Supplemental Digital Content is available in the text.
Abstract
Background.
Liver transplantation (LT) has been employed for hepatic adenoma (HA) on a case-oriented basis. We aimed to describe the characteristics, waitlist, and post-LT outcomes of patients requiring LT for HA.
Methods.
All patients listed or transplanted for HA in the United States were identified in the United Network for Organ Sharing (UNOS) database (1987–2020). A systematic literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement.
Results.
A total of 199 HA patients were listed for LT in UNOS and the crude waitlist mortality was 9.0%. A total of 142 HA patients underwent LT; 118 of these were among those listed with an indication of HA who underwent LT, and 24 were diagnosed incidentally. Most did not experience hepatocellular carcinoma transformation (89.4%). Over a median follow-up of 62.9 mo, death was reported in 18.3%. The 1-, 3-, and 5-y patient survival rates were 94.2%, 89.7%, and 86.3% in the UNOS cohort. The systematic review yielded 61 articles reporting on 99 nonoverlapping patients undergoing LT for HA and 2 articles reporting on multicenter studies. The most common LT indications were suspected malignancy (39.7%), unresectable HA (31.7%), and increasing size (27.0%), whereas 53.1% had glycogen storage disease. Over a median follow-up of 36.5 mo, death was reported in 6.0% (n=5/84). The 1-, 3-, and 5-y patient survival rates were all 95.0% in the systematic review.
Conclusions.
LT for HA can lead to excellent long-term outcomes in well-selected patients. Prospective granular data are needed to develop more optimal selection criteria and further improve outcomes.
Hepatic adenoma (HA) is a benign liver tumor that most commonly occurs in women of reproductive age, classically receiving oral contraceptives.1 Development of HA has also been associated with underlying diseases of the liver, including glycogen storage disease (GSD) and absence of the portal vein (Abernethy malformation).2,3 In 1985, Flejou et al4 described liver adenomatosis, a variant of HA, referring to patients with >10 HAs in an otherwise normal liver parenchyma. The clinical presentation of HA is variable, with the most noticeable features being abdominal pain and bloating, hepatomegaly, or rupture with intraperitoneal bleeding, whereas some patients may be asymptomatic and have an incidental diagnosis of HA.5-7 The management of HA is also variable and relies mostly on symptom severity, the risk of hemorrhage or rupture, and the risk of malignant transformation.7-9 Treatment options range from regular follow-up accompanied with imaging and withdrawal of hormone-containing pills to liver resection or liver transplantation (LT).5,7-10
Despite the broadening of indications of LT for liver neoplasms, particularly of malignant nature,11-13 LT has been only rarely used for the management of HA. The current United Network for Organ Sharing (UNOS) guidance for granting exception points for HA includes having HA with risk of malignant transformation, not amenable to resection, and either or both (1) biopsy-proven malignancy or (2) presence of GSD. The available evidence is limited mostly to case reports or small case series. The only large report is a multicenter study from Europe reporting on <50 cases of LT for liver adenomatosis that span between 1986 and 2013.14 On account of the importance and rarity of employing LT for HA and the inability to draw solid conclusions based on the currently available level of evidence, revisiting this topic is of paramount importance. Therefore, we aimed to describe the demographics, clinical characteristics, indications, and outcomes of LT for HA through a retrospective analysis of a US transplant registry and a systematic review of the global literature.
MATERIALS AND METHODS
UNOS Analysis
Data Source, Patient Identification, Data Encoding
The UNOS database administers the Organ Procurement and Transplantation Network under contract with the US Department of Health and Human Services. This database contains data on all transplant candidates undergoing listing for solid organ transplantation in the United States since October 1987. All data were deidentified, and thus no Institutional Review Board approval was required.
Patient pretransplant, transplant, and follow-up data were obtained from the UNOS Standard Transplant Analysis and Research data file (released on September 4, 2020). In this retrospective cohort study, we included patients of all ages listed for LT or undergoing LT for HA between October 1987 and September 2020 in the United States. To avoid the introduction of bias by intrapatient correlation, when patients had multiple listings, only the most recent listing of each patient was used. Patients were identified using the primary and secondary listing diagnosis codes and transplant diagnosis codes from UNOS for HA (code 4450). In addition, to identify potentially missed patients with HA, we searched in the free-text field of the diagnosis codes using the word fragment “aden.” Recipients were considered to have a nonincidental HA, if HA was a listing diagnosis, whereas they were considered to have an incidental HA if they were listed for another indication, but HA was identified only among transplant recipient diagnosis variables. Recipients were considered to have a listing hepatocellular carcinoma (HCC) codiagnosis if HCC was a listing diagnosis, whereas they were considered to have an incidental HCC if it was identified only among transplant recipient diagnosis variables (codes 4400 or 4401). Recipients were also considered to have a codiagnosis of GSD if they had any GSD diagnosis code (code 4303 or 4304) or if GSD was present in any free-text field.
Statistical Analysis
Continuous variables were presented using medians and interquartile ranges (IQRs), whereas categorical variables were presented using frequencies and percentages. Univariable linear regression was used to assess the number of patients listed for LT over the study period.
For the waitlist outcomes analysis, there are 2 competing risks/outcomes: (1) waitlist mortality, defined as death/delisting due to being too sick, which was the main risk/outcome of interest, and (2) other reason for waitlist removal (including LT), which was the competing risk/outcome. Patients who were still on the waitlist by the date of the last follow-up were censored. Survival was measured from the date of listing for LT until the date of removal from the waitlist for any reason or until the date of the last follow-up. The effect of patient characteristics on waitlist mortality was explored using Fine-Gray15 competing risks regression modeling for competing risks analysis. In contrast to the cause-specific hazards estimates provided by the Cox regression models, the Fine-Gray15 competing risks regression models provide the subdistribution hazard ratios and 95% confidence intervals (95% CIs) of each competing outcome.
Post-LT patient and graft survival were defined as the duration from the date of LT until the date of last patient contact or patient death/graft loss, respectively. The Kaplan-Meier method was used to determine the 1-, 3-, and 5-y patient and graft survival rates. Cox regression models were also fitted to estimate the hazard ratio and 95% CI and to determine the effect of patient characteristics on patient mortality and graft loss. Cohort development and statistical analyses were performed using Stata IC 16.0 (StataCorp LLC, College Station, TX). All tests were 2-sided and P < 0.05 was considered statistically significant.
Systematic Review
Study Design, Search Strategy, Study Eligibility, and Selection
A systematic review of the literature was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement.16 No Institutional Review Board approval or patient written consent was necessary, since the systematic review used only published data. Articles published in English reporting on demographic, clinical characteristics, and outcomes of patients undergoing LT for HA were searched through the MEDLINE (via PubMed), Embase, Scopus, and Cochrane Library databases (last search date: March 2, 2021) using the following algorithm: (transpl*) AND (hepatic adenoma OR liver adenoma OR hepatic adenomatosis OR liver adenomatosis). Two researchers (I.A.Z., P.T.T.) performed the title/abstract and full-text screening stages of the literature search independently. The citations of the systematically reviewed studies were hand-searched for potentially eligible, missed studies using the snowball methodology.17 No sample size restriction or other search filters were applied. Any conflicts were resolved through quality control discussions. The Covidence reference and article manager software was used for all stages of the database search and study selection.18
Data Extraction and Tabulation
A standardized, prepiloted form was used for data tabulation and extraction from the included studies for evidence synthesis. Two reviewers (P.T.T., N.S.) extracted the data independently and any discrepancies were identified and resolved through quality control discussions with another author (I.A.Z.). The following data were extracted for each eligible study: first author, year of publication, transplant center and country, number of patients, age at diagnosis and at LT, sex, time of HA diagnosis (pre-LT or incidentally on explant pathology), clinical presentation and laboratory results, HA characteristics (number, size, liver location), indication for LT, underlying liver disease, immunohistochemistry findings, previous interventions, graft type and multiorgan transplant type, post-LT complications, and survival data (patient status, follow-up time, cause of death).
Data on characteristics and outcomes of interest were tabulated and analyzed cumulatively, whereas all relative rates were estimated based on the availability of data for each variable of interest. In case of overlapping study populations, only the larger study was included. When analyses on additional outcomes were presented in several eligible articles, data were extracted from all as their population was not summed in the overall subject numbers, but rather represented additional analyses on the same cohorts. Univariable linear regression was used to assess the number of patients undergoing LT for HA outside the United States (since the data in the United States were assessed using the UNOS database) over the study publication date.
RESULTS
UNOS Analysis
Waitlist Cohort
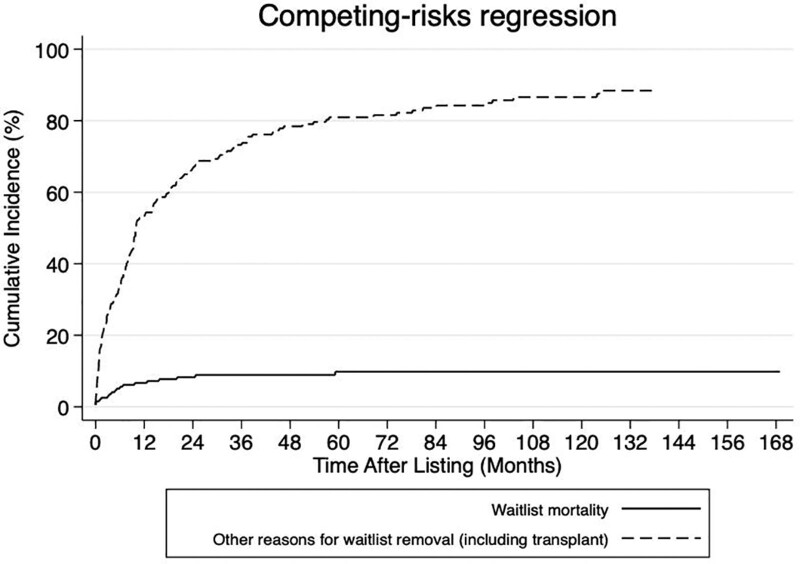
A total of 199 patients were listed for LT with an indication of HA between January 1994 and August 2020. No patient listed for HA was identified between 1987 and 1994. The number of patients listed for HA increased over the study period (P = 0.003). Data on number of HAs and HA size were not available. The overall crude waitlist mortality was 9.0% (n = 18 of 199), with the cause of death being available for 7 patients and that included cardiac arrest (n = 3), hemorrhage (n = 2), multiple organ failure (n = 1), infection/sepsis (n = 1); 118 patients underwent LT (Figure 1). The median age at listing was 36 y, 81.9% were female, the median waitlist time was 8.7 mo, and previous ablative therapy was administered in 4.7% (n = 6 of 128). Detailed characteristics for the patients with HA listed for LT in the UNOS database are presented in Table 1.
FIGURE 1.
Cumulative incidence competing risk curves of waitlist removal due to death or being too sick and due to other reasons in patients listed for liver transplant for hepatic adenoma from the United Network for Organ Sharing database.
TABLE 1.
Characteristics of waitlisted patients in the UNOS database
| Characteristic at listing a | Total (n = 199) |
|---|---|
| Age, y | 36.0 (22.0–46.0) |
| Waitlist time, mo | 8.7 (2.0–23.8) |
| Sex | |
| Female | 163 (81.9%) |
| Male | 36 (18.1%) |
| Race | |
| White | 126 (63.3%) |
| Black | 37 (18.6%) |
| Hispanic | 20 (10.1%) |
| Asian | 11 (5.5%) |
| American Indian/Alaska Native | 2 (1.0%) |
| Multiracial | 3 (1.5%) |
| Blood type | |
| A | 67 (33.7%) |
| AB | 6 (3.0%) |
| B | 26 (13.1%) |
| O | 100 (50.3%) |
| Height, cm (n = 193) | 162.6 (155.0–170.2) |
| Weight, kg (n = 198) | 71.6 (58.1–87.0) |
| BMI, kg/m2 (n = 193) | 26.4 (21.9–31.7) |
| Laboratory PELD/MELD score (n = 155) | 7.0 (6.0–9.0) |
| Albumin, g/dL (n = 164) | 4.0 (3.5–4.4) |
| Bilirubin, mg/dL (n = 164) | 0.6 (0.4–1.2) |
| INR (n = 164) | 1.0 (1.0–1.2) |
| Serum creatinine, mg/dL (n = 163) | 0.6 (0.5–0.8) |
| Serum sodium, mEq/L (n = 144) | 139.0 (137.5–141.0) |
| Ascites (n = 160) | 15 (9.4%) |
| Encephalopathy (n = 159) | 13 (8.2%) |
| PVT (n = 173) | 14 (8.1%) |
| Dialysis within prior wk (n = 161) | 3 (1.9%) |
| On life support | 4 (2.0%) |
| Exception points given | 91 (45.7%) |
| Previous ablative therapy (n = 128) | 6 (4.7%) |
| Outcome | |
| Died | 12 (6.0%) |
| Delisted for being too sick | 6 (3.0%) |
| Condition improved | 23 (11.6%) |
| Transplanted | 118 (59.3%) |
| Refused transplant | 2 (1.0%) |
| Still waiting | 16 (8.0%) |
| Other | 17 (8.5%) |
| Unable to contact | 5 (2.5%) |
aThe data are available for the whole cohort of 199 patients unless otherwise specified.
Continuous variables are presented as medians and interquartile ranges. Categorical variables are presented as frequencies and percentages.
BMI, body mass index; INR, international normalized ratio; MELD, model for end-stage liver disease; PELD, pediatric end-stage liver disease; PVT, portal vein thrombosis; UNOS, United Network for Organ Sharing.
Univariable Fine-Gray15 competing risks regression modeling showed that increasing age, laboratory model for end-stage liver disease (MELD)/pediatric end-stage liver disease (PELD) score, international normalized ratio, serum creatinine, decreasing albumin, ascites, and being on life support were associated with an increased risk of waitlist mortality (Table S1, SDC, http://links.lww.com/TXD/A391).
Transplant Cohort
A total of 142 patients with HA underwent LT; 118 of these were among those mentioned previously who were listed for LT with an indication of HA and ended up undergoing LT, and 24 were diagnosed incidentally. The median age at LT was 34.5 y with 32 (22.5%) patients being children (<18 y), the majority (79.6%) were female, and 9 patients had a codiagnosis of GSD (6.3%). Data on number of HAs and HA size were not available. The median laboratory MELD/PELD score was 8 and 61.3% received exception points. Most patients received a deceased donor whole graft (90.1%), and 4 patients underwent simultaneous liver-kidney transplant (2.8%), whereas 1 patient underwent simultaneous liver-pancreas-small bowel transplant (0.7%). The majority did not experience HCC transformation (89.4%), whereas 7 (4.9%) had a listing codiagnosis of HCC, and 8 (5.6%) had an incidental HCC noted on explant. Detailed characteristics for the patients with HA undergoing LT in the UNOS database are presented in Table 2.
TABLE 2.
Characteristics of transplanted patients in the UNOS database
| Characteristic at transplant a | Total (n = 142) |
|---|---|
| Time of HA diagnosis | |
| Pre-LT/listing | 118 (83.1%) |
| Incidental (explant pathology) | 24 (16.9%) |
| Age, y | 34.5 (19.0–47.0) |
| Sex | |
| Female | 113 (79.6%) |
| Male | 29 (20.4%) |
| Race | |
| White | 96 (67.6%) |
| Black | 23 (16.2%) |
| Hispanic | 15 (10.6%) |
| Asian | 4 (2.8%) |
| American Indian/Alaska Native | 2 (1.4%) |
| Multiracial | 2 (1.4%) |
| Blood type | |
| A | 50 (35.2%) |
| AB | 6 (4.2%) |
| B | 18 (12.7%) |
| O | 68 (47.9%) |
| Height, cm | 162.6 (154.9–170.0) |
| Weight, kg | 68.1 (55.1–84.5) |
| BMI, kg/m2 | 24.9 (21.0–30.8) |
| Laboratory PELD/MELD score (n = 110) | 8.0 (6.0–13.0) |
| Albumin, g/dL (n = 140) | 3.9 (3.2–4.4) |
| Bilirubin, mg/dL (n = 141) | 0.7 (0.4–1.8) |
| INR (n = 116) | 1.1 (1.0–1.3) |
| Serum creatinine, mg/dL | 0.7 (0.5–0.9) |
| Serum sodium, mEq/L (n = 103) | 139.0 (137.0–141.0) |
| Ascites (n = 107) | 19 (17.8%) |
| Encephalopathy (n = 107) | 15 (14.0%) |
| PVT (n = 121) | 18 (14.9%) |
| Dialysis within prior wk (n = 131) | 5 (3.8%) |
| On life support (n = 141) | 8 (5.7%) |
| Exception points given | 87 (61.3%) |
| Previous ablative therapy (n = 92) | 3 (3.3%) |
| Malignant transformation/HCC | |
| No | 127 (89.4%) |
| Pre-LT/listing HCC | 7 (4.9%) |
| Incidental HCC | 8 (5.6%) |
| Graft type | |
| Deceased donor whole graft | 128 (90.1%) |
| Deceased donor partial/split graft | 5 (3.5%) |
| Living donor graft | 9 (6.3%) |
aThe data are available for the whole cohort of 142 patients unless otherwise specified.
Continuous variables are presented as medians and interquartile ranges. Categorical variables are presented as frequencies and percentages.
BMI, body mass index; HA, hepatic adenoma; HCC, hepatocellular carcinoma; INR, international normalized ratio; LT, liver transplantation; MELD, model for end-stage liver disease; PELD, pediatric end-stage liver disease; PVT, portal vein thrombosis; UNOS, United Network for Organ Sharing.
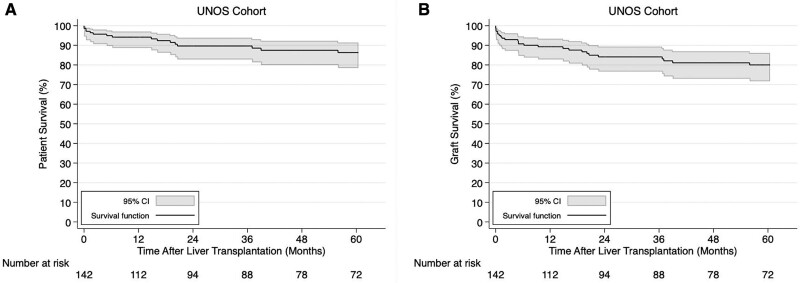
Over a median follow-up of 62.9 (IQR, 13.6–124.1) mo, death was reported in 18.3% (n = 26 of 142) and the cause of death was liver malignancy (n = 3), secondary extrahepatic cancer unrelated to the liver (n = 6), infection (n = 7; 6 had sepsis, 1 had multifocal pneumonia), pulmonary edema/respiratory failure (n = 3; 2 also had pneumonia), operative mortality/cardiac arrest (n = 2), primary graft failure with concomitant renal failure (n = 1), renal failure (n = 1), congestive heart failure (n = 1), hemorrhagic shock (n = 1), and unknown (n = 1). The 1-, 3-, and 5-y patient survival rates were 94.2% (95% CI, 88.7%-97.0%), 89.7% (95% CI, 82.8%-93.9%), and 86.3% (95% CI, 78.5%-91.5%) (Figure 2A). The 1-, 3-, and 5-y graft survival rates were 89.3% (95% CI, 82.8%-93.4%), 84.1% (95% CI, 76.6%-89.4%), and 80.0% (95% CI, 71.7%-86.1%) (Figure 2B). Univariable Cox regression modeling showed that increasing age and laboratory MELD/PELD score, male sex, decreasing albumin and serum sodium, ascites, encephalopathy, and being on life support were associated with an increased risk of post-LT patient mortality (Table S2, SDC, http://links.lww.com/TXD/A391). However, only increasing laboratory MELD/PELD score, decreasing albumin and serum sodium, and being on life support were associated with an increased risk of post-LT graft loss in univariable Cox regression (Table S2, SDC, http://links.lww.com/TXD/A391).
FIGURE 2.
Kaplan-Meier patient (A) and graft (B) survival curves in hepatic adenoma liver transplant recipients from the United Network for Organ Sharing (UNOS) database. CI, confidence interval.
Systematic Review
Study Characteristics
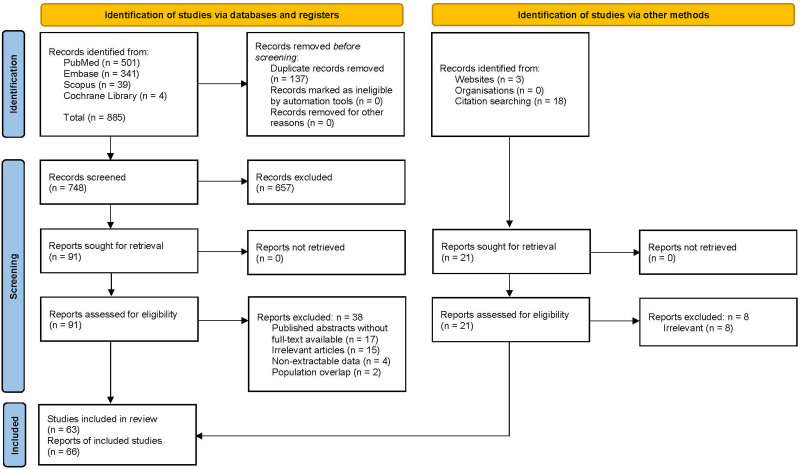
Our initial database search yielded 748 unique records, 91 of which were retrieved for full-text assessment, 53 of which fulfilled the inclusion criteria and were ultimately included. Another 21 records were retrieved for full-text assessment, 13 of which were ultimately included. Overall, 66 reports were included2,10,14,19-81; since 4 of them reported on overlapping patients,39,50,61,72 only the largest series of 4 was included,39 whereas data were extracted from all as their population represented additional analyses on the same patients, yielding a total of 63 included studies (Figure 3). Two of the 63 studies were multicenter14,79 and reported on 5 patients79 and 49 patients14 (that may be overlapping to a certain extent), respectively, whereas the rest 61 studies reported on 99 nonoverlapping (unique) patients who underwent LT for HA (Table 3). Sixty-seven patients underwent LT for HA outside the United States, and the number of patients did not appear to change over the publication date (P = 0.62).
FIGURE 3.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow diagram of the search strategy and study selection.
TABLE 3.
Characteristics of studies included in the systematic review
| Author, year | Center, city | Country | Number of patients |
|---|---|---|---|
| Registry studies | |||
| Current UNOS analysis | Multicenter | United States | 142 |
| Baiges, 202079 | Multicenter | International | 5 |
| Chiche, 201614 | Multicenter | European | 49 |
| Unique patients | |||
| Intaraprasong et al, 202157 | Ramathibodi Hospital Mahidol University, Bangkok | Thailand | 1 |
| Singh et al, 202030 | The Ohio State University Wexner Medical Center, Columbus | United States | 1 |
| Barbier et al, 201936 | University of Tours, Tours | France | 1 |
| Salhanick et al, 201931 | University of Texas Southwestern Medical Center, Dallas | United States | 1 |
| Timothy et al, 201924 | Mayo Clinic, Rochester | United States | 1 |
| Vali et al, 201975 | Tartu University Hospital, Tartu | Estonia | 1 |
| Mohkam et al, 201742 | Croix-Rousse University Hospital, Lyon | France | 1 |
| Leone et al, 201634 | Tampa General Hospital, Tampa | United States | 1 |
| Samuk et al, 201632 | University of Miami Miller School of Medicine, Miami | United States | 1 |
| Sorkin et al, 201669 | King’s College Hospital, London | United Kingdom | 1 |
| Szili et al, 201674 | Semmelweis University, Budapest | Hungary | 1 |
| Brasoveanu et al, 201545 | Fundeni Clinical Institute, Bucharest | Romania | 1 |
| Nacif et al, 201547 | University of São Paulo School of Medicine, São Paulo | Brazil | 1 |
| Oterdoom et al, 201576 | Erasmus Medical Center, Rotterdam | Netherlands | 1 |
| Sanada et al, 201570 | Jichi Medical University, Shimotsuke | Japan | 5 |
| Solbach et al, 201571 | Medizinische Hochschule, Hannover | Germany | 1 |
| Fernández-Vega et al, 201455 | Hospital Universitario Central de Asturias, Oviedo | Spain | 1 |
| Gordon-Burroughs et al, 201427 | Houston Methodist Hospital, Houston | United States | 1 |
| Burgis et al, 201319 | Lucile Packard Children’s Hospital, Palo Alto | United States | 1 |
| Carvalho et al, 201349 | Coimbra University Hospital, Coimbra | Portugal | 1 |
| Colle et al, 201353 | Ghent University Hospital, Ghent | Belgium | 1 |
| Maya Aparicio et al, 201356 | Virgen del Rocio University Hospitals, Seville | Spain | 1 |
| Vennarecci et al, 201364 | San Camillo Hospital, Rome | Italy | 1 |
| Sakellariou et al, 20122 | King’s College Hospital, London | United Kingdom | 7 |
| Manzia et al, 201168 | Tor Vergata University, Rome | Italy | 1 |
| Marega et al, 201163 | Universitaria S. Maria della Misericordia, Udine | Italy | 1 |
| Franchi-Abella et al, 201044 | Hopital Bicetre, Le Kremlin Bicetre | France | 1 |
| Raphe et al, 201048 | Hospital de Base, São José do Rio Preto | Brazil | 1 |
| Wellen et al, 201022 | Washington University School of Medicine, St. Louis | United States | 1 |
| Bioulac-Sage et al, 200943 | Centre Hospitalier Universitaire (CHU) Bordeaux, Bordeaux | France | 3 |
| Di Sandro et al, 200962 | Niguarda Ca’ Granda Hospital, Milan | Italy | 1 |
| Dokmak et al, 200910 | University of Paris 7 and Beaujon Hospital, Clichy | France | 1 |
| Ji et al, 200960 | Zhejiang University School of Medicine, Hangzhou | China | 1 |
| Reddy et al, 200921 | Duke University Medical Center, Durham | United States | 5 |
| Santambrogio et al, 200965 | University of Milan, Milan | Italy | 1 |
| Sibulesky et al, 200929 | Mayo Clinic, Jacksonville | United States | 1 |
| Di Rocco et al, 200867 | Gaslini Institut, Genoa | Italy | 2 |
| Carreiro et al, 200746 | Clementino Fraga Filho University Hospital, Rio de Janeiro | Brazil | 1 |
| Davis and Weinstein, 200726 | University of Florida College of Medicine, Gainesville | United States | 1 |
| Iyer et al, 200758 | Chang Gung Memorial Hospital-Kaohsiung Medical Center, Niao-Sung | Taiwan | 3 |
| Morotti et al, 200728 | Mount Sinai School of Medicine, New York City | United States | 1 |
| Fujita et al, 200625 | University of Florida College of Medicine, Gainesville | United States | 1 |
| Panaro et al, 200466 | St. Martino Hospital University of Genoa, Genoa | Italy | 1 |
| Wojcicki et al, 200477 | University Hospital Groningen, Groningen | Netherlands | 1 |
| Lerut et al, 200354 | Universite´ catholique de Louvain, Brussels | Belgium | 3 |
| Liu et al, 200359 | Kaohsiung Medical Center, Kaohsiung | Taiwan | 2 |
| Chiche et al, 200041 | University Hospital of Caen, Caen | France | 2 |
| Koestinger et al, 200078 | Lausanne University School of Medicine, Lausanne | Switzerland | 1 |
| Weimann et al, 200073 | Medizinische Hochschule Hannover, Hannover | Germany | 4 |
| Faivre et al, 199938 | Hopital Bicetre, Le Kremlin Bicetre | France | 3 |
| Matern et al, 199980 | Duke University Medical Center, Durham | United States | 2 |
| Reid and Hebert, 199652 | Hospital for Sick Children, Toronto | Canada | 2 |
| Mueller et al, 199520 | California Pacific Medical Center, San Francisco | United States | 2 |
| Tepetes et al, 199539 | University of Pittsburgh Medical Center, Pittsburgh | United States | 6 |
| Marino et al, 199250 | |||
| Malatack et al, 198361 | |||
| Selby et al, 199372 | |||
| Alshak et al, 199481 | Cedars-Sinai Medical Center, Los Angeles | United States | 1 |
| Bernard et al, 199437 | University of Bordeaux, Bordeaux | France | 1 |
| Janes et al, 199323 | Mayo Clinic, Rochester | United States | 1 |
| Kirschner et al, 199133 | University of Chicago Hospitals, Chicago, | United States | 1 |
| Leese et al, 198840 | Hopital Paul Brousse, Villejuif | France | 2 |
| Poe and Snover, 198835 | University of Minnesota Medical School, Minneapolis | United States | 2 |
| Coire et al, 198751 | Henderson General Hospital and McMaster University, Toronto | Canada | 1 |
| Total | 99 | ||
UNOS, United Network for Organ Sharing.
Patient Characteristics
As not all reports contained all variables of interest, relative rates were estimated based on the availability of data. The median age at diagnosis and at LT in the nonoverlapping systematically reviewed patients was 19.5 and 25.0, respectively, with 24 of 86 (27.9%) patients being children at the time of LT. Most patients were female (60.7%), and most had multiple HAs (75.3%), whereas 93.5% were diagnosed before LT and only 6.5% were incidental. The most common indications for LT were suspected malignancy (39.7%), unresectable HA (31.7%), and increasing HA size (27.0%). Biopsy-confirmed HCC transformation pre-LT was an indication in 9.5% (n = 6), whereas more than half of the patients (53.1%) had GSD. The graft type for LT was reported in only 27 of the 99 cases (27.3%). Thirteen were performed using whole deceased donor graft, 1 using reduced-size deceased donor graft, 1 using split deceased donor graft, and 12 using living donor graft. Multiorgan transplant was performed in 10.7% (n = 9 of 84; 8 were simultaneous liver-kidney transplants and 1 was simultaneous liver-small bowel transplant). Malignant transformation was reported in 18 (23.1%) with 6 (7.7%) being biopsy-confirmed HCC transformation pre-LT and 12 (15.4%) incidental HCC on explant pathology. Detailed characteristics for the systematically reviewed patients with HA undergoing LT are presented in Table 4.
TABLE 4.
Characteristics of patients included in the systematic review
| Characteristic | Total (n = 99) |
|---|---|
| Age at diagnosis (n = 52) | 19.5 (14.8–31.0) |
| Age at LT (n = 86) | 25.0 (17.0–31.0) |
| Sex (n = 84) | |
| Female | 51 (60.7%) |
| Male | 33 (39.3%) |
| Number of HAs (n = 77) | |
| 1 | 14 (18.2%) |
| 2 | 5 (6.5%) |
| Multiple (>2) | 58 (75.3%) |
| Size of largest HA (n = 43) | 5.0 (2.0-10.0) |
| Location of HA (n = 40) | |
| Left lobe | 4 (10.0%) |
| Right lobe | 9 (22.5%) |
| Both lobes | 27 (67.5%) |
| Time of diagnosis (n = 92) | |
| Pre-LT | 86 (93.5%) |
| Incidental (explant pathology) | 6 (6.5%) |
| Indication (n = 63)a | |
| Suspected malignancy | 25 (39.7%) |
| Unresectable HA | 20 (31.7%) |
| Increasing HA size | 17 (27.0%) |
| Clinical deteriorationb | 14 (22.2%) |
| Recurrent HA after resection | 10 (15.9%) |
| Biopsy-confirmed HCC transformation pre-LT | 6 (9.5%) |
| Risk of rupture | 5 (7.9%) |
| Encephalopathy | 3 (4.8%) |
| Rupture | 2 (3.2%) |
| Lipoprotein lipase deficiency | 1 (1.6%) |
| Underlying liver disease (n = 98) | |
| No | 27 (27.6%) |
| Glycogen storage disease | 52 (53.1%) |
| Abernethy malformation/absence of portal vein | 14 (14.3%) |
| Steatosis | 4 (4.1%) |
| Idiopathic portal hypertension with portal vein patency | 1 (1.0%) |
| Malignant transformation/HCC (n = 78) | |
| No | 60 (76.9%) |
| Biopsy-confirmed HCC transformation pre-LT | 6 (7.7%) |
| Incidental HCC (explant pathology) | 12 (15.4%) |
Continuous variables are presented as medians and interquartile ranges. Categorical variables are presented as frequencies and percentages.
aThe numbers do not add up to 63 because many patients had >1 indication for LT.
bRefers to worsening abdominal pain, end-stage liver disease–related symptoms, metabolic derangement, or hormonal disturbances.
HA, hepatic adenoma; HCC, hepatocellular carcinoma; LT, liver transplantation.
Clinical Presentation
Patients with HA presented with abdominal pain, hepatomegaly, pruritus, subclinical hemorrhage, hepatic encephalopathy, whereas few of the patients were asymptomatic. Five of the 99 patients (5.1%) presented initially with HA intraperitoneal rupture; 2 of them underwent upfront LT during the same admission, 1 was managed conservatively and underwent interval LT, 1 was managed with left lobectomy but 5 y after presentation the patient underwent LT for suspected malignant transformation, and 1 patient underwent left lateral segmentectomy followed by a second liver resection for HA recurrence and finally underwent interval LT due to recurrence of numerous HAs. Another 2 patients presented with intratumoral rupture and underwent interval LT due to increased risk of hemorrhage and worsening symptoms.
Immunohistochemistry
Immunohistochemistry post-LT was reported in only 21 of the 99 patients (21.2%) and had positive findings in 18 of them. One patient had HA positive to both hepatocyte nuclear factor-1 alpha and beta-catenin (b-CAT), 3 patients had HA positive to both inflammatory hepatocellular adenoma (IHCA) immunohistochemistry and b-CAT, 3 patients had HA positive to only IHCA, and 11 patients had HA positive to only b-CAT. Pretransplant histology was not reported.
Previous Interventions
Thirty-three patients had a previous intervention (39.3%, n = 33 of 84). Twenty-seven of them underwent a previous liver resection (32.1%; 3 patients underwent previous liver resection twice), 4 patients underwent portocaval shunt (4.8%; 1 of them had undergone a previous liver resection), 3 patients underwent previous embolization (3.6%; 2 of them had undergone a previous liver resection), 1 mesocaval anastomosis (1.2%), and 1 patient underwent diagnostic laparotomy (1.2%).
Outcomes
Retransplantation was required in 2.3% (n = 2 of 87). Post-LT complications were reported in 25.0% (n = 21 of 84). These included acute rejection (13.1%, n = 11 of 84), renal failure (4.8%, n = 4 of 84; 2 patients also experienced acute rejection, whereas 1 patient also experienced arthritis secondary to tacrolimus, left upper extremity deep vein thrombosis, incisional hernia, diabetes mellitus, and osteopenia), cytomegalovirus infection (n = 3; 1 of them also experienced acute rejection), tremor secondary to tacrolimus (n = 1), ischemic stroke (n = 1), hepatitis C virus infection (n = 1), portal vein thrombosis (n = 2; 1 of them also experienced bile leak and subphrenic hematoma), and hepatic vein thrombosis (n = 1).
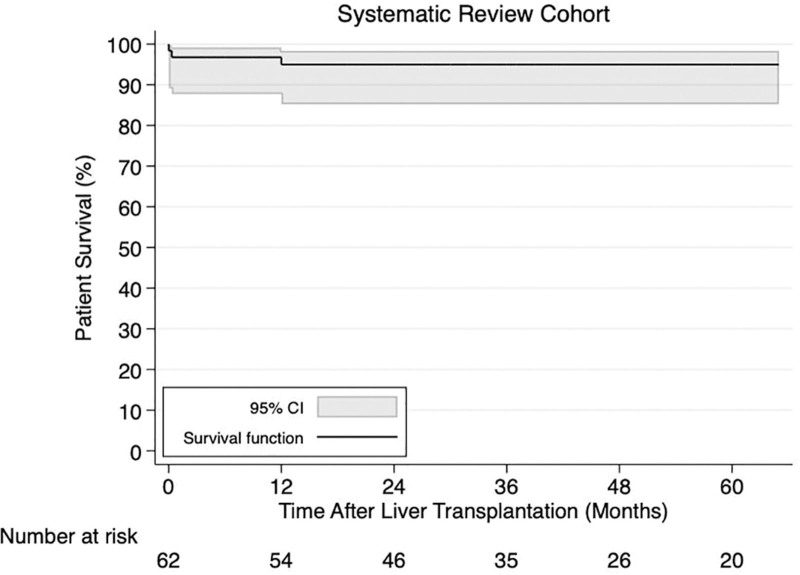
Over a median follow-up of 36.5 (IQR, 19.0–72.0) mo, death was reported in 6.0% (n = 5 of 84) and the cause of death was necrotizing pancreatitis (n = 1), postoperative complications (n = 1), multiorgan failure (n = 1), septic organ failure (n = 1), and recurrent HCC (n = 1). Complete survival data (patient status and follow-up) were available for 62 patients. The 1-, 3-, and 5-y patient survival rates after LT were all 95.0% (95% CI, 85.2%-98.4%) (Figure 4).
FIGURE 4.
Kaplan-Meier patient survival curve in hepatic adenoma liver transplant recipients from the systematically reviewed articles. CI, confidence interval.
DISCUSSION
HA constitutes an entity with heterogeneous clinical presentation that requires a case-oriented management approach.9 LT has been proposed as a treatment option in certain patients with HA, yet the indications and outcomes have been unclear. In the present study, we are the first to use the UNOS data to examine the characteristics and waitlist outcomes of patients with HA listed for LT and showed that <1 out of 10 patients with HA experience waitlist mortality, and the main causes include cardiac arrest and hemorrhage with excellent post-LT outcomes. Interestingly, majority of patients do not fit the UNOS Regional Board Guidelines of awarding exception points for HA because 62.5% of listed patients received MELD exception points, but only 6.3% had diagnosis of GSD and 4.9% had listing codiagnosis of HCC. Furthermore, only 5.6% had additional incidental explant finding of HCC.
We also compiled all the previously published data through a systematic literature review to examine the characteristics and outcomes of patients with HA undergoing LT. Our findings suggest that the typical LT recipient is a White woman of reproductive age with multiple HAs diagnosed pre-LT (nonincidental) and a low laboratory MELD score. The most common reasons for LT appear to be the inability to surgically resect the HA, suspicion for malignancy, increase in size and risk of rupture, underlying liver disease, or recurrence after liver resection.
The only other large study in the literature is a 2016 European report by Chiche et al14 on LT for 49 patients with liver adenomatosis. About one-third of that cohort had underlying GSD, which is lower than the respective proportion of our systematic review, whereas a similar proportion of patients had underlying vascular abnormality (14%–15%). Although confirmed pre-LT HCC was reported in a smaller proportion of patients in our UNOS analysis and systematic review (5%–8%), a similar proportion of patients had suspected malignancy in both our systematic review and in the European multicenter study.14 A similar proportion of patients underwent prior embolization in our systematic review and in the European report (both 4%), whereas a slightly higher proportion of patients underwent prior liver resection in the European report (43%) compared with our systematic review (32%). These differences might reflect a difference in practices among healthcare systems but may also be subject to reporting bias or heterogeneity. Although a similar post-LT mortality was described in the European report (16.3%) and in our UNOS analysis (18.3%), the respective proportion was lower in the systematic review (6%), which can be attributed to either publication bias (cases with dismal outcomes are less likely to be published) or the shorter follow-up period in the systematic review given the fact that the most common causes of death in this population are recurrence of liver malignancy or unrelated extrahepatic post-LT malignancy. Additionally, in an international observational study, Baiges et al79 described 5 patients with Abernethy malformation undergoing LT for HA and reported only 1 death, which was due to septic complications.
The current study represents the largest cohort of LT recipients for HA, as well as the first systematic review of the literature to synthesize the currently available data on the demographic, clinicopathological characteristics, and outcomes in these patients. Although there is the possibility of patient overlap between the systematic review and the UNOS cohort, the value of the systematic review lies in its role to supplement the UNOS analysis and provide further insight and granularity into important parameters of interest that are currently not captured in US transplant registries. Although nearly 5% of patients in the UNOS cohort had a listing codiagnosis of HCC, more granular data on the specific indications for LT for HA are not available in the registry. However, according to the systematic review, the most common specific indications for LT for HA were suspected malignancy (39.7%), unresectable HA (31.7%), and increasing HA size (27.0%) with about 9.5% of patients having been diagnosed with HCC pre-LT. Given the limitation of underreporting liver codiagnoses in US transplant registries, the systematic review data showed that more than half of the patients had GSD and nearly 15% had Abernethy malformation or absence of the portal vein.
Given the favorable outcomes of LT for HA and the limited use of grafts from living donors not only in our analysis of the UNOS data and our systematic review, but also in the European experience,14 we believe that earlier LT evaluation of patients at high risk of requiring LT for HA—including patients with HA and suspected malignancy, patients with HA of increasing size or multiple and unresectable HAs, or even patients with GSD under surveillance who are at risk for developing HA—will allow for more time to identify a potential living donor and take advantage of this important yet underutilized source of grafts in the future. Moreover, advancements in the risk assessment for malignant transformation of HA could contribute to an efficient selection system of patients in need of LT and to an improved system for allocation of deceased donor grafts in this patient population. HA is a heterogeneous disease comprising of different molecular subtypes, such as the hepatocyte nuclear factor-1 alpha (30%–35%), b-CAT (10%–15%), IHCA (40%–50%), and unclassified (<10%) subtypes.82,83 In addition to biopsy, MRI-based approaches of HA subtyping have been described and, although the accuracy for b-CAT—which is associated with an increased risk of malignant transformation—may be limited, further refinement and validation of these methods can individualize the surveillance and management of HA in the future, as well as provide further insight about the role of LT in our armamentarium against HA.83,84
Our study has some limitations. These include the potential miscoding of retrospective US transplant registry data (HA or HCC may be underreported or misclassified as another pretransplant diagnosis in some patients with HA or HCC) and paucity of data on clinically important parameters relevant to the disease process, such as pre-LT management and more specific listing indication data for HA. Regarding the systematic review, all included articles were retrospective case reports or series, and thus impart a degree of inherent selection bias and heterogeneity. It is likely that some patients in the systematic review cohort may also be represented in the UNOS cohort. In addition, the published articles did not report on the same variables that are available in UNOS, and therefore some data are presented only in the UNOS cohort and some only in the systematic review cohort. Moreover, not all studies reported on all variables of interest, and therefore, all analyses were performed according to the availability of data. For instance, laboratory values were only available for ≈10%–20% of the patients at the time of diagnosis, at the time of LT, and post-LT, and thus no meaningful analyses could be pursued. Clinical manifestations were also significantly underreported in the literature (<25% of the patients), and because we could not accurately quantify the respective relative rates, these findings were presented qualitatively. Finally, the number of LTs for HA performed outside the United States did not change over publication date according to our systematic review, but this is subject to publication bias and may not necessarily reflect what happens in the real world.
In conclusion, LT constitutes a feasible option for patients with HAs at high risk of malignant transformation, proven malignancy, and underlying diagnosis of GSD with very low waitlist mortality and excellent post-LT outcomes. The rarity of this condition precludes any definitive conclusions from available data that may change current allocation guidelines. Development of a multicenter registry collecting prospective granular data is warranted to develop more optimal patient selection criteria and further improve outcomes in this complex patient population.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
I.A.Z. contributed to conception and design, analysis and interpretation of data, drafting of the article, final approval of the article submitted, and agreement to be accountable for all aspects of the work. P.T.T. contributed to analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the article submitted, and agreement to be accountable for all aspects of the work. N.S. contributed to analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the article submitted, and agreement to be accountable for all aspects of the work. S.P.A. contributed to analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the article submitted, and agreement to be accountable for all aspects of the work. M.I.M. contributed to analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the article submitted, and agreement to be accountable for all aspects of the work. A.S. contributed to conception and design, analysis and interpretation of data, drafting of the article, final approval of the article submitted, and agreement to be accountable for all aspects of the work.
Part of the findings were presented at the American Association for the Study of Liver Diseases, The Liver Meeting Digital Experience™, November 12–15, 2021.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. [PubMed] [Google Scholar]
- 2.Sakellariou S, Al-Hussaini H, Scalori A, et al. Hepatocellular adenoma in glycogen storage disorder type I: a clinicopathological and molecular study. Histopathology. 2012;60:E58–E65. [DOI] [PubMed] [Google Scholar]
- 3.Benedict M, Rodriguez-Davalos M, Emre S, et al. Congenital extrahepatic portosystemic shunt (Abernethy Malformation Type Ib) with associated hepatocellular carcinoma: case report and literature review. Pediatr Dev Pathol. 2017;20:354–362. [DOI] [PubMed] [Google Scholar]
- 4.Flejou JF, Barge J, Menu Y, et al. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89:1132–1138. [PubMed] [Google Scholar]
- 5.Cherqui D, Rahmouni A, Charlotte F, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674–1681. [PubMed] [Google Scholar]
- 6.Marrero JA, Ahn J, Rajender Reddy K; Americal College of Gastroenterology. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109:1328–1347; quiz 1348. [DOI] [PubMed] [Google Scholar]
- 7.Klompenhouwer AJ, de Man RA, Thomeer MG, et al. Management and outcome of hepatocellular adenoma with massive bleeding at presentation. World J Gastroenterol. 2017;23:4579–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farges O, Ferreira N, Dokmak S, et al. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. [DOI] [PubMed] [Google Scholar]
- 9.van der Windt DJ, Kok NF, Hussain SM, et al. Case-orientated approach to the management of hepatocellular adenoma. Br J Surg. 2006;93:1495–1502. [DOI] [PubMed] [Google Scholar]
- 10.Dokmak S, Paradis V, Vilgrain V, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–1705. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 12.Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201–207. [DOI] [PubMed] [Google Scholar]
- 13.Ziogas IA, Giannis D, Economopoulos KP, et al. Liver transplantation for intrahepatic cholangiocarcinoma: a meta-analysis and meta-regression of survival rates. Transplantation. 2021;105:2263–2271. [DOI] [PubMed] [Google Scholar]
- 14.Chiche L, David A, Adam R, et al. Liver transplantation for adenomatosis: European experience. Liver Transpl. 2016;22:516–526. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In: Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering (EASE ’14), London, United Kingdom. Association for Computer Machinery; May 13–14, 2014:1–10. [Google Scholar]
- 18.Covidence. Covidence Systematic Review Software. Available at www.covidence.org. Accessed March 2, 2021.
- 19.Burgis JC, Pratt CA, Higgins JP, et al. Multiple hepatic adenomas in a child with microvillus inclusion disease. Dig Dis Sci. 2013;58:2784–2788. [DOI] [PubMed] [Google Scholar]
- 20.Mueller J, Keeffe EB, Esquivel CO. Liver transplantation for treatment of giant hepatocellular adenomas. Liver Transpl Surg. 1995;1:99–102. [DOI] [PubMed] [Google Scholar]
- 21.Reddy SK, Austin SL, Spencer-Manzon M, et al. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483–490. [DOI] [PubMed] [Google Scholar]
- 22.Wellen JR, Anderson CD, Doyle M, et al. The role of liver transplantation for hepatic adenomatosis in the pediatric population: case report and review of the literature. Pediatr Transplant. 2010;14:E16–E19. [DOI] [PubMed] [Google Scholar]
- 23.Janes CH, McGill DB, Ludwig J, et al. Liver cell adenoma at the age of 3 years and transplantation 19 years later after development of carcinoma: a case report. Hepatology. 1993;17:583–585. [DOI] [PubMed] [Google Scholar]
- 24.Timothy LD, Lehrke HD, Chandan VS, et al. Diffuse adenomatosis and hepatocellular carcinoma treated with liver transplantation in an adolescent female with kabuki syndrome with a novel KMT2D gene mutation. Case Rep Pediatr. 2019;2019:7983824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita S, Mekeel KL, Fujikawa T, et al. Liver-occupying focal nodular hyperplasia and adenomatosis associated with intrahepatic portal vein agenesis requiring orthotopic liver transplantation. Transplantation. 2006;81:490–492. [DOI] [PubMed] [Google Scholar]
- 26.Davis MK, Weinstein DA. Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant. 2008;12:137–145. [DOI] [PubMed] [Google Scholar]
- 27.Gordon-Burroughs S, Balogh J, Weiner MA, et al. Liver transplantation in an adult with adenomatosis and congenital absence of the portal vein: a case report. Transplant Proc. 2014;46:2418–2421. [DOI] [PubMed] [Google Scholar]
- 28.Morotti RA, Killackey M, Shneider BL, et al. Hepatocellular carcinoma and congenital absence of the portal vein in a child receiving growth hormone therapy for turner syndrome. Semin Liver Dis. 2007;27:427–431. [DOI] [PubMed] [Google Scholar]
- 29.Sibulesky L, Burcin Taner C, Willingham DL, et al. Orthotopic liver transplantation for hepatic adenoma in a patient with portal vein agenesis. Open Transplant J. 2009;3:22–25. [Google Scholar]
- 30.Singh N, El-Hinnawi A, Hill B, et al. Hepatic resection and transplant in glycogen storage diseases. Exp Clin Transplant. [Epub ahead of print. February 7, 2020]. doi: 10.6002/ect.2019.0313 [DOI] [PubMed] [Google Scholar]
- 31.Salhanick M, MacConmara MP, Pedersen MR, et al. Two-stage liver transplant for ruptured hepatic adenoma: a case report. World J Hepatol. 2019;11:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuk I, Tekin A, Tryphonopoulos P, et al. Abdominal transplantation for unresectable tumors in children: the zooming out principle. Pediatr Surg Int. 2016;32:337–346. [DOI] [PubMed] [Google Scholar]
- 33.Kirschner BS, Baker AL, Thorp FK. Growth in adulthood after liver transplantation for glycogen storage disease type I. Gastroenterology. 1991;101:238–241. [DOI] [PubMed] [Google Scholar]
- 34.Leone JP, Santos MG, Finan J, et al. Liver transplantation recipient with malignant transformation of hepatic adenomas. Case Reports Clin Pathol. 2016;3:8–14. [Google Scholar]
- 35.Poe R, Snover DC. Adenomas in glycogen storage disease type 1. Two cases with unusual histologic features. Am J Surg Pathol. 1988;12:477–483. [DOI] [PubMed] [Google Scholar]
- 36.Barbier L, Nault JC, Dujardin F, et al. Natural history of liver adenomatosis: a long-term observational study. J Hepatol. 2019;71:1184–1192. [DOI] [PubMed] [Google Scholar]
- 37.Bernard PH, Carles J, Dumas F, et al. Liver transplantation for voluminous hepatocellular adenoma in a patient with idiopathic portal hypertension previously treated by a portacaval shunt. Eur J Gastroenterol Hepatol. 1994;6:269–274. [Google Scholar]
- 38.Faivre L, Houssin D, Valayer J, et al. Long-term outcome of liver transplantation in patients with glycogen storage disease type Ia. J Inherit Metab Dis. 1999;22:723–732. [DOI] [PubMed] [Google Scholar]
- 39.Tepetes K, Selby R, Webb M, et al. Orthotopic liver transplantation for benign hepatic neoplasms. Arch Surg. 1995;130:153–156. [DOI] [PubMed] [Google Scholar]
- 40.Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiche L, Dao T, Salamé E, et al. Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohkam K, Darnis B, Cazauran JB, et al. Polymorphic multiple hepatocellular adenoma including a non-steatotic HNF1α-inactivated variant. Hepatobiliary Pancreat Dis Int. 2017;16:552–555. [DOI] [PubMed] [Google Scholar]
- 43.Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. [DOI] [PubMed] [Google Scholar]
- 44.Franchi-Abella S, Branchereau S, Lambert V, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322–330. [DOI] [PubMed] [Google Scholar]
- 45.Brasoveanu V, Ionescu MI, Grigorie R, et al. Living donor liver transplantation for unresectable liver adenomatosis associated with congenital absence of portal vein: a case report and literature review. Am J Case Rep. 2015;16:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carreiro G, Villela-Nogueira CA, Coelho Hu, et al. Orthotopic liver transplantation in glucose-6-phosphatase deficiency–Von Gierke disease–with multiple hepatic adenomas and concomitant focal nodular hyperplasia. J Pediatr Endocrinol Metab. 2007;20:545–549. [DOI] [PubMed] [Google Scholar]
- 47.Nacif LS, Paranaguá-Vezozzo DC, Galvão FH, et al. Significance of CT scan and color Doppler duplex ultrasound in the assessment of Abernethy malformation. BMC Med Imaging. 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raphe R, Felício HC, Rocha MF, et al. Histopathologic characteristics of incidental hepatocellular carcinoma after liver transplantation. Transplant Proc. 2010;42:505–506. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho PM, Silva NJ, Dias PG, et al. Glycogen storage disease type 1a - a secondary cause for hyperlipidemia: report of five cases. J Diabetes Metab Disord. 2013;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marino IR, Scantlebury VP, Bronsther O, et al. Total hepatectomy and liver transplant for hepatocellular adenomatosis and focal nodular hyperplasia. Transpl Int. 1992;5(Suppl 1):S201–S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coire CI, Qizilbash AH, Castelli MF. Hepatic adenomata in type Ia glycogen storage disease. Arch Pathol Lab Med. 1987;111:166–169. [PubMed] [Google Scholar]
- 52.Reid CJ, Hebert D. Acute renal failure complicating liver transplantation in twin sisters with glycogen storage disease type Ia. Transplant Proc. 1996;28:3629–3631. [PubMed] [Google Scholar]
- 53.Colle I, Laureys G, Raevens S, et al. Living related liver transplantation in an adult patient with hepatocellular adenoma and carcinoma 13 years after bone marrow transplantation for Fanconi anemia: A case report. Hepatol Res. 2013;43:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerut JP, Ciccarelli O, Sempoux C, et al. Glycogenosis storage type I diseases and evolutive adenomatosis: an indication for liver transplantation. Transpl Int. 2003;16:879–884. [DOI] [PubMed] [Google Scholar]
- 55.Fernández-Vega I, Santos-Juanes J, García-Pravia C, et al. Hepatic adenomatosis: a rare cause of liver transplant. Rev Esp Enferm Dig. 2014;106:494–496. [PubMed] [Google Scholar]
- 56.Maya Aparicio AC, Bernal Bellido C, Tinoco González J, et al. Fifteen years of follow-up of a liver transplant recipient with glycogen storage disease type Ia (Von Gierke disease). Transplant Proc. 2013;45:3668–3669. [DOI] [PubMed] [Google Scholar]
- 57.Intaraprasong P, Kitiyakara T, Angkathunyakul N, et al. Liver transplantation for hepatic adenomatosis: the first case report in Thailand and literature review. J Med Assoc Thail. 2021;104:320–325. [Google Scholar]
- 58.Iyer SG, Chen CL, Wang CC, et al. Long-term results of living donor liver transplantation for glycogen storage disorders in children. Liver Transpl. 2007;13:848–852. [DOI] [PubMed] [Google Scholar]
- 59.Liu PP, de Villa VH, Chen YS, et al. Outcome of living donor liver transplantation for glycogen storage disease. Transplant Proc. 2003;35:366–368. [DOI] [PubMed] [Google Scholar]
- 60.Ji HF, Wang WL, Shen Y, et al. Reduced-size liver transplantation for glycogen storage disease. Hepatobiliary Pancreat Dis Int. 2009;8:106–108. [PubMed] [Google Scholar]
- 61.Malatack JJ, Finegold DN, Iwatsuki S, et al. Liver transplantation for type I glycogen storage disease. Lancet. 1983;1:1073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Sandro S, Slim AO, Lauterio A, et al. Liver adenomatosis: a rare indication for living donor liver transplantation. Transplant Proc. 2009;41:1375–1377. [DOI] [PubMed] [Google Scholar]
- 63.Marega A, Fregonese C, Tulissi P, et al. Preemptive liver-kidney transplantation in von Gierke disease: a case report. Transplant Proc. 2011;43:1196–1197. [DOI] [PubMed] [Google Scholar]
- 64.Vennarecci G, Santoro R, Antonini M, et al. Liver transplantation for recurrent hepatic adenoma. World J Hepatol. 2013;5:145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santambrogio R, Marconi AM, Ceretti AP, et al. Liver transplantation for spontaneous intrapartum rupture of a hepatic adenoma. Obstet Gynecol. 2009;113:508–510. [DOI] [PubMed] [Google Scholar]
- 66.Panaro F, Andorno E, Basile G, et al. Simultaneous liver-kidney transplantation for glycogen storage disease type IA (von Gierke’s disease). Transplant Proc. 2004;36:1483–1484. [DOI] [PubMed] [Google Scholar]
- 67.Di Rocco M, Calevo MG, Taro’ M, et al. Hepatocellular adenoma and metabolic balance in patients with type Ia glycogen storage disease. Mol Genet Metab. 2008;93:398–402. [DOI] [PubMed] [Google Scholar]
- 68.Manzia TM, Angelico R, Toti L, et al. Glycogen storage disease type Ia and VI associated with hepatocellular carcinoma: two case reports. Transplant Proc. 2011;43:1181–1183. [DOI] [PubMed] [Google Scholar]
- 69.Sorkin T, Strautnieks S, Foskett P, et al. Multiple β-catenin mutations in hepatocellular lesions arising in Abernethy malformation. Hum Pathol. 2016;53:153–158. [DOI] [PubMed] [Google Scholar]
- 70.Sanada Y, Mizuta K, Niki T, et al. Hepatocellular nodules resulting from congenital extrahepatic portosystemic shunts can differentiate into potentially malignant hepatocellular adenomas. J Hepatobiliary Pancreat Sci. 2015;22:746–756. [DOI] [PubMed] [Google Scholar]
- 71.Solbach P, Potthoff A, Raatschen HJ, et al. Testosterone-receptor positive hepatocellular carcinoma in a 29-year old bodybuilder with a history of anabolic androgenic steroid abuse: a case report. BMC Gastroenterol. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Selby R, Starzl TE, Yunis E, et al. Liver transplantation for type I and type IV glycogen storage disease. Eur J Pediatr. 1993;152 (Suppl 1):S71–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weimann A, Ringe B, Klempnauer J, et al. Critical issues in the diagnosis and treatment of hepatocellular adenoma. HPB. 2000;2:25–32. [Google Scholar]
- 74.Szili B, Görög D, Gerlei Z, et al. Rapid height growth after liver transplantation in adulthood. Growth Horm IGF Res. 2016;29:1–3. [DOI] [PubMed] [Google Scholar]
- 75.Väli T, Tein A, Uksov A. Liver transplantation for hepatic tumors: a small center experience. Transplant Reports. 2019;4:100033. [Google Scholar]
- 76.Oterdoom LH, Verweij KE, Biermann K, et al. Hepatocellular adenomas and carcinoma in asymptomatic, non-cirrhotic type iii glycogen storage disease. J Gastrointestin Liver Dis. 2015;24:515–518. [DOI] [PubMed] [Google Scholar]
- 77.Wojcicki M, Haagsma EB, Gouw AS, et al. Orthotopic liver transplantation for portosystemic encephalopathy in an adult with congenital absence of the portal vein. Liver Transpl. 2004;10:1203–1207. [DOI] [PubMed] [Google Scholar]
- 78.Koestinger A, Gillet M, Chioléro R, et al. Effect of liver transplantation on hepatic glucose metabolism in a patient with type I glycogen storage disease. Transplantation. 2000;69:2205–2207. [DOI] [PubMed] [Google Scholar]
- 79.Baiges A, Turon F, Simón-Talero M, et al. ; REHEVASC, VALDIG an EASL consortium, Abernethy group. Congenital extrahepatic portosystemic shunts (Abernethy Malformation): an international observational study. Hepatology. 2020;71:658–669. [DOI] [PubMed] [Google Scholar]
- 80.Matern D, Starzl TE, Arnaout W, et al. Liver transplantation for glycogen storage disease types I, III, and IV. Eur J Pediatr. 1999;158 (Suppl 2):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alshak NS, Cocjin J, Podesta L, et al. Hepatocellular adenoma in glycogen storage disease type IV. Arch Pathol Lab Med. 1994;118:88–91. [PubMed] [Google Scholar]
- 82.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. [DOI] [PubMed] [Google Scholar]
- 83.Mittal S, Gopal P, Khatri G, et al. Evaluation and management of hepatocellular adenomas. Clin Liver Dis (Hoboken). 2021;17:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronot M, Bahrami S, Calderaro J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182–1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.