Supplemental Digital Content is available in the text.
Keywords: emergency department, epidemiology, infectious disease, influenza, intensive care unit
OBJECTIVES:
Demonstrate the feasibility of weekly data collection and analysis of public health emergency (PHE) data. Assess fluctuations in, and challenges of, resource matching and potential effect on patient care for influenza in ICUs.
DESIGN:
Multicenter prospective noninterventional study testing effectiveness of leveraging the Discovery Critical Care Research Network Program for Resilience and Emergency Preparedness (Discovery-PREP) in performing PHE research. A 20-question internet survey was developed to prospectively assess ICU influenza-related resource stress. An informatics tool was designed to track responses; data were analyzed within 24 hours of weekly survey completion by the team biostatistician for timely reporting.
PARTICIPANTS:
Critical care and Emergency Medicine Discovery-PREP network investigators self-selected to participate in the voluntary query.
SETTING:
ICUs of 13 hospitals throughout the United States, 12 academic, and one community.
INTERVENTIONS:
ICU physicians were electronically surveyed weekly over 17 weeks during the influenza season (January 2018–April 2018). Responses were collected for 48 hours after each email query.
MEASUREMENTS AND MAIN RESULTS:
The average weekly response among the sites was 79% (range, 65–100%). Significant stress, defined as alterations in ICU staffing and/or resource allocation, occurred in up to 41% of sites during the national peak of influenza activity. These alterations included changes in staffing, not accepting external patient transfers, and canceling elective surgery. During this same period, up to 17% of the sites indicated that these changes might not have been sufficient to prevent potentially avoidable patient harm.
CONCLUSIONS:
This novel approach to querying ICU operational stress indicated that almost half of participating sites experienced critical care resource limitations during peak influenza season and required process and/or staffing changes to better balance resources with patient care demands. This weekly national reporting infrastructure could be adapted and expanded to better inform providers, hospital emergency management teams, and government leaders during PHEs.
Influenza resulted in an estimated 4.3–23 million medical visits, 140,000–960,000 hospitalizations, and 12,000–79,000 respiratory and circulatory deaths annually in the United States during 2010–2018 (1). Despite this burden, data on the optimum treatment for severe influenza remain limited to antiviral treatment and nonstandardized supportive care (2, 3). In addition, the high frequency of patients with severe influenza places a heavy, albeit partially predictable, strain on ICUs during winter months (4, 5). This strain—including higher patient-to-nurse ratios, shortfalls in needed equipment such as mechanical ventilators, and potential shortages of needed medications may have adverse effects on patient care. Ideally, health systems should be able to monitor system stress related to influenza patients and promptly respond to overwhelmed medical centers. Improved situational awareness of healthcare system stress can enable timely resource allocation.
Unfortunately, detailed clinical data collection may be challenging during a public health crisis, as clinicians are focused on alleviating high patient care demands and less likely to have the time for detailed documentation and reporting (6). To be most useful, any monitoring of healthcare system stress needs to be minimally disruptive to clinical care of patients and yet collect sufficient data to measure space, staff, and/or supply resource challenges. Such monitoring requires that data collection and analysis are performed rapidly in real time so that decision-makers are informed regarding the status of a given ICU and can respond to needs. For example, one study reported much higher requirements for sedatives and analgesics required for critically ill patients during the 2009 H1N1 pandemic compared to patients with respiratory failure from other causes (7). Nationwide surges of the coronavirus disease 2019 (COVID-19) pandemic have underscored the need for data sharing and understanding of health system stress to improve resiliency (8, 9).
In previous work with federal partners, we developed an electronic tool to assess emergency department (ED) and ICU system stress that might rise to the level of affecting patient care during a public health emergency. The goal was to use this tool, together with a national database of clinician investigators, to assess feasibility of weekly healthcare systems stress measurement and reporting, in effect, keeping one’s “finger on the pulse” of acute and critical care clinician needs during an emergent event.
We leveraged the Society of Critical Care Medicine (SCCM) Discovery Critical Care Research Network Program for Resilience and Emergency Preparedness (Discovery PREP [10]) to pilot a weekly “Pulse” of system stress on ICU’s related to influenza patients during the 2017–2018 influenza season. Our primary aim was to determine the feasibility of collecting and reporting on prospective, weekly data about the impact of seasonal influenza upon ICU patient care during January 2018–April 2018. Reporting tools were developed to allow weekly assessment of: 1) system stress in the preceding 24 hours and 7 days, 2) patient treatment protocols being used, and 3) key patient characteristics and needs that could impact patient care.
METHODS
This was a prospective, noninterventional longitudinal study that surveyed Discovery PREP physician-scientist participants who volunteered to responding to weekly electronic surveys regarding ICU stress related to care of patients with laboratory-confirmed influenza. Each site had a designated primary responder, with secondary responders at six sites (46%). The University of Southern California Institutional Review Board approved the study protocol under exempt review (HS-16-00948).
An electronic case report form (eCRF) was developed on Meridian, a web-based electronic data capture platform (11). Twenty questions specific to ICUs assessed aggregate site-level resource stress, including: 1) the ability to accept or transfer patients to/from other hospitals, 2) staffing needs, 3) whether available supplies matched patient care demands, and 4) processes or access to advanced organ support (e.g., mechanical ventilation, extracorporeal membrane oxygenation [ECMO], dialysis) (Supplementary Fig. 1, http://links.lww.com/CCX/A879). The specific data elements in the eCRF are reported in Supplementary Table 2 (http://links.lww.com/CCX/A879). Institutional review board (IRB) approval was obtained at all 13 participating sites. The survey excluded collection of protected health information (PHI) on individual patients; thus, informed consent was not required by any site.
Responders at each site received automated weekly emails that contained a web link to the eCRF. Responders were ED or ICU physicians. The individual receiving the query was able to forward the query to a colleague or administrative staff if they were unable to respond given clinical duties and hospital stress. Data were collected between Wednesday 8 am and Friday 8 am Pacific Standard Time (PST) weekly. A follow-up email was sent by the research coordinator to nonresponsive sites if the survey response was not received by 4:30 pm PST daily during the 48-hour data collection period. If by Thursday afternoon sites had still not responded, then a second email reminder was sent at 1 pm PST to nonresponsive sites. Every Friday, after 8 am PST, the weekly data were analyzed by the study biostatistician and a report was created (process time approximately 45 min/wk). This report was sent to the study investigators for review and monthly reports of progress and key findings were distributed to the sites. After the data collection window ended, we queried the sites ad hoc for additional information regarding: 1) the number of ICUs at each site from which data were obtained, 2) whether the number of ICUs included in the query varied week-by-week, and 3) how many ICU beds are in each ICU(s). Analyses were descriptive and included measures of ICU stress, including changes to normal procedures, increases inpatient risk due to increased patient acuity, and the number of patients with influenza receiving treatment. Weekly participation (i.e., response compliance) overall and by site were tracked. Additionally, responses to the eCRF items (n and %) were computed weekly and tracked over time. Analyses were performed using IBM SPSS Statistics (v.24).
RESULTS
Thirteen of 17 (76%) Discovery PREP sites participated in this pilot study (Supplementary Table 1, http://links.lww.com/CCX/A879). Out of the 13, 12 of the sites were academic medical centers in metropolitan areas and one was a community-based hospital. Sites choosing not to participate cited a lack of time and/or funding for research assistant support to conduct the study. On the designated initiation date of the study, nine sites had IRB approval and were considered active; by week 5, all 13 sites were active. The number of ICU beds per site ranged from 16 to 101 (median, 36; 25th–75th percentile, 20–76). All but one site submitted data regarding the same ICUs each week. The weekly response of participating sites varied from 69% to 100% across the 17 weeks of the study. Levels of participation varied by site, with four sites completing all surveys once they were IRB approved; six sites missed only 1 to 3 weeks once activated, while three sites missed 7 or 8 weeks with a total weekly response of 50–100% for individual sites.
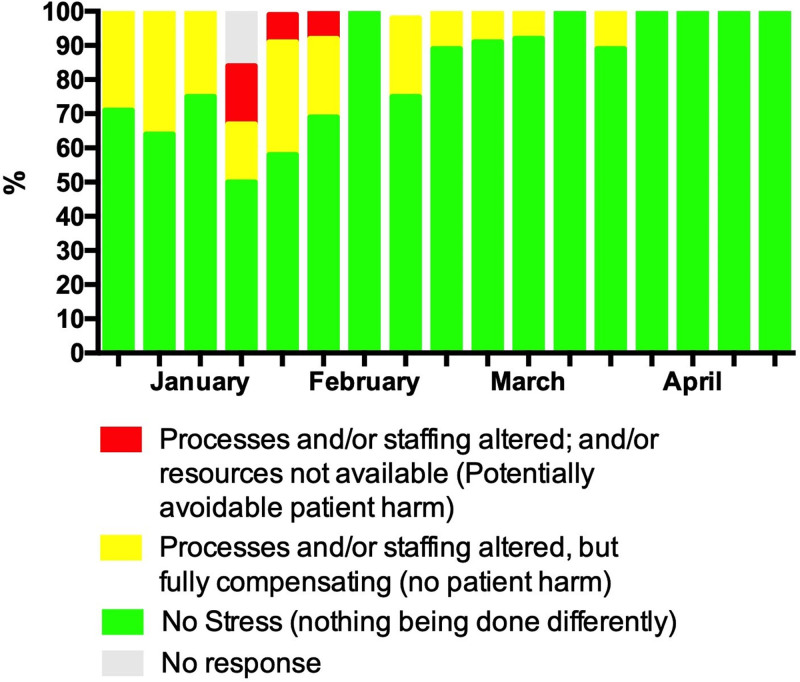
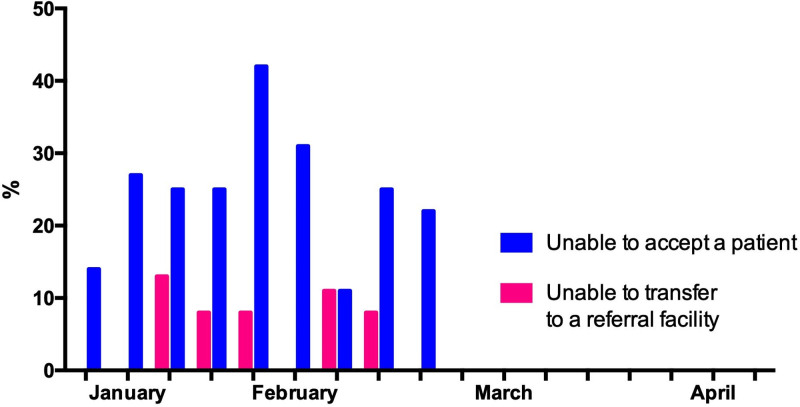
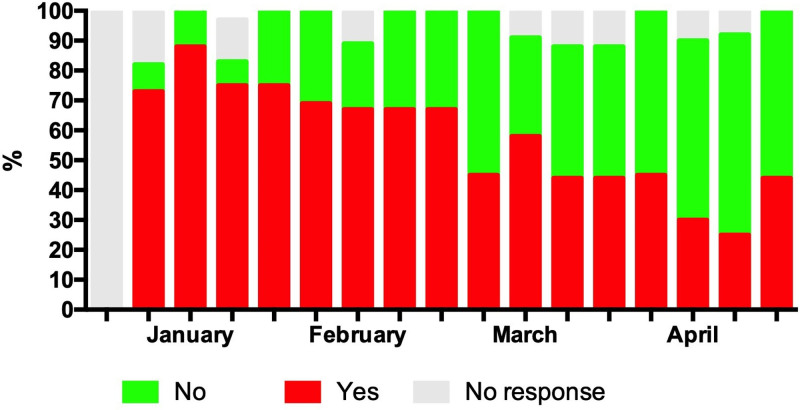
From first survey in the beginning of January 2018, responses indicated that clinical processes and staffing were altered compared to usual practice. Figure 1 demonstrates that these resources were limited during the end of January through February, with 3 weeks in early February having reports of processes or staffing being altered to the point of potentially avoidable patient harm (range 8–17% of sites during the weeks January 24, 2018, to February 7, 2018). This time period coincided with the national peak of seasonal influenza in the United States during the 2017–2018 season (12) (Details about specific changes are included in Supplementary Table 2, http://links.lww.com/CCX/A879). Of particular note are reports of the inability to accept and/or transfer patients, even if their condition was life-threatening (Fig. 2). Reporting at participating sites indicated that system stress resolved completely by early April 2018. Across sites, 25–88% of ICU patients were reported as high risk for influenza-related complications (13) and during January through March at least 50% of the sites reported a predominance of high-risk patients (Fig. 3). Information regarding patients’ influenza vaccination status was limited, with increased reporting of unvaccinated patients occurring in the later months of observation.
Figure 1.
ICU stress for active sites in past 24 hr.
Figure 2.
Ability to transfer patients to or from ICUs at 13 hospitals, January 2018–March 2018.
Figure 3.
High-risk patients. High risk was defined as infants less than 2 yr old, adults 65 yr and older, pregnant women, persons with chronic medical conditions (i.e., pulmonary, cardiac, neurologic, renal, hepatic, endocrine, metabolic, hematologic disorders, immunosuppression), persons less than 19 yr old receiving long-term aspirin therapy, extreme obesity (body mass index > 40), residents of long-term care facilities, and American Indians or Alaska Natives (13).
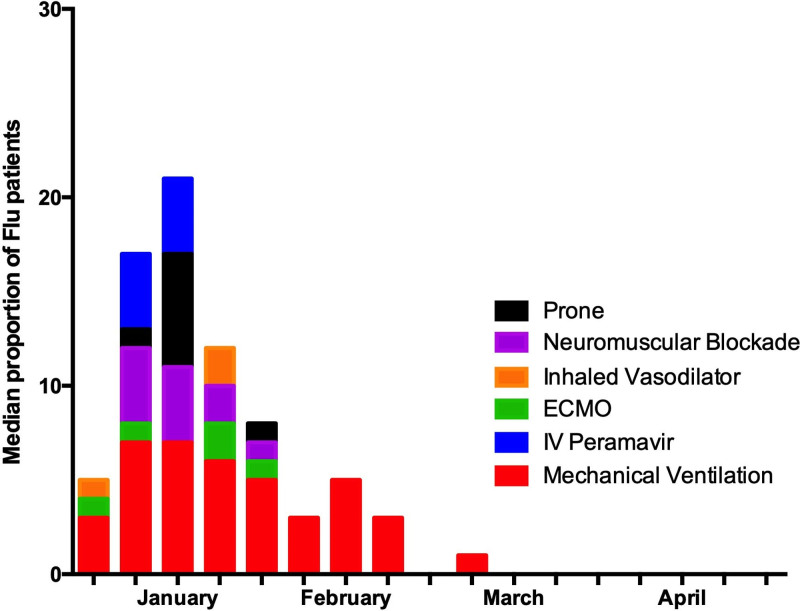
The proportions of patients per site receiving specific treatments are presented in Figure 4, including peramivir, mechanical ventilation, ECMO, neuromuscular blockade, prone positioning, and inhaled vasodilators. Mechanical ventilation was the most commonly reported treatment, with median prevalence of patients across centers on mechanical ventilation ranging from 3% to 7% from January to February. At the highest point, up to 50% of beds at one site were occupied by influenza patients on mechanical ventilation with peaks that coincided with the periods of highest reported stress. Other treatments fluctuated in reported frequency throughout the study period (data not shown).
Figure 4.
Treatments for influenza patients. ECMO = extracorporeal membrane oxygenation.
DISCUSSION
Discovery PREP clinical research network demonstrated that it was feasible to deploy an electronic weekly survey to clinicians; most responded quickly and completed the questionnaire in full. This novel approach to querying ICU operational stress indicated that a large percentage of participating sites experienced critical care resource limitations during the severe 2017–2018 influenza season that required staffing and/or process changes to better balance supplies with patient care demands. Notably, the estimated number of influenza-related hospitalizations and deaths in the United States during 2017–2018 was much higher than during the 2009 H1N1 pandemic and was the most severe influenza season during 2010–2020 (1, 14). Despite these staffing and process changes, a significant percentage of the sites indicated that they were at risk for potentially avoidable patient harm.
Increased critical care resource utilization and challenges due to outbreaks of respiratory disease are incompletely described in the United States. Statistical modeling studies of the impact of a severe influenza pandemic have projected a need for up to 60,000 mechanical ventilators to support critically ill patients in order to avert 300,000 deaths, assuming a sufficient amount of consumables and trained staff (15). Similar models have been used to predict ICU demand during the 2009 A/H1N1 pandemic, in Canada (16), Australia (17), The Netherlands (18), the United Kingdom (19, 20), and France (21).
Despite the existence of large surveillance networks and detailed retrospective cohorts of influenza patients, timely surveillance providing actionable data remains limited, especially regarding critically ill patients. A recent pilot study from North Carolina analyzed influenza-related demands on ICU’s in a three-hospital network during the 2013–2014 season (4). The investigators noted a significant lag time between the initial surge in influenza-associated hospitalizations and increases in ICU-specific utilization, such as mechanical ventilation and ECMO. Furthermore, the SCCM Discovery PREP successfully secured Center for Disease Control funding to apply lessons learned from COVID-19 to Severe Acute Respiratory Infection Preparedness to mitigate the lack of capability to collect data in the wake of COVID-19 (22).
We evaluated not only the interventions patients required but also the more subjective and variable issues of healthcare system stress recognizing that hospitals differ in their resources and limitations. For example, a small number of patients requiring ECMO may be a minor imposition on some hospitals but a massive commitment of resources at others. Identifying stress points in the system frequently and regularly, as determined by the staff working in situ, has the potential to permit on-demand resource allocation that mitigates systems stress and could improve patient care. For example, less-stressed high-acuity units in a health system may be able to provide an appropriate level of clinical support. Trauma systems, for instance, could be a source of ICU beds, trained staffing, and critical care support (23). Conversely, a reduction in less-urgent admissions or elective surgical cases may be necessary in times of high-system stress, as was done in Toronto during the severe acute respiratory syndrome outbreak in 2003 (24, 25) and is increasingly a routine hospital management strategy during times of high demand for hospital beds. Assuming geographic proximity, ICU stress could also be mitigated by directing and diverting patients to ICUs in less-stressed hospitals in a geographic area. The COVID-19 pandemic has highlighted how hospital surge can affect patient outcomes. One large U.S. study of data from 558 hospitals reported that hospital surge was associated with an increased risk of in-hospital mortality across wards, ICUs, and intubated COVID-19 patients (26).
A strength of this study was the use of a simple data entry tool that was quick to complete and allowed for regular, automated notification and data collection, even during the busiest weeks of influenza season. One of the major limitations of this research is that we did not ask for many details about the types of resource limitations. As a result, many of the responses solicited are incomplete and/or not internally consistent (Supplementary Table 2, http://links.lww.com/CCX/A879). Such knowledge would be helpful in identifying specific needs and limitations (e.g., availability of space, staff, life support devices, and medications). While compliance with survey completion was very high, we did not collect information about why missing data were not reported. However, we observed in Figure 1 that the week with the most missing data/no response was the national peak of seasonal influenza that year, supporting the anecdotal feedback from nonresponders that they felt the query was onerous and chose not to participate. Additionally, because the goal of this study was to assess system level stress, we did not include PHI; therefore, the factors we evaluated provided limited knowledge about the impact of stress on individual patient care and outcomes. Without individual patient-level data, we could not evaluate treatment effectiveness; more detailed data collection may be helpful in identifying specific needs and limitations (e.g., availability of space, life support devices, and medications).
As this was a feasibility study with primarily academic hospitals, data are likely not representative of ICUs across the United States. Further work is ongoing expanding the reach to a wider range of ICUs and patients, as well as expansion to EDs who also see an influx of influenza patients most of whom will not require transfer to ICU. As this work expands, examining non-ICU hospital inpatient and outpatient settings, such as private practices, urgent care centers, and long-term care facilities would undoubtedly provide insight into stressors and resource limitations across the spectrum of healthcare as well as aggregate information on impact of influenza on healthcare systems and society. Collection of patient-level data will likely not only help clinicians and decision-makers to make improved resource allocations but would also allow observational or interventional treatment effectiveness studies. Collecting patient-level information would require additional IRB considerations that may impact time required for IRB approval as well and/or research personnel resources required to perform the study, particularly if patient consent is required. Such efforts would ideally include an expansion to a central IRB so that identified changes in the eCRFs could be instituted swiftly. Additional limitations of the study included understanding seasonality due to an increase in influenza compared to an increase in other respiratory-related disorders, investigating changes inpatient flow and the impact on ICU resources, gathering data beyond the ICU and EDs, and obtaining data in an automated fashion across sites. Exploring these areas could provide a more comprehensive view of the impact of the influenza epidemic in future studies.
In summary, our study suggests that it is feasible to rapidly collect and analyze data to closely monitor ICU stress weekly during regarding influenza, which was used as a proxy for a public health emergency. However, room for improvement in data collection was an identified need to ensure consistent, reliable, and expanded data capture. In future studies, responses from other acute care environments should be included, such as non-ICU inpatient units, progressive care (step-down) units, EDs, private practices, and urgent care centers. This national reporting infrastructure, with appropriate funding, could be adapted and scaled to generate actionable information for providers, hospital emergency management teams, and government leaders that could be used to mitigate human, healthcare system, and societal impact during public health emergencies.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by a contract from the Food and Drug Administration and the Biomedical Advanced Research and Development Authority, FDA BAA-12-00118 (HHSF223201400115C and HHSF223201810034C).
Presented in abstract form at the 48th Annual Critical Care Congress of the Society of Critical Care Medicine, San Diego, CA, February 17–20, 2019.
Dr. Cobb also directs the National Resilience Intelligence Network. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Some of the authors are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, opinions or policies of the Centers for Disease Control and Prevention, the Department of Health and Human Services, Department of the Navy, the Department of Defense, or the U.S. Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
REFERENCES
- 1.Centers for Disease Control and Prevention: Disease Burden of Influenza. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed December 20, 2018
- 2.Uyeki TM, Bernstein HH, Bradley JS, et al. : Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2018; 68:e1–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uyeki TM, Fowler RA, Fischer WA: Gaps in the clinical management of influenza: A century since the 1918 pandemic. JAMA 2018; 320:755–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker AW, Edmond MB, Herwaldt LA, et al. : Real-time surveillance of influenza morbidity: Tracking intensive care unit resource utilization. Ann Am Thorac Soc 2017; 14:1810–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng C, Ye L, Noorduyn SG, et al. ; Serious Outcomes Surveillance Network of the Canadian Immunization Research Network (CIRN) Investigators; Toronto Invasive Bacterial Diseases Network (TIBDN) Investigators: Resource utilization and cost of influenza requiring hospitalization in Canadian adults: A study from the serious outcomes surveillance network of the Canadian Immunization Research Network. Influenza Other Respir Viruses 2018; 12:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy DJ, Rubinson L, Blum J, et al. ; United States Critical Illness and Injury Trials Group–Program for Emergency Preparedness: Development of a core clinical dataset to characterize serious illness, injuries, and resource requirements for acute medical responses to public health emergencies. Crit Care Med 2015; 43:2403–2408 [DOI] [PubMed] [Google Scholar]
- 7.Olafson K, Ramsey CD, Ariano RE, et al. : Sedation and analgesia usage in severe pandemic H1N1 (2009) infection: A comparison to respiratory failure secondary to other infectious pneumonias. Ann Pharmacother 2012; 46:9–20 [DOI] [PubMed] [Google Scholar]
- 8.Peiffer-Smadja N, Maatoug R, Lescure FX, et al. : Machine learning for COVID-19 needs global collaboration and data-sharing. Nat Mach Intell 2020; 2:293–294 [Google Scholar]
- 9.Moorthy V, Henao Restrepo AM, Preziosi MP, et al. : Data sharing for novel coronavirus (COVID-19). Bull World Health Organ 2020; 98:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb JP: Clinical investigation during public health emergencies: The resilience intelligence network. Am J Public Health 2019; 109:S268–S270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akido Labs Inc: Project Meridian, Version 2.0. Los Angeles, CA, Akido Labs, 2018 [Google Scholar]
- 12.Garten R, Blanton L, Elal AIA, et al. : Update: Influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. Morb Mortal Wkly Rep 2018; 67:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention: People at High Risk of Flu Complications. Available at: https://www.cdc.gov/flu/about/disease/high_risk.htm. Accessed September 10, 2021
- 14.Shrestha SS, Swerdlow DL, Borse RH, et al. : Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009-April 2010). Clin Infect Dis 2011; 52(Suppl 1):S75–S82 [DOI] [PubMed] [Google Scholar]
- 15.Meltzer MI, Patel A, Ajao A, et al. : Estimates of the demand for mechanical ventilation in the United States during an influenza pandemic. Clin Infect Dis 2015; 60(Suppl 1):S52–S57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Zarychanski R, Pinto R, et al. ; Canadian Critical Care Trials Group H1N1 Collaborative: Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879 [DOI] [PubMed] [Google Scholar]
- 17.Lum ME, McMillan AJ, Brook CW, et al. : Impact of pandemic (H1N1) 2009 influenza on critical care capacity in Victoria. Med J Aust 2009; 191:502–506 [DOI] [PubMed] [Google Scholar]
- 18.Nap RE, Andriessen MP, Meessen NE, et al. : Pandemic influenza and excess intensive-care workload. Emerg Infect Dis 2008; 14:1518–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ercole A, Taylor BL, Rhodes A, et al. : Modelling the impact of an influenza A/H1N1 pandemic on critical care demand from early pathogenicity data: The case for sentinel reporting. Anaesthesia 2009; 64:937–941 [DOI] [PubMed] [Google Scholar]
- 20.Rowan KM, Harrison DA, Walsh TS, et al. : The Swine Flu Triage (SwiFT) study: Development and ongoing refinement of a triage tool to provide regular information to guide immediate policy and practice for the use of critical care services during the H1N1 swine influenza pandemic. Health Technol Assess 2010; 14:335–492 [DOI] [PubMed] [Google Scholar]
- 21.Giovannelli J, Loury P, Lainé M, et al. : Forecasts of health care utilization related to pandemic A(H1N1)2009 influenza in the Nord-Pas-de-Calais region, France. Public Health 2015; 129:493–500 [DOI] [PubMed] [Google Scholar]
- 22.Society of Critical Care Medicine: SARI-PREP. Available at: https://www.sccm.org/Research/Discovery-Research-Network/Discovery-Activities/Severe-Acute-Respiratory-Infection-Preparedness. Accessed September 10, 2020
- 23.Michaels AJ, Hill JG, Bliss D, et al. : Pandemic flu and the sudden demand for ECMO resources: A mature trauma program can provide surge capacity in acute critical care crises. J Trauma Acute Care Surg 2013; 74:1493–1497 [DOI] [PubMed] [Google Scholar]
- 24.Schull MJ, Stukel TA, Vermeulen MJ, et al. : Surge capacity associated with restrictions on nonurgent hospital utilization and expected admissions during an influenza pandemic: Lessons from the Toronto severe acute respiratory syndrome outbreak. Acad Emerg Med 2006; 13:1228–1231 [DOI] [PubMed] [Google Scholar]
- 25.Stukel TA, Schull MJ, Guttmann A, et al. : Health impact of hospital restrictions on seriously ill hospitalized patients: Lessons from the Toronto SARS outbreak. Med Care 2008; 46:991–997 [DOI] [PubMed] [Google Scholar]
- 26.Kadri SS, Sun J, Lawandi A, et al. : Association between caseload surge and COVID-19 survival in 558 U.S. hospitals, March to August 2020. Ann Intern Med 2021; 174:1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.