible (e.g., 197 are visible in Fig. 2G). In this particular view, the membrane of the cyst cell is traceable over practically the entire field, confirming the integrity of the somatic cell envelope and the continuity of the cell contents, thereby ruling out a mechanism by which the somatic cyst cell and the germ line simply coil up on themselves to form the characteristic ball-of-yarn structure. Thus, the absence of RnRacGAP causes premature arrest of cyst elongation and the uncoupling of flagellar growth from membrane extension.
FIG. 1.
rn null mutant testicular and seminal vesicle phenotypes. The apical side is to the left, and the basal side is to the right. (A, B, E) Wild type. (C, D, F) Null mutant rn20 red e/rn20 pp. (A, C) Testes; the seminal vesicle protrudes from the basal end of the testes in panel A. (B, D) Seminal vesicles. Spermatozoa are visible as fine coils exterior to the vesicle in panel B. (E, F) Testes have been teased apart near the apical end at a point corresponding to about one-third of the length of the testes. Germaria are to the left. Boxes mark areas of the testes that have been enlarged (3×) as insets to show cyst morphology. The arrowhead in panel E points to a seminal vesicle which protrudes from the basal end of the testis, and spermatozoa are visible escaping from its free end. Bar, 80 μm (A); 25 μm (B); 100 μm (E). This and all following figures have been processed with Adobe Photoshop 5.0 software.
FIG. 2.
Rescue by genomic rnRacGAP transgene of testicular cyst length and termination defect phenotypes. (A, D) Wild type. (B, E, F, G) Null mutant rn20 red e/rn20 pp. (C) Transgenic fly P(rnRacGAP)13 rn20/rn20 pp. (A, B, C) Fluorescence microscopy of isolated DAPI-stained testicular cysts. Bar, 180 μm. Inset, gel analysis of RT-PCR products from isolated testis RNA, assayed with rnRacGAP sequence-specific primers. Lanes: 1, wild type; 2, null mutant rn20 red e/rn20 pp; 3, transgenic flies P(rnRacGAP)10/+; rn20/rn20 pp; 4, transgenic flies P(rnRacGAP)13 rn20/rn20 pp; 5, genomic DNA assayed with the same primers. For lanes 1, 3, and 4 a single band appears with the predicted length of 274 bp; for lane 5 the band at 394 bp reflects the presence of an intron of 120 bp. (D, E) Phase-contrast microscopy of cyst terminations. Bar, 7.5 μm. In panel E, rn null mutant cysts show the characteristic ball-of-yarn termination phenotype. Bracket F, terminal bulge; bracket G, area proximal to the terminal bulge. (F, G) Transmission electron microscopy of rn null mutant cysts. In panel F mature flagella are visible in various orientations within the terminations. Bar, 2.2 μm. In panel G arrows mark the membrane of the cyst cell, which is visible in its integrity over practically the entire field of view, confirming the continuity of the somatic cell contents and envelope. Bar, 1.2 μm.
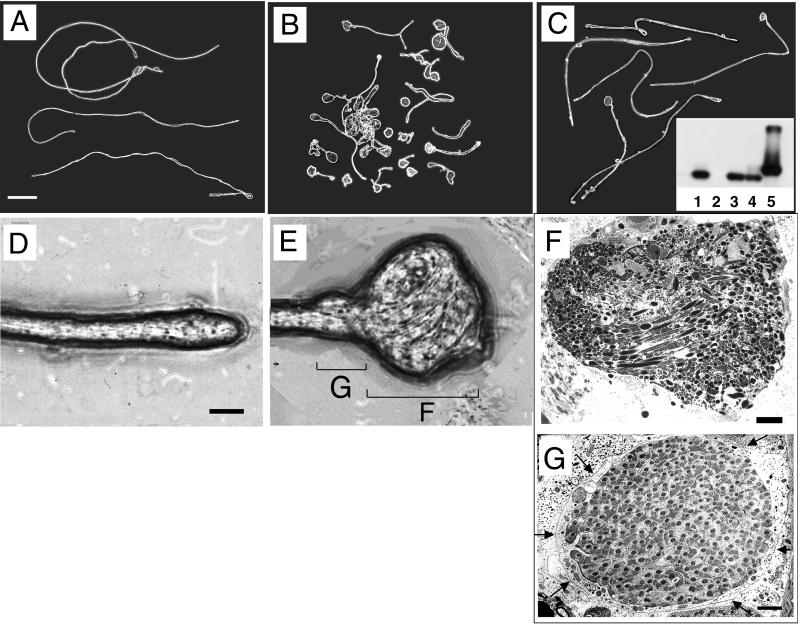
We tested whether lack of RnRacGAP was specifically at the origin of these morphogenetic defects by transgenic rescue experiments. We constructed a P element transposon carrying a 5.0-kb genomic fragment including the rnRacGAP coding sequence. When placed in an rn null mutant background in two independently isolated transgenic strains, P(rnRacGAP)10/+; rn20/rn20 pp and P(rnRacGAP)13 rn20/rn20 pp, this genomic transgene directed rnRacGAP expression in the testes as assayed by RT-PCR amplification (Fig. 2C, inset).
Observation of the isolated cysts from these two transgenic strains established that rnRacGAP expression conferred a significant increase in the overall length of the cysts; while the extremities were rarely as uniformly slender as in the wild type, the ball-of-yarn phenotype was significantly reduced (Fig. 2C). Where wild-type length was unambiguously attained (approximately 5% of the cysts), flagellar termination was invariably normal. Thus, rnRacGAP expression in both independent transgenic strains substantially rescued rn-induced abnormal cyst morphology, confirming that RnRacGAP function is critical to sperm differentiation. These rescued sperm were indeed transferred to the seminal vesicles of the transgenic flies, as dissection of their vesicles released motile sperm (data not shown); however, while motility had been partially restored, fertility assays revealed only marginal rescue of fertility in the transgenic males, reaching only 5 to 10% in tube assays (for description, see Materials and Methods).
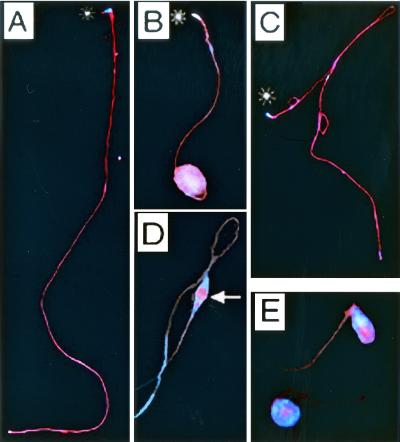
Since we had previously demonstrated that overexpression of RnRacGAP affected the distribution of polymerized actin (F-actin) during embryonic cellularization (22), we examined the effects of the absence of RnRacGAP on the localization of F-actin within the cysts. Staining with rhodamine-coupled phalloidin, we found F-actin to be uniformly distributed in wild-type cysts (Fig. 3A), with the exception of an expected intense staining at the level of the ICs (Fig. 3D). In normal development, these actin-rich structures traverse the cyst from head to tail, isolating each spermatid from the syncytium by breaking the interspermatid cytoplasmic bridges and sealing membrane gaps (50). In rn null cysts, we found no localized accumulations of actin, either in the form of ICs or in any abnormal configuration. Even in the most severely affected tail regions, overall F-actin staining remained homogeneously distributed (Fig. 3B). The absence of signal in the rn null mutants was not due to physical masking of the F-actin in the ball-of-yarn configuration, as we detected single, localized accumulations of actin resembling ICs in morphologically indistinguishable cysts which persisted in the testes of the transgenic flies, P(rnRacGAP)10/+; rn20/rn20 pp and P(rnRacGAP)13 rn20/rn20 pp (Fig. 3E). In contrast, in those cysts in which the rnRacGAP transgene had completely restored both length and morphology, F-actin staining was indistinguishable from that of the wild type (Fig. 3C).
FIG. 3.
Localized F-actin accumulation in testicular cysts depends upon RnRacGAP. Superposition of DAPI (blue) and rhodamine-coupled phalloidin (red) staining visualized by UV fluorescence microscopy. Asterisks mark the positions of the heads. (A, D) Wild type. The arrow marks the position of an actin-rich IC. (B) Null mutant rn20 red pp/rn20 red. (C, E) Transgenic strain P(rnRacGAP) 13 rn20/rn20 pp. For wild-type and transgenically rescued cysts, overlapping images taken along the 1.8-mm cyst length were superimposed and fused to create the full-length composites shown here.
RnRacGAP is necessary for spermatid membrane deposition.
We analyzed the morphological defects induced by the absence of RnRacGAP by transmission electron microscopy (for reviews of spermatogenesis, see references 17 and 50). In semithin sections of wild-type testes, germ cells and spermatocytes filled the apical third of the testes and stained only lightly with toluidine blue during early stages (Fig. 4A), whereas mature cysts stained progressively darker, appearing in cross section as dense hexagonal structures or longitudinally as long fine parallel fibers. Seminal vesicles were filled with darkly staining mature spermatozoa. The testes of rn null mutants were distinctive (Fig. 4B), appearing generally less full and containing large, vacuolated oval bodies, often densely staining. Fibrous flagella were clearly visible within these giant cysts, indicating that significant spermatid maturation had taken place, but the seminal vesicles were empty and were identifiable only by their relative position (data not shown). One copy of the rnRacGAP transgene in the rn null background substantially rescued these phenotypes and restored normal internal cyst morphology (Fig. 4C). Seminal vesicles were fuller and released motile sperm (data not shown). Thus, both independent rnRacGAP genomic inserts rescued abnormal cyst morphology and induced the production of motile sperm, confirming that RnRacGAP function is critical to sperm differentiation.
FIG. 4.
Rescue by genomic rnRacGAP transgene of membrane and mitochondrial defects in rn null mutants. (A, D, G) Wild type. (B, E, H) Null mutant rn20 red pp/rn20 red. (C, F, I) Transgenic fly P(rnRacGAP)13 rn20/rn20 pp. (A, B, C) Optical microscopy of semithin sections of dissected reproductive organs stained with toluidine blue. White arrows, early-stage spermatocytes; black arrows, mature cysts in longitudinal section, with long, darkly staining flagella; black arrowheads, hexagonally shaped cysts in transverse section; white arrowhead, section of seminal vesicle; black-and-white arrowheads, abnormal vacuolated cysts. Bar, 10 μm. (D to I) Transmission electron microscopy of ultrathin sections of testes. (D to F) In comparison to wild-type cysts, rn null cysts are disorganized and developing spermatids are randomly oriented. The rnRacGAP transgene restores spermatid orientation, resulting in a more orderly distribution. Bar, 1.2 μm. (G to I) Magnified views of mature sperm from panels D to F, respectively. Bar, 230 nm. The white arrow in panel H points to a fully retracted minor mitochondrial derivative.
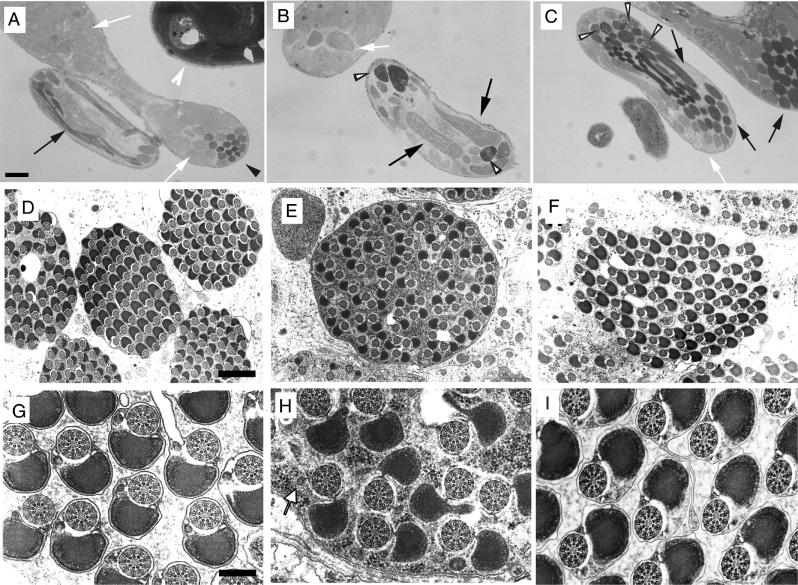
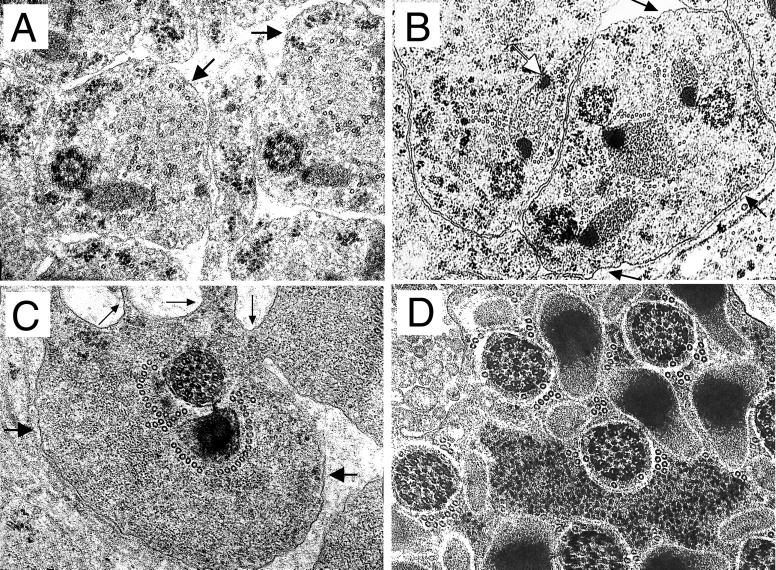
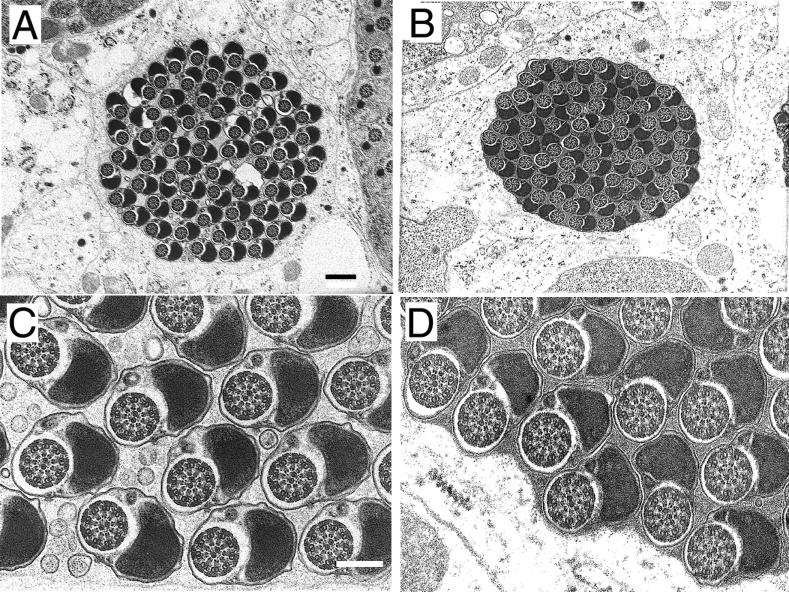
Comparison of the ultrastructure of the testes from wild-type, null mutant, and rescued males defined the underlying cellular basis of the observed phenotypes as a severe reduction in the amount of interspermatid membranes. In the wild type, spermatids are tightly packed and highly oriented, producing a paracrystalline array when cysts are viewed in transverse section (Fig. 4D). At higher magnification, each flagellum is seen to be composed of an axoneme-mitochondrion couple completely surrounded by an integral membrane (Fig. 4G). These membranes are largely absent in mature null mutant cysts (Fig. 4E and H). The spermatids are randomly oriented, being separated by an excess of granular cytoplasmic material. A similar cytoplasmic phenotype has been described for a semilethal allele of the clathrin heavy chain gene which is defective in spermatid individualization (15), although membrane deposition was not reported to be abnormal. Mitochondrial condensation in the rn null mutants was also affected, as portions of the major mitochondrial derivative were incompletely retracted (Fig. 4H). Interestingly, the extent of condensation of the minor mitochondrial derivative as well as axonemal decoration, morphological characteristics usually associated with the passage of the IC, was appreciable.
In rescue experiments, the rnRacGAP transgene restores near-normal spermatid orientation, although cysts remain somewhat larger than in the wild type and fail to attain paracrystalline packing (Fig. 4F). The extent of rescue is even more complete at the level of the individual spermatid (Fig. 4I): an integral membrane surrounds each axonemal-mitochondrial unit, the mitochondria regain their compact regular shape, and interspermatid cytoplasmic material is reduced. Membrane deposition is occasionally imperfect, however, allowing vacuolar blebs or supplementary membranous material to accumulate between some spermatids.
We examined whether membrane deposition defects were apparent at earlier developmental stages. An example of a preindividualization wild-type spermatid is shown in Fig. 5A: the elaboration of the microtubules of the outer singlet ring of the axoneme is still incomplete and the condensing mitochondrial derivatives, which have taken on an oval shape, show only a very localized intense staining adjacent to the axoneme. Each axonemal-mitochondrial unit is similarly oriented, resides singly within the cytoplasmic ground substance, and is surrounded by an integral membrane. At a comparable developmental stage in the rn null mutant as shown in Fig. 5B, three randomly oriented spermatids are present within a shared cytoplasm defined by a single enveloping membrane. Mitochondrial condensation is defective, the size and orientation of the derivatives vary even within the same cytoplasm, and in one mitochondrion regions of condensation exist at both poles, whereas compaction is normally restricted to the region closest to the axoneme. An example of a mature wild-type spermatid is shown in Fig. 5C: the mitochondrion, surrounded by a highly organized sheath of microtubules, has appreciably condensed, and the cytoplasmic ground substance has become more dense; however, a number of interspermatid bridges are still present extending from the interspermatid membrane. Several differences are apparent in the rn null mutant judged to be at a similar developmental stage by the extent of axonemal decoration and the intensity of mitochondrial condensation (Fig. 5D): the mitochondria are abnormally shaped, microtubules are scattered randomly in the cytoplasm, even occasionally invading the peripheral territory close to the axoneme usually devoid of these structures, and finally, high numbers of spermatids are present within a single cytoplasm without intervening membranes. These observations suggest that the absence of interspermatid membranes in mature rn null cysts results at least in part from problems in sperm membrane deposition and/or stability during preindividualization stages rather than simply from a late-stage degeneration of disorganized membranes already in place.
FIG. 5.
Defective membrane deposition during spermatid differentiation in rn null mutants. (A, C) Early and late-intermediate stages of wild-type spermatid differentiation. (B, D) Early stages of rn null mutant spermatid differentiation. (A) Arrows point to interspermatid membranes. Bar, 245 nm. (B) Black arrows point to a single membrane surrounding three developing spermatids; the white arrow points to a mitochondrion in which condensation has initiated at both poles. (C) Thin arrows point to interspermatid cytoplasmic bridges; thick arrows point to the single interspermatid membrane. Bar, 240 nm. (D) Multiple mature spermatids without intervening membrane. Developmental staging was based upon the extent of axonemal decoration and the staining intensity of condensed portions of the mitochondria.
RacGAP-Ras cross talk in the rescue of rn-induced testicular phenotypes.
Ras has been described to activate Rac signaling in cells in culture (41, 45, 49), and recently in Drosophila cross talk between DRacGAP, a Drosophila RnRacGAP homologue, and Ras has been shown to function during wing morphogenesis (47). By analogy, the absence of RnRacGAP as a putative negative regulator of Rac might be expected to induce hyperactivity of its substrate Rac that might be restored to more normal levels in rn null flies by a concomitant decrease in Ras activity. In order to test this hypothesis, we assayed for suppression of the rn-induced testicular phenotypes by a hypomorphic mutation of the Dras1 gene (Ras1E1B) within the rn null mutant background. While homozygous mutation of the Dras1 gene is lethal, heterozygous Ras1E1B/+ flies are viable and fully fertile. When introduced into the rn null mutant background, the hypomorphic heterozygous Ras1E1B mutation induced a significant restoration of cyst organization (Fig. 6A) and membrane deposition (Fig. 6C) in the rn null mutants. However, the integrity of the restored membranes may have been less than that provided by the rnRacGAP transgene; though completely surrounding each spermatid, membranes showed an inflated, irregular outline. We were unable to assay the fertility of the Ras1E1B rn20/rn20 flies due to their reduced eclosion and short life span (2 to 3 days).
FIG. 6.
Suppression of rn null cyst and sperm membrane phenotypes by heterozygous hypomorphic mutations in genes coding for Ras and Drk. (A, C) Double mutant Ras1E1B rn20/rn20 red. (B, D) Double mutant E(sev)EDA/CyO; rn20red/rn20 red. (A, B) Testicular cysts. (C, D) Magnified views of mature sperm from panels A and B, respectively. (A) rn null cysts suppressed by a heterozygous hypomorphic mutation in Dras1 are more compact, although spermatids are not perfectly oriented and the surrounding membrane is somewhat irregular. Bar, 520 nm. (C) rn null cysts suppressed by a heterozygous hypomorphic mutation in drk present the same aspect as cysts in panel B, but the interspermatid material is more condensed and the spermatid membrane is more regular. Bar, 260 nm.
As the Drk (Downstream receptor kinase) adapter (38) and Sos (Son of sevenless) RasGEF activator (8) proteins are necessary to transmit the signal from an activated receptor tyrosine kinase through to Ras, similar mutations in the Dras1, drk, and sos genes may be expected to act comparably. Like mutations in Dras1, homozygous mutations of either of these genes are lethal. We found that a heterozygous hypomorphic mutation in the sos gene rendered the stock poorly viable and difficult to maintain. Unfortunately, flies carrying both this mutation and the deletion of the rn locus (sose2H; rn20/rn20) did not eclose. Flies carrying a heterozygous hypomorphic mutation in the drk gene were of normal viability and fertility, and this mutation in combination with the deletion of the rn locus (E(sev)EDA/CyO; rn20/rn20) survived to about the same extent as the Ras1E1 rn20/rn20 flies; thus, morphological analyses were feasible. As predicted, a reduction in the amount of the Drk protein in the rn null mutant background restored cyst organization (Fig. 6B) and spermatid membrane deposition (Fig. 6D) to the same extent that the hypomorphic heterozygous mutation of Dras1 did. Thus, downregulation of the activity of the Ras signaling pathway by reducing the level of either Ras or its upstream activator Drk suppressed the morphological effects of RnRacGAP absence during spermatid differentiation.
RacGAP-Ras cross talk in the rescue of rn-induced retinal phenotypes.
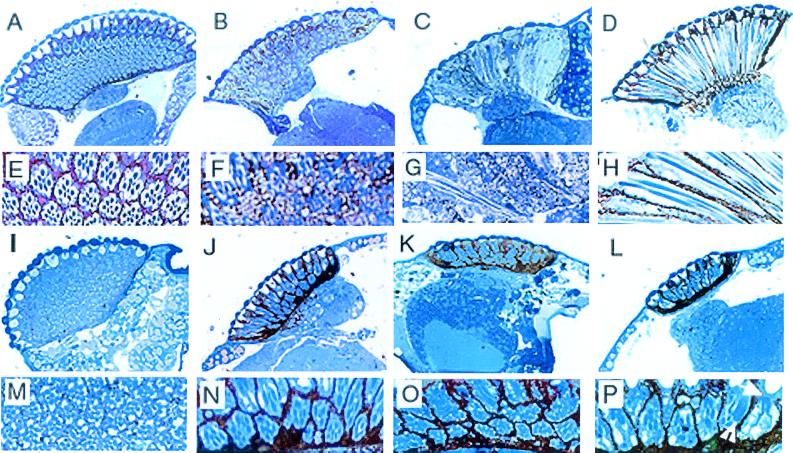
The precise regularity of the wild-type fly eye is due to the precise packing of about 800 ommatidia into a hexagonal array. Internally, each wild-type ommatidium is composed of eight photoreceptor cells, four cone cells, and pigment cells, eight of which are unshared and three of which are shared with neighboring ommatidia (for review, see reference 33). Each photoreceptor cell projects a dense, actin- and membrane-enriched, light-gathering structure, the rhabdomere, into the central core (Fig. 7A). The rhabdomeres appear in transverse section as round organelles, the size and disposition of which are characteristic of each photoreceptor cell (Fig. 7E). This internal organization of the retina is severely disrupted by the rn null mutation (Fig. 7B and C). A reduced number of ill-defined ommatidia are randomly dispersed in a nondescript, vacuolated substance with some dispersed membrane. The photoreceptor cells are fewer in number, rhabdomeres are malformed, and some irregularly disposed pigment cells are present (Fig. 7F). During development, the thickness of the retina is determined by postmeiotic elongation of the retinal cells. In the rn null mutant, retinal depth was variably reduced with ommatidia that spanned the entire distance (Fig. 7C and G).
FIG. 7.
Modification of rn null internal eye phenotypes by heterozygous hypomorphic mutations in genes coding for Ras and Drk. Optical microscopy of semithin sections of eyes stained with toluidine blue. (A, E) Wild type. (B, C, F, G) Null mutant rn20 red e/rn20 pp. (B, C) Sagittal and longitudinal sections through the rn null mutant eye. Note that even though ommatidia are malformed, they tend to span the entire retinal distance. (D, H) Transgenic fly P(rnRacGAP)10/+; rn20/rn20 pp. (I, M) Double mutant E(sev)EDA/CyO; rn20 red/rn20 red. (J, N) Double mutant Ras1E1B rn20/rn20 red. (K, O) Triple mutant E(sev)EDA/CyO; Ras1E1B rn20/rn20 red. (L, P) Transgenic double mutant P(rnRacGAP)10/+; Ras1E1B rn20/rn20 red. Arrow, folded rhabdomere; arrowhead, fused rhabdomere.
The absence of RnRacGAP is specifically responsible for the observed disorder, as shown by the restoration of the internal architecture by the rnRacGAP transgene (Fig. 7D). The retinas of transgenic flies were indistinguishable from those of the wild type in that the ommatidia were well organized and contained the normal number of full-length photoreceptor cells surrounded by pigment cells. The only difference was that the pigment cell layer seemed sparser than that in the wild type (Fig. 7H).
Heterozygous hypomorphic mutations of either the Dras1 or drk gene in a wild-type background have no effect on the eye (data not shown), but when placed in the rn null background, both radically modifed the rn null phenotype, though in different ways. As it had in spermatogenesis, a hypomorphic mutation in the drk gene suppressed the defects caused by the absence of RnRacGAP (Fig. 7I and M) to fully the same extent as had the rnRacGAP transgene (Fig. 7D and H). The drk-suppressed rn null retinas showed a variable reduction in the number of pigment cells, occasionally reaching levels even lower than those observed in the transgenically rescued flies. In contrast, hypomorphic mutation of the Dras1 gene in the rn null background caused a significant increase in the number of pigment cells and closer packing than had been present in the rn null mutant (Fig. 7J and N). The ommatidia remained strangely shaped and carried variable numbers of outer and inner photoreceptor cells (Fig. 7N). Secondly, there was a drastic reduction in the depth of the retina, as though this usually mild and variable aspect of the rn null phenotype had been much enhanced. Similar reduction in retinal deepening has also been observed in the eye-directed overexpression of Cdc42 (37) or Rho1 (6, 26). Furthermore, the interaction between Dras1 and rn mutations was epistatic to all other combinations tested. For example, the Ras-associated phenotype dominated when the Dras1 mutation was combined with those of the drk and rn genes in the triple mutant, E(sev)EDA/CyO; Ras1E1B rn20/rn20 (Fig. 7K and O); similarly, the Dras1 mutation prevented rescue by the rnRacGAP transgene of the rn20/rn20 phenotypes (Fig. 7L and P) in flies of the genotype P(rnRacGAP)10/+; Ras1E1B rn20/rn20. In fact, rhabdomere structure may have been adversely affected: a significant number of rhabdomeres became fused or folded (Fig. 7P) in these flies.
DISCUSSION
The complex rn locus in Drosophila contains two major transcripts, one of which encodes a RacGAP protein expressed principally in the testes (3). We have shown that deletion of the rn locus and neighboring genes induced complete male sterility associated with pleiotropic effects in the male reproductive system. The testes were small and the seminal vesicles empty of sperm. Internally, the testicular cysts, developmental units formed by the association of a syncytial germ line and two enveloping somatic cells, attained only a fraction of their normal 1.8-mm length. In mature spermatids, these global effects were correlated at the cellular level with drastic reduction in interspermatid and cyst membranes, the absence of ICs, and incomplete mitochondrial condensation. All of these morphological phenotypes were substantially rescued by expression of RnRacGAP from genomic rnRacGAP transgenes. Thus, we define RnRacGAP function as critical to spermatid bundle elongation and individualization.
Developmental analyses of rn null mutant phenotypes established that the observed scarcity of membranes in mature mutant cysts was not simply due to degeneration of preexisting membranes, as we observed multiple spermatids developing within a single plasma membrane even in preindividualized cysts. As such, this phenotype is in accord with the putative role of RnRacGAP as a regulator of the Rac and/or Cdc42 proteins (44). The function of these GTPases in remodeling the membrane through specific rearrangements of subcortical actin network has been well documented in cells of diverse origin in culture (for review, see reference 23) and in several in vivo systems: for example, in Drosophila altered forms of Drac1 disrupt the actin cytoskeleton in apical regions of the wing imaginal disk (14) and in the epidermis (25). In cellularizing embryos ectopic expression of RnRacGAP itself displaced F-actin from its normal subcortical position to a uniform apical distribution, inducing abnormally smooth membrane surfaces and altered cell packing at the embryo surface (22).
In both cellularization and spermatogenesis, massive amounts of membrane are needed to isolate the developing cells from their respective syncytia, thereby necessitating precise actin mobilization. In embryonic cellularization, membrane deposition is coordinated with the formation of numerous microvillous projections stabilized by central actin cores (18, 54). In spermatogenesis, membranes also invaginate, progressing tailward from the head region to envelop each of the elongating spermatids, but their deposition must also integrate localized membrane fusion events to generate the intercellular bridges which maintain the syncytium. While the mechanics of membrane fusion are not yet well understood, the formation of actin-filled microvillous projections on apposing membrane surfaces appears as a common strategy (55), with the resulting configuration apparently inducing local stress on the membranes to favor their union (12, 43). Recently, directed actin polymerization was proposed to be the driving force for the development of membrane junctions in epithelial cell-cell adhesion (53). Thus, regulation of localized F-actin accumulation and that of membrane synthesis are likely to be tightly coupled; as RnRacGAP absence affects the level of membrane synthesis during development, it may provide a critical switch in such a regulatory process.
Conversely, at the end of spermatid differentiation, the spermatids must be individualized. Existing cytoplasmic bridges are broken and the membranes are sealed by the passage of actin-rich ICs (50). Preindividualized stages can be identified according to four criteria (15): rudimentary axonemal decoration, light-staining mitochondrial derivatives, enlarged minor mitochondrial derivatives, and the presence of external cytoplasmic ground substance. The rn null cysts clearly present only the last of these characteristics; otherwise, they display highly decorated axonemes; fairly densely staining, though incompletely condensed, mitochondria; and compact minor mitochondrial derivatives. These characteristics are more consistent with the abortive passage of ICs than with their total blockage. Thus, RnRacGAP absence may cause the defective localization of F-actin to ICs, leading to their incomplete function and, presumably, also reduced stability, as we detected no localized actin in rn null mutant cysts. In contrast, morphologically indistinguishable cysts from partially rescued transgenic flies showed actin deposits in their bulbous ends. Thus, in these transgenic tails, RnRacGAP levels were not sufficient to restore the normal phenotype, but they apparently permitted the formation of IC-like structures which may have become trapped as they entered the defective extremities. As such, this function is consistent with dosage-dependent regulation by RnRacGAP of F-actin localization during actin-directed morphogenetic movements (22).
The rn null testicular phenotypes also provided a means to analyze the interdependence of different spermatogenic processes. For example, in the rn mutants flagellar growth became uncoupled from cyst length determination, continuing despite the severely limited membranous compartment and creating massive cyst ends containing intertwined flagella. These flagella apparently reached variable lengths, as we found no strict correlation between cyst length and the diameter of the tail ends, suggesting that the signal for flagellar growth arrest was also deregulated in the rn null mutants. In contrast, coordination of the elongation of the somatic cyst cell and the germ line was complete: however short the germ line remained, extension of its companion cyst cell was equally limited. Developmental analysis further revealed disorganization of microtubules around condensing mitochondria in intermediate stages of rn null mutant spermiogenesis, and this was correlated with incomplete mitochondrial condensation. If actin is indeed the principal target of RnRacGAP-regulated signaling, then actin and microtubule networks may cooperate to guide and/or stabilize mitochondrial fusion and condensation during spermiogenesis.
The total sterility of rn null males was reflected in the observed absence of sperm from the seminal vesicles. We used this phenotype as the basis of a simple and rapid assay to test for partners of RnRacGAP function during sperm differentiation. As Ras activation of Rac signaling had been proposed from previous studies (41, 45, 49) and since cross talk between DRacGAP, a Drosophila RnRacGAP homologue, and Dras has recently been shown to regulate wing morphogenesis (47), we tested for genetic interaction between the rn null mutation and heterozygous hypomorphic mutations in the genes Dras1 and drk. We found that each mutation suppressed the rn null phenotype, causing the characteristic empty vesicles to fill with detectable amounts of motile sperm. Ultrastructural analysis of the testes of these double mutants confirmed an equivalent return to normal cellular morphology. These results defined cross talk between RnRacGAP-regulated signaling and the Ras pathway in the rn null mutants and provided the first demonstration of Ras function in the testes. These data are consistent with a model in which Rac signaling is activated by Ras and negatively regulated by RnRacGAP during spermatid differentiation. Recently, a homologue of RnRacGAP, DRacGAP, has been shown to negatively regulate Drac and Dcdc42 in the wing while itself being negatively regulated by the epidermal growth factor receptor-Ras pathway (47).
As the Ras signaling pathway had been particularly well characterized in the eye (11), we examined the effects of RnRacGAP absence on both external and internal eye differentiation. We found that in addition to causing small, roughened eyes, deletion of the rn locus severely perturbed retinal organization. The external phenotype of rn null mutants was not rescued by the presence of the rnRacGAP transgene, suggesting that this phenotype may be induced by the deletion of another gene uncovered by the rn locus. Dosage sensitivity of rnRacGAP rescue cannot be ruled out, but it would not seem to be due to complete failure of expression of the transgene in the eye tissue as the rnRacGAP transgenes restored a high degree of internal order in these same eyes. This phenotypic rescue defined RnRacGAP function as critical to retinal differentiation.
In a separate study (K. Raymond, E. Bergeret, R. Griffin-Shea, and M.-O. Fauvarque, unpublished data) we obtained results consistent with a model in which RnRacGAP specifically functions in the eye through modulation of Rac activity. Using the UAS-GAL4 system (9), we found that GMRGal4-driven ectopic expression in the eye of wild-type RnRacGAP or its GAP domain induced phenotypes similar to those induced by the dominant-negative mutant form of Rac, DracN17. In addition, phenotypes resulting from the overexpression of Drac were strongly enhanced by a reduction in rnRacGAP dosage. A similar approach was not possible in the testes, as the GAL4-UAS system available at the time of these experiments was not adapted for directed expression in the testes. We also could not employ ectopic expression driven by a heat shock promoter as spermatogenesis is quite sensitive to temperature (4) and we found the wild-type controls to be adversely affected in several protocols involving either constantly elevated temperatures or heat shock pulses.
With respect to Ras signaling in the retina, the observed genetic interactions were partially consistent with a model in which Ras activates Rac. As in spermatogenesis, a heterozygous hypomorphic drk mutation suppressed the effects of putative Rac hyperactivity induced by RnRacGAP absence. However, a heterozygous mutation of the Dras1 gene caused a qualitatively different phenotype, a drastic reduction of retinal deepening and an increase in pigment cell differentiation. These phenotypes remained unchanged when the heterozygous hypomorphic drk mutation was added to the same background. This divergence may simply reflect the fundamentally different natures of the two morphogenetic systems. In Drosophila spermatogenesis, it has been shown that almost all transcription is shut off upon entry into the meiotic divisions (21, 39). Thus, our analysis of postmeiotic spermatid differentiation may have allowed us to dissect the purely cytoskeletal aspects of RacGAP function and cross talk with the Ras pathway from transcriptional considerations. If so, the testes model suggests that at the level of the cytoskeleton, an equivalent signal is produced by a reduction in the quantity of either the upstream activator Drk or that of Ras itself, each presumably reducing Ras activity sufficiently to counteract the absence of RnRacGAP. In contrast, in retinal differentiation, the two proteins also play a critical role in signaling to the nucleus where certain effectors may be sensitive to the actual quantity of Ras, with the resulting imbalances generating novel phenotypes; such effectors being situated downstream of Drk would explain the observed epistatic effect of the Ras mutation over that of drk in the rn null mutants. Furthermore, the reintroduction of the rnRacGAP transgene into Ras1E1B rn20/rn20 flies did not restore the normal phenotype, suggesting that Dras1 may have rnRacGAP-independent interactions, e.g., with another gene uncovered by the rn locus, which might themselves be sensitive to Ras dosage.
However, not all the effects may be exclusively due to transcriptional regulation, as evidence of functional, and even structural, links between cytoskeletal components other than actin and the Ras-Rac pathways is accumulating. These interactions may activate downstream effectors in the morphogenetic processes, leading to retinal deepening, pigment cell differentiation, or rhabdomere formation sensitive to changes in the Ras/RacGAP ratio. For example, retinal deepening depends upon sealing of adherens junctions (AJs) between the photoreceptor membrane and the basal feet of the nonneuronal cone cells that form the retinal floor (33, 56). In epithelial cells, AJ formation and stability depend both upon initial actin localization to punctate structures (53) and complicated Ras-Rac cross talk: in several cell lines, constitutively activated Ras and Rac cooperated to decrease AJ stability (42), whereas in MDCK cells they acted antagonistically, with oncogenic Ras inhibiting enhanced AJ formation induced by activated Rac (40). Furthermore, during retinal deepening, the actin-rich microvilli that constitute the rhabdomeres must increase their surface area substantially (33). While defects in rhabdomere shape and organization in rn null mutants were indeed rescued by the rnRacGAP transgene, a reduction in the dose of Ras in this same genetic background produced significant numbers of folded and fused rhabdomeres. A similar folded phenotype was shown to be due to local collapse along the apical-distal axis in mutants of the Drosophila bifocal gene, which encodes a protein that colocalizes with actin (5). Partial loss-of-function mutations in the canoe locus or overexpression of wild-type Canoe protein induced twisted, branched, and often fused rhabdomeres. Canoe protein, which accumulates in the AJs of rhabdomeres and pigment cells, binds to both F-actin and Ras (34).
Finally, despite the fundamental differences in the sperm and eye morphogenetic systems, we have demonstrated that a hypomorphic heterozygous mutation of drk consistently acted to suppress the rn-induced morphological phenotypes. This suggests that across tissues, certain primary cytoskeletal responses may be coordinated by cross talk between specific upstream components of the Ras signaling pathway and putative downstream targets regulated by RnRacGAP. The simple sperm release assay that we have developed should be of value in testing this hypothesis and identifying other genes that may act at this level.
ACKNOWLEDGMENTS
We thank Paul Andreassen for technical assistance with the confocal microscope and helpful advice, Philippe Huber for the use of the fluorescence microscope, Jim Fabrizio for informative and enthusiastic discussion, Marie-Claire Joseph for media preparation and stock maintenance, and Marie-Joseph Rabiet for critical reading of the manuscript. We also thank Pierre Vignais and Michel Sartre for the installation and continued support of our group.
This work was supported by grants from the Ministère de la Recherche et de l'Enseignement (ACCSV-4) and from L'Association pour la Recherche Contre le Cancer (no. 9392), as well as a fellowship to A.G. from the Commissariat à l'Énergie Atomique (C.F.R).
REFERENCES
- 1.Agnel M, Kerridge S, Vola C, Griffin-Shea R. Two transcripts from the rotund region of Drosophila show similar positional specificities in imaginal disc tissues. Genes Dev. 1989;3:85–95. doi: 10.1101/gad.3.1.85. [DOI] [PubMed] [Google Scholar]
- 2.Agnel M, Roder L, Griffin-Shea R, Vola C. The spatial expression of Drosophila rotund gene reveals that the imaginal discs are organized in domains along the proximal-distal axis. Roux's Arch Dev Biol. 1992;201:284–295. doi: 10.1007/BF00592109. [DOI] [PubMed] [Google Scholar]
- 3.Agnel M, Roder L, Vola C, Griffin-Shea R. A Drosophila rotund transcript expressed during spermatogenesis and imaginal disc morphogenesis encodes a protein which is similar to human Rac GTPase-activating (racGAP) proteins. Mol Cell Biol. 1992;12:5111–5122. doi: 10.1128/mcb.12.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburner M. Drosophila: a laboratory handbook. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. 198. [Google Scholar]
- 5.Bahri S M, Yang X, Chia W. The Drosophila bifocal gene encodes a novel protein which colocalizes with actin and is necessary for photoreceptor morphogenesis. Mol Cell Biol. 1997;17:5521–5529. doi: 10.1128/mcb.17.9.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 7.Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 8.Bonfini L, Karlovich C A, Dasgupta C, Banerjee U. The son of sevenless gene product: a putative activator of Ras. Science. 1992;255:603–606. doi: 10.1126/science.1736363. [DOI] [PubMed] [Google Scholar]
- 9.Brand A H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 10.Cavener D R, Otteson D C, Kaufman T C. A rehabilitation of the genetic map of the 84B-D region in Drosophila melanogaster. Genetics. 1986;114:111–123. doi: 10.1093/genetics/114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H C, Karim F D, O'Neill E M, Rebay I, Solomon N M, Therrien M, Wassarman D A, Wolff T, Rubin G M. Ras signal transduction pathway in Drosophila eye development. Cold Spring Harbor Symp Quant Biol. 1994;59:147–153. doi: 10.1101/sqb.1994.059.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Dai J, Sheetz M P. Regulation of endocytosis, exocytosis, and shape by membrane tension. Cold Spring Harbor Symp Quant Biol. 1995;60:567–571. doi: 10.1101/sqb.1995.060.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Diekmann D, Brill S, Garrett M D, Totty N, Hsuan J, Monfries C, Hall C, Lim L, Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991;351:400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- 14.Eaton S, Auvinen P, Luo L, Jan Y N, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabrizio J J, Hime G, Lemmon S K, Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development. 1998;125:1833–1843. doi: 10.1242/dev.125.10.1833. [DOI] [PubMed] [Google Scholar]
- 16.Fauvarque M O, Dura J M. Polyhomeotic regulatory sequences induce developmental regulator-dependent variegation and targeted P-element insertions in Drosophila. Genes Dev. 1993;7:1508–1520. doi: 10.1101/gad.7.8.1508. [DOI] [PubMed] [Google Scholar]
- 17.Fuller M. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin Cell Dev Biol. 1998;9:433–444. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- 18.Fullilove S, Jacobson A. Nuclear elongation and cytokinesis in Drosophila montana. Dev Biol. 1971;26:560–577. doi: 10.1016/0012-1606(71)90141-2. [DOI] [PubMed] [Google Scholar]
- 19.Gaul U, Chang H, Choi T, Karim F, Rubin G M. Identification of ras targets using a genetic approach. Ciba Found Symp. 1993;176:85–92. doi: 10.1002/9780470514450.ch6. [DOI] [PubMed] [Google Scholar]
- 20.Gonczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- 21.Gould-Somero M, Hardy R, Holland L. The Y chromosome and sperm length in D. melanogaster. Exp Cell Res. 1974;87:397–398. doi: 10.1016/0014-4827(74)90500-x. [DOI] [PubMed] [Google Scholar]
- 22.Guichard A, Bergeret E, Griffin-Shea R. Overexpression of RnRacGAP in Drosophila melanogaster deregulates cytoskeletal organization in cellularising embryos and induces discrete imaginal phenotypes. Mech Dev. 1997;61:49–62. doi: 10.1016/s0925-4773(96)00619-3. [DOI] [PubMed] [Google Scholar]
- 23.Hall A. G proteins and small GTPases: distant relatives keep in touch. Science. 1998;280:2074–2075. doi: 10.1126/science.280.5372.2074. [DOI] [PubMed] [Google Scholar]
- 24.Hall C, Monfries C, Smith P, Lim H H, Kozma R, Ahmed S, Vanniasingham V, Leung T, Lim L. Novel human brain cDNA encoding a 34,000 Mr protein n-chimaerin, related to both the regulatory domain of protein kinase C and BCR, the product of the breakpoint cluster region gene. J Mol Biol. 1990;211:11–16. doi: 10.1016/0022-2836(90)90006-8. [DOI] [PubMed] [Google Scholar]
- 25.Harden N, Loh H Y, Chia W, Lim L. A dominant inhibitory version of the small GTP-binding protein Rac disrupts cytoskeletal structures and inhibits developmental cell shape changes in Drosophila. Development. 1995;121:903–914. doi: 10.1242/dev.121.3.903. [DOI] [PubMed] [Google Scholar]
- 26.Hariharan I K, Hu K Q, Asha H, Quintanilla A, Ezzell R M, Settleman J. Characterization of rho GTPase family homologues in Drosophila melanogaster: overexpressing Rho1 in retinal cells causes a late developmental defect. EMBO J. 1995;14:292–302. doi: 10.1002/j.1460-2075.1995.tb07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazelrigg T, Watkins W S, Marcey D, Tu C, Karow M, Lin X R. The exuperantia gene is required for Drosophila spermatogenesis as well as anteroposterior polarity of the developing oocyte, and encodes overlapping sex-specific transcripts. Genetics. 1990;126:607–617. doi: 10.1093/genetics/126.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoemann C D, Bergeret E, Guichard A, Griffin-Shea R. Alternative splicing of the Drosophila melanogaster rotundRacGAP gene. Gene. 1996;168:135–141. doi: 10.1016/0378-1119(95)00747-4. [DOI] [PubMed] [Google Scholar]
- 29.Jullien Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis J H. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. J Biol Chem. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 30.Kozma R, Ahmed S, Best A, Lim L. The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia. Mol Cell Biol. 1996;16:5069–5080. doi: 10.1128/mcb.16.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn R, Kuhn C, Borsch D, Glatzer K H, Schafer U, Schafer M. A cluster of four genes selectively expressed in the male germ line of Drosophila melanogaster. Mech Dev. 1991;35:143–151. doi: 10.1016/0925-4773(91)90064-d. [DOI] [PubMed] [Google Scholar]
- 32.Leung T, How B E, Manser E, Lim L. Germ cell beta-chimaerin, a new GTPase-activating protein for p21rac, is specifically expressed during the acrosomal assembly stage in rat testis. J Biol Chem. 1993;268:3813–3816. [PubMed] [Google Scholar]
- 33.Longley R L, Jr, Ready D F. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo T, Takahashi K, Kondo S, Kaibuchi K, Yamamoto D. Regulation of cone cell formation by Canoe and Ras in the developing Drosophila eye. Development. 1997;124:2671–2680. doi: 10.1242/dev.124.14.2671. [DOI] [PubMed] [Google Scholar]
- 35.Matunis E, Tran J, Gonczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- 36.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 37.Nolan K M, Barrett K, Lu Y, Hu K Q, Vincent S, Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivier J P, Raabe T, Henkemeyer M, Dickson B, Mbamalu G, Margolis B, Schlessinger J, Hafen E, Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993;73:179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- 39.Olivieri G, Olivieri A. Autoradiographic study of nucleic acid synthesis during spermatogenesis in Drosophila melanogaster. Mutat Res. 1965;2:366–380. doi: 10.1016/0027-5107(65)90072-2. [DOI] [PubMed] [Google Scholar]
- 40.Potempa S, Ridley A J. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu R G, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinlan M P. Rac regulates the stability of the adherens junction and its components, thus affecting epithelial cell differentiation and transformation. Oncogene. 1999;18:6434–6442. doi: 10.1038/sj.onc.1203026. [DOI] [PubMed] [Google Scholar]
- 43.Raucher D, Sheetz M P. Membrane expansion increases endocytosis rate during mitosis. J Cell Biol. 1999;144:497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridley A J. Signal transduction through the GTP-binding proteins Rac and Rho. J Cell Sci Suppl. 1994;18:127–131. doi: 10.1242/jcs.1994.supplement_18.19. [DOI] [PubMed] [Google Scholar]
- 45.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 46.Simon M A, Bowtell D D, Dodson G S, Laverty T R, Rubin G M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 47.Sotillos S, Campuzano S. DRacGAP, a novel Drosophila gene, inhibits EGFR/Ras signalling in the developing imaginal wing disc. Development. 2000;127:5427–5438. doi: 10.1242/dev.127.24.5427. [DOI] [PubMed] [Google Scholar]
- 48.Tan P B O, Kim S K. Signaling specificity of the RTK/MAP kinase pathway in metazoans. Trends Genet. 1999;15:145–149. doi: 10.1016/s0168-9525(99)01694-7. [DOI] [PubMed] [Google Scholar]
- 49.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs J B, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokayasu K, Peacock W, Hardy R. Dynamics of spermiogenesis in Drosophila melanogaster. 1. Individualization process. Z Zellforsch. 1972;124:479–506. doi: 10.1007/BF00335253. [DOI] [PubMed] [Google Scholar]
- 51.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneige S A, Alberts A S. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–26. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 52.Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, Reibel L, Gacon G. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 53.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 54.Warn R M, Robert-Nicoud M. F-actin organization during the cellularization of the Drosophila embryo as revealed with a confocal laser scanning microscope. J Cell Sci. 1990;96:35–42. doi: 10.1242/jcs.96.1.35. [DOI] [PubMed] [Google Scholar]
- 55.Wilson N F, Snell W J. Microvilli and cell-cell fusion during fertilization. Trends Cell Biol. 1998;8:93–96. doi: 10.1016/s0962-8924(98)01234-3. [DOI] [PubMed] [Google Scholar]
- 56.Zusman S, Grinblat Y, Yee G, Kafatos F C, Hynes R O. Analyses of PS integrin functions during Drosophila development. Development. 1993;118:737–750. doi: 10.1242/dev.118.3.737. [DOI] [PubMed] [Google Scholar]