Abstract
Inflammatory bowel disease (IBD) is caused by the activation of an abnormal immune response in the intestinal mucosa; the spleen is involved in the main immune response. Ulcerative colitis (UC) and Crohn disease (CD) have different inflammatory mechanisms; this study aimed to quantitatively measure and compare the spleen volumes between patients with UC and CD and examine the relationship between spleen volume and disease activity in both.
We retrospectively analyzed 44 patients with IBD aged 30–60 years (UC group, n = 24; CD group, n = 20). The control group comprised 19 patients with pancreatic cysts that did not affect the spleen volume. All patients underwent computed tomography (CT) between April 2014 and March 2019. Using the Image J software, spleen volumes in the UC, CD, and control groups were measured accurately from the CT images and adjusted for the body weight.
No significant differences in the sex, age, or body weight were noted between the UC and CD groups and the control group. The spleen volumes, adjusted for the body weight, were 2.2 ± 1.0 cm3/kg, 2.0 ± 1.0 cm3/kg, and 3.6 ± 1.7 cm3/kg in the control, UC, and CD groups, respectively. The volumes differed significantly between the CD and control groups (P = .01), but not between the UC and control groups (P = .43). Furthermore, a significant strong correlation was found between the disease activity and the body weight-adjusted spleen volume in patients with CD (P < .01).
The spleen volume, adjusted for the body weight, was significantly larger in patients with CD than in the controls and was also strongly correlated with the CD activity. These results suggest that the immune response in CD may affect the spleen volume.
Keywords: computed tomography, Crohn disease, disease activity, inflammatory bowel disease, spleen volume, ulcerative colitis
1. Introduction
Genetic and environmental factors are believed to be involved in the pathophysiology of Crohn disease (CD) and ulcerative colitis (UC); however, the detailed mechanisms remain unclear.[1–4] In inflammatory bowel disease (IBD), various cytokines are thought to be involved in excessive T-cell activation in the intestinal mucosa.[5–9] These inflammatory substances may affect not only the intestinal tract, but also other organs.
Lymphoid tissue aggregates in the spleen; this is strongly correlated with an immune response.[10] Splenomegaly has various etiologies, such as portal hypertension, infectious diseases, blood diseases, and metabolic disorders. According to previous case reports, patients with CD have a large spleen.[11,12] However, details on the correlation between IBD and the spleen remain unknown because this clinical presentation was not explored in detail due to its rarity and difficulties in the accurate measurement of the spleen size at that time. Conversely, IBD is known to be associated with a decreased splenic function; however, the relationship between disease activity and the spleen volume is unknown.[13] Furthermore, the spleen volume is strongly affected by age and body weight; therefore, these factors should be considered when comparing the spleen volumes between CD and UC.[14] This study aimed to calculate the accurate spleen volume of patients with IBD using the Image J software (a science image analysis program) and to examine the relationship between spleen volume and IBD activity.
2. Materials and methods
2.1. Study group
We identified 24 and 20 patients diagnosed with UC (UC group) and CD (CD group), respectively, at the Fukushima Medical University and the Ohta Nishinouchi Hospital between April 2014 and March 2019. In this study, only patients between 30 and 60 years of age were included. An age-matched control group was also maintained; this comprised 19 patients who were diagnosed with pancreatic cysts that did not affect the spleen volume. Patients with factors that affected the spleen volume were excluded from the study. These factors comprised liver disease, portal hypertension, heart disease, infectious disease, blood disease, and cancer. Additionally, patients receiving tumor necrosis factor (TNF)-α antagonists or immunomodulators were also excluded from this study. The study protocol and design were approved by the Ethics Committee of the Fukushima Medical University. Informed consent was obtained in an opt-out manner on the institutional website.
2.2. Measurement of the spleen volume
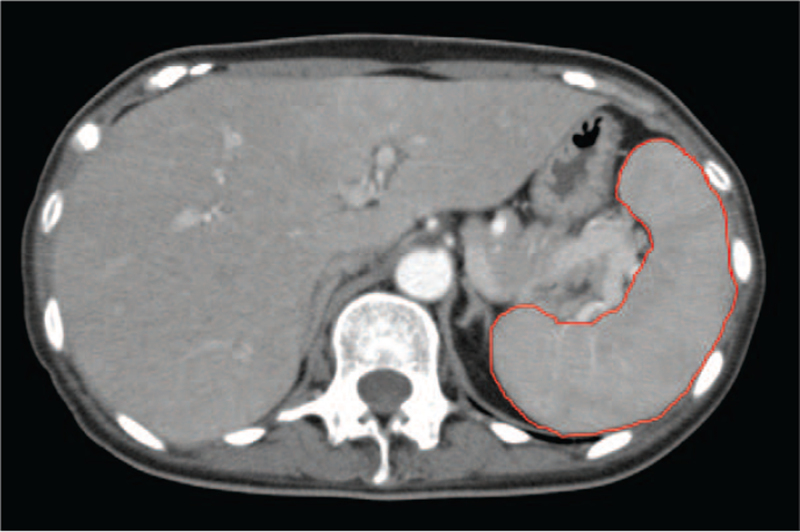
All computed tomography (CT) images that were taken with a slice width of 5–7 mm were analyzed with the Image J software (National Institute of Health, Bethesda, MD).[14] An outline of the spleen was made on each slice, and the spleen area was measured accordingly; the spleen volume in each slice was calculated by multiplying the spleen area by the corresponding slice thickness. The total spleen volume in each patient was calculated by combining the total spleen volumes of all slices (Fig. 1).
Figure 1.
Spleen volume measurement using computed tomography (CT) in a patient with Crohn disease. Using the Image J software, the outline of the spleen is drawn (red line) on each slice of the CT image, and the spleen area is calculated. The thickness of each CT slice is multiplied by the corresponding spleen area on the slice; summation of the volumes of all slices gives the total spleen volume.
2.3. Evaluation of disease activity in IBD
For each patient, the condition of the large intestinal mucosa was evaluated from the findings of the lower gastrointestinal endoscopy performed at the same time as the CT. CD activity was scored using the Crohn's Disease Activity Index (CDAI)[15]; this tool includes weight, general health status, stool frequency, degree of abdominal pain, hematocrit, and complications. Conversely, UC activity was scored using the partial Mayo score[16]; this tool includes stool frequency, rectal bleeding, and the physician's general assessment. Using electronic medical records, we collected the blood test data that were obtained closest to when CT was performed.
2.4. Receiver operating characteristic curve
The receiver operating characteristic curve (ROC) for the spleen volume (adjusted for body weight) in the prediction between UC and CD is evaluated to determine cut-off value.
2.5. Statistical analysis
Categorical variables were compared using the chi-square test or the Fisher's exact test. Quantitative variables were compared using the Mann–Whitney U test. The Pearson's correlation coefficient was used for the comparative study of inflammatory activity and spleen volume after adjusting for the body weight. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).[17] More precisely, EZR is a modified version of the R commander that was designed to add statistical functions frequently used in biostatistics. A P-value <.05 was considered statistically significant.
3. Results
3.1. Patients’ characteristics
The characteristics of the control, UC, and CD groups are shown in Table 1. The mean ages of the UC and CD groups were 42.5 ± 8.1 years and 44.2 ± 8.7 years, respectively; these did not differ significantly from the mean age of the control group (44.7 ± 4.9 years; control vs. UC, P = .39; control vs. CD, P = .60). The mean body weights of the UC and CD groups were 63.0 ± 12.5 kg and 55.2 ± 13.1 kg, respectively; these did not differ significantly from the mean body weight of the control group (62.6 ± 12.7 kg; control vs. UC, P = .91; control vs. CD, P = .11). The disease classification was determined based on the extent of the disease. Accordingly, 2, 11, and 11 patients from the UC group had a rectal-type, left-side-type, and total colon-type disease, respectively; furthermore, 4, 12, and 4 patients from the CD group had a small intestine-type, small intestine colon-type, and colon-type disease, respectively. The mean disease durations in the UC and CD groups were 10.8 ± 10.1 years and 7.6 ± 6.6 years, respectively. Furthermore, 3 (15.0%) and 5 (20.8%) patients in the CD and UC groups were taking prednisolone at the time of the CT examination, respectively.
Table 1.
Characteristics of patients with inflammatory bowel disease and the controls.
| Control (n = 19) | UC (n = 24) | CD (n = 20) | P-value UC vs Control∗/CD vs Control† | |
| Age | 44.7 ± 4.9 | 42.5 ± 8.1 | 44.2 ± 8.7 | 0.39∗/0.60† |
| Sex, n (%) | 0.22∗/0.65† | |||
| Male/Female | 13/6 | 12/12 | 15/5 | |
| Body weight (kg) | 62.6 ± 12.7 | 63.0 ± 12.5 | 55.2 ± 13.1 | 0.91∗/0.11† |
| Period of illness (years) | – | 10.8 ± 10.1 | 7.6 ± 6.6 | – |
| Disease extent, n (%) | – | |||
| – | Rectal, 2 | Ileal, 4 | ||
| – | Left-sided, 11 | Ileocolonic, 12 | ||
| – | Pancolitis, 11 | Colonic, 4 | ||
| 5-aminosalicylic acid, n (%) | – | 23 (95.8) | 10 (50.0) | – |
| Prednisolone, n (%) | – | 5 (20.8) | 3 (15.0) | – |
Table 2 shows the blood test results. All blood data for the control group were within the normal ranges. However, the platelet counts were significantly higher in the UC (33.1 ± 10.3 × 104/μL) and CD (35.6 ± 13.1 × 104/μL) groups than in the control group (22.6 ± 5.9 × 104/μL). The albumin levels were significantly lower in the UC (3.6 ± 0.7 g/dL) and CD (3.3 ± 0.7 g/dL) groups than in the control group (4.2 ± 0.4 g/dL) (control vs UC, P < .01; control vs. CD, P < .01). The serum C-reactive protein (CRP) level in the UC group (1.6 ± 3.3 mg/dL) did not differ significantly from that in the control group (0.2 ± 0.2 mg/dL); however, the CRP level in the CD group (7.2 ± 9.6 mg/dL) was significantly higher than that in the control group (P < .001).
Table 2.
Results of blood tests in patients with inflammatory bowel disease and the controls.
| Control (n = 19) | UC (n = 24) | CD (n = 20) | P-value UC vs Control∗/CD vs Control† | |
| WBC count (/μl) | 6868 ± 3075 | 7592 ± 3114 | 9165 ± 3257 | .43∗/.03† |
| Plt count (104/ μl) | 22.6 ± 5.9 | 33.1 ± 10.3 | 35.6 ± 13.1 | <.01∗/<.01† |
| CRP level (mg/dl) | 0.2 ± 0.2 | 1.6 ± 3.3 | 7.2 ± 9.6 | .18∗/<.001† |
| Alb level (g/dl) | 4.2 ± 0.4 | 3.6 ± 0.7 | 3.3 ± 0.7 | <.01∗/<.01† |
| TB level (mg/dl) | 0.9 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.4 | .19∗/.18† |
| AST level (U/L) | 17.5 ± 5.0 | 18.6 ± 10.0 | 19.8 ± 12.2 | .65∗/.73† |
| ALT level (U/L) | 19.1 ± 12.9 | 21.5 ± 19.4 | 21.8 ± 15.0 | .93∗/.96† |
| ALP level (U/L) | 197.6 ± 37.3 | 203.7 ± 67.0 | 281.5 ± 146.2 | .98∗/.02† |
| GTP level (U/L) | 36.5 ± 33.6 | 31.2 ± 24.8 | 41.7 ± 47.6 | .78∗/.92† |
3.2. Spleen volume
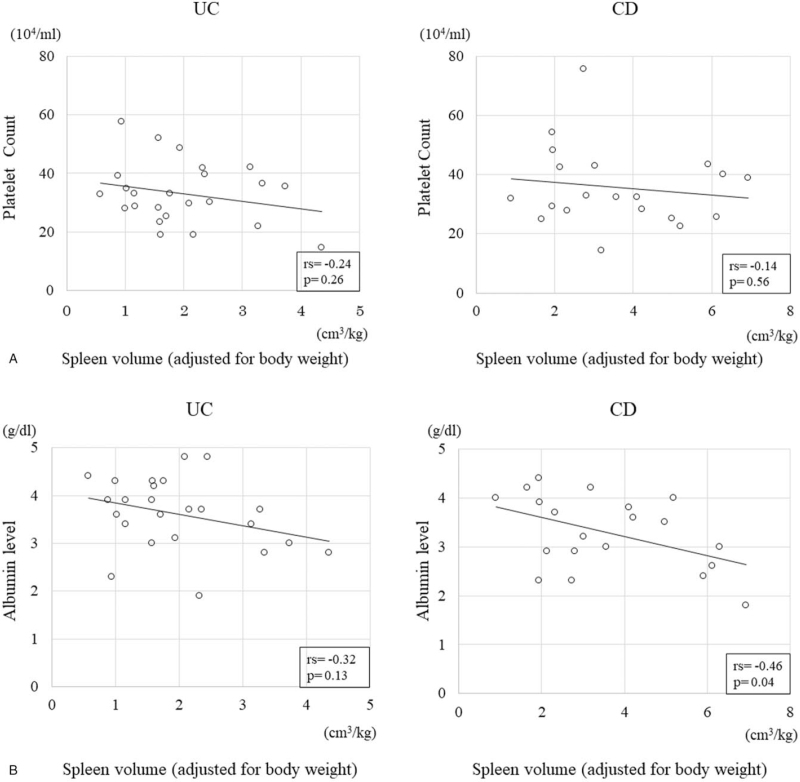
The mean spleen volume in the control group was 134.6 ± 65.2 cm3 (Table 3). The mean spleen volumes in the UC and CD groups were 124.3 ± 61.2 cm3 and 194.2 ± 103.4 cm3, respectively; these did not differ significantly from that of the control group (P = .69 and P = .06, respectively). In contrast, after adjusting for the body weight, the spleen volumes in the control group and the CD group were 2.2 ± 1.0 cm3/kg and 3.6 ± 1.7 cm3/kg, respectively; this difference was significant (P = .01). Figure 2A & B show the relationships of the spleen volume, adjusted for the body weight, with the albumin level and platelet count in the UC and CD groups; no significant correlations between the platelet count and the spleen volume were observed in either group. However, the albumin level in the CD group showed a significantly strong negative correlation with the spleen volume (rs = -0.46, P = .04), but these in the UC group did not.
Table 3.
The correlation between disease activity and splenic volume.
| Control (n = 19) | UC (n = 24) | CD (n = 20) | P-value UC vs Control∗/CD vs Control† | |
| Partial Mayo score | – | 4.9 ± 3.8 | – | NS |
| CDAI | – | – | 104.1 ± 60.6 | NS |
| Spleen volume (cm3) | 134.6 ± 65.2 | 124.3 ± 61.2 | 194.2 ± 103.4 | .69∗/.06† |
| Spleen volume adjusted for body weight (cm3/kg) | 2.2 ± 1.0 | 2.0 ± 1.0 | 3.6 ± 1.7 | .43∗/.01† |
Figure 2.
Relationship between the spleen volumes (adjusted for the body weight) and the platelet counts and albumin levels in patients with ulcerative colitis (UC) and Crohn disease (CD). (A).No significant negative correlation is observed between the spleen volume (adjusted for the body weight) and the platelet count in the UC and CD groups (rs = -0.24, P = .26 and rs = -0.14, P = .56, respectively). (B). No significant correlation is found between the spleen volume (adjusted for the body weight) and the albumin level in the UC group (rs = -0.32, P = .13). However, a significant negative correlation is found between the two in the CD group (rs = -0.46, P = .04).
3.3. The relationship between disease activity and the spleen volume
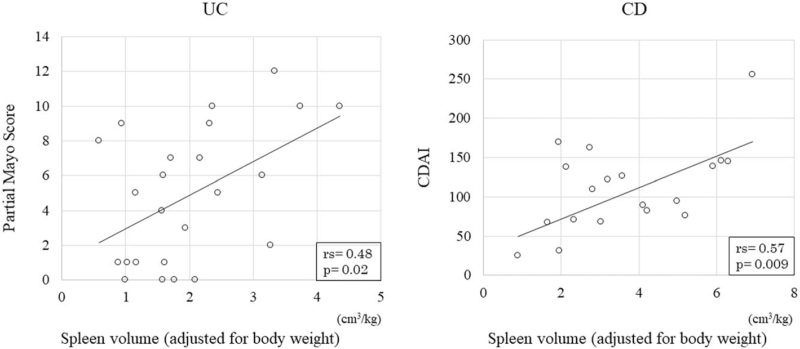
The relationship between disease activity and the spleen volume (adjusted for the body weight) was compared between the UC and CD groups using the Pearson's correlation coefficient. The mean partial Mayo score on CT was 4.9 ± 3.8, indicating a mild UC activity. However, the mean CDAI was 104.1 ± 60.6, indicating inactive CD. Figure 3 shows the relationships of the partial Mayo score in the UC group and the CDAI in the CD group with the spleen volume (adjusted for the body weight). The partial Mayo score and the CDAI showed a significantly positive (rs = 0.48, P = .02) and a strong, significantly positive (rs = 0.57, P < .01) correlation with the spleen volume. No significant correlations were found between the CRP level and the spleen volume, adjusted for body weight, in either group (UC: rs = 0.33, P = .12; CD: rs = 0.16, P = .51; data not shown).
Figure 3.
Relationship between the disease activity and the spleen volume (adjusted for the body weight) in patients with ulcerative colitis (UC) and Crohn disease (CD). The partial Mayo score in patients with UC shows a significant positive correlation with the spleen volume adjusted for the body weight (rs = 0.48, P = .02). The Crohn disease activity index in patients with CD shows a significant, strong positive correlation with the spleen volume adjusted for the body weight (rs = 0.57, P = .009).
3.4. Receiver operating characteristic curve
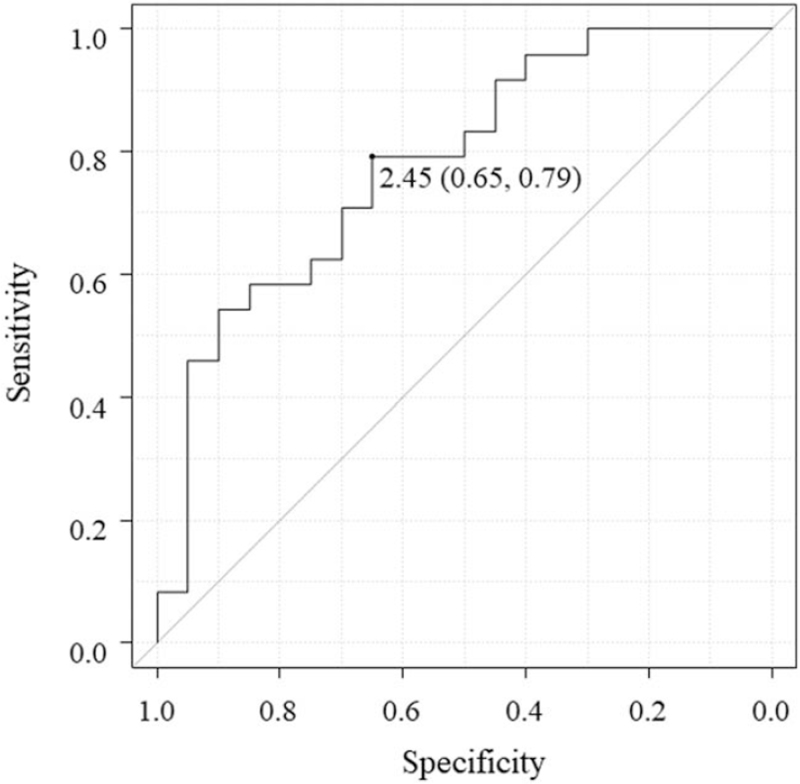
ROC curve analysis was performed for determining the predictive cut-off values of the spleen volume (adjusted for body weight) for UC and CD. The cut-off value, specificity, and sensitivity were found to be 2.5 cm3/kg, 0.65, and 0.79, respectively, for both UC and CD. The area under the ROC curve was 0.78 (95% confidence interval: 0.64–0.92) (Fig. 4).
Figure 4.
Receiver operating characteristic curve for determining the spleen volume (adjusted for the body weight) predictive for ulcerative colitis and Crohn disease. The cut-off value, specificity, and sensitivity of the spleen volume (adjusted for the body weight) are 2.5 cm3/kg, 0.65, and 0.79, respectively. The area under the curve is 0.78 (0.64–0.92).
4. Discussion
By accurately measuring the spleen volume using the Image J software, this study identified the relationship between IBD activity and the spleen volume (adjusted for body weight) in patients with UC and CD; it is the first to do so. It was found that this adjusted spleen volume did not differ significantly between the UC and control groups. However, it was significantly larger in the CD group than in the control group; accordingly, we identified a significant and strong positive correlation between the CD activity and the spleen volume (adjusted for the body weight). These results suggest that the immune response in CD may affect the spleen volume. The cut-off value of the spleen volume (adjusted for the body weight) for both UC and CD was 2.5 cm3/kg, which may be useful in their diagnosis.
The mechanism underlying IBD involves an abnormal immune response in the intestinal tract. The intestinal mucosa is exposed to feces and dietary antigens every day; it is precisely controlled to prevent an excessive immune response. The onset of IBD is thought to be a result of the breakdown of this precise control of the immune response.[1–4] We hypothesized that the abnormal immune response in the intestinal mucosa in patients with IBD may affect other organs, especially the spleen, which is strongly involved in the immune response.
The mean spleen volume in healthy individuals aged 40–49 years has been reported to be 131.0 ± 83.0 cm; this is extremely similar to the spleen volume of the control group in this study (134.6 ± 65.2 cm3).[3,14] Therefore, our method of measuring the spleen was considered acceptable. The spleen volume is positively associated with the body weight; thus, a body-weight adjusted spleen volume was compared between the IBD groups and the control group in this study.[14] This study showed that only the body weight-adjusted spleen volume in the CD group was significantly larger than that in the control group. We believe that there was no significant difference in the spleen volume (unadjusted for body weight) between in the CD and the control groups due to the relatively small body weight in the CD group. In this study, no pathological swelling of the lymph nodes was found near the intestine in both the UC and CD groups. However, it was suspected that the inflammatory cells and cytokines entered the blood vessels and affected the spleen due to the breakdown of the intestinal tract barrier in CD. Inflammation in UC is limited to the large intestinal mucosa; however, in CD, the inflammation affects the entire length and all layers of the digestive tract. This may be the reason why the body weight-adjusted spleen volume was different between the UC and CD groups.[18] Our study may show one of the essential differences in the pathogenic mechanisms of UC and CD in the entire body. According to previous publications, the platelet counts were significantly higher in patients with IBD than in healthy individuals; furthermore, they were elevated in patients with CD even when the CRP levels were not.[19,20] In this study, the platelet counts were significantly higher in the UC and CD groups than in the control group; however, no significant correlation was identified between the platelet count and the spleen volume, adjusted for the body weight, in either group.
Splenomegaly occurs with various etiologies, including portal hypertension, infectious diseases, blood disorders, and metabolic disorders. The spleen size in patients with portal hypertension is correlated with the portal pressure,[21] and splenomegaly due to portal hypertension increases the splenic function and is accompanied by thrombocytopenia.[22] Conversely, IBD is accompanied by a decreased splenic function,[13] and TNF-α (a major cytokine in IBD) damages the marginal zone of the spleen.[23] Regarding spleen dysfunction in IBD, Muller et al. used differential interference contrast microscopy and showed that pitted erythrocytes, which reflect the reticuloendothelial function in UC, are increased in IBD.[24] Sabatino et al reported that immunoglobulin M memory B cells, which are correlated with the splenic function, were reduced in IBD.[25,26] Immune diseases, including rheumatism, have been reported to be associated with hyposplenism[10]; however, the association between the spleen volume and immune disease activity was unclear. This study provides the first statistical evidence of the association between IBD disease activity and the spleen volume. The immune response in CD, specifically, was thought to have led to spleen swelling and an elevated number of platelets due to decreased spleen function; the detailed mechanisms remain unclear.[9]
This study had some limitations. First, this was a single-center retrospective study, and the sample size was small. The CRP level in the CD group was significantly higher than that in the control group; however, the CD activity was evaluated as “inactive” according to the CDAI. This discrepancy may be because the evaluation of CDAI was subjective. Second, Di Sabatino et al reported that TNF-α antagonists improve the splenic function in patients with CD; however, in this study, patients taking TNF-α antagonists or immunomodulators were excluded.[27] Therefore, we could not investigate the effects of these drugs on the spleen volume. It will be necessary to investigate the relationship between changes in the disease activity due to treatments and corresponding changes in the spleen volume in the future. Finally, this study only included a short-term analysis, which revealed that the body weight-adjusted spleen volume was associated with inflammatory activity; however, the long-term outcomes, including the remission state, were not analyzed due to the small sample size. A prospective, multi-center study with a large sample size and further studies on the relationship between the spleen and IBD may help provide evidence to clarify the inflammatory mechanism of IBD.
5. Conclusions
We performed accurate spleen volume measurements, after considering the age and body weight, in patients with IBD. The spleen volume, adjusted for the body weight, was significantly larger in patients with CD than in the control group and was strongly correlated with the disease activity. We speculate that the immune response in CD may affect the spleen volume.
Acknowledgments
We wish to express our deep appreciation to the entire Gastroenterology medical staff and ward staff of the Fukushima Medical University Hospital and the Ohta General Hospital for their assistance with the endoscopic procedures and care of the study patients.
Author contributions
Writing – original draft: Kazumasa Kawashima.
Writing – review & editing: Michio Onizawa, Tatsuo Fujiwara, Naohiko Gunji, Hidemichi Imamura, Kyoko Katakura, Hiromasa Ohira.
Footnotes
Abbreviations: CD = Crohn disease, CDAI = Crohn Disease Activity Index, IBD = inflammatory bowel disease, ROC = receiver operating characteristic curve, TNF = tumor necrosis factor, UC = ulcerative colitis.
How to cite this article: Kawashima K, Onizawa M, Fujiwara T, Gunji N, Imamura H, Katakura K, Ohira H. Evaluation of the relationship between the spleen volume and the disease activity in ulcerative colitis and Crohn disease. Medicine. 2022;101:1(e28515).
The authors have no funding to disclose.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Statistical analysis was conducted using Mann–Whitney's U test and Fisher's exact test with P-values <.05 being considered statistically significant.
CD = Crohn disease, NS = not significant, UC = ulcerative colitis, vs = versus.
The P-value∗ was compared between the UC and control groups, and the P-value† was compared between the CD and control groups.
Data are expressed as mean ± standard deviation.
Statistical analysis was conducted using Mann–Whitney's U text with P-values <.05 being considered statistically significant.
Alb = albumin, ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, CD = Crohn disease, CRP = C-reactive protein, GTP = γ-glutamyltransferase, Plt = platelet, TB = total bilirubin, UC = ulcerative colitis, WBC = white blood cell.
The P-value∗ was compared between the UC and control groups, and P-value† was compared between the CD and control group.
Data are expressed as mean ± standard deviation.
Statistical analysis was conducted using Mann–Whitney's U test with P-values <.05 being considered statistically significant.
CD = Crohn disease, CDAI = Crohn Disease Activity Index, NS = not significant, UC = ulcerative colitis.
The P-value∗ was compared between the UC and control groups, and the P-value† was compared between the CD and control groups.
Data are expressed as mean ± standard deviation.
References
- [1].Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol 2010;45:09–16. [DOI] [PubMed] [Google Scholar]
- [2].Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 2014;20:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol 2007;42:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:01–10. [DOI] [PubMed] [Google Scholar]
- [5].Andreou NP, Legaki E, Gazouli M. Inflammatory bowel disease pathobiology: the role of the interferon signature. Ann Gastroenterol 2020;33:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology 2005;128:1868–78. [DOI] [PubMed] [Google Scholar]
- [7].Takahashi M, Nakamura K, Honda K, et al. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci 2006;51:677–86. [DOI] [PubMed] [Google Scholar]
- [8].Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- [9].Di Sabatino A, Biancheri P, Rovedatti L, MacDonald TT, Corazza GR. New pathogenic paradigms in inflammatory bowel disease. Inflam Bowel Dis 2012;18:368–71. [DOI] [PubMed] [Google Scholar]
- [10].de Porto AP, Lammers AJ, Bennink RJ, ten Berge IJ, Speelman P, Hoekstra JB. Assessment of splenic function. Eur J Clin Microbiol Infect Dis 2010;29:1465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rozen P, Flatau E, Schujman E, Gefel A. Variability of splenomegaly in Crohn's disease. Am J Gastroenterol 1977;67:498–3. [PubMed] [Google Scholar]
- [12].Pereira JL, Hughes LE, Young HL. Spleen size in patients with inflammatory bowel disease. Does it have any clinical significance? Dis Colon Rectum 1987;30:403–9. [DOI] [PubMed] [Google Scholar]
- [13].Ryan FP, Smart RC, Holdsworth CD, Preston FE. Hyposplenism in inflammatory bowel disease. Gut 1978;19:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harris A, Kamishima T, Hao HY, et al. Splenic volume measurements on computed tomography utilizing automatically contouring software and its relationship with age, gender, and anthropometric parameters. Eur J Radiol 2010;75:e97–101. [DOI] [PubMed] [Google Scholar]
- [15].Best WR, Becktel JM, Singleton JW, Kern F, Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's disease study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- [16].Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflam Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- [19].Nakarai A, Kato J, Hiraoka S, et al. Slight increases in the disease activity index and platelet count imply the presence of active intestinal lesions in C-reactive protein-negative Crohn's disease patients. Intern Med 2014;53:1905–11. [DOI] [PubMed] [Google Scholar]
- [20].Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol 2001;96:776–81. [DOI] [PubMed] [Google Scholar]
- [21].Shah SH, Hayes PC, Allan PL, Nicoll J, Finlayson ND. Measurement of spleen size and its relation to hypersplenism and portal hemodynamics in portal hypertension due to hepatic cirrhosis. Am J Gastroenterol 1996;91:2580–3. [PubMed] [Google Scholar]
- [22].Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008;48:1000–7. [DOI] [PubMed] [Google Scholar]
- [23].Engwerda CR, Ato M, Cotterell SE, et al. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am J Pathol 2002;161:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Muller AF, Cornford E, Toghill PJ. Splenic function in inflammatory bowel disease: assessment by differential interference microscopy and splenic ultrasound. Q J Med 1993;86:333–40. [PubMed] [Google Scholar]
- [25].Di Sabatino A, Carsetti R, Rosado MM, et al. Immunoglobulin M memory B cell decrease in inflammatory bowel disease. Eur Rev Med Pharmacol Sci 2004;8:199–203. [PubMed] [Google Scholar]
- [26].Di Sabatino A, Rosado MM, Ciccocioppo R, et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 2005;100:1788–95. [DOI] [PubMed] [Google Scholar]
- [27].Di Sabatino A, Rosado MM, Cazzola P, et al. Splenic function and IgM-memory B cells in Crohn's disease patients treated with infliximab. Inflam Bowel Dis 2008;14:591–6. [DOI] [PubMed] [Google Scholar]