Abstract
Background:
To assess the effect of obesity or a high body mass index (BMI) on the risk of severe outcomes in patients with coronavirus disease 2019 (COVID-19).
Methods:
Studies on the relationship between BMI or obesity and COVID-19 since December 2019. The odds ratio (OR) and weighted mean difference (WMD) with their 95% confidence intervals (CIs) were used to assess the effect size.
Results:
BMI was significantly increased in COVID-19 patients with severe illness (WMD: 1.18; 95% CI: 0.42–1.93), who were admitted to an intensive care unit (ICU) (WMD: 1.46; 95% CI: 0.96–1.97), who required invasive mechanical ventilation (IMV) (WMD: 2.70, 95% CI: 1.05–4.35) and who died (WMD: 0.91, 95% CI: 0.02–1.80). In Western countries, obesity (BMI of ≥30 kg/m2) increased the risk of hospitalization (OR: 2.08; 95% CI: 1.22–3.54), admission to an ICU (OR: 1.54; 95% CI: 1.29–1.84), need for IMV (OR: 1.73, 95% CI: 1.38–2.17), and mortality (OR: 1.43; 95% CI: 1.17–1.74) of patients with COVID-19. In the Asian population, obesity (BMI of ≥28 kg/m2) increased the risk of severe illness (OR: 3.14; 95% CI: 1.83–5.38). Compared with patients with COVID-19 and a BMI of <25 kg/m2, those with a BMI of 25–30 kg/m2 and ≥30 kg/m2 had a higher risk of need for IMV (OR: 2.19, 95% CI: 1.30–3.69 and OR: 3.04; 95% CI: 1.76–5.28, respectively). The risk of ICU admission in patients with COVID-19 and a BMI of ≥30 kg/m2 was significantly higher than in those with a BMI of 25–30 kg/m2 (OR: 1.49; 95% CI: 1.00–2.21).
Conclusion:
As BMI increased, the risks of hospitalization, ICU admission, and need for IMV increased, especially in COVID-19 patients with obesity.
Ethics and dissemination:
This systematic review and meta-analysis does not require an ethics approval as it does not collect any primary data from patients.
Keywords: BMI, COVID-19, intensive care unit, invasive mechanical ventilation, mortality, obesity
1. Introduction
Coronavirus disease (COVID-19) has spread to several countries around the world since 2019 and poses a significant threat to the health and property of people around the world. COVID-19 is caused by infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a membrane-wrapped, single-stranded ribonucleic acid virus.[1]
COVID-19 mainly manifests with respiratory symptoms (fever, fatigue, and cough), but some patients experience gastrointestinal symptoms, such as diarrhea, vomiting, and anorexia. Approximately 10% of patients with COVID-19 who present with gastrointestinal symptoms have no signs of fever or respiratory infections.[2] Patients with severe COVID-19 can experience respiratory failure and multiple organ failure, leading to death. COVID-19 is primarily symptomatic, with no specific drug for treatment currently identified.
Humans are generally susceptible to SARS-CoV-2 infections. The World Health Organization declared COVID-19 a global pandemic on March 11, 2020.[1] As it is an emerging infectious disease, many mechanisms of COVID-19 remain unknown. The understanding of the disease and its risk factors are key factors for implementing public health policies at present. Previous studies have shown that hypertension, diabetes mellitus, cardiovascular and cerebrovascular diseases, pulmonary diseases, age, and gender affect the prognosis and outcome of patients with COVID-19.[3] Obesity is a risk factor for many diseases such as heart disease, diabetes, and hypertension, with the number of people with obesity worldwide increasing annually. The prevalence of obesity in the United States was 39.8% from 2015 to 2016 and is projected to be 48.9% by 2030.[4] In developing countries such as China also, obesity is on the rise, with a considerable number of patients with COVID-19 being obese.[5] Therefore, at present, researchers need to pay attention to whether obesity in patients with COVID-19 increases the risk of adverse outcomes and whether a high BMI will affect the outcomes in patients with COVID-19. In this meta-analysis, the influence of different BMI values of patients with COVID-19 on their adverse clinical outcomes was investigated to provide a reference for the treatment of these patients in clinical practice.
2. Methods
2.1. Literature retrieval
We performed this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,[6] and it was registered on the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42021260770). This systematic review and meta-analysis does not require an ethics approval as it does not collect any primary data from patients. The PubMed, Embase, and China National Knowledge Infrastructure databases were searched for all studies since December 2019. We also included the relevant references of selected studies. The search strategy of matching keywords with free words was adopted, and the search terms were as follows: (“coronavirus disease 2019” or “2019 novel coronavirus” or “covid-19” or “2019-ncov” or “novel coronavirus 2019 infection” or “severe acute respiratory syndrome coronavirus 2” or “sars-cov-2”) AND (“obesity” or “overweight” or “body mass index” or “BMI” or “risk factors” or “factor” or “risk factor” or “clinical characteristics” or “clinical features”)
2.2. Inclusion and exclusion criteria
The following studies were included:
-
(1)
studies on patients diagnosed with COVID-19 confirmed via polymerase chain reaction,
-
(2)
retrospective or prospective studies without language restrictions,
-
(3)
studies containing information on COVID-19 and BMI or obesity,
-
(4)
studies with original data provided and the indicators, such as the number of patients with obesity in the experimental and control groups, not adjusted for, and
-
(5)
studies mentioning the clinical outcomes of patients with COVID-19.
The following studies were excluded:
-
(1)
studies involving populations without COVID-19,
-
(2)
studies based on animals,
-
(3)
case reports or reviews, and
-
(4)
studies with incomplete data or poor quality (Newcastle–Ottawa Scale [NOS] score of ≤3).
2.3. Data extraction
Two researchers independently searched for relevant literature and decided whether the selected studies met the inclusion criteria. Any discrepancies were resolved via discussion. The following variables were extracted from each study if available: first author's name, country, single-center or multi-center, age of the study population, percentage of men in the population, sample of participants, different outcomes of patients with COVID-19, BMI levels with different clinical outcomes, number of patients with obesity who had different clinical outcomes, number of patients with different clinical outcomes at different BMI classes, rates of different comorbidities such as hypertension, diabetes mellitus, heart disease, dyslipidemia, and chronic kidney disease in patients with COVID-19.
2.4. Quality assessment
The NOS was used to assess the quality of the studies included in the meta-analysis. The scale consists of eight items under three dimensions: selection (4 items, maximum score 4), comparability (1 item, maximum score 2), and outcome (3 items, maximum score 3).[7] The highest score was 9. A total score of higher than 7 indicates high quality, 5–6 indicates moderate quality, and 0–4 indicates low quality.
2.5. Statistical analysis
In this meta-analysis, the following clinical adverse outcome events in patients with COVID-19 patients were used:
-
(1)
hospitalization,
-
(2)
severe illness,
-
(3)
invasive mechanical ventilation (IMV) needed during hospitalization,
-
(4)
admission to an intensive care unit (ICU) during hospitalization, and
-
(5)
mortality during hospitalization.
First, the total number of patients in the experimental group (with the above outcome events) and the control group (without the above outcome events) were extracted, as well as their respective BMI; the original data in the included studies were mostly given as median values with interquartile ranges of BMI. We used the statistical method described by Luo et al[8] to calculate the mean and standard deviation (mean ± SD) of BMI based on the median values with interquartile ranges. The weighted mean difference (WMD) and 95% confidence interval (CI) were calculated according to the mean ± SD of BMI in each group. When the WMD was 0 or its 95% CI contained 0, the diamond-shaped box representing the combined effect size intersected with the equivalent line in the forest map, suggesting that there was no statistically significant difference between the experimental and control groups in terms of the relevant outcome indicators. When the WMD was greater than 0 and the lower limit of 95% CI was greater than 0, the diamond-shaped box of the forest map was located to the right of the equivalent line, indicating that BMI was higher in the experimental group than in the control group. When the WMD was less than 0 and the upper limit of the 95% CI was less than 0, the diamond-shaped box of the forest map was located to the left of the equivalent line, indicating that BMI in the experimental group was lower than that in the control group.
Second, the criterion for obesity in the Asian population was a BMI of ≥28 kg/m2,[9] whereas for non-Asians, obesity was defined as a BMI of ≥30 kg/m2.[10] We analyzed the studies from these two populations according to the respective obesity standards. The total number of patients in the experimental group and control groups (without relevant outcome events) and the number of patients with obesity in the experimental group and the control group were extracted. Using STATA 12.0, odds ratios (OR) and 95% CIs were calculated. When the OR value was equal to 1 or the 95% CI was 1, BMI was not associated with the risk of adverse outcomes in patients with COVID-19. When the OR value was greater than 1 or the lower limit of 95% CI was greater than 1, BMI was positively associated with the risk of adverse outcomes in patients with COVID-19. When the OR value was less than 1 or the upper limit of the 95% CI was less than 1, BMI was inversely associated with the risk of adverse outcomes in patients with COVID-19.
At present, BMI is used to measure the degree of body fat and thinness and whether a person is healthy or not. In our study, a BMI of ≥30 kg/m2 in Western countries and a BMI of ≥28 kg/m2 in the Asian population were considered to indicates obesity, and the risks of relevant clinical outcomes were analyzed. In addition, BMI was stratified into three classes: <25 kg/m2, 25–30 kg/m2, and ≥30 kg/m2.[11] BMI ≥30 kg/m2 by extracting the number of COVID-19 patients at different BMI classes who had related clinical outcomes and those who did not have relevant clinical outcomes in relevant studies. The three different classes were compared, and the OR values and 95% CI for different clinical outcomes were calculated using STATA 12.0 software.
In this meta-analysis, STATA 12.0 was used to draw forest maps. A fixed effect model or a random effect model was adopted according to the heterogeneity of the included studies, and Cochran Q and I2 statistics were used to test the heterogeneity. A fixed-effects model was used when I2 < 50%. Otherwise, a random-effects model was chosen. In the subgroup analysis, P < .05 was considered statistically significant.
3. Results
3.1. Search results
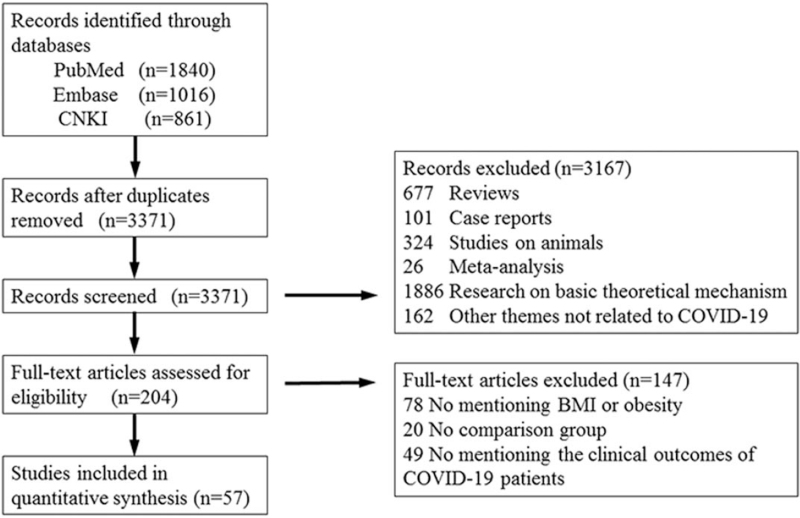
A total of 3717 studies describing the correlations between COVID-19 and BMI or obesity were retrieved (1840 in PubMed, 1016 in Embase, and 861 in the China National Knowledge Infrastructure databases). By reading the titles and abstracts, we screened the studies, and 346 duplicates were eliminated. As a result, 3371 studies remained, and after skimming the titles, abstracts, and reading the full texts, 204 studies were obtained. After carefully reading the 204 studies, 57 studies were finally selected.[12–67] The details of the screening process are presented in Figure 1.
Figure 1.
Flow diagram of the study selection.
3.2. Basic characteristics of the included studies
The basic characteristics of 57 studies are shown in Table 1. The studies were from different parts of the world including China, the United States, and Europe. There were 49 retrospective cohort studies,[12–25,27–30,32–38,40–41,43–60,62,64,65,67] 8 prospective cohort studies,[26,31,39,42,51,61,63,66] 43 single-center studies,[12,15,16,18–21,23–47,49,52,53,55–58,60–62,66] and 14 multi-center studies.[13,14,17,22,48,50,51,54,59,63–65,67] Among these, 29 studies[13,24,31–34,36,47–67] with original data related to the dichotomous variable (obesity), 36 studies[12–22,25–49] with original data related to continuous data (BMI), and 9 studies[23,32–34,49,55,57,60,67] with BMI stratification could be carried out. The clinical outcomes of patients with COVID-19 in the included studies were hospitalization, ICU admission, need for IMV, and mortality; in 14 studies,[12–22,25–27] the clinical outcome was severe illness. In three of these studies,[25–27] the criteria for severe illness was based on the American Thoracic Society/Infectious Diseases Society of America guidelines,[68] while in the other 11 studies,[12–22] the criteria for severe illness was according to the National Health Commission of China classification.[69] In two studies,[31,47] the population was divided into two cohorts according to the time of hospitalization and age; therefore, we analyzed the two cohorts separately.
Table 1.
Basic characteristics of the included studies.
| Study | Country | Study type | Center (single-/multi-) | Sample (severe group/non severe group) | Primary outcomes | Age (yr) | Male (%) | Comorbidities (%) |
| Cai[25]2020 | China | Retrospective | Single | 298 (58/240) | Severe illness | 47.5 | 48.7 | HTN 15.8, DM 6.04, CVD 8.39 |
| Bhatla[28]2020 | USA | Retrospective | Single | 700 (79/621) | ICU | 50 | 45 | HTN 50, CHD 11, DM 26, CKD 11 |
| Chao[29]2020 | USA | Retrospective | Single | 46 (13/33) | ICU | 13.1 | 69.6 | NA |
| Wei[12]2020 | China | Retrospective | Single | 276 (14/262) | Severe illness | 51.0 | 56.2 | HTN 17, DM 5.1, CHD 4.0, CVD 2.2 |
| Li[26]2020 | China | Prospective | Single | 548 (269/279) | Severe illness | 60 | 50.9 | HTN 30.3, DM 15.1, CHD 6.2, CKD 1.8 |
| Almazeedi[30]2020 | Kuwait | Retrospective | Single | 1096 (42/1054) | ICU | 41 | 81 | HTN 16.1, DM 14.1, Dyslipidemia 5.9, CAD 3.7, CKD 1.0, CVD 0.6 |
| Huang[13]2020 | China | Retrospective | Multi | 202 (23/179) | Severe illness | 44 | 57.4 | HTN 14.4, DM 9.4, CAD 2.5, CVD 1.5 |
| Wu[14]2020 | China | Retrospective | Multi | 280 (83/197) | Severe illness | 43.1 | 53.9 | CVD20.36, CKD 1.07 |
| Xiang[15]2020 | China | Retrospective | Single | 49 (9/40) | Severe illness | 42.9 | 67.3 | HTN 12.2, DM 4.1 |
| Chen[16]2020 | China | Retrospective | Single | 145 (43/102) | Severe illness | 47.5 | 54.4 | HTN 15.2, DM 9.7, CKD 2.1, Hyperlipidemia 2.3 |
| Xiong[17]2020 | China | Retrospective | Multi | 131 (30/101) | Severe illness | 63.2 | 57.3 | CAD 68.7, DM 22.9 |
| Sun[18]2020 | China | Retrospective | Single | 57 (45/12) | Severe illness | NA | 50.9 | Chronic disease history 59.6 |
| Mejía-Vilet[31]2020 | Mexico | Prospective | Single | 329 (115/214) | ICU | 49 | 64 | HTN 27, DM 24, CKD 6 |
| Mejía-Vilet[31]2020 | Mexico | Prospective | Single | 240 (115/125) | ICU | 52 | 69 | HTN 31, DM 33, CKD 5 |
| Liu[19]2020 | China | Retrospective | Single | 30 (4/26) | Severe illness | 35 | 33 | NA |
| Peng[20]2020 | China | Retrospective | Single | 112 (16/96) | Severe illness | 62 | 47.3 | HTN 82.14, DM 20.54, CHD 55.36 |
| Simonnet[34]2020 | France | Retrospective | Single | 124 (85/39) | IMV | 60 | 73 | HTN 49, DM 23, Dyslipidemia 28 |
| Dreher[35]2020 | Germany | Retrospective | Single | 50 (24/26) | IMV | 65 | 66 | HTN 70, DM 59, CKD 20, CVD 14 |
| Regina[36]2020 | Switzerland | Retrospective | Single | 200 (37/163) | IMV | 70 | 60 | HTN 43.5, DM 21.5, CAD 17.5, CKD 14 |
| Zhang[21]2020 | China | Retrospective | Single | 52 (21/31) | Severe illness | 65.5 | 63.5 | HTN 65.4, DM 100, CHD 26.9, CKD 5.8 |
| Huang[22]2020 | China | Retrospective | Multi | 60 (8/52) | Severe illness | 57 | 58.3 | HTN 23.3, DM 16.7, CAD 5.0, CKD 1.7 |
| Petrey[27]2021 | USA | Retrospective | Single | 22 (8/14) | Severe illness | NA | 59 | HTN 50, DM 50 |
| Argenziano[33]2020 | USA | Retrospective | Single | 850 (236/614) | ICU | 63 | 60.1 | HTN 59.8, DM 39.2, CKD 13.7, CAD 13.5 |
| Brill[37]2020 | UK | Retrospective | Single | 410 (173/237) | Death | 72 | 60 | HTN 43, DM 30 |
| Cao[38]2020 | China | Retrospective | Single | 102 (17/85) | Death | 54 | 52 | HTN 27.5, DM 10.8, CAD 4.9, CKD 3.9 |
| Garcia[39]2020 | Switzerland | Prospective | single | 398 (97/301) | Death | 63 | 75.1 | HTN 44.1, DM 23, CHD 23.8 |
| Gayam[40]2021 | USA | Retrospective | Single | 408 (132/276) | Death | 67 | 56.6 | HTN 66.42, DM 43.24, CAD 13.24, Dyslipidemia 16.18 |
| Krishnan[41]2020 | USA | Retrospective | Single | 152 (92/60) | Death | 68 | 62.5 | HTN 73, DM 65, CAD 15, Hypercholesterolemia 61, CKD 14 |
| Masetti[42]2020 | Italy | Prospective | Single | 229 (33/196) | Death | 60.7 | 64.6 | HTN 38, DM 18.8, CHD 9.2, CKD 4.8 |
| Salacup[43]2021 | USA | Retrospective | Single | 242 (52/190) | Death | 66 | 49 | HTN 74, DM 49, CAD 19, CKD 17 |
| Auld[44]2020 | USA | Retrospective | Single | 209 (62/147) | Death | 64.0 | 54.2 | HTN 61.7, DM 45.6, CAD 14.3, CKD 26.7 |
| Luo[45]2021 | China | Retrospective | Single | 85 (12/73) | Death | 63.0 | 56.5 | HTN 35.29, CHD 11.76, DM 14.12 |
| Zhang[46]2020 | China | Retrospective | Single | 43 (12/31) | Death | NA | NA | NA |
| Klang[47]2020 | USA | Retrospective | Single | 572 (60/512) | Death | NA | 69.4 | HTN 29.5, DM 25.2, CAD 5.1, CKD 10.4, Hyperlipidemia 12.3 |
| Klang[47]2020 | USA | Retrospective | Single | 2834 (1076/1758) | Death | NA | 58.9 | HTN 71.7, DM 47.7, CAD 20.4, CKD 17.0, Hyperlipidemia 40.0 |
| Halvatsiotis[48]2020 | Greece | Retrospective | Multi | 86 (26/60) | Death | 65.5 | 80 | HTN 50, DM 18.9, CAD 21.1, CKD 4.4 |
| Halasz[49]2020 | Italy | Retrospective | Single | 242 (78/164) | Death | 64 | 80.2 | HTN 45.5, DM 15.3, CAD 14.5 |
| Giacomelli[61]2020 | Italy | Prospective | Single | 233 (48/185) | Death | 61 | 69.1 | NA |
| Borobia[62]2020 | Spain | Retrospective | Single | 2226 (460/1766) | Death | 61 | 48.2 | HTN 41.3, DM 17.1, CKD 7.8, CHD 19.3 |
| Rossi[63]2020 | Italy | Prospective | Multi | 1292 (217/1075) | Death | 63.2 | 50.1 | HTN 18.1, DM 12, CKD 2.5, CHD 12.9, Dyslipidemia 5 |
| Carrillo-Vega[50]2020 | Mexico | Retrospective | Multi | 9946 (963/8983) | Death | 48.15 | 57.7 | HTN 21.74, DM 17.65, CHD 2.99, CKD 2.13 |
| Murillo-Zamoraa[64]2021 | Mexico | Retrospective | Multi | 5393 (1735/3658) | Death | NA | 63.6 | HTN 36.6,DM 31.1,CKD 5.5 |
| Baqui[65]2020 | Brazil | Retrospective | Multi | 7371 (3328/4043) | Death | NA | 58.2 | DM 25.7, CHD 33.9, CKD 5.3 |
| Rodríguez[66]2020 | Spain | Prospective | Single | 38 (10/28) | Death | NA | NA | DM 18.6, CHD 9.3, CKD 4.7 |
| Amit[67]2020 | USA | Retrospective | Multi | 109 (56/53) | Death | 72 | 69 | HTN 54.5, DM 39.7, CHD 32.1, CKD 15.4, Dyslipidemia 15.4 |
| Goyal[58]2020 | USA | Retrospective | Single | 380 (129/251) | IMV | 62.2 | 60.6 | HTN 50.1,DM 25.2 |
| Hur[59]2020 | USA | Retrospective | Multi | 486 (138/348) | IMV | 59 | 55.8 | HTN 54.9, DM 32.9, CHD 22.8, CKD 8.6 |
| Carrillo-Vega[50]2020 | Mexico | Retrospective | Multi | 9946 (3922/6024) | Hospitalization | 48.15 | 57.7 | HTN 21.74, DM 17.65, CHD 2.99, CKD 2.13 |
| Shekhar[52]2020 | USA | Retrospective | Single | 39 (27/12) | ICU | 55 | 46 | HTN 34 |
| Ebinger[53]2020 | USA | Retrospective | Single | 214 (77/137) | ICU | 52.72 | 63.1 | HTN 36.4 |
| Ebinger[53]2020 | USA | Retrospective | Single | 77 (52/25) | IMV | 52.72 | 74.0 | HTN 36.4 |
| Ferguson[54]2020 | USA | Retrospective | Multi | 72 (21/51) | ICU | 60.4 | 52.8 | CHD 59.7, CKD 5.6 |
| Lodigiani[55]2020 | Italy | Retrospective | Single | 363 (57/306) | ICU | 66 | 68 | HTN 47.2, DM 22.7, CHD 13.9, CKD 15.1, Dyslipidemia 19.6 |
| Hu[23]2020 | China | Retrospective | Single | 294 (164/130) | Severe illness | 61 | 51.4 | HTN 32.5, DM 14.6,CKD 2.2 |
| Itelman[56]2020 | Israel | Retrospective | Single | 162 (26/136) | ICU | 52 | 65 | HTN 30.2, DM 18.5, CHD 7.4, CKD 1.2 |
| Petrilli[51]2020 | USA | Prospective | Multi | 5279 (2741/2538) | Hospitalization | 54 | 49.5 | HTN 42.7, DM 22.6, CHD 52.1, CKD 12.3, Dyslipidemia 32.5 |
| Petrilli[51]2020 | USA | Retrospective | Multi | 4103 (1999/2104) | Hospitalization | NA | 50.5 | HTN 24, CKD 5.2, DM 15, CHD 8.9, CKD 12.3, Dyslipidemia 18 |
| Al-Sabah[32]2020 | Kuwait | Retrospective | Single | 1158 (104/1054) | ICU | 40.5 | 81.6 | HTN 20.4,DM 23.4 |
| Caussy[60]2020 | France | Retrospective | Single | 291 (170/121) | IMV | NA | NA | NA |
| Kalligeros[57]2020 | USA | Retrospective | Single | 103 (44/59) | ICU | 60 | 61.1 | HTN 64, DM 36.8, CHD 24.2, CKD 10.6 |
| Wang[24]2021 | China | Retrospective | Single | 482 (93/389) | Severe illness | 52 | 54.7 | HTN 24.77, DM 8.69, CAD 5.36 |
3.3. Quality assessment of the included studies
The NOS was used to assess the quality of the studies. The results are shown in Table 2. All studies included in this study were of moderate or high quality.
Table 2.
Quality assessment of included studies (NOS).
| Study | Selection | Demonstration that outcome of interest was not present at start of study | Comparability | Outcome | Total | ||||
| Representation of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up Long enough for outcomes to occur | Adequacy of follow-up of cohorts | |||
| Cai[25]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Bhatla[28]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Chao[29]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Wei[12]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Li[26]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Almazeedi[30]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Huang[13]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Wu[14]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Xiang[15]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Chen[16]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Xiong[17]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Sun[18]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Mejía-Vilet[31]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Liu[19]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Peng[20]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Simonnet[34]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Dreher[35]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Regina[36]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhang[21]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Huang[22]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Al-Sabah[32]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Petrey[27]2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Argenziano[33]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Brill[37]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cao[38]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Garcia[39]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Gayam[40]2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Krishnan[41]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Masetti[42]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Salacup[43]2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Auld[44]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Luo[45]2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhang[46]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Klang[47]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Halvatsiotis[48]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Halasz[49]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Giacomelli[61]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 8 | |
| Borobia[62]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Rossi[63]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Murillo-Zamoraa[64]2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Baqui[65]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Rodríguez[66]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Amit[67]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Goyal[58]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Petrilli[51]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Hur[59]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Carrillo-Vega[50]2020 | 1 | 1 | 1 | 2 | 1 | 6 | |||
| Shekhar[52]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Petrilli[51]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Ebinger[53]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Ferguson[54]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||
| Lodigiani[55]2020 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Hu[23]2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Itelman[56]2020 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Caussy[60]2020 | 1 | 1 | 1 | 1 | 1 | 5 | |||
| Kalligeros[57]2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Wang[24]2021 | 1 | 1 | 2 | 1 | 1 | 6 | |||
3.4. Associations between elevated BMI and different clinical outcomes
A total of 36 studies[12–22,25–49] mentioned BMI and different clinical outcomes in patients with COVID-19. Among them, 14 studies[12–22,25–27] compared BMI of severe illness group and non-severe illness group, and 6 studies[28–33] were on the difference in BMI between the ICU group and non-ICU group. There were 3 studies[34–36] comparing BMI of patients with COVID-19 requiring IMV and not requiring IMV, while there were 14 studies[30,37–49] comparing BMI between the death group and survival group. The WMD and 95% CI were calculated according to the total number of patients in the experimental and control groups in each study and the mean ± SD of BMI in each group by the random effect model.
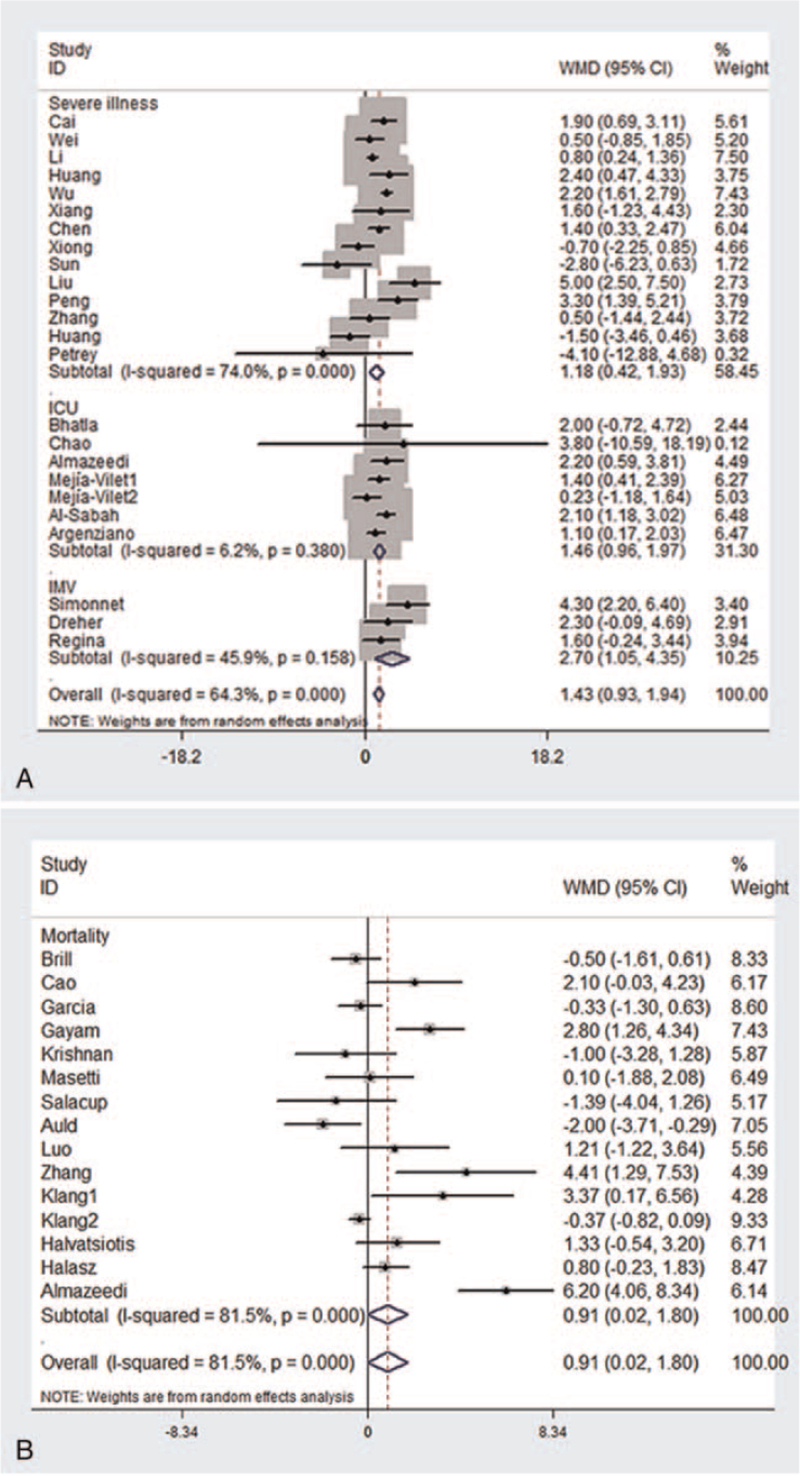
As shown in Figure 2A, compared with patients in the control group, those who had severe illness, were admitted to an ICU, and required IMV had significantly higher BMI (severe illness: WMD: 1.18, 95% CI: 0.42–1.93; admission to ICU: WMD: 1.46, 95% CI: 0.96–1.97; IMV acquirement: WMD: 2.70, 95% CI: 1.05–4.35), there was also significant difference in BMI between the death group and survival group (WMD: 0.91, 95% CI: 0.02–1.80) (Figure 2B).
Figure 2.
Forest plots of the weighted mean difference of elevated BMI and different clinical outcomes. (A) Severe illness; admission to an ICU; need for IMV; (B) mortality. ICU = intensive care unit; IMV = invasive mechanical ventilation.
3.5. Associations between obesity and different clinical outcomes
A total of 29 studies[13,24,31–34,36,47–67] were included in this meta-analysis of association between different clinical outcomes and obesity in patients with COVID-19. In 2 of these studies,[13,24] the obesity standard was a BMI of ≥28 kg/m2, and the clinical outcomes described in these 2 studies were severe illness. In the remaining 27 studies,[31–34,36,47–67] the obesity criteria was a BMI of ≥30 kg/m2. Among them, there were 3 studies[50,51] on hospitalization, 9[31–33,52–57] on admission to an ICU, 6[34,36,53,58–60] on need for IMV, and 11 on mortality.[47–50,61–67] These studies all directly provided the total number of patients with relevant clinical outcome events and those without relevant outcome events and the number of patients with obesity who showed relevant outcome events and those who did not. The random-effects model was used to calculate the OR values and 95% CIs for different clinical outcomes in Western patients with obesity, while in the Asian population, the fixed-effects model was used.
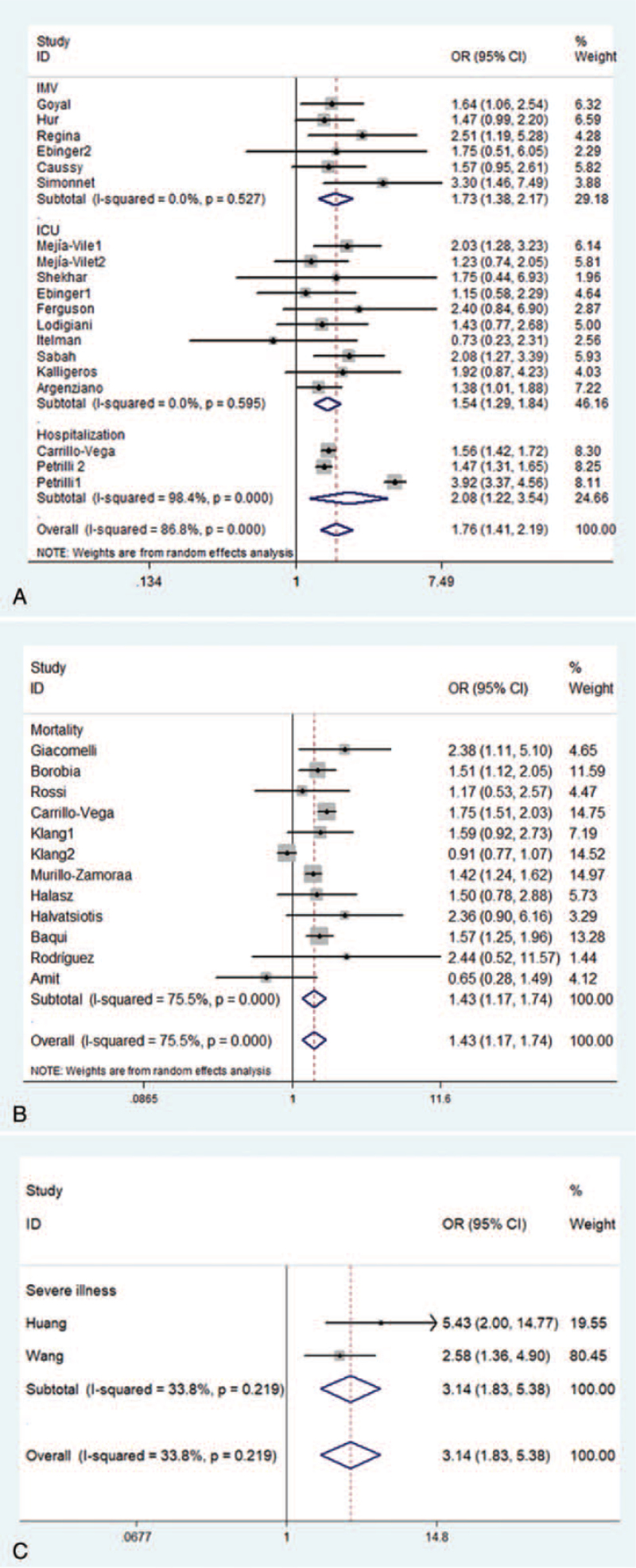
As shown in Figure 3A, in Western countries, obesity (BMI of ≥30 kg/m2) not only increased the risk of hospitalization (OR: 2.08, 95% CI: 1.22–3.54), but also increased the risk of ICU admission and need for IMV in patients with COVID-19 (OR: 1.54, 95% CI: 1.29–1.84 and OR: 1.73, 95% CI: 1.38–2.17). Obesity (BMI of ≥30 kg/m2) also increased the risk of mortality in patients with COVID-19 (OR: 1.43, 95% CI: 1.17–1.74) (Figure 3B). Figure 3C showed that in the Asian population, obesity (BMI of ≥28 kg/m2) increased the risk of severe illness (OR: 3.14, 95% CI: 1.83–5.38).
Figure 3.
Forest plots of the odds ratios of obesity and different clinical outcomes. Need for IMV; admission to an ICU; hospitalization (Western population). Mortality (Western population). (C) Severe illness (Asian population). ICU = intensive care unit; IMV = invasive mechanical ventilation.
3.6. Associations between different BMI classes and adverse clinical outcomes in patients with COVID-19
In our study, BMI was divided into 3 classes: <25 kg/m2, 25–30 kg/m2, and ≥30 kg/m2. A total of 9 relevant studies[23,32–34,49,55,57,60,67] were included in this analysis. Of these studies, 3[32,55,57] reported on ICU admission, 2[34,60] on need for IMV, and 2[49,67] on mortality. For the outcome event of hospitalization,[33] severe illness,[23] and critical illness,[33] there was only 1 study each. Therefore, we did not perform a meta-analysis on these 3 outcomes. The relevant data were extracted and pairwise comparisons were made according to different BMI classes (BMI: 25–30 kg/m2 vs <25 kg/m2, ≥30 kg/m2 vs <25 kg/m2, ≥30 kg/m2 vs 25–30 kg/m2), and OR values and 95% CI for different clinical outcomes were calculated.
As shown in Table 3, compared with patients with COVID-19 and a BMI of <25 kg/m2, those with a BMI of 25–30 kg/m2 had an increased risk of need for IMV (OR: 2.19, 95% CI: 1.30–3.69). Meanwhile, compared with patients with COVID-19 and a BMI of <25 kg/m2, those with a BMI of ≥30 kg/m2 had an increased risk of ICU admission and need for IMV (OR: 2.32, 95% CI: 1.20–4.47; OR: 3.04, 95% CI: 1.76–5.28).
Table 3.
Associations between different BMI levels and adverse clinical outcomes in patients with COVID-19.
| BMI (kg/m2) | ICU | IMV | Death |
| BMI 25–30 vs BMI <25 | 1.55 (0.75,3.17) | 2.19 (1.30, 3.69) | 0.50 (0.09, 2.65) |
| BMI >30 vs BMI <25 | 2.32 (1.20,4.47) | 3.04 (1.76, 5.28) | 0.63 (0.09, 4.53) |
| BMI >30 vs BMI 25–30 | 1.49 (1.00,2.21) | 1.69 (0.69, 4.15) | 1.30 (0.75, 2.28) |
At the same time, patients with COVID-19 and a BMI of ≥30 kg/m2 had an increased risk of ICU admission compared with those with a BMI of 25–30 kg/m2 (OR: 1.49, 95% CI: 1.00–2.21).
4. Discussion
In this study, we used 3 different methods from 3 different perspectives to explore the effects of BMI and obesity on the clinical outcomes of patients with COVID-19. First, we found that patients with COVID-19 who had severe illness, were admitted to an ICU, and needed IMV had higher BMI than that of patients without these clinical outcome events. Second, patients with COVID-19 and obesity had an increased risk of hospitalization, admission to an ICU, need for IMV, and mortality compared to their counterparts. Finally, we found that compared with that in the normal BMI group, there was an increased risk of hospitalization and need for IMV in the overweight group. Meanwhile, the obesity group was associated with an increased risk of hospitalization, ICU admission, and need for IMV compared with the normal BMI group.
Previous studies have shown that BMI is an independent risk factor for influenza, and obesity increases the severity of influenza and other respiratory infectious diseases.[70] Some researchers have also reported that obesity is associated with an increased risk of ICU admission, need for IMV, and death.[71] Another study found that half of the patients over the age of 20 years in California who were infected with H1N1 were obese.[72] Similarly, it was found that COVID-19 patients with obesity have a poor prognosis, with a significant proportion of patients in the ICU being overweight.[73]
However, there is still no clear explanation of why obesity increases the risk of adverse outcome events in patients with COVID-19. The possible reasons are as follows.
Patients with obesity, especially those with abdominal obesity, are likely to have limited diaphragm and chest wall movement, which reduces respiratory compliance. Moreover, they often have narrow airways in the nose and pharynx, which further increases respiratory resistance and aggravates the symptoms of dyspnea in patients with COVID-19.
Patients with obesity are likely to develop obstructive sleep apnea hypoventilation syndrome, wherein the body is in a state of chronic hypoxia for a long time, which can cause a series of target organ function damage, making patients in a state of long-term cardiopulmonary impairment, causing coronary heart disease, pulmonary hypertension and cerebral stroke and other diseases. When these patients are infected with SARS-CoV-2, they are likely to develop heart and respiratory failure, which can lead to worsening of the illness and even death. Similarly, obesity is a risk factor for diabetes mellitus, which is reportedly associated with an increased risk of adverse outcome events in patients with COVID-19.
Adipose tissue not only stores energy but also has endocrine functions. It can secrete a variety of inflammatory factors, participate in inflammatory responses, and regulate immunity. Obesity is a chronic metabolic disease that is associated with chronic inflammation, oxidative stress, and changes in hormone levels in the body. Inflammatory factors produced by adipose tissue include leptin, adiponectin, resistin, and visfatin. Individuals with obesity tend to have higher leptin levels[74] and lower adiponectin levels[75] compared to those with normal BMI. Studies have shown that leptin level can affect the proliferation of effector T cells, thereby influencing the function of immune system.[76] Adiponectin is an inflammatory factor secreted by bronchial epithelial cells; it plays an important role in preventing airway smooth muscle thickening, airway hyper reactivity, and bronchial inflammation.[77] At the same time, macrophages, as a component of adipose tissue, can secrete a variety of cytokines and chemokines, such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and monocyte chemotactic protein-1. These factors can damage the patient's immune system, and immune dysfunction and excessive immune system activation can cause cytokine storms, further aggravating the disease and even leading to life-threatening events.[78] Many patients with severe COVID-19 have significantly increased serum levels of inflammatory factors, especially TNF-alpha, IL-6, IL-8, and IL-17.[79] Moreover, studies have found that in the treatment of COVID-19, cytoinflammatory factor antagonists, particularly anti-interleukin-6 drugs, can effectively improve the prognosis of patients with severe COVID-19,[80] suggesting that chronic inflammation may play an important role in the progression of COVID-19.
SARS-CoV-2 is a virus that uses angiotensin-converting enzyme 2 (ACE2) as an invasion receptor. SARS-CoV-2 spike protein receptor binding domain interacts with ACE2 to invade cells.[81] ACE2 is highly expressed in adipose tissue, and patients with obesity tend to have more adipose tissue than that observed in the general population. This may explain why patients with COVID-19 and obesity are more likely to have serious clinical outcomes than are patients with a normal BMI. ACE2 plays an extremely important regulatory role in the renin-angiotensin-aldosterone system, and infection with SARS-CoV-2 reduces the activity of ACE 2, leading to increased levels of angiotensin II, further causing lung damage.[82]
Previous studies have shown that compared with normal weight mice, obese mice are more prone to lung injury, pulmonary edema, and inflammatory reactions, and obese mice require a longer recovery period during the process of tissue repair.[83] Additionally, studies have shown that obesity prolongs the time required for virus shedding from the body.[84] This may explain the increased severity of the COVID-19 in patients with obesity.
Patients with obesity usually present with impaired T or B cell immunity. Previous studies on obese mice have found that the cytotoxicity and levels of influenza-specific CD8+ memory T cells in obese mice are significantly reduced, while neutrophil counts increase, which usually indicates severe disease status and poor prognosis.[85]
Finally, studies have confirmed that moderate aerobic exercise has a certain anti-inflammatory effect.[86] Patients with obesity often have a sedentary lifestyle, which increases their chance of being infected with SARS-CoV-2.
5. Limitation
Most of the studies included in this meta-analysis were single-center retrospective studies, while multi-center and large-sample studies were rarely included. This study only used BMI as a single indicator to define obesity and lacked data on multi-dimensional indicators such as waist circumference, waist-to-hip ratio, and the distribution of visceral fat.
In this meta-analysis, the heterogeneity of the research results may be related to race, comorbidities, age, and gender distribution of the patients. The lack of subgroup analyses is another limitation of this study.
6. Conclusions
This meta-analysis suggests that BMI is closely related to COVID-19 severity. As BMI increases, especially in COVID-19 patients with obesity, the risks of hospitalization, ICU admission, and need for IMV increase. Therefore, during the COVID-19 epidemic, the protection of people with obesity should be strengthened. Clinicians should take into consideration the impact of BMI when assessing the risks of COVID-19 in patients and determining the next treatment steps.
Author contributions
Conceptualization: Jingfang Liu.
Data curation: Yaxian Yang, Liting Wang, Songbo Fu.
Methodology: Yaxian Yang, Jingfang Liu, Songbo Fu.
Resources: Jingfang Liu, Songbo Fu, Liyuan Zhou.
Software: Liting Wang.
Writing – original draft: Yaxian Yang.
Writing – review & editing: Yaxian Yang, Jingfang Liu, Songbo Fu, Liyuan Zhou, Yan Wang.
Footnotes
Abbreviations: ACE2 = angiotensin-converting enzyme 2, BMI = body mass index, CAD = coronary artery disease, CHD = coronary heart disease, CIs = confidence intervals, CKD = chronic kidney diseases, COVID-19 = coronavirus disease 2019, CVD = cerebrovascular disease, DM = diabetes mellitus, HTN = hypertension, ICU = intensive care unit, IL = interleukin, IMV = invasive mechanical ventilation, NA = not available, NOS = Newcastle–Ottawa Scale, OR = odds ratio, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, SD = standard deviation, TNF = tumor necrosis factor, WMD = weighted mean difference.
How to cite this article: Yang Y, Wang L, Liu J, Fu S, Zhou L, Wang Y. Obesity or increased body mass index and the risk of severe outcomes in patients with COVID-19: A protocol for systematic review and meta-analysis. Medicine. 2022;101:1(e28499).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CAD = coronary artery disease, CHD = coronary heart disease, CKD = chronic kidney diseases, CVD = cerebrovascular disease, DM = diabetes mellitus, HTN = hypertension, ICU = intensive care unit, IMV = invasive mechanical ventilation, and NA = not available.
The number in the table represents the score.
BMI = body mass index, ICU = intensive care unit, and IMV = invasive mechanical ventilation.
References
- [1].Giovanetti M, Benedetti F, Campisi G, et al. Evolution patterns of SARS-CoV-2: snapshot on its genome variants. Biochem Biophys Res Commun 2021;538:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J 2021;23:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 2021;76:428–55. [DOI] [PubMed] [Google Scholar]
- [4].Tchang BG, Saunders KH, Igel LI. Best practices in the management of overweight and obesity. Med Clin North Am 2021;105:149–74. [DOI] [PubMed] [Google Scholar]
- [5].de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis 2020;20:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [8].Luo D, Wan X, Liu J, Tong T. How to estimate the sample mean and standard deviation from the sample size, median, extremes or quartiles? Chin J Evid-Based Med 2017;17:1350–6. [Google Scholar]
- [9].Liu C, Wang C, Guan S, et al. The prevalence of metabolically healthy and unhealthy obesity according to different criteria. Obes Facts 2019;12:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Phillips CM, Dillon C, Harrington JM, et al. Defining metabolically healthy obesity: role of dietary and lifestyle factors. PLoS One 2013;8:e76188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol 2016;22:681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wei Y, Zeng W, Huang X, et al. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect Dis 2020;20:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl Trop Dis 2020;14:e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med 2020;288:128–38. [DOI] [PubMed] [Google Scholar]
- [15].Xiang T, Liu J, Xu F, et al. Analysis of clinical characteristics of 49 patients with coronavirus disease 2019 in Jiangxi. Chin J Resp Crit Care Med 2020;19:154–60. [Google Scholar]
- [16].Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection 2020;48:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 2020;31:1387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun DW, Zhang D, Tian RH, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: a sentinel? Clin Chim Acta 2020;508:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu M, He P, Liu HG, et al. [Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:209–14. [DOI] [PubMed] [Google Scholar]
- [20].Peng YD, Meng K, Guan HQ, et al. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:450–5. [DOI] [PubMed] [Google Scholar]
- [21].Zhang N, Wang C, Zhu F, et al. Risk factors for poor outcomes of diabetes patients with COVID-19: a single-center, retrospective study in early outbreak in China. Front Endocrinol (Lausanne) 2020;11:571037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang M, Yang Y, Shang F, et al. Clinical characteristics and predictors of disease progression in severe patients with COVID-19 infection in Jiangsu Province, China: a descriptive study. Am J Med Sci 2020;360:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis 2020;71:2089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang M, Yang F, Zhu X, et al. Clinical characteristics and outcome of novel coronavirus pneumonia patients with different body mass index. Chin J Endocrinol Metab 2021;37:17–21. [Google Scholar]
- [25].Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020;75:1742–52. [DOI] [PubMed] [Google Scholar]
- [26].Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020;146:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petrey AC, Qeadan F, Middleton EA, Pinchuk IV, Campbell RA, Beswick EJ. Cytokine release syndrome in COVID-19: innate immune, vascular, and platelet pathogenic factors differ in severity of disease and sex. J Leukoc Biol 2021;109:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020;17:1439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr 2020;223:14–9. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Almazeedi S, Al-Youha S, Jamal MH, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine 2020;24:100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mejia-Vilet JM, Cordova-Sanchez BM, Fernandez-Camargo DA, Mendez-Perez RA, Morales-Buenrostro LE, Hernandez-Gilsoul T. A risk score to predict admission to the intensive care unit in patients with COVID-19: the ABC-GOALS score. Salud Publica Mex 2020;63:01–11. [DOI] [PubMed] [Google Scholar]
- [32].Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: impact of obesity and diabetes on disease severity. Clin Obes 2020;10:e12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 Patients with COVID-19 in New York: retrospective case series. BMJ 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dreher M, Kersten A, Bickenbach J, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int 2020;117:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Regina J, Papadimitriou-Olivgeris M, Burger R, et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: an observational retrospective study. PLoS One 2020;15:e0240781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brill SE, Jarvis HC, Ozcan E, et al. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC Med 2020;18:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wendel Garcia PD, Fumeaux T, Guerci P, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020;25:100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gayam V, Chobufo MD, Merghani MA, Lamichhane S, Garlapati PR, Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol 2021;93:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Krishnan S, Patel K, Desai R, et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth 2020;67:110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Masetti C, Generali E, Colapietro F, et al. High mortality in COVID-19 patients with mild respiratory disease. Eur J Clin Invest 2020;50:e13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Salacup G, Lo KB, Gul F, et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: a single tertiary center cohort. J Med Virol 2021;93:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020;48:e799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Luo HC, You CY, Lu SW, Fu YQ. Characteristics of coagulation alteration in patients with COVID-19. Ann Hematol 2021;100:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang F, Xiong Y, Wei Y, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol 2020;92:2536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28:1595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Halvatsiotis P, Kotanidou A, Tzannis K, et al. Demographic and clinical features of critically ill patients with COVID-19 in Greece: the burden of diabetes and obesity. Diabetes Res Clin Pract 2020;166:108331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Halasz G, Leoni ML, Villani GQ, Nolli M, Villani M. Obesity, overweight and survival in critically ill patients with SARS-CoV-2 pneumonia: is there an obesity paradox? Preliminary results from Italy. Eur J Prev Cardiol 2020;28:e15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carrillo-Vega MF, Salinas-Escudero G, Garcia-Peña C, Gutierrez-Robledo LM, Parra-Rodriguez L. Early estimation of the risk factors for hospitalisation and mortality by COVID-19 in México. PLoS One 2020;15:e0238905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shekhar R, Upadhyay S, Sheikh A, Atencio J, Kapuria D. Early experience with COVD-19 patients at academic hospital in Southwestern United States. Infect Dis (Lond) 2020;52:596–9. [DOI] [PubMed] [Google Scholar]
- [53].Ebinger JE, Achamallah N, Ji H, et al. Pre-existing traits associated with Covid-19 illness severity. PLoS One 2020;15:e0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ferguson J, Rosser JI, Quintero O, et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March–April 2020. Emerg Infect Dis 2020;26:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:09–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Edward I, Yishay W, Amitai S, et al. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J 2020;22:271–4. [PubMed] [Google Scholar]
- [57].Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring) 2020;28:1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hur K, Price CPE, Gray EL, et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg 2020;163:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Caussy C, Wallet F, Laville M, et al. Obesity is associated with severe forms of COVID. Obesity 2020;28:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Giacomelli A, Ridolfo AL, Milazzo L, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res 2020;158:104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Borobia AM, Carcas AJ, Arnalich F, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med 2020;9:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rossi PG, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia, Italy. PLoS One 2020;15:e0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult inpatients with COVID-19. Public Health 2021;190:01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Global Health 2020;8:e1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rodriguez A, Moreno G, Gomez J, et al. Severe infection due to the SARS-CoV-2 coronavirus: Experience of a tertiary hospital with COVID-19 patients during the 2020 pandemic. Med Intensiva (Engl Ed) 2020;44:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Amit M, Sorkin A, Chen J, et al. Clinical course and outcomes of severe Covid-19: a National Scale study. J Clin Med 2020;9:2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].China National Health Commission. Diagnosis and treatment of pneumonitis caused by new coronavirus (trial version 7) Beijing: China National Health Commission; March 3, 2020. Available at: http://en.nhc.gov.cn/2020-03/29/c_78469.htm. [Google Scholar]
- [70].Moser JS, Galindo-Fraga A, Ortiz-Hernandez AA, et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir Viruses 2019;13:03–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fuhrman C, Bonmarin I, Bitar D, et al. Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol Infect 2011;139:1202–9. [DOI] [PubMed] [Google Scholar]
- [72].Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011;52:301–12. [DOI] [PubMed] [Google Scholar]
- [73].Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med (Lond) 2020;20:e109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Friedman MHJL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- [75].Salvator H, Grassin-Delyle S, Naline E, et al. Contrasting effects of adipokines on the cytokine production by primary human bronchial epithelial cells: inhibitory effects of adiponectin. Front Pharmacol 2020;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fischer HJ, Sie C, Schumann E, et al. The insulin receptor plays a critical role in T cell function and adaptive immunity. J Immunol 2017;198:1910–20. [DOI] [PubMed] [Google Scholar]
- [77].Zhu XL, Qin XQ, Xiang Y, Tan YR, Qu XP, Liu HJ. Adipokine adiponectin is a potential protector to human bronchial epithelial cell for regulating proliferation, wound repair and apoptosis: comparison with leptin and resistin. Peptides 2013;40:34–41. [DOI] [PubMed] [Google Scholar]
- [78].Picchianti Diamanti A, Rosado MM, Pioli C, Sesti G, Lagana B. Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci 2020;21:3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 2020;9:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shimizu Y. Understanding the immunopathogenesis of COVID-19: its implication for therapeutic strategy. World J Clin Cases 2020;8:5835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Barnes CO, Jette CA, Abernathy ME, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020;588:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Furuhashi M, Moniwa N, Takizawa H, Ura N, Shimamoto K. Potential differential effects of renin-angiotensin system inhibitors on SARS-CoV-2 infection and lung injury in COVID-19. Hypertens Res 2020;43:837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus 1,2. J Nutr 2007;137:1236–43. [DOI] [PubMed] [Google Scholar]
- [84].Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018;218:1378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020;81:e6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Codella R, Luzi L, Inverardi L, Ricordi C. The anti-inflammatory effects of exercise in the syndromic thread of diabetes and autoimmunity. Eur Rev Med Pharmacol Sci 2015;19:3709–22. [PubMed] [Google Scholar]