Abstract
Antimicrobial-lock therapy is an economically viable strategy to prevent/reduce the catheter-related bloodstream infections (CRBSI) that are associated with central venous catheters (CVCs). Herein, we report the synthesis and characterization of the S-nitroso-N-acetyl-l-cysteine ethyl ester (SNACET), a nitric oxide (NO)-releasing molecule, and for the first time its application as a catheter lock solution to combat issues of bacterial infection associated with indwelling catheters. Nitric oxide is an endogenous gasotransmitter that exhibits a wide range of biological properties, including broad-spectrum antimicrobial activity. The storage stability of the SNACET and the NO release behavior of the prepared lock solution were analyzed. SNACET lock solutions with varying concentrations exhibited tuneable NO release at physiological levels for >18 h, as measured using chemiluminescence. The SNACET lock solutions were examined for their efficacy in reducing microbial adhesion after 18 h of exposure to Staphylococcus aureus (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria). SNACET lock solutions with 50 and 75 mM concentrations were found to reduce >99% (ca. 3-log) of the adhered S. aureus and E. coli adhesion to the catheter surface after 18 h. The SNACET lock solutions were evaluated in a more challenging in vitro model to evaluate the efficacy against an established microbial infection on catheter surfaces using the same bacteria strains. A >90% reduction in viable bacteria on the catheter surfaces was observed after instilling the 75 mM SNACET lock solution within the lumen of the infected catheter for only 2 h. These findings propound that SNACET lock solution is a promising biocidal agent and demonstrate the initiation of a new platform technology for NO-releasing lock solution therapy for the inhibition and treatment of catheter-related infections.
Keywords: catheter-related bloodstream infections (CRBSI), antimicrobial-lock therapy (ALT), S-nitroso-N-acetylcysteine (SNACET), nitric oxide (NO), antibacterial interfaces
Graphical Abstract
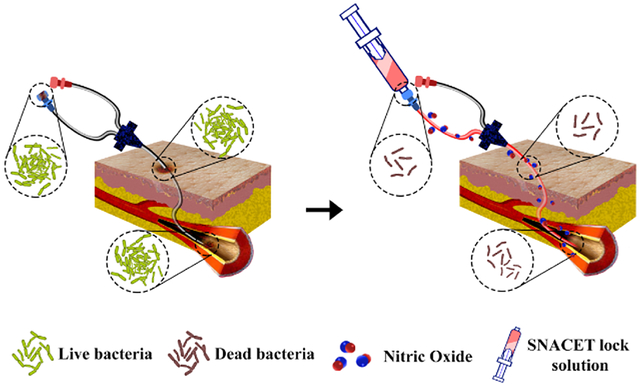
1. INTRODUCTION
Central venous catheters (CVCs) have become indispensable in managing critical-care patients. However, intravascular catheter-related bloodstream infections (CRBSIs) are one most serious and perpetual complications associated with CVCs.1,2 The CRBSIs can originate from different sites such as an infected catheter hub, transferred bacteria from skin microbiota, or hematogenous seeding from other infected sites.3–5 Once the biofilm matures on the catheter surface, the planktonic bacteria can be deployed from the biofilm matrix causing bloodstream infection (BSI). In many cases, the “gold standard” is to remove the catheter and treat the patient with high doses of systematic antibiotics.6,7 CRBSIs that originate from the catheter can spread to the bloodstream or other organs, leading to morbidity and mortality in both hospitalized as well as nonhospitalized patients. According to reports from the Centers for Disease Control and Prevention (CDC), approximately 30 000 to more than 200 000 patients suffering from CRBSIs each year in the United States are forced to extended hospitalization time and extensive healthcare costs totalling billions of dollars for the whole healthcare system.1 The cost of these bacterial infections in the healthcare system can expand from $2 billion to more than $40 billion annually.4,8,9
Over the past few years, various approaches have been attempted to prevent and treat the CRBSIs, including the antimicrobial-lock therapy (ALT).5,10–12 The antimicrobial-lock therapy is an economically viable strategy to prevent/reduce rates of CRBSIs.13 In this approach, a highly concentrated solution of antimicrobial agents (typically 100–1000 times higher than the planktonic MIC) is instilled within an intravascular catheter for up to 24–48 h while it is not in use to inhibit colonization or to eradicate bacteria on the inner lumen of the infected catheter. Various antibiotics, such as vancomycin,14,15 daptomycin,16 gentamicin,17 minocycline,18 trimethoprim,9,19 teicoplanin,20 and nonantibiotic solutions, such as citrate,21 ethanol,22,23 tetrasodium ethylenediaminetetraacetic acid (EDTA),8 taurolidine,21,24 nitroglycerin,25 sodium bicarbonate,26 have been reported as antimicrobial-lock solutions either alone or in combination for the prevention of CRBSIs.27,28 However, persistent use of these antibiotic lock solutions may lead to the emergence of antibiotic resistance and thus limits the long-term prevention of CRBSIs. Further, antibiotic-based lock solutions only have the potential to eradicate microbes present on the inner luminal surfaces, are limited in their ability to penetrate established biofilms on surfaces, and cannot diffuse through the catheter wall to eradicate microbes that may contaminate the outer luminal surfaces (e.g., microbial contamination from skin).3,29 Therefore, the development of a new class of nonantibiotic antimicrobial-lock solutions is a prerequisite to replace the antibiotic-based lock solutions in the near future.
Nitric oxide (NO), an endogenous gaseous signaling molecule, has gained great scientific attention in recent years due to involvement in various biological processes, such as vasodilation, immune modulation, prevention of platelet activation and adhesion, and wound healing.30–35 The bactericidal activity of NO against both Gram-positive and Gram-negative bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), has also gained great attention in the last two decades.36 Nitric oxide is a gasotransmitter that has two predominant mechanisms for killing bacteria.37–40 First, NO can induce oxidative stress to the bacteria cells through lethal peroxynitrite (OONO−) generation upon reaction with superoxides (O2−), which leads to cell membrane disruption and DNA damage. Second, NO is a highly reactive free radical, which can react with the available O2 to form N2O3 and eventually react with sulfhydryl groups on cysteine residues of membrane proteins, leading to membrane disruption and cell death.41,42 Many research groups have given considerable effort to the development of various pharmacologically active NO donors, such as nitrates, N-diazeniumdiolates (NON-Oates), and S-nitrosothiols (RSNOs), and evaluated them for their prolonged and controlled NO delivery, antibacterial, and antithrombotic efficacy.42–51 NO-releasing polymeric biomaterials have been developed by incorporating such NO donor molecules by either physically incorporating into a suitable polymer matrix or tethered covalently to the polymer chain and fabricated into biomedical devices to bestow prolonged and controlled NO release behavior.46 Nitric oxide can diffuse through the microbial biofilm matrix as well as through a wide range of biomedical polymers (e.g., silicone), and also its short half-life inhibits the potential development of resistance.52,53 These NO-releasing biomaterials have shown promising antimicrobial activity but do possess a limited NO payload, which has limited their long-term applications.
The NO-releasing donor molecules can be a promising alternative to the conventional antibacterial lock solutions as they can easily be freshly instilled and provide long-term NO availability at the catheter interfaces. However, to date, NO donating molecules have not been explored as lock solutions to combat bacterial growth on CVCs. One potential NO donor candidate for the lock solution therapy is a NO-releasing cysteine-derived small molecule, S-nitroso-N-acetyl-l-cysteine ethyl ester (SNACET), which has been previously studied for its pharmacologic properties as an oral therapeutic.54 SNACET is a charge-free, highly lipophilic, and water-soluble NO donor molecule. Metal ions (e.g., Cu+), light, and heat can catalyze NO release from SNACET and formation of the corresponding disulfide (RSSR) product (Figure 1a). The water solubility and NO release properties of SNACET make it a suitable material for the development of a nonantibiotic lock solutions. Herein, for the first time, SNACET was synthesized via nitrosation reaction and was isolated in a solid state from the reaction mixture. The molecular structure of SNACET was also confirmed by various spectroscopic methods. SNACET was used to formulate a lock solution using phosphate-buffered saline (PBS) (10 mM, pH 7.4, with 100 μM EDTA) to combat issues of bacterial infection associated with CVCs. The storage stability of the SNACET at −20 and −80 °C was analyzed and the NO release behavior of the SNACET lock solution with varying concentrations filled in the silicone catheter tubing was quantified. Finally, the SNACET lock solutions were examined for their efficacy in reducing microbial adhesion after 18 h of exposure to S. aureus (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria). Further, the antibacterial activity of the SNACET lock solution was also evaluated in a more challenging model of established S. aureus and E. coli infection on the catheter surfaces (Figure 1b).
Figure 1.
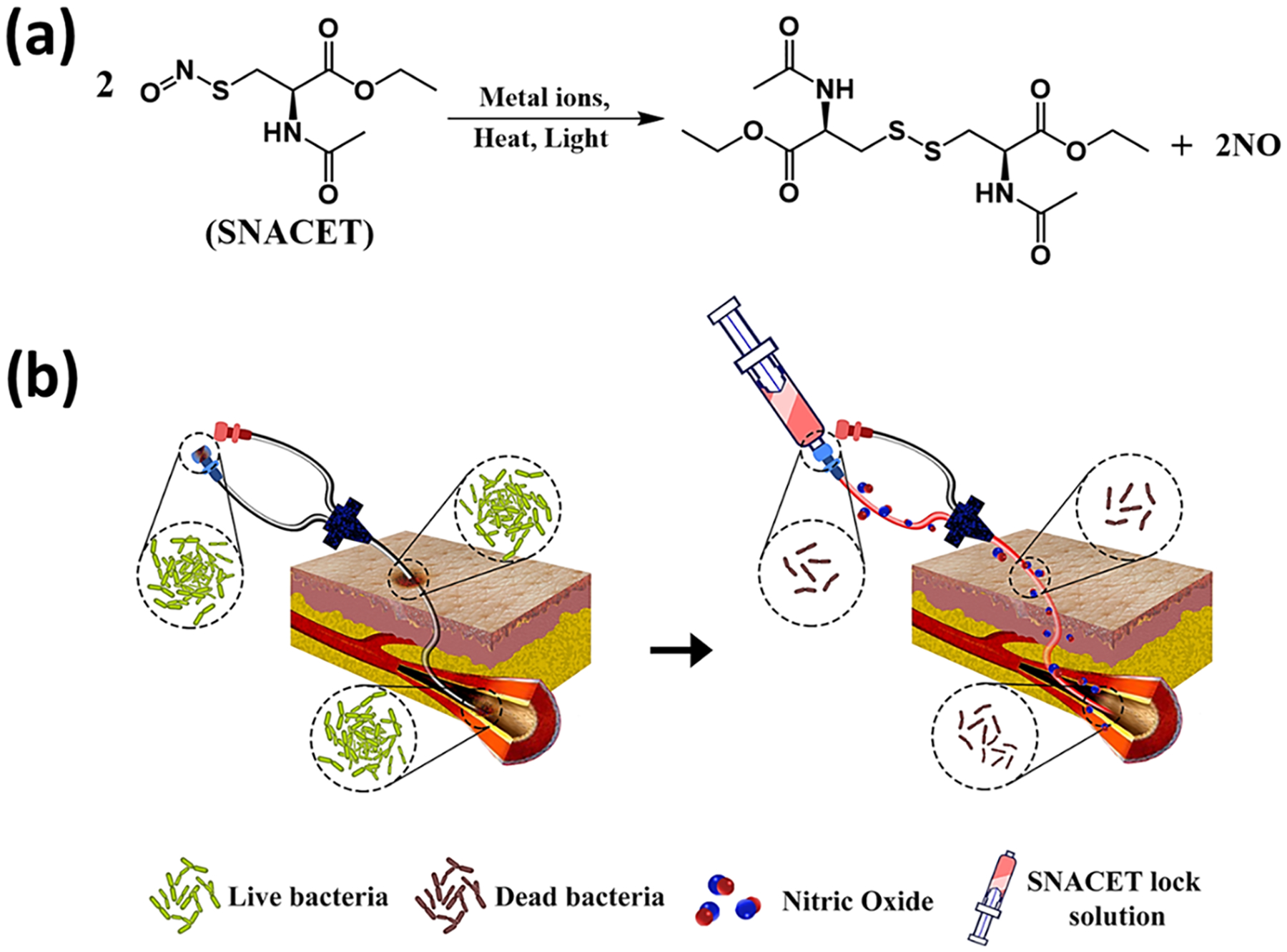
(a) Structure of S-nitroso-N-acetylcysteine ethyl ester (SNACET) and its decomposition in the presence of metal ions, heat, or light, which yields the disulfide (RSSR) product and nitric oxide (NO). (b) Schematic representation of SNACET lock solution eradicating bacterial infection associated with CVCs.
2. EXPERIMENTAL SECTION
2.1. Materials and Chemicals.
N-Acetyl-l-cysteine (NAC), sodium nitrite (NaNO2), anhydrous ethanol (EtOH), copper(II) chloride (CuCl2), ethylenediaminetetraacetic acid (EDTA), phosphate-buffered saline (PBS) (10 mM, pH 7.4) containing 100 μM EDTA, Luria–Bertani (LB) broth microbial growth medium, and LB broth with agar were purchased from Sigma-Aldrich (St. Louis, MO). Hydrochloric acid (HCl) and Fisherbrand Disposable Cuvettes (1.5 mL capacity and 1 cm path length) were obtained from Fisher Scientific (Hampton, NH). The HelixMark silicone catheter tubing (60-011-05) with 1.02 mm I.D., 2.16 mm O.D, and a wall thickness of 0.58 mm was purchased from VWR International. DOWSIL 3-1944 RTV Coating was purchased from DOW Corporation. Female Luer Lock to Barb Connector (11733) was purchased from Qosina Corp. All aqueous solutions were prepared using Milli-Q water for experimental usage. The bacterial strains S. aureus ATCC 6538 and E. coli ATCC 25922 were purchased from American Type Culture Collection (ATCC). All of the liquid buffers and media, as well as solid apparatus, were sterilized prior to biological studies using autoclave sterilization cycles with saturated steam at 121 °C under 15 psi pressure for 30 min.
2.2. Synthesis of N-Acetylcysteine Ethyl Ester (NACET).
The parent thiol, NACET, was synthesized by following the reported literature.55 Briefly, 10 g (61.3 mmol) of NAC was first dissolved in 500 mL of absolute ethanol and subsequently slowly added the anhydrous hydrochloric acid (generated by 24.2 mL of acetylchloride + 30 mL of absolute ethanol) with stirring at room temperature. The reaction mixture was left for stirring for 4 h at 25 °C and then the solvent was evaporated to give a crude residue, which was purified by silica gel chromatography (40–60 nm) by using CHC13 and MeOH (95:5 v/v) as the eluent to give analytically pure NACET as a white solid (7 g).
1H NMR and 13C NMR were obtained on a Varian Mercury 300 MHz NMR spectrometer using CDCl3 and DMSO-d6 as the solvent and TMS as an internal standard. Chemical shifts were reported in parts per million (ppm). The following abbreviations were used to describe the peak multiplicities: s, singlet; d, doublet; dd, double doublet; t, triplet; and m, multiplet. High-resolution mass spectra (HRMS) were recorded on the Bruker Impact II mass spectrometer. 1H NMR (300 MHz, CDCl3, 25 °C, TMS): δ = 6.42 (s, 1H), 4.88–4.81 (m, 1H), 4.30–4.18 (m, 2H), 3.00 (dd, J = 9.0, 4.1 Hz, 2H), 2.05 (s, 3H), 1.29 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3, 25 °C, TMS): δ = 170.09, 169.80, 62.01, 53.52, 26.88, 23.14, 14.18; HRMS (ESI) m/z [M + Na]+ calculated for C7H13NNaO3S: 214.0514, found: 214.0509; Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra were collected between 700 and 4000 cm−1 on a PerkinElmer Spectrum 3 FTIR spectrometer. ATR-IR, cm−1: 3315, 2988, 2946, 2541, 1734, 1626, 1541, 1370, 1346, 1225, 1198, 1055, 983, 867, 704, 664.
2.3. Synthesis of S-Nitroso-N-acetylcysteine Ethyl Ester (SNACET).
The NO-releasing SNACET was synthesized by dissolving 0.5 g (2.61 mmol) of NACET in 10 mL of DI water followed by the addition of 1.0 mL of 2 M HCl. The reaction mixture was cooled in ice bath for 30 min prior to the addition of 0.2 g of (2.89 mmol) NaNO2. The reaction mixture was stirred for 10 min to obtain SNACET as a precipitate, which was filtered, washed with ice-cold water, and dried in a vacuum desiccator overnight to obtain the SNACET as a pink solid (0.3 g) with purity > 95%, as tested using the Zysense chemiluminescence Nitric Oxide Analyzer (NOA) 280i using a previously reported method.56 The SNACET product was characterized by NMR and FTIR, as described above. 1H NMR (300 MHz, DMSO-d6, 25 °C, TMS): δ = 8.44 (d, J = 7.6 Hz, 1H), 4.49 (dd, J = 13.0, 7.2 Hz, 1H), 4.10 (q, J = 7.1 Hz, 2H), 3.99 (d, 2H), 1.81 (s, 3H), 1.19 (t, J = 7.1 Hz, 3H).; 13C NMR (75 MHz, DMSO-d6, 25 °C, TMS): δ = 170.26, 169.88, 61.56, 51.62, 34.32, 22.56, 14.39.; HRMS (ESI) m/z [M + Na]+ calculated for C7H12N2NaO4S: 243.0415, found: 243.0416; ATR-IR, cm−1: 3311.13, 2985.28, 2938.32, 1730.27, 1620.70, 1528.21, 1370.27, 1313.25, 1216.59, 1171.06, 1034.46, 860.86, 705.77, 663.08.
2.4. Absorption Spectroscopic Analysis.
UV–vis spectra were recorded in the wavelength range of 250–650 nm using an Agilent Cary 60 UV–vis Spectrometer at room temperature. A 1 mM SNACET solution in PBS (10 mM, pH 7.4) containing 100 μM EDTA was freshly prepared and absorbance values were measured at 334 and 545 nm using a cuvette with the 1 cm path length. The decomposition of SNACET at physiological conditions was determined by recording the UV–vis spectra of 1 mM of SNACET solution in PBS (10 mM, pH 7.4) containing 100 μM EDTA at various time points over 24 h.
2.5. SNACET Storage Stability and Half-life Analysis.
The synthesized SNACET powder was stored in sealed glass vials at −20 and −80 °C in dark within a desiccated container. The storage stability (%) of the SNACET in a solid state was determined by measuring the amount of SNACET remaining in the samples after 1, 2, 4, and 8 weeks of storage. A 1 mM SNACET solution in PBS (10 mM, pH 7.4) containing 100 μM EDTA was freshly prepared at each time point and the UV–vis spectra were recorded using a UV–vis spectrophotometer.
2.6. Preparation of SNACET Lock Solutions for NO Release Characterization.
Lock solutions of four different concentrations (25, 50, 75, and 100 mM) were prepared by dissolving 5.5, 11, 16.5, or 22 mg of SNACET in 1 mL of 10 mM PBS, pH 7.4, with 100 μM EDTA. The Biomedical grade HelixMark silicone catheter tubing (60-011-05) with 1.02 mm I.D., 2.16 mm O.D., and 3 cm length was used for NO release analysis. One end of the silicone catheter tubing was sealed (0.4 cm filling) with RTV silicone rubber and dried under ambient conditions for at least 24 h. Then, the silicone tubing was filled with approximately 20 μL of freshly prepared SNACET lock solution using a 25 μL syringe and then the end was capped with a Qosina barbed cap. The catheter samples were used immediately after instilling with the SNACET lock solutions.
2.7. Nitric Oxide Release Analysis.
Nitric oxide release from the SNACET lock solution was analyzed by a Zysense chemiluminescence Nitric Oxide Analyzer (NOA) 280i. An amber glass sample cell was filled with 5 mL of PBS (10 mM, pH 7.4) containing 100 μM EDTA and incubated at 37 °C to mimic physiological conditions. The silicone catheter tubing filled with SNACET lock solution of a known concentration described in Section 2.6 was submerged in the buffer within the sample vessel, which was purged with N2 bubbling to sweep the NO released from the catheter sample from the sample cell to the chemiluminescence detection chamber. The nitrogen flow rate that purged the sample vessel was set to 200 mL/min with a reaction chamber pressure of 9.8 Torr and an oxygen pressure of 6.5 psi. NO release was normalized by the O.D. surface area of the cylindrical tubing samples (×10−10 mol min−1 cm−2). Samples were incubated in PBS, 100 μM EDTA at 37 °C between measurements.
2.8. Determining the Minimum Inhibitory Concentration (MIC) of SNACET.
To determine the MIC of SNACET, S. aureus and E. coli were inoculated in LB broth and grown to the mid-log phase in a shaking incubator at 120 rpm, 37 °C for 5 h. The initial absorbance for each bacterial strain was adjusted to 0.2 OD600 (optical density at 600 nm wavelength) using a UV–vis spectrophotometer (Cary 60, Agilent Technologies) before commencing the experiment. Bacterial suspensions were incubated in a flat-bottom 96-well plate with different concentrations of the SNACET lock solution ranging from 0.1 to 10 mM (n = 3) for 18 h at 37 °C under continual agitation in a microplate reader (Cytation 5 imaging multi-mode reader, BioTek). The OD600 of the bacteria was monitored in 1 h intervals. For each concentration of SNACET, 50 μL of the bacterial suspension was combined with 50 μL of the SNACET solution in a 96-well plate such that the final OD600 of the bacteria was ~0.1. Corresponding blank controls were prepared for each concentration of SNACET along with the media (without bacteria) to obtain the final absorbances of bacteria. Previous studies have shown the MIC of RSNO donors for bacteria ranging from 3 to 5 mM.57 Therefore, the range of 0.1–10 mM was utilized in this study (data shown for the range of 0.1–4 mM).
2.9. In Vitro Prevention of Bacterial Adhesion Inhibition Assay.
2.9.1. SNACET Catheter Lock Solution Preparation.
Antibacterial efficacy of SNACET lock solution was determined by quantification of viable bacteria adhered to the surface of the silicone tubing filled with the lock solution. Briefly, the silicone tubing was cut into 3 cm pieces, rinsed carefully with DI water and 70% ethanol, and dried at room temperature. One end of the tubing was sealed using RTV silicone coating and cured at room temperature for 24 h prior to 20 min of UV sterilization. After sterilization, SNACET lock solutions with different concentrations (25, 50, and 75 mM) were instilled within the catheter tubing using a sterile syringe, and the other end was capped using a sterile Qosina barbed cap. The catheter tubing filled with only PBS (10 mM, pH 7.4) containing 100 μM EDTA was used as control.
2.9.2. Bacterial Culture Preparation and Exposure.
S. aureus and E. coli strains are the two most commonly reported strains in CRBSIs and were used to evaluate the bactericidal efficacy of the SNACET lock solution. The culture medium was prepared following the previously established protocol with minor modifications.58 Briefly, an isolated single colony of bacteria was inoculated in 10 mL of sterilized LB broth media for 15 h at 37 °C in a shaker incubator with a rotation speed of 120 rpm. The logarithmic bacterial growth phase was confirmed by identifying the optical density of the inoculum at 600 nm wavelength (OD600) using the Cary UV–vis spectrophotometer (Agilent Technologies) to be approximately 0.5–0.6. The inoculum was centrifuged for 7 min in 3500 rpm and washed with sterile PBS, pH 7.4, to remove dead cells and debris. The supernatant was decanted and the bacteria pellet was resuspended in sterile PBS, using a vortex mixer for 60 s. The suspension was diluted with PBS to obtain a concentration of ~108 colony-forming units per milliliter (CFU mL−1). Each catheter sample was incubated in 2 mL of the bacterial culture individually in a shaker incubator at 37 °C with a rotation speed of 120 rpm, while protected from light. After 18 h, the samples were removed from the bacterial solution. The capped ends were removed from the catheter sample and the lock solution was discarded. The samples were gently rinsed in sterile PBS twice to detach loosely adhered bacteria from the surface, transferred into 2 mL of fresh sterile PBS, homogenized using sonication prob (Omni-TH sonicator) for 60 s at 25 000 rpm, and vortexed for 60 s to detach the adhered bacteria from the surface. The resulting suspension was plated on solid LB agar plates using a spiral plater (EDDY JET 2W, IUL Instruments, Cincinnati, OH). Plates were incubated at 37 °C for 24 h prior to counting the CFUs (SphereFlash IUL Instruments, Cincinnati, OH). The CFU counts on the plates were used to calculate the viable bacteria reduction of SNACET lock solutions, normalized by the surface area, using the following equation where C = CFU cm−2.
| (1) |
2.10. In Vitro Antimicrobial Evaluation in an Infected Catheter Model.
The SNACET lock solution was also evaluated for its ability to eradicate established microbial infection on catheter surfaces. The silicone catheter tubing (7 cm length) was capped on both ends with Qosina caps and incubated in S. aureus and E. coli bacteria culture (prepared as described in Section 2.9) for 48 h to establish microbial infection on the outer lumen surface of the catheter samples. The catheter samples were incubated under static conditions at 37 °C and the LB medium was changed approximately every 4 h during the 48 h incubation. After 48 h, the samples were removed and rinsed twice in fresh sterile PBS to detach loosely bounded, planktonic bacteria from the surfaces. Approximately 45 μL of SNACET lock solution (50 or 75 mM) was injected into the inner lumen of the catheter tubing and then recapped. Sterile PBS (10 mM, pH 7.4) containing 100 μM EDTA was used as control. The infected catheter tubing samples instilled with SNACET or control lock solutions were incubated for 2 h in LB medium in a static condition at 37 °C in darkness. After the 2 h lock solution exposure, the caps were removed from the catheter tubing and the lock solution was discarded. The samples were rinsed in sterile PBS to detach the loosely adhered cells, transferred into 2 mL of sterile PBS, homogenized for 60 s, and vortexed for 60 s to detach the adhered bacteria on the catheter surface. The resulting suspensions were plated on agar plates and bacteria eradication (%) was calculated by using the plating and counting protocol described in Section 2.9.
2.11. Statistical Analysis.
All of the experiments were done n ≥ 3 for each sample group. Data are expressed as mean ± standard deviation (SD). The results between the control and SNACET catheter lock solutions were analyzed by a comparison of means using a two-tailed Student’s t-test with equal variance. Values of p < 0.05 were considered statistically significant for all tests.
3. RESULTS AND DISCUSSION
3.1. Synthesis of NACET and SNACET.
S-Nitroso-N-acetyl-l-cysteine ethyl ester (SNACET) was synthesized in two steps (Scheme 1). In the first step, N-acetyl-l-cysteine ethyl ester (NACET) was synthesized by protecting the carboxylic (−COOH) group of N-acetyl-l-cysteine (NAC) using anhydrous HCl in the presence of ethanol. The synthesized NACET was confirmed by NMR (Figures S1–S3). In the second step, SNACET was synthesized by nitrosation of an ice-cold acidic aqueous solution of NACET using sodium nitrite. For the first time, to the best of our knowledge, SNACET was synthesized via nitrosation reaction and the SNACET was isolated in the solid state. The synthesized NACET and SNACET were characterized by NMR, mass spectrometry, and ATR-FTIR spectroscopic techniques. From the 1H NMR spectra of SNACET, the peaks for hydrogen atoms attached to the carbon atoms marked as 3 (−CH2-SNO) and 4 (−CH2-O−) were merged at ca. 4 ppm (Figure 2a). Therefore, HSQC NMR analysis was performed to determine the proton–carbon single-bond correlation for the hydrogen atoms attached to the carbon atoms marked as 3 and 4 (Figure 2b). Two distinct peaks for carbon and hydrogen atoms marked as 3 and 4 were observed at 34.32 and 61.56 ppm, respectively (y-axis). From the 13C NMR spectra of NACET, a chemical shift for the carbon atom (marked as 3) adjacent to the thiol (−SH) group was observed at δ 26.90 (Figure S2), whereas for SNACET, it was deshielded to δ 34.32 due to the negative inductive (−I) effect induced by the SNO group (Figure 2c). From the mass spectrometry spectra (ESI), the molecular ion peak for the NACET and its corresponding disulfide (RSSR) product peak were observed at m/z 214.0509 (M + Na+) and 403.0906 (2M − 2H + Na+), respectively (Figure S3). However, for SNACET, the molecular ion peak was observed at m/z 243.0416 (M + Na+) (Figure 3a). SNACET released one NO molecule and formed a NACET thiyl radical that further reacted with another NACET thiyl radical to form a disulfide product, as presented in Figure 1a. The peak for both the NACET free radical and the corresponding disulfide product was observed at m/z 213.0433 (M − NO + Na+) and 403.0977 (2M − 2NO + Na+), respectively (Figure 3a). The ATR-IR spectra depicted the disappearance of the characteristic thiol (−SH) peak observed at 2541 cm−1 for NACET after its conversion to nitrosothiol (−SNO) forming SNACET (Figure 3b). The other characteristic IR bands for different functional groups present in NACET and SNACET are summarized in Table S1. The purity of the SNACET, in terms of conversion of the −SH to the NO-rich −SNO functionality, was found to be >95%, as calculated using Nitric Oxide Analyzer (NOA) 280i. The protection of carboxylic acid and the amine group of the cysteine molecule induced amphiphilicity to the charge-free SNACET molecule, making it soluble in water as well as in most common organic solvents corresponding to the previous reports.59
Scheme 1.
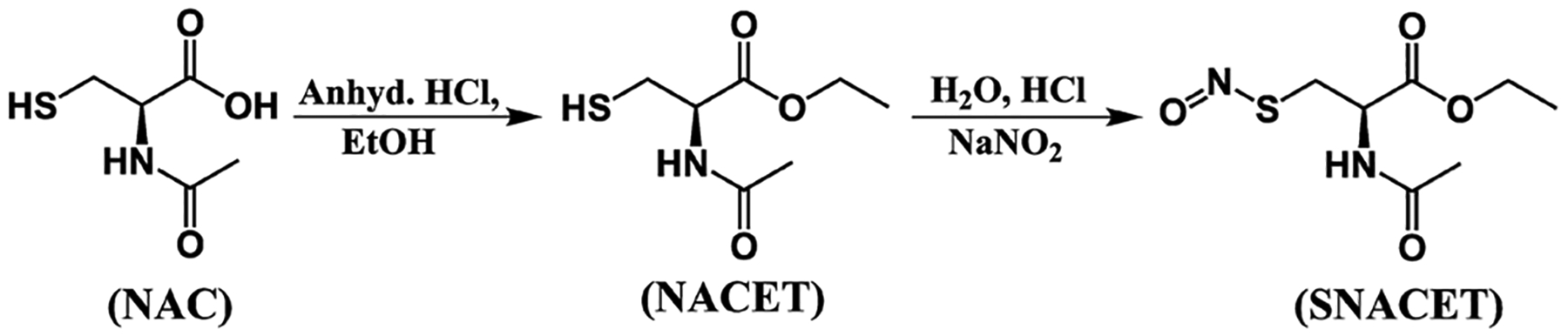
Synthesis of S-Nitroso-N-acetyl-l-cysteine Ethyl Ester (SNACET), where the carboxylic acid group of N-Acetylcysteine (NAC) was protected using anhydrous HCl in the presence of ethanol forming the N-Acetylcysteine Ethyl Ester (NACET), followed by nitrosation using acidified nitrite.
Figure 2.
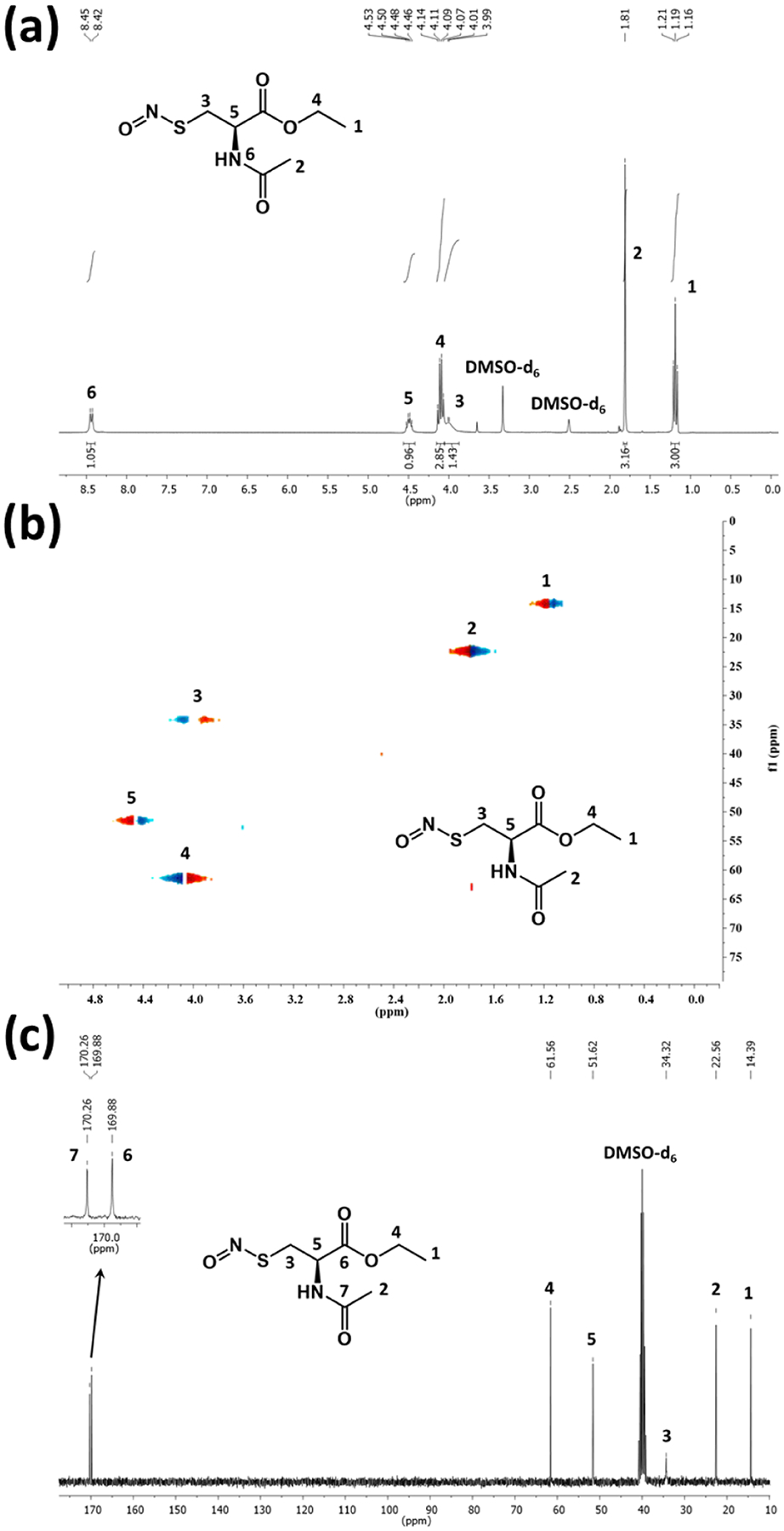
(a) 1H NMR, (b) 13C NMR, and (c) HSQC NMR spectra of SNACET. DMSO-d6 was used as a deuterated solvent for NMR analysis.
Figure 3.
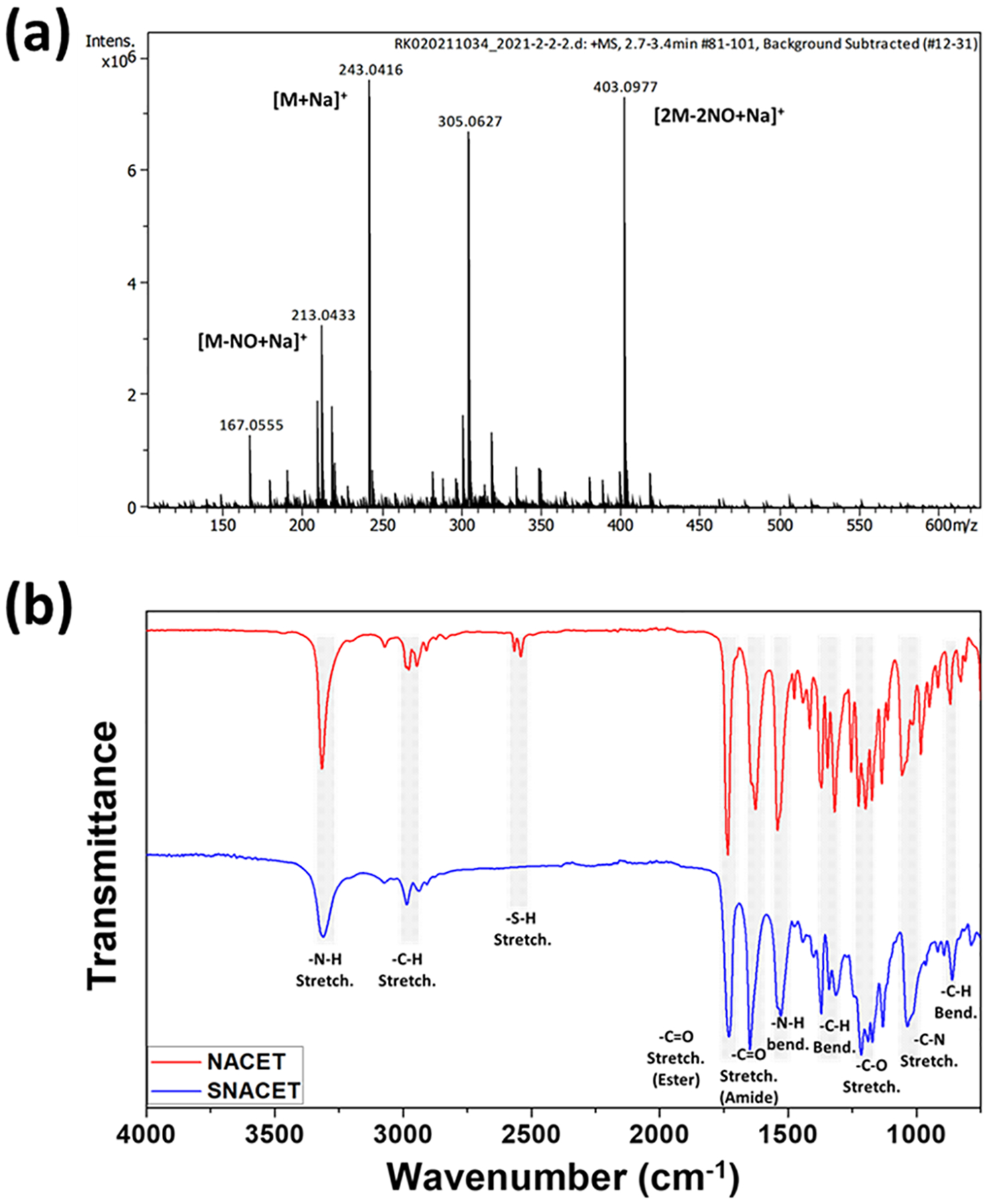
(a) Mass spectrometry spectra (ESI) of SNACET showing the peak for the molecular ion and its corresponding disulfide product. (b) ATR-FTIR spectra of NACET and SNACET depicting the characteristic IR bands.
3.2. Absorption Spectroscopic Analysis.
The absorption spectra of the SNACET with varying concentrations were analyzed in the wavelength range of 250–650 nm, by dissolving various concentrations of SNACET (0.25–1.5 mM) in PBS (10 mM, pH 7.4) containing 100 μM EDTA. The SNACET molar extinction coefficient for SNACET was determined by the correlation of UV–vis spectroscopy. The SNACET showed two characteristic absorption peaks for the S-nitroso group (S-NO) at 334 and 545 nm corresponding to the nO−π* and nN−π* transitions,60 respectively (Figure 4a,b). The absorbance of dissolved SNACET in PBS (10 mM, pH 7.4) containing 100 μM EDTA was obtained at 334 and 543 nm by UV–vis spectroscopy for six different concentrations (0.25–1.5 mM) within the linear range. The molar absorption coefficient was found to be ε334 = 780 M−1cm−1 and ε543 = 13.8 M−1cm−1 (Figure 4c,d).
Figure 4.
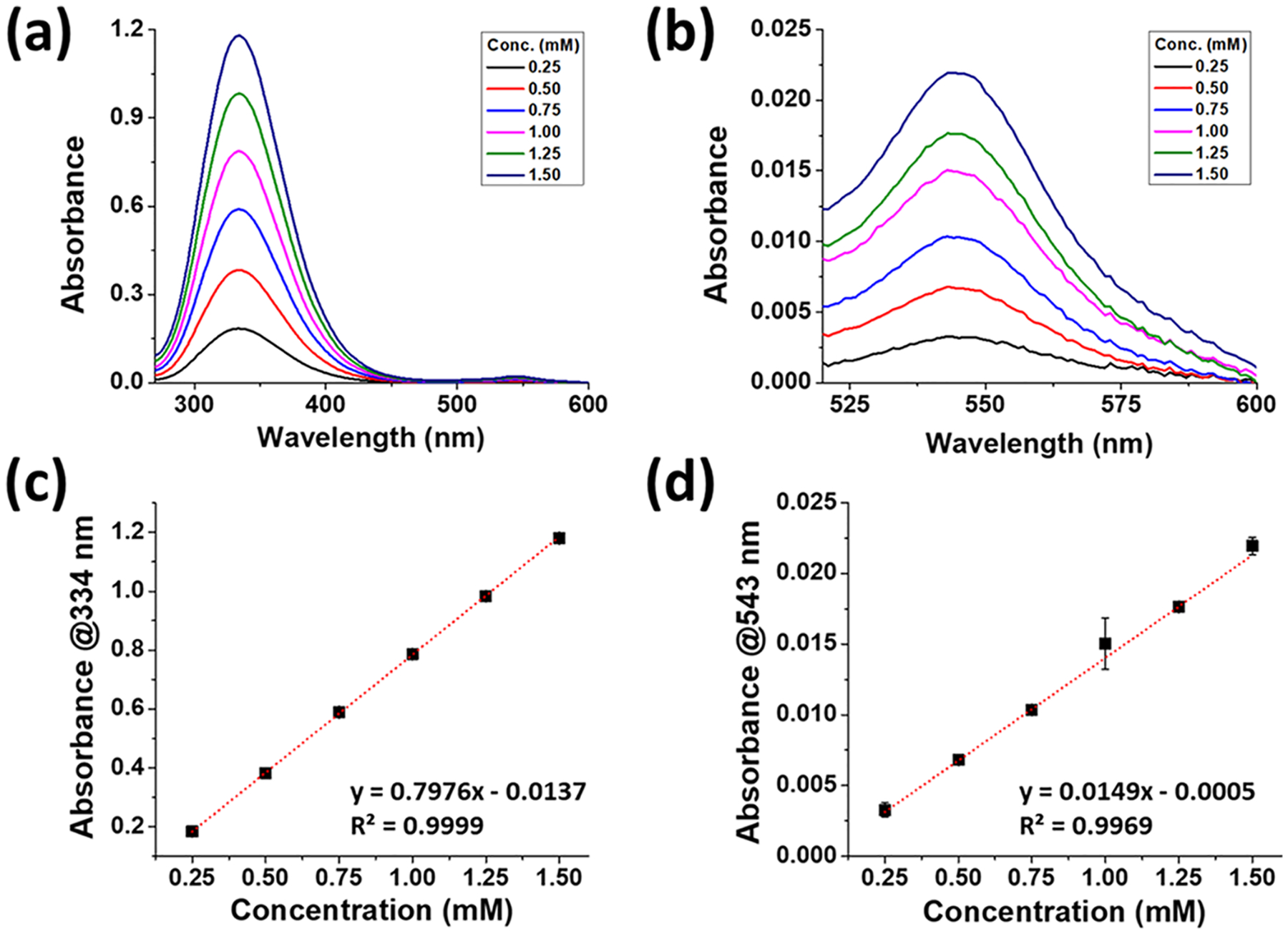
Representative UV–vis spectra of SNACET with varying concentrations in PBS (10 mM, pH 7.4) containing 100 μM EDTA, depicting absorption maxima (a) at 334 nm and (b) at 543 nm. The calibration curve of SNACET (c) at 334 nm. Equation of line: y = 0.7976x − 0.0137 (R2 = 0.9997) and (d) at 543 nm. Equation of line: y = 0.0149x − 0.0005 (R2 = 0.9969). Data reported as mean ± SD of n ≥ 3 replicate measurements.
3.3. Storage Stability of SNACET and Decomposition under Physiological Conditions.
The storage stability of NO donors plays a decisive role in determining their clinical applicability. Other cysteine-derived NO donors have been reported to exhibit short half-lives and limited stability.61 The synthesized SNACET powder was stored at −20 and −80 °C in the dark (Figure 5a). The storage stability of the SNACET in a solid-state powder was determined by measuring the amount of SNACET remaining in the samples after 1, 2, 4, and 8 weeks of storage. A 1 mM SNACET solution in PBS (10 mM, pH 7.4) containing 100 μM EDTA was freshly prepared from the stored powder at each time point and the absorbance was measured using a UV–vis spectrophotometer. SNACET was found to be poorly stable at −20 °C, where ~20 and ~90% of SNACET were degraded after 3 days and 3 weeks, respectively. However, at −80 °C, it showed excellent stability, and only ~2 and ~7% of degradation were observed after 3 and 8 weeks, respectively. The decomposition of SNACET was also monitored under physiological conditions for 24 h to estimate the dwelling period of SNACET lock solution in catheters to prevent infection (Figure 5b). SNACET exhibited a half-life (t1/2) of around 15 h in PBS (10 mM, pH 7.4) containing 100 μM EDTA at 37 °C.
Figure 5.
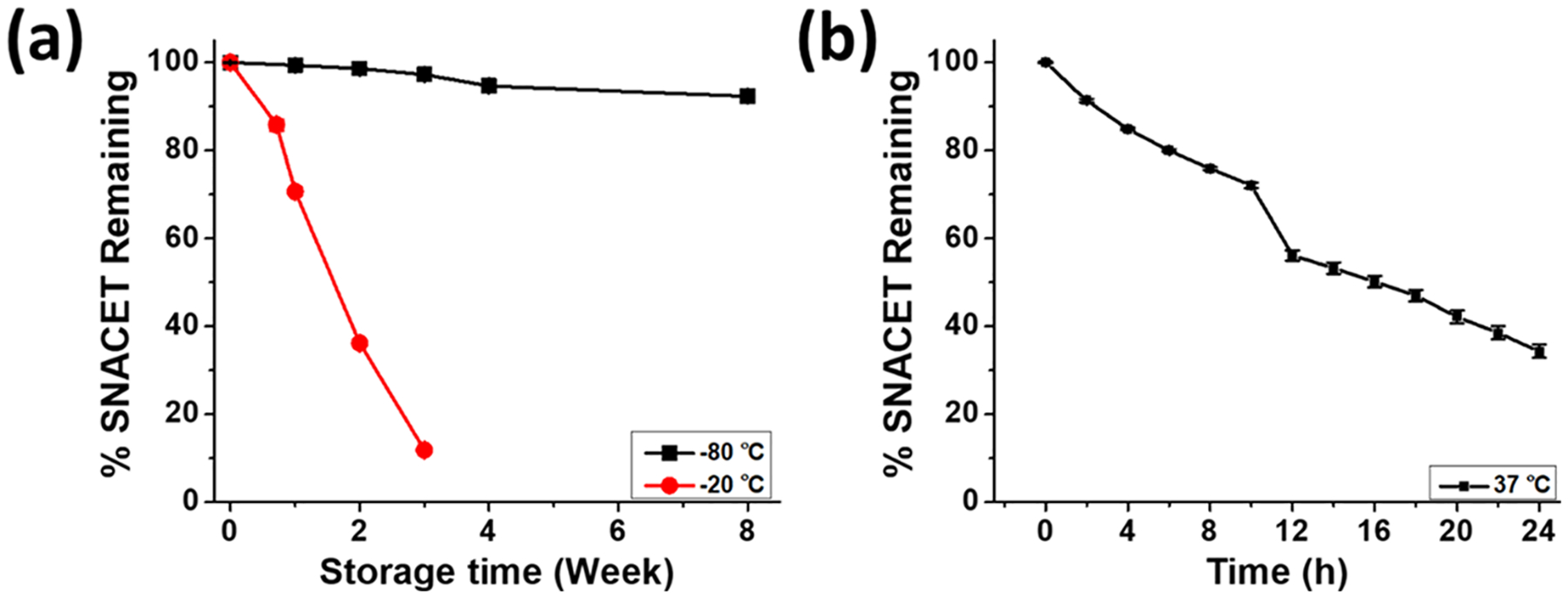
(a) Storage stability of SNACET powder stored at −80 or −20 °C. (b) Decomposition of 1 mM SNACET in PBS (10 mM, pH 7.4) containing 100 μM EDTA at 37 °C. Data reported as mean ± SD of n≥3 replicate measurements.
3.4. NO Release Analysis of SNACET Lock Solution.
SNACET lock solutions of four varying concentrations (25, 50, 75, 100 mM) were prepared by dissolving SNACET in PBS (10 mM, pH 7.4) containing 100 μM EDTA. The lock solutions were instilled within the silicone catheter tubing and the real-time NO release kinetics were measured using a chemiluminescence NO analyzer over 24 h under physiological conditions. A tunable, concentration-dependent NO release behavior was observed for all of the SNACET lock solution samples (Figure 6). The initial release of NO for all of the samples was found to be in the range of ca. 3−12 ×10−10 mol min−1 cm−2 and a time-dependent attenuation in NO release levels was observed as the SNACET decomposed over 24 h. During the initial hours (0–3 h), the NO release levels for 25, 50, 75, and 100 mM concentration were observed to be ca. 2.9, 6.6, 9.4, and 12.1 × 10−10 mol min−1 cm−2, respectively. After 18 h, the NO flux value was observed to be ca. 1.1, 3.8, 6.0, and 8.1 × 10−10 mol min−1 cm−2, respectively. Although all of the samples maintained physiologically relevant levels of NO for at least 18 h, the SNACET lock solution concentrations of 25, 50, and 75 mM were further evaluated for in vitro antimicrobial activity due to their ability to mimic endothelial levels of NO.62 In clinical practice, fresh SNACET lock solutions could be instilled within catheters every ca. 18 h to maintain physiological levels of NO for the indwelling catheter’s implantation timeframe.
Figure 6.
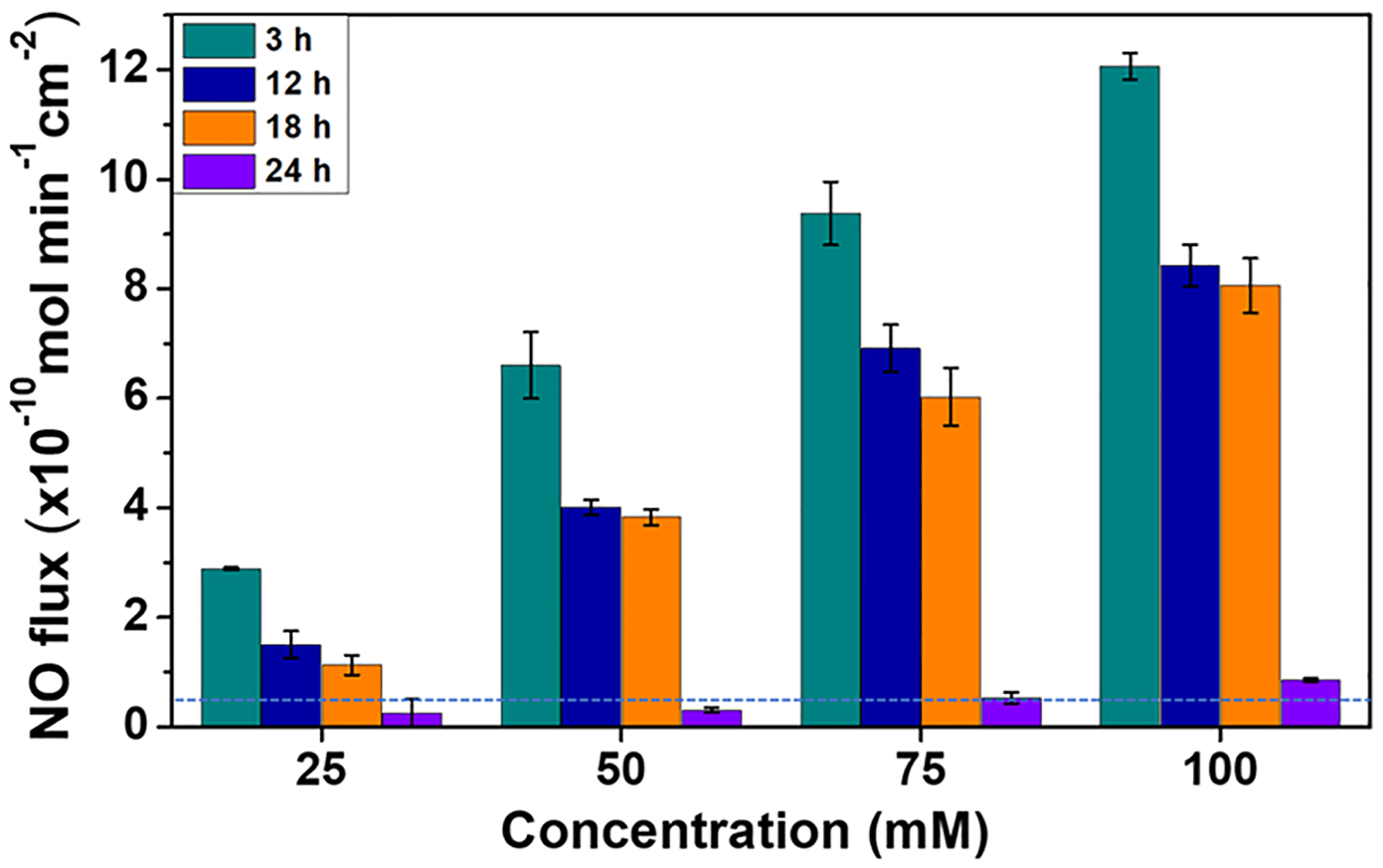
Real-time NO release from SNACET lock solutions with varying concentrations as measured by chemiluminescence in PBS (10 mM, pH 7.4) containing 100 μM EDTA at 37°C. The dotted blue line represents the lower range of endothelial NO release. Data are the mean ± standard error of the mean (SEM) (n = 3).
3.5. Minimum Inhibitory Concentration (MIC) of SNACET.
Minimum inhibitory concentration (MIC) refers to the lowest concentration of a drug required to prevent the visible growth of microorganisms after overnight incubation. MIC values are used as a research tool to characterize the in vitro function of antimicrobial agents, which are used by clinicians to identify the resistance/susceptibility patterns of microorganisms toward various antibiotics. A lower MIC indicates that less of the drug is required to prevent the growth of microorganisms. It is well understood that antimicrobial agents with lower MIC values are far more efficient than their counterparts that require high dosages of a drug to kill the same number of microorganisms. MIC values can help enhance the outcomes for patients and have the ability to inhibit the rapid progression of multidrug-resistant bacterial species by classifying suitable drugs for their appropriate usage quantity. For this reason, the MIC of SNACET was studied in both Gram-positive and Gram-negative bacteria (Figure 7). Both S. aureus and E. coli were exposed to various concentrations of SNACET up to 18 h to identify their dynamics in the presence of SNACET. The MIC was determined to be the lowest concentration that prevented visible growth, defined as an OD600 of 0.05 for up to 18 h. Bacteria growth kinetics revealed 3 ± 0.002 and 3 ± 0.003 mM as the MIC of SNACET against S. aureus and E. coli, respectively. While a few concentrations lower than 3 mM also showed some inhibitory effects for a certain period, these levels were not sufficient enough to continuously inhibit the growth of bacteria for the entire 18 h. In contrast, SNACET at or above 3 mM concentration continuously showed inhibitory effect against both Gram-positive S. aureus and Gram-negative E. coli. Comparable results were observed for similar RSNO donors such as S-nitroso-N-acetylcysteine (SNAC) and S-nitrosoglutathione (GSNO) in PBS that exhibited MIC values ranging between 3 and 5 mM for clinical isolates of S. aureus and E. coli bacteria.57
Figure 7.
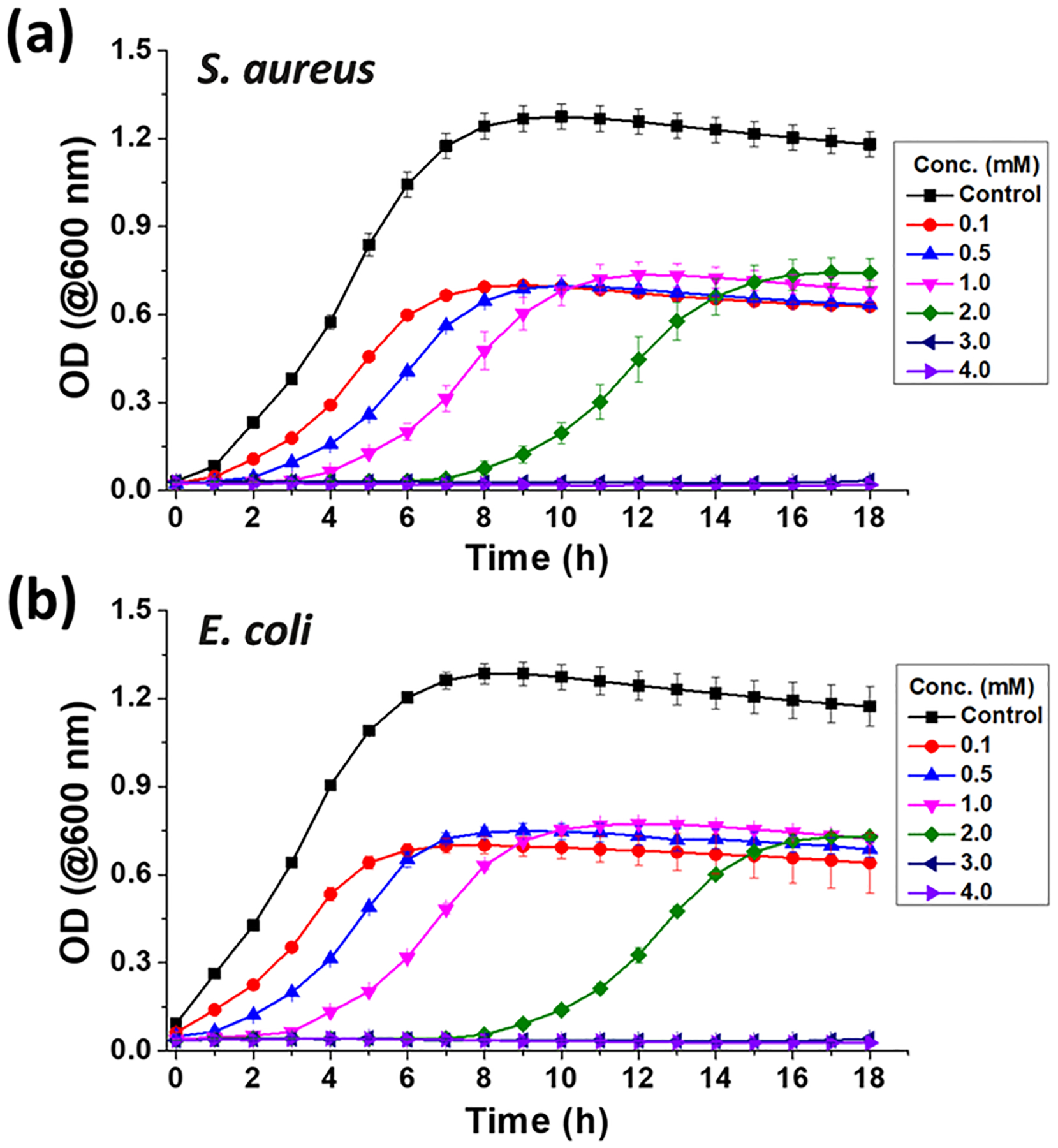
Bacteria growth curve against SNACET lock solution. The MIC values of S. aureus (a) and E. coli (b) were determined to be the lowest concentration of SNACET that prevented visible growth of bacteria, defined as an optical density at 600 nm of 0.05 up to 18 h. The experiment was done in triplicates and data are reported as mean ± SD.
3.6. In Vitro Analysis of Biocidal Activity to Reduce CRBSIs.
3.6.1. Quantification of Ability to Prevent Viable Bacterial Adhesion on Surfaces.
In this study, SNACET lock solutions with three different concentrations were tested against two common microorganisms that are involved with CRBSIs, S. aureus and E. coli. The antimicrobial activity was evaluated over 18 h to ensure that all lock solutions maintained physiological levels of NO release (>0.5 × 10−10 mol cm−2 min−1)62 during the bacteria exposure. All concentrations of the SNACET lock solutions exhibited bactericidal effects in comparison with the control catheter instilled with PBS, where the antimicrobial efficiency increased with the increasing concentration of SNACET in the lock solution (Figure 8). Greater concentrations of SNACET release higher levels of NO, leading to greater biocidal efficiency. Even the lowest SNACET concentration of 25 mM exhibited 85.6 ± 2.1 and 77.6 ± 0.1% reduction in viable CFUs for both S. aureus and E. coli, respectively, after 18 h exposure. A >99% (approximately 3-log) reduction of the adhered S. aureus was observed with the 50 mM SNACET lock solution (Figure 8a). The same bactericidal efficacy (>99% reduction in viable CFUs) was observed for the E. coli with the 75 mM SNACET lock solution (Figure 8b). While Gram-positive S. aureus is typically more susceptible to antibacterial agents, Gram-negative E. coli often needs a slightly higher dose to be eradicated.63 These results demonstrate the ability of these levels of NO to impart potent broad-spectrum antimicrobial properties at the catheter surface, especially given that in this study the lock solution never came in direct contact with the bacteria. Bacteria were exposed to the outer surface of the catheter, while the lock solutions were instilled within the catheter lumen and the NO released from the solution diffused through the silicone catheter wall to eradicate the bacteria. Prior studies have demonstrated the ability of NO to efficiently diffuse through polymers such as silicone,53 and the NO release reported in this study demonstrates that levels of NO on the outer catheter surfaces mimic endogenous levels. Greater antimicrobial activity was observed with increasing NO flux levels released from the SNACET lock solutions, which corresponds with previous literature reports of other NO-releasing technologies.46
Figure 8.
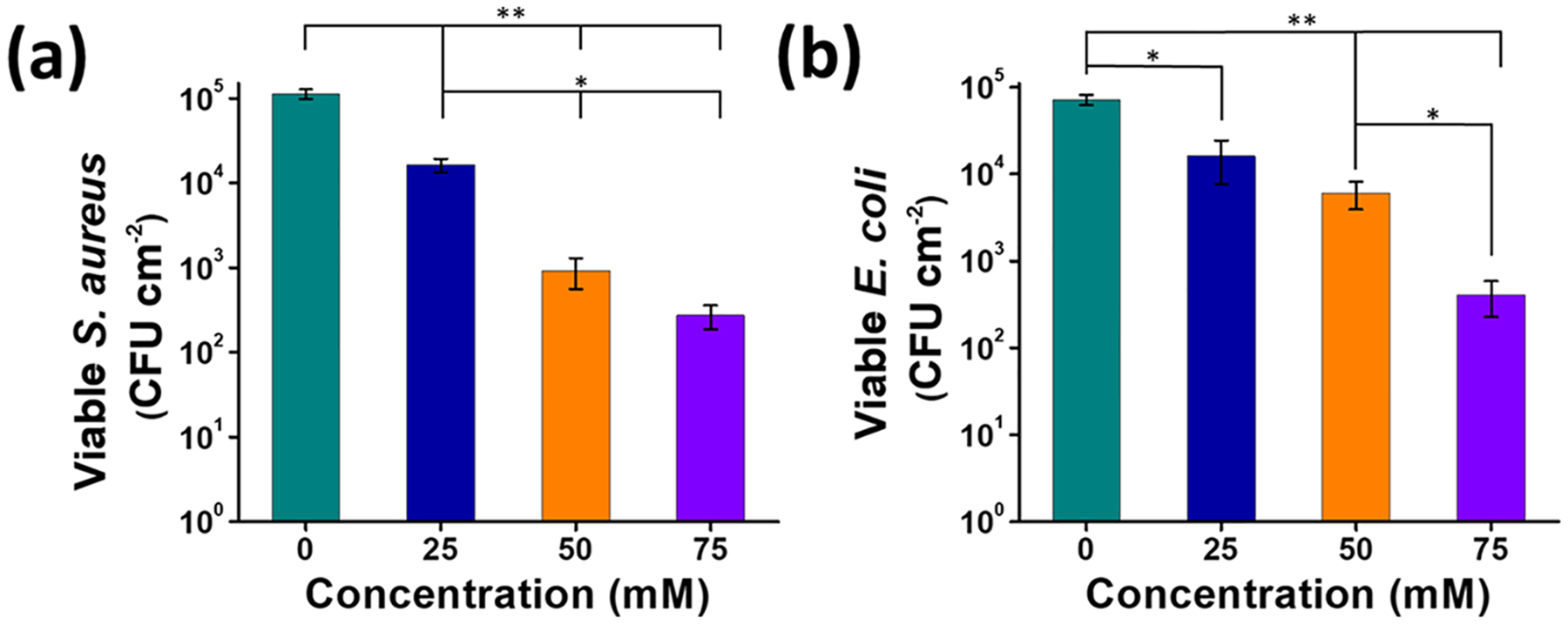
Bactericidal activity of the SNACET lock solutions showed concentration-dependent effect against (a) S. aureus and (b) E. coli. Data represent the mean ± SD (n = 6). p-values ≤ 0.01 and ≤ 0.005 were indicated with * and **, respectively.
3.6.2. In Vitro Evaluation of SNACET Lock Solution Antimicrobial Efficacy against Established Catheter Infections.
In addition to studying the ability of antimicrobial-lock solutions to prevent viable bacterial adhesion on catheter surfaces, it is also important to evaluate their ability to eradicate established microbial infections on the catheter surface that can continue to proliferate and form a biofilm. Bacteria growth and biofilm formation on the catheter surface can lead to clinical challenges such as CRBSIs or sepsis.7,64 Microbial biofilms are notoriously difficult to eradicate because the bacteria produce an extracellular polysaccharide matrix that protects them from antibiotics or immune cells.65 However, bacteria within the biofilm are still vulnerable to NO because NO can diffuse through the biofilm matrix and actively kill the bacteria as well as act as a signaling agent to initiate biofilm dispersion.42,66 The 50 and 75 mM SNACET lock solutions that exhibited the highest antimicrobial activity over 18 h as described above were evaluated in this in vitro model of established catheter infections (Figure 9a). In this study, silicone catheters were exposed to S. aureus or E. coli, which are both known to establish biofilms on medical device interfaces,67–69 for 48 h to establish a microbial infection on the outer surfaces of the catheter samples. Then, the SNACET or control lock solutions were instilled within the inner lumen of the catheter and incubated for 2 h. The NO released from the SNACET lock solution quickly diffused through the tubing wall to eradicate the bacteria adhered to the outer lumen surface, demonstrating the ability of the SNACET lock solution to simultaneously provide antimicrobial activity on both the outer and inner luminal surfaces. The viable bacteria on catheter surfaces after 2 h exposure to the SNACET or control lock solutions instilled within the catheter lumen was quantified by plate counting (Table 1). The starting amount of each strain of bacteria that adhered to the catheter surfaces was different, likely due to differences in their motility as observed in other reports.70 Both the 50 and 75 mM SNACET lock solutions exhibited significant antimicrobial activity in comparison to the control lock solution, as demonstrated by the percent reduction of viable bacteria on the catheter surfaces after 2 h exposure to the lock solutions (Figure 9b,c). The 75 mM SNACET lock solution exhibited >96.4 and 90.3% reduction in the viable S. aureus and E. coli CFUs, respectively, on catheter surfaces with an established microbial infection. Increased levels of NO released by increasing concentrations of SNACET in the lock solution exhibited more potent antimicrobial properties. These antimicrobial studies demonstrate that SNACET lock solutions have the ability to prevent viable bacteria from proliferating on catheter surfaces and also have the ability to significantly reduce the viable bacteria on catheter surfaces with an established microbial infection. The SNACET lock solutions exhibit physiological NO release levels for at least 18 h, and such lock solutions could be replaced periodically to maintain antimicrobial activity on the catheter surfaces.
Figure 9.
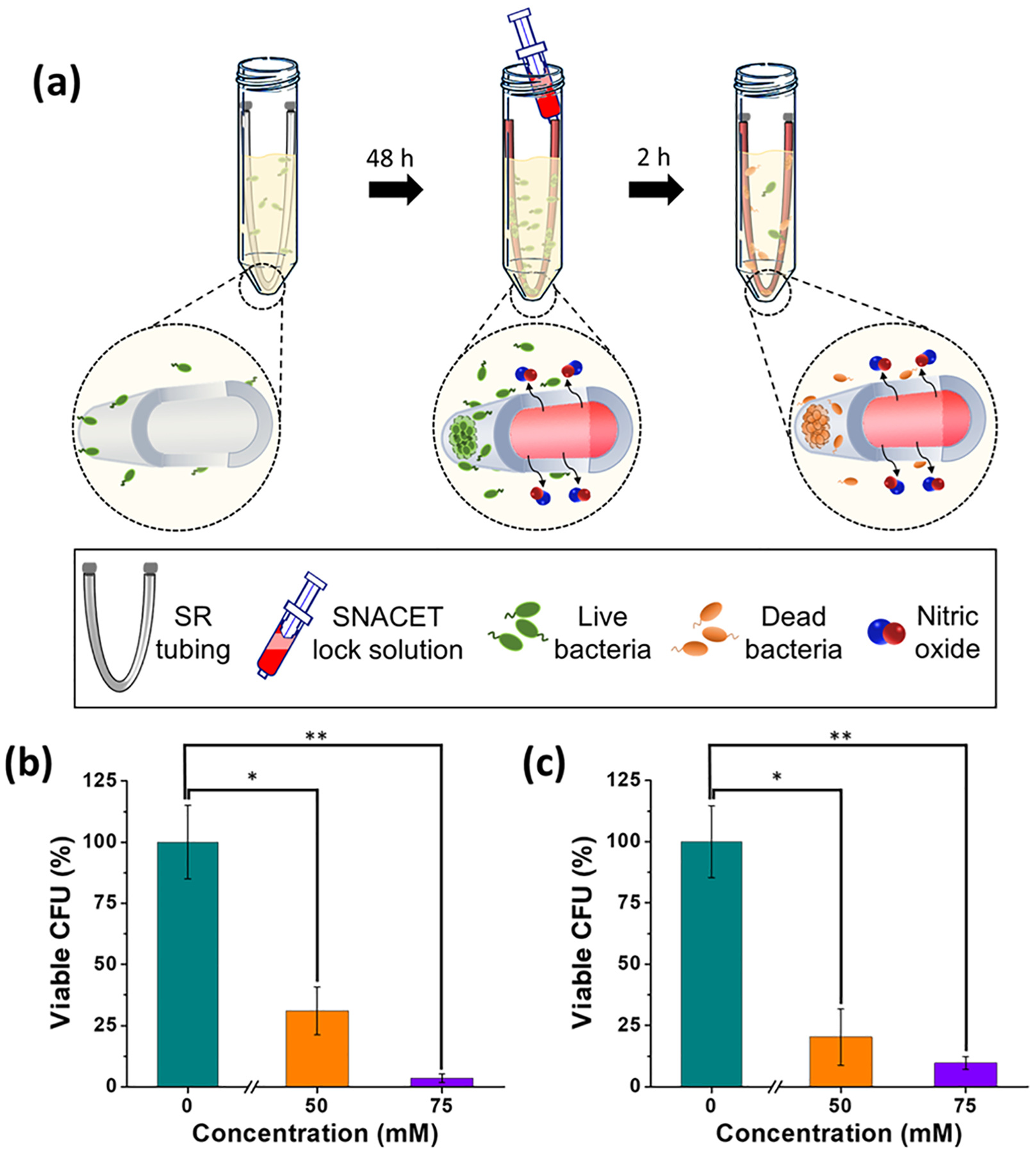
(a) Schematic representation of the in vitro experiment to evaluate the efficacy of the SNACET lock solutions against established catheter infections. The outer surfaces of the catheters were infected by incubating in the bacterial inoculum for 48 h, followed by infusion of the SNACET or control lock solutions within the inner lumen of the catheter. After 2 h exposure to the lock solutions, the viable bacteria on catheter surfaces was determined by plate counting. Percent reduction in viable (b) S. aureus and (c) E. coli CFUs on preinfected catheters after exposure to the SNACET or control lock solutions that were instilled within the catheter samples for 2 h. Data represent the mean ± standard error of the mean (n = 3). p-values ≤0.05 and ≤0.01 were indicated with * and **, respectively.
Table 1.
Viable CFU Counts after Catheter Samples With Established S. aureus or E. coli Infection were Exposed to the SNACET Lock Solution Instilled Within the Catheter Tubing for 2 h.a
| Viable CFU Concentration | ||
|---|---|---|
| Lock Solution Conc. (mM) | S. aureus (105 × CFU cm−2) | E. coli (107 × CFU cm−2) |
| 0 | 3.76 ± 0.56 | 22.58 ± 3.33 |
| 50 | 1.17 ± 0.37 | 4.58 ± 2.59 |
| 75 | 0.13 ± 0.06 | 2.19 ± 0.59 |
All data points show significant differences between SNACET and control (p < 0.05) and are expressed as the mean ± SD (n = 3).
4. CONCLUSIONS
In this work, SNACET, a cysteine derivative NO donor, was synthesized by protecting both the amine and carboxylic groups of cysteine. The synthesized SNACET powder that was isolated was characterized by NMR, mass spectrometry, and FTIR spectroscopic methods. The SNACET powder exhibited better stability when stored at −80 °C compared to −20 °C. The synthesized amphiphilic SNACET molecule was used to formulate a lock solution in PBS (10 mM, pH 7.4) containing 100 μM EDTA, and the NO release behavior was analyzed using chemiluminescence. The SNACET lock solutions exhibited tunable NO release levels by modulating the concentration of SNACET (25–100 mM), and all samples released physiologically relevant levels of NO for >18 h. The MIC value and antibacterial activity of the SNACET lock solution were evaluated against Gram-positive S. aureus and Gram-negative E. coli, two of the most common microorganisms that result in medical device and hospital-acquired infections. The 50 and 75 mM SNACET lock solutions exhibited excellent broad-spectrum antibacterial properties and a >99% (approximately 3-log) reduction of viable S. aureus and E. coli adhered to the silicone catheter tubing after 18 h exposure. Further, the lock solutions also exhibited the ability to eradicate established microbial infections on catheter surfaces. A >90% reduction in the viable S. aureus and E. coli was observed after the catheters with established microbial infection were exposed to the 75 mM SNACET lock solution for only 2 h. These promising results demonstrate the ability of NO-releasing molecules, such as SNACET, to be utilized as nonantibiotic catheter lock solutions, which can prevent and treat infections that develop on a wide range of catheter surfaces (e.g., intravascular, urinary, hemodialysis).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the National Institutes of Health (R01HL151473).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.1c06427.
Additional NMR data for the NACET molecule characterization and characteristic FTIR bands for the SNACET and NACET characterization (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsami.1c06427
The authors declare no competing financial interest.
Contributor Information
Rajnish Kumar, School of Chemical, Materials and Biomedical Engineering, University of Georgia, Athens, Georgia 30602, United States.
Hamed Massoumi, School of Chemical, Materials and Biomedical Engineering, University of Georgia, Athens, Georgia 30602, United States.
Manjyot Kaur Chug, School of Chemical, Materials and Biomedical Engineering, University of Georgia, Athens, Georgia 30602, United States.
Elizabeth J. Brisbois, School of Chemical, Materials and Biomedical Engineering, University of Georgia, Athens, Georgia 30602, United States.
REFERENCES
- (1).Rupp ME; Karnatak R Intravascular Catheter-Related Bloodstream Infections. Infect. Dis. Clin. North Am 2018, 32, 765–787. [DOI] [PubMed] [Google Scholar]
- (2).Zhang HH; Cortes-Penfield NW; Mandayam S; Niu J; Atmar RL; Wu E; Chen D; Zamani R; Shah MK Dialysis Catheter-Related Bloodstream Infections in Patients Receiving Hemodialysis on an Emergency-Only Basis: A Retrospective Cohort Analysis. Clin. Infect. Dis 2019, 68, 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Safdar N; Maki DG The Pathogenesis of Catheter-Related Bloodstream Infection with Noncuffed Short-Term Central Venous Catheters. Intensive Care Med. 2004, 30, 62–67. [DOI] [PubMed] [Google Scholar]
- (4).Shah CB; Mittelman MW; Costerton JW; Parenteau S; Pelak M; Arsenault R; Mermel LA Antimicrobial Activity of a Novel Catheter Lock Solution. Antimicrob. Agents Chemother 2002, 46, 1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Del Pozo JL; Alonso M; Serrera A; Hernaez S; Aguinaga A; Leiva J Effectiveness of the Antibiotic Lock Therapy for the Treatment of Port-Related Enterococci, Gram-Negative, or Gram-Positive Bacilli Bloodstream Infections. Diagn. Microbiol. Infect. Dis 2009, 63, 208–212. [DOI] [PubMed] [Google Scholar]
- (6).Wu H; Moser C; Wang H-Z; Høiby N; Song Z-J Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral Sci 2015, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gominet M; Compain F; Beloin C; Lebeaux D Central Venous Catheters and Biofilms: Where Do We Stand in 2017? APMIS 2017, 125, 365–375. [DOI] [PubMed] [Google Scholar]
- (8).Percival SL; Kite P; Eastwood K; Murga R; Carr J; Arduino MJ; Donlan RM Tetrasodium Edta as a Novel Central Venous Catheter Lock Solution against Biofilm. Infect. Control Hosp. Epidemiol 2005, 26, 515–519. [DOI] [PubMed] [Google Scholar]
- (9).Chandra J; Long L; Isham N; Mukherjee PK; DiSciullo G; Appelt K; Ghannoum MA In Vitro and in Vivo Activity of a Novel Catheter Lock Solution against Bacterial and Fungal Biofilms. Antimicrob. Agents Chemother 2018, 62, No. e00722–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Vassallo M; Dunais B; Roger PM Antimicrobial Lock Therapy in Central-Line Associated Bloodstream Infections: A Systematic Review. Infection 2015, 43, 389–398. [DOI] [PubMed] [Google Scholar]
- (11).Zhang J; Wang B; Li R; Ge L; Chen KH; Tian J Does Antimicrobial Lock Solution Reduce Catheter-Related Infections in Hemodialysis Patients with Central Venous Catheters? A Bayesian Network Meta-Analysis. Int. Urol. Nephrol 2017, 49, 701–716. [DOI] [PubMed] [Google Scholar]
- (12).Zembles TN; Flannery LS; Huppler AR Development and Implementation of an Antimicrobial Lock Therapy Guideline in a Pediatric Hospital. Am. J. Health-Syst. Pharm 2018, 75, 299–303. [DOI] [PubMed] [Google Scholar]
- (13).Pliakos EE; Andreatos N; Ziakas PD; Mylonakis E The Cost-Effectiveness of Antimicrobial Lock Solutions for the Prevention of Central Line-Associated Bloodstream Infections. Clin. Infect. Dis 2019, 68, 419–425. [DOI] [PubMed] [Google Scholar]
- (14).Garland JS; Alex CP; Henrickson KJ; McAuliffe TL; Maki DG A Vancomycin-Heparin Lock Solution for Prevention of Nosocomial Bloodstream Infection in Critically Ill Neonates with Peripherally Inserted Central Venous Catheters: A Prospective, Randomized Trial. Pediatrics 2005, 116, e198–205. [DOI] [PubMed] [Google Scholar]
- (15).Safdar N; Maki DG Use of Vancomycin-Containing Lock or Flush Solutions for Prevention of Bloodstream Infection Associated with Central Venous Access Devices: A Meta-Analysis of Prospective, Randomized Trials. Clin. Infect. Dis 2006, 43, 474–484. [DOI] [PubMed] [Google Scholar]
- (16).LaPlante KL; Mermel LA In Vitro Activity of Daptomycin and Vancomycin Lock Solutions on Staphylococcal Biofilms in a Central Venous Catheter Model. Nephrol., Dial., Transplant 2007, 22, 2239–2246. [DOI] [PubMed] [Google Scholar]
- (17).Nori US; Manoharan A; Yee J; Besarab A Comparison of Low-Dose Gentamicin with Minocycline as Catheter Lock Solutions in the Prevention of Catheter-Related Bacteremia. Am. J. Kidney Dis 2006, 48, 596–605. [DOI] [PubMed] [Google Scholar]
- (18).Raad I; Chaftari AM; Zakhour R; Jordan M; Al Hamal Z; Jiang Y; Yousif A; Garoge K; Mulanovich V; Viola GM; Kanj S; Pravinkumar E; Rosenblatt J; Hachem R Successful Salvage of Central Venous Catheters in Patients with Catheter-Related or Central Line-Associated Bloodstream Infections by Using a Catheter Lock Solution Consisting of Minocycline, Edta, and 25% Ethanol. Antimicrob. Agents Chemother 2016, 60, 3426–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ghannoum MA; Isham N; Jacobs MR Antimicrobial Activity of B-Lock against Bacterial and Candida Spp. Causing Catheter-Related Bloodstream Infections. Antimicrob. Agents Chemother 2011, 55, 4430–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Del Pozo JL; Garcia Cenoz M; Hernaez S; Martinez A; Serrera A; Aguinaga A; Alonso M; Leiva J Effectiveness of Teicoplanin Versus Vancomycin Lock Therapy in the Treatment of Port-Related Coagulase-Negative Staphylococci Bacteraemia: A Prospective Case-Series Analysis. Int. J. Antimicrob. Agents 2009, 34, 482–485. [DOI] [PubMed] [Google Scholar]
- (21).Chong C-Y; Ong RY; Seah VX; Tan NW; Chan MY; Soh SY; Ong C; Lim AS; Thoon KC Taurolidine-Citrate Lock Solution for the Prevention of Central Line-Associated Bloodstream Infection in Paediatric Haematology-Oncology and Gastrointestinal Failure Patients with High Baseline Central-Line Associated Bloodstream Infection Rates. J. Paediatr. Child Health 2020, 56, 123–129. [DOI] [PubMed] [Google Scholar]
- (22).Balestrino D; Souweine B; Charbonnel N; Lautrette A; Aumeran C; Traore O; Forestier C Eradication of Microorganisms Embedded in Biofilm by an Ethanol-Based Catheter Lock Solution. Nephrol., Dial., Transplant 2009, 24, 3204–3209. [DOI] [PubMed] [Google Scholar]
- (23).Cober MP; Kovacevich DS; Teitelbaum DH Ethanol-Lock Therapy for the Prevention of Central Venous Access Device Infections in Pediatric Patients with Intestinal Failure. J. Parenter. Enteral Nutr 2011, 35, 67–73. [DOI] [PubMed] [Google Scholar]
- (24).Touré A; Lauverjat M; Peraldi C; Boncompain-Gerard M; Gelas P; Barnoud D; Chambrier C Taurolidine Lock Solution in the Secondary Prevention of Central Venous Catheter-Associated Bloodstream Infection in Home Parenteral Nutrition Patients. Clin. Nutr 2012, 31, 567–570. [DOI] [PubMed] [Google Scholar]
- (25).Reitzel RA; Rosenblatt J; Hirsh-Ginsberg C; Murray K; Chaftari AM; Hachem R; Raad I In Vitro Assessment of the Antimicrobial Efficacy of Optimized Nitroglycerin-Citrate-Ethanol as a Nonantibiotic, Antimicrobial Catheter Lock Solution for Prevention of Central Line-Associated Bloodstream Infections. Antimicrob. Agents Chemother 2016, 60, 5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).El-Hennawy AS; Frolova E; Romney WA Sodium Bicarbonate Catheter Lock Solution Reduces Hemodialysis Catheter Loss Due to Catheter-Related Thrombosis and Blood Stream Infection: An Open-Label Clinical Trial. Nephrol., Dial., Transplant 2019, 34, 1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yahav D; Rozen-Zvi B; Gafter-Gvili A; Leibovici L; Gafter U; Paul M Antimicrobial Lock Solutions for the Prevention of Infections Associated with Intravascular Catheters in Patients Undergoing Hemodialysis: Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Clin. Infect. Dis 2008, 47, 83–93. [DOI] [PubMed] [Google Scholar]
- (28).Zacharioudakis IM; Zervou FN; Arvanitis M; Ziakas PD; Mermel LA; Mylonakis E Antimicrobial Lock Solutions as a Method to Prevent Central Line-Associated Bloodstream Infections: A Meta-Analysis of Randomized Controlled Trials. Clin. Infect. Dis 2014, 59, 1741–1749. [DOI] [PubMed] [Google Scholar]
- (29).Abad CL; Safdar N Catheter-Related Bloodstream Infections. Infect. Dis. Special Ed 2011, 14, 84–98. [Google Scholar]
- (30).Radomski MW; Palmer RM; Moncada S The Role of Nitric Oxide and Cgmp in Platelet Adhesion to Vascular Endothelium. Biochem. Biophys. Res. Commun 1987, 148, 1482–1489. [DOI] [PubMed] [Google Scholar]
- (31).Fang FC Perspectives Series: Host/Pathogen Interactions. Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J. Clin. Invest 1997, 99, 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zamora R; Vodovotz Y; Billiar TR Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med 2000, 6, 347–373. [PMC free article] [PubMed] [Google Scholar]
- (33).Bogdan C Nitric Oxide and the Immune Response. Nature 2001, 2, 907–916. [DOI] [PubMed] [Google Scholar]
- (34).Schwentker A; Vodovotz Y; Weller R; Billiar TR Nitric Oxide and Wound Repair: Role of Cytokines? Nitric Oxide 2002, 7, 1–10. [DOI] [PubMed] [Google Scholar]
- (35).Moncada S; Higgs EA The Discovery of Nitric Oxide and Its Role in Vascular Biology. Br. J. Pharmacol 2006, 147, S193–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Jones ML; Ganopolsky JG; Labbé A; Wahl C; Prakash S Antimicrobial Properties of Nitric Oxide and Its Application in Antimicrobial Formulations and Medical Devices. Appl. Microbiol. Biotechnol 2010, 88, 401–407. [DOI] [PubMed] [Google Scholar]
- (37).Halpenny GM; Mascharak PK Emerging Antimicrobial Applications of Nitric Oxide (No) and No-Releasing Materials. Anti-infect. Agents Med. Chem 2010, 9, 187–197. [Google Scholar]
- (38).Fang FC Perspectives Series: Host/Pathogen Interactions. Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J. Clin. Invest 1997, 99, 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Brown GC; Borutaite V Interactions between Nitric Oxide, Oxygen, Reactive Oxygen Species and Reactive Nitrogen Species. Biochem. Soc. Trans 2006, 34, 953–956. [DOI] [PubMed] [Google Scholar]
- (40).Rouillard KR; Novak OP; Pistiolis AM; Yang L; Ahonen MJR; McDonald RA; Schoenfisch MH Exogenous Nitric Oxide Improves Antibiotic Susceptibility in Resistant Bacteria. ACS Infect. Dis 2021, 7, 23–33. [DOI] [PubMed] [Google Scholar]
- (41).Schairer DO; Chouake JS; Nosanchuk JD; Friedman AJ The Potential of Nitric Oxide Releasing Therapies as Antimicrobial Agents. Virulence 2012, 3, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wo Y; Brisbois EJ; Bartlett RH; Meyerhoff ME Recent Advances in Thromboresistant and Antimicrobial Polymers for Biomedical Applications: Just Say Yes to Nitric Oxide (No). Biomater. Sci 2016, 4, 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Brisbois EJ; Handa H; Major TC; Bartlett RH; Meyerhoff ME Long-Term Nitric Oxide Release and Elevated Temperature Stability with S-Nitroso-N-Acetylpenicillamine (Snap)-Doped Elast-Eon E2as Polymer. Biomaterials 2013, 34, 6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Brisbois EJ; Kim M; Wang X; Mohammed A; Major TC; Wu J; Brownstein J; Xi C; Handa H; Bartlett RH; Meyerhoff ME Improved Hemocompatibility of Multilumen Catheters Via Nitric Oxide (No) Release from S-Nitroso-N-Acetylpenicillamine (Snap) Composite Filled Lumen. ACS Appl. Mater. Interfaces 2016, 8, 29270–29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Brisbois EJ; Major TC; Goudie MJ; Meyerhoff ME; Bartlett RH; Handa H Attenuation of Thrombosis and Bacterial Infection Using Dual Function Nitric Oxide Releasing Central Venous Catheters in a 9 Day Rabbit Model. Acta Biomater. 2016, 44, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chug MK; Feit C; Brisbois EJ Increasing the Lifetime of Insulin Cannula with Antifouling and Nitric Oxide Releasing Properties. ACS Appl. Bio Mater 2019, 2, 5965–5975. [DOI] [PubMed] [Google Scholar]
- (47).Malone-Povolny MJ; Schoenfisch MH Extended Nitric Oxide-Releasing Polyurethanes Via S-Nitrosothiol-Modified Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 12216–12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Doverspike JC; Mack SJ; Luo A; Stringer B; Reno S; Cornell MS; Rojas-Pena A; Wu J; Xi C; Yevzlin A; Meyerhoff ME Nitric Oxide-Releasing Insert for Disinfecting the Hub Region of Tunnel Dialysis Catheters. ACS Appl. Mater. Interfaces 2020, 12, 44475–44484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Jeakle MM; Major TC; Meyerhoff ME; Bartlett RH Comparison of Diazeniumdiolated Dialkylhexanediamines as Nitric Oxide Release Agents on Nonthrombogenicity in an Extracorporeal Circulation Model. ACS Appl. Bio Mater 2020, 3, 466–476. [DOI] [PubMed] [Google Scholar]
- (50).Singha P; Goudie MJ; Liu Q; Hopkins S; Brown N; Schmiedt CW; Locklin J; Handa H Multipronged Approach to Combat Catheter-Associated Infections and Thrombosis by Combining Nitric Oxide and a Polyzwitterion: A 7 Day in Vivo Study in a Rabbit Model. ACS Appl. Mater. Interfaces 2020, 12, 9070–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Devine R; Goudie M; Singha P; Douglass M; Schmiedt CW; Brisbois EJ; Handa H Mimicking the Endothelium: Dual Action Heparinized Nitric Oxide Releasing Surface. ACS Appl. Mater. Interfaces 2020, 12, 20158–20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Privett BJ; Broadnax AD; Bauman SJ; Riccio DA; Schoenfisch MH Examination of Bacterial Resistance to Exogenous Nitric Oxide. Nitric Oxide 2012, 26, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Ren H; Bull JL; Meyerhoff ME Transport of Nitric Oxide (No) in Various Biomedical Grade Polyurethanes: Measurements and Modeling Impact on No Release Properties of Medical Devices. ACS Biomater. Sci. Eng 2016, 2, 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Tsikas D; Dehnert S; Urban K; Surdacki A; Meyer HH Gc–Ms Analysis of S-Nitrosothiols after Conversion to S-Nitroso-N-Acetyl Cysteine Ethyl Ester and in-Injector Nitrosation of Ethyl Acetate. J. Chromatogr. B 2009, 877, 3442–3455. [DOI] [PubMed] [Google Scholar]
- (55).Van den Broeke L; Van Henegouwen GB Uv Radiation Protecting Efficacy of Cysteine Derivatives, Studies with Uva-Induced Binding of 8-Mop and Cpz to Rat Epidermal Biomacromolecules in Vivo. Int. J. Radiat. Biol 1995, 67, 411–420. [DOI] [PubMed] [Google Scholar]
- (56).Colletta A; Wu J; Wo Y; Kappler M; Chen H; Xi C; Meyerhoff ME S-Nitroso-N-Acetylpenicillamine (Snap) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device to Prevent Catheter Associated Urinary Tract Infections. ACS Biomater. Sci. Eng 2015, 1, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Cariello AJ; Bispo PJM; de Souza GFP; Pignatari ACC; de Oliveira MG; Hofling-Lima AL Bactericidal Effect of S-Nitrosothiols against Clinical Isolates from Keratitis. Clin. Ophthalmol 2012, 6, 1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Feit CG; Chug MK; Brisbois EJ Development of S-Nitroso-N-Acetylpenicillamine Impregnated Medical Grade Polyvinyl Chloride for Antimicrobial Medical Device Interfaces. ACS Appl. Bio Mater 2019, 2, 4335–4345. [DOI] [PubMed] [Google Scholar]
- (59).Tsikas D; Schwedhelm KS; Surdacki A; Giustarini D; Rossi R; Kukoc-Modun L; Kedia G; Uckert S S-Nitroso-N-Acetyl-L-Cysteine Ethyl Ester (Snacet) and N-Acetyl-L-Cysteine Ethyl Ester (Nacet)-Cysteine-Based Drug Candidates with Unique Pharmacological Profiles for Oral Use as No, H2s and Gsh Suppliers and as Antioxidants: Results and Overview. J. Pharm. Anal 2018, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zhang C; Biggs TD; Devarie-Baez NO; Shuang S; Dong C; Xian M S-Nitrosothiols: Chemistry and Reactions. Chem. Commun 2017, 53, 11266–11277. [DOI] [PubMed] [Google Scholar]
- (61).Mathews WR; Kerr SW Biological Activity of S-Nitrosothiols: The Role of Nitric Oxide. J. Pharmacol. Exp. Ther 1993, 267, 1529–1537. [PubMed] [Google Scholar]
- (62).Vaughn MW; et al. Estimation of Nitric Oxide Production and Reaction Rates in Tissue by Use of a Mathematical Model. Am. J. Physiol.: Heart Circ. Physiol 1998, 274, H2163–H2176. [DOI] [PubMed] [Google Scholar]
- (63).Friedman A; Blecher K; Sanchez D; Tuckman-Vernon C; Gialanella P; Friedman JM; Martinez LR; Nosanchuk JD Susceptibility of Gram-Positive and-Negative Bacteria to Novel Nitric Oxide-Releasing Nanoparticle Technology. Virulence 2011, 2, 217–221. [DOI] [PubMed] [Google Scholar]
- (64).Vilela SFG; Junqueira JC; Barbosa JO; Majewski M; Munin E; Jorge AOC Photodynamic Inactivation of Staphylococcus aureus and Escherichia coli Biofilms by Malachite Green and Phenothiazine Dyes: An in Vitro Study. Arch. Oral Biol 2012, 57, 704–710. [DOI] [PubMed] [Google Scholar]
- (65).Ren H; Colletta A; Koley D; Wu J; Xi C; Major TC; Bartlett RH; Meyerhoff ME Thromboresistant/Anti-Biofilm Catheters Via Electrochemically Modulated Nitric Oxide Release. Bioelectrochemistry 2015, 104, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Arora DP; Hossain S; Xu Y; Boon EM Nitric Oxide Regulation of Bacterial Biofilms. Biochemistry 2015, 54, 3717–3728. [DOI] [PubMed] [Google Scholar]
- (67).Wo Y; Li Z; Brisbois EJ; Colletta A; Wu J; Major TC; Xi C; Bartlett RH; Matzger AJ; Meyerhoff ME Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of Carbosil Polymer Doped with S-Nitroso-N-Acetyl-D-Penicillamine. ACS Appl. Mater. Interfaces 2015, 7, 22218–22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Leslie DC; Waterhouse A; Berthet JB; Valentin TM; Watters AL; Jain A; Kim P; Hatton BD; Nedder A; Donovan K; et al. A Bioinspired Omniphobic Surface Coating on Medical Devices Prevents Thrombosis and Biofouling. Nat. Biotechnol 2014, 32, 1134–1140. [DOI] [PubMed] [Google Scholar]
- (69).Sotiri I; Overton JC; Waterhouse A; Howell C Immobilized Liquid Layers: A New Approach to Anti-Adhesion Surfaces for Medical Applications. Exp. Biol. Med 2016, 241, 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Lee WH; Ren H; Wu J; Novak O; Brown RB; Xi C; Meyerhoff ME Electrochemically Modulated Nitric Oxide Release from Flexible Silicone Rubber Patch: Antimicrobial Activity for Potential Wound Healing Applications. ACS Biomater. Sci. Eng 2016, 2, 1432–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


