Supplemental Digital Content is available in the text.
Keywords: biomarkers, burn, endothelium, glycocalyx, inflammation, sepsis
OBJECTIVES:
To compare the diagnostic value of clinical sepsis criteria to novel protein biomarkers in the burn patient.
DESIGN:
Prospective observational study.
SETTING:
American Burn Association verified Burn Center ICU.
PATIENTS:
Burn patients (n = 24) and healthy volunteers (n = 10).
INTERVENTIONS:
Enrolled burn patients (n = 24) were stratified based on whether or not they met a clinical definition of sepsis. Four separate clinical criteria for sepsis were analyzed for their diagnostic sensitivity and specificity, which were compared to a panel of protein biomarkers. The most significant protein biomarkers were further analyzed via the area under the receiver operating characteristic curves (AUROCs).
MEASUREMENTS AND MAIN RESULTS:
Of the clinical criteria, SEPSIS-2 criteria led to the highest AUROC (0.781; p < 0.001), followed by the quick Sequential Organ Failure Assessment score (AUROC = 0.670; p = 0.022). Multiplexing revealed a number of inflammatory proteins (complement C5) and matrix metalloproteinases (MMP1, MMP7) that were significantly elevated in septic samples compared with both healthy controls and nonseptic burn samples. Furthermore, three proteins associated with endothelial dysfunction and glycocalyx shedding revealed diagnostic potential. Specifically, syndecan-1, p-selectin, and galectin-1 were all significantly elevated in sepsis, and all resulted in an AUROC greater than 0.7; analyzing the sum of these three markers led to an AUROC of 0.808.
CONCLUSIONS:
These data reveal several potential biomarkers that may help with sepsis diagnosis in the burn patient. Furthermore, the role of endotheliopathy as a mechanistic etiology for sepsis after burns warrants further investigation.
Patients with severe burns (e.g., > 20% total body surface area [TBSA]) are confronted with a unique pathophysiology that is often accompanied by a profound and sustained host response with clinical sequelae such as prolonged ICU stays. After the initial loss of the skin barrier function, this extended care can be confounded by organ dysfunction resulting in the need for the use of ventilators and both vascular and urinary catheters. Together, these factors predispose burn patients to infectious complications and sepsis, which drastically increases mortality (1, 2). Indeed, sepsis is the leading cause of death in patients that survive the initial burn injury, with estimates of sepsis as the cause of death approaching 2/3 of decedent burn patients (3, 4). Additionally, the burden of injury combined with iatrogenic influences such as surgical intervention is confounded by a profound and sustained hypermetabolic state, which persists for the duration of the hospitalization and beyond.
To improve outcomes, early recognition of sepsis and subsequent intervention is of the utmost importance. While extensive efforts such as the Surviving Sepsis Campaign have improved sensitivity and specificity of sepsis diagnosis, these criteria did not derive from burn patients. Due to the host response to the burn itself and the sequelae that follow, many clinical sepsis criteria (including SEPSIS-3) are often met in burn patients in the absence of true clinical sepsis (5). Specifically, the original criteria set forth by the Society of Critical Care Medicine have used systemic inflammatory response syndrome (SIRS) criteria (i.e., temperature, pulse, respiratory rate, WBCs) that are relatively nonspecific and are met in almost all severely burned patients. While the quick Sequential Organ Failure Assessment (qSOFA) score may provide diagnostic value, it has also proven ineffective at predicting bacteremia in burn patients (6). Furthermore, newer SEPSIS-3 criteria also take into account organ dysfunction; however, this scoring system was shown to not improve mortality prediction in burn patients (7). In fact, one of the main conclusions from Yoon et al was the emphasis that biomarkers warrant further study.
While bacteremia can signal sepsis in the general ICU population, culture-negative sepsis can also occur. This is especially frequent in burn patients, where clinical decompensation can occur in the absence of positive blood cultures (8). For these reasons, the American Burn Association has sought to develop specific criteria for the diagnosis of sepsis in burn patients (9). These clinical parameters have been compared with other criteria, all of which have demonstrated suboptimal specificity and sensitivity (10). A simple-to-measure biomarker that can foretell the development of sepsis in burn patients has the potential to improve outcomes by facilitating early recognition and treatment. Burn sepsis diagnosis has evolved to consider this possibility (11), with procalcitonin being one such candidate (12). However, markers of inflammation and endotheliopathy may provide value in burn sepsis and are increasingly of interest in burn pathophysiology (13). To this end, we aimed to examine four different clinical criteria of sepsis: qSOFA, SEPSIS-2, the American Burn Association (ABA) sepsis criteria, and the Mann-Salinas criteria, in a prospective observational study. Furthermore, we aimed to compare the diagnostic accuracy of these criteria to various proteins involved in inflammation and endotheliopathy. We hypothesized that at least one biomarker would perform with sensitivity and specificity equal to clinical scoring systems for sepsis diagnosis in burns.
METHODS
Study Design
This prospective, observational pilot study was conducted at the Burn ICU (BICU) at the U.S. Army Institute of Surgical Research (USAISR) from January 2018 to January 2020. The study was reviewed and approved by the U.S. Army Medical Research and Development Command Institutional Review Board under approval number M-10659. Written informed consent was obtained prior to performing any study-related procedures, activities, or data collection. The study aimed to enroll 10 patients in each of the following groups: septic, nonseptic, and incipient (enrolled as nonseptic but developing sepsis during the study period).
Patient Population
Healthy donors were screened by the research staff and selected based on the following inclusion criteria: healthy adult males (≥ 18 yr) from the community willing to donate blood. Research staff screened the medical records of all burn patients admitted to the BICU daily. Subjects were selected on the following inclusion criteria: adult patients (≥ 18 yr) admitted to the BICU with a thermal injury of greater than or equal to 15% TBSA with or without traumatic injuries. Patients who were prisoners, pregnant, had nonthermal burn injury (chemical, electrical, dermatological injury), or used total parenteral nutrition were excluded.
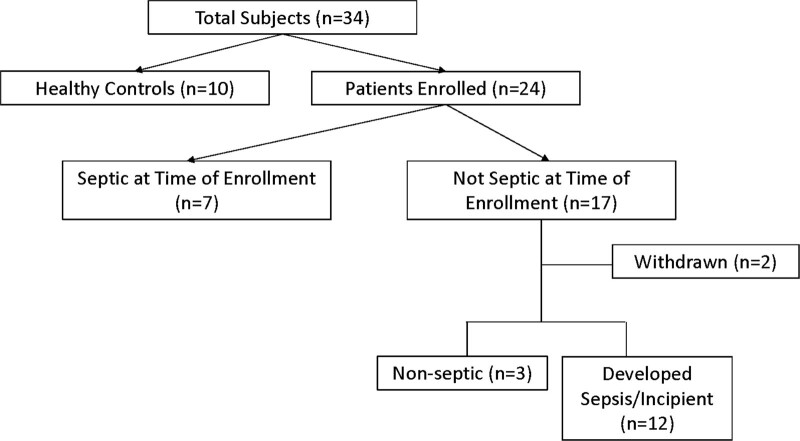
The subject population for this study included the following four groups: Healthy Blood Donor Group, Burn Sepsis Group, Burn Nonseptic Group, and Burn Incipient Group. Healthy volunteers were recruited for a one-time blood specimen from the USAISR Blood Donation Program (SOP Number BRP-001). Burn subjects were recruited for blood sampling over a 14-day period or until completion of prescribed antimicrobial therapy. A clinical definition of sepsis was used that reflected clinicians’ interpretations of a patients’ condition. Sepsis was defined as a new empiric prescription of at least one broad-spectrum IV antibiotics as well as one of the following: initiation of vasopressor agents, hypotension requiring bolus IV fluids, or blood cultures drawn. We enrolled a total of 10 healthy volunteers and 24 burn patients that are represented in a Consolidated Standards of Reporting Trials diagram (Fig. 1). Upon enrollment, each patient provided an initial blood draw regardless of their sepsis classification, which continued daily on weekdays until completion of antimicrobial therapy, or 14 days for uninfected individuals. Two subjects from the burn noninfected group were withdrawn from the study and did not contribute any blood samples. Seven patients met the criteria for sepsis at the time of enrollment, while the remainder did not. Of the 17 patients without evidence of infections/sepsis at enrollment, 12 subsequently developed sepsis at some point during the 14-day sampling period and were transferred to the Burn Incipient Group.
Figure 1.
Consolidated Standards of Reporting Trials diagram for patient inclusion.
Sample and Patient Data Collection
Universal precautions were used while handling all samples to prevent transmission of bloodborne pathogens to research personnel. Authorized research staff obtained a sample of undiluted whole blood directly from the subjects through an existing vascular catheter (preferred) or via venous phlebotomy with processing occurring within 24 hours of collection by the USAISR Research Laboratory. The sample was collected into Lithium-Heparin coated tubes for anticoagulation. In the event that collection of a venous whole blood sample from the subject was not possible due to, for example, subject unavailability due to procedures, the remnant blood from the current day’s complete blood count was sought from the Clinical Hematology laboratory. Specimens were stored at 4°C to prevent RBC hemolysis until transport to the USAISR Laboratory.
Data were extracted from the electronic medical record onto a standardized form and later compiled using Microsoft Access database (Redmond, WA). Variables collected include demographics such as height, weight, body mass index (BMI), burn injury and admission date/time, as well as clinical and laboratory parameters such as heart rate, respiratory rate, blood pressure, temperature, Glasgow Coma Scale, glucose, leukocytes, antibiotic use, use of vasopressors, and/or IV fluid boluses, prescribed antimicrobials, platelets, etc. The definitions for each of the clinical scoring criteria are shown in Supplemental Table 1 (http://links.lww.com/CCX/A891). For incipient patients, these data were pulled from the timepoint preceding diagnosis and the timepoint after the diagnosis of sepsis.
Multiplex Assay
Plasma from patients and healthy volunteers were isolated by spinning Lithium-Heparin anticoagulated blood at 1,000 × g for 10 minutes and aliquotting for storage at –80°C until use. Plasma was diluted 1:2 dilution for running on a custom, human-specific magnetic Luminex assay (26-plex) according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). In addition to this, a procalcitonin enzyme-linked immunosorbent assay (Catalog Number ab221828; Abcam, Cambridge, MA) was performed with a 1:20 dilution according to the manufacturer’s instruction. For measured biomarkers, incipient patient samples were separated into nonseptic and septic groups based on whether that particular sample was taken before or after the diagnosis of sepsis.
Statistical Analysis
Statistics were calculated by using Prism v.7.05 (GraphPad Software, La Jolla, CA) and Stata 15 (StataCorp, College Station, TX) (14). Data were tested for normality by both the D’Agostino and Pearson and Shapiro-Wilk normality tests. One-way analysis of variances or Kruskal-Wallis analysis generated overall p values given, with corresponding Fisher exact or Dunn tests for multiple comparisons used to determine differences between groups, as appropriate. All data are presented as the mean ± sem. To assess the diagnostic ability of protein biomarkers, nonparametric Somer’s D statistics for pairwise comparison were used, with ses robust to the clustering of observations within each patient. Receiver operating characteristic curves were generated. The area under the receiver operating characteristic curves (AUROCs) and their 95% CIs were estimated. For the clinical criteria, AUROC analysis includes a binary outcome (i.e., sepsis yes/no) and a binary predictor (e.g., qSOFA), which results in three points in the plot. The CI estimation used a Jackknife resampling approach. For diagnostic value, the number of patients needed to misdiagnose (NNM) was determined, as has been described previously (15). All statistics used the alpha level of 0.05.
RESULTS
Patient and volunteer characteristics are shown in Table 1. A total of 22 patients enrolled in the study were sampled, with only 3 (13.6%) not diagnosed with sepsis throughout any time during their hospital stay. A total of 12 patients (55%) were enrolled in the incipient group, thus providing samples before and after the diagnosis of sepsis. There were no differences across groups in age (p = 0.361), %TBSA (p = 0.448), or %TBSA full thickness (p = 0.896), which represents the largest predictors of mortality in burn patients (inhalation injury not reported herein). Additionally, there were no differences in weight or BMI across the groups.
TABLE 1.
Patient Characteristics
| Demographics | Healthy (n = 10) | Nonseptic (n = 3) | Septic (n = 7) | Incipient (n = 12) | p |
|---|---|---|---|---|---|
| Age (yr) | 26.4 ± 5.1 | 30.3 ± 9.8 | 40.0 ± 7.0 | 30.9 ± 2.7 | 0.361 |
| Number of male | 10 | 3 | 4 | 6 | |
| Number of military | 9 | 0 | 0 | 1 | |
| Total body surface area (%) | NA | 34.3 ± 16.9 | 53.0 ± 10.3 | 42.8 ± 5.1 | 0.448 |
| Full thickness % | NA | 20.1 ± 11.9 | 30.8 ± 14.0 | 16.8 ± 5.1 | 0.896 |
| Total days sampled (d) | 1 | 3–6 | 4–12 | 11–41 |
NA = not available.
Patient data reveal no differences in age, total body surface area, full thickness surface area involved.
Clinical Sepsis Criteria
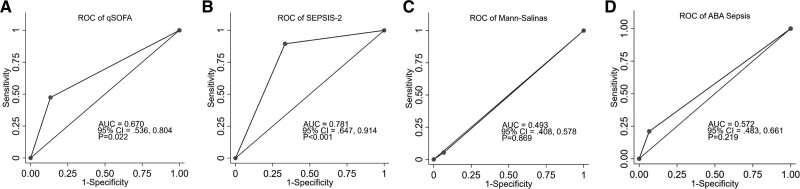
Of the 19 patients diagnosed with sepsis, 10 had no positive blood culture, one had Gram-positive sepsis, four presented with Gram-negative species, two presented with fungal culture, and two had mixed bacterial and fungal species. The majority of patients (13/19) received vancomycin with or without other IV antibiotics. Other systemic antibiotics given included meropenem, ciprofloxacin, and cefepime. To examine the validity of previously published sepsis criteria (Supplemental Table 1, http://links.lww.com/CCX/A891) in our patient population, the AUROC curves were generated (Fig. 2). Of the clinical criteria, the qSOFA (Fig. 2A) and SEPSIS-2 (Fig. 2B) criteria were significant against the reference line with an AUROC of 0.67 and 0.78, respectively. The Mann-Salinas criteria (Fig. 2C) and ABA sepsis criteria (Fig. 2D) were not significant.
Figure 2.
Receiver operating characteristic (ROC) curves for clinical criteria. Validation of different clinical scoring criteria in burn patients revealed an area under the receiver operating characteristic curve (AUROC) of 0.67, 0.78, 0.49, and 0.57 for quick Sequential Organ Failure Assessment (qSOFA) (A), SEPSIS-2 (B), Mann-Salinas (C), and ABA sepsis criteria (D), respectively. ABA = American Burn Association, AUC = area under the curve.
Protein Biomarkers
A multiplex assay was performed on plasma samples, to include a number of proteins involved with inflammation and vascular dysfunction, and all concentrations of these shown in Supplemental Table 2 (http://links.lww.com/CCX/A892). Levels of inflammatory cytokines (e.g., interleukin [IL]-1β, IL-6, IL-17, IL-27, IL-33) were variable and revealed no significant differences between septic and nonseptic burn patients, with the exception of IL-6 that was slightly significant (p = 0.014). Similarly, procalcitonin levels were not different between septic and nonseptic burn patients (p = 0.284) and led to a nonsignificant AUROC (Supplemental Fig. 1, http://links.lww.com/CCX/A893). There were statistically higher levels of a number of other inflammatory proteins in septic samples when compared with healthy and nonseptic samples, including complement component 5a (C5a), C-C motif chemokine ligand 8 (CCL8), matrix metalloproteinases (MMP1, MMP7), and tenascin (Supplemental Table 2, http://links.lww.com/CCX/A892). Similarly, levels of several proteins that play a role in endotheliopathy and/or vascular dysfunction (i.e., galectin-1, syndecan-1, and P-selectin) were significantly elevated in septic burn patients but not nonseptic patients when compared with healthy subjects. Furthermore, all three of these proteins were significantly elevated in septic samples when compared with samples from nonseptic and healthy subjects and were not different between healthy and nonseptic subjects.
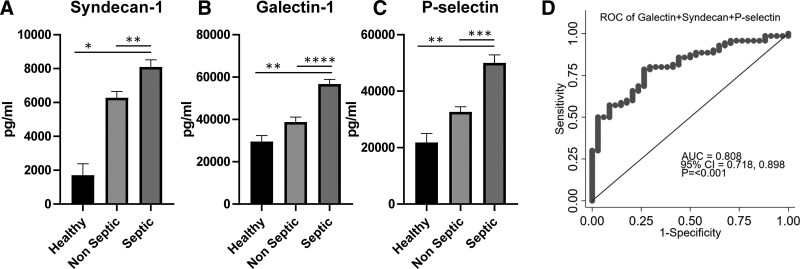
The most highly significant biomarkers (i.e., p < 0.001) were also subjected to AUROC analysis, with the AUROC, CI, and significance levels reported in Table 2. A number of inflammatory biomarkers (e.g., MMP-1, MMP-7. C5a, and Tenascin) revealed significant AUROC compared with reference. Furthermore, all three of the endotheliopathy biomarkers led to statistically significant AUROCs compared with reference (p < 0.001) with an AUROC of 0.7 or greater. The highest of these was galectin-1 with an AUROC of 0.797. Figure 3 graphically depicts these concentrations in healthy volunteers and burn patients to display the magnitude of changes due to burn and sepsis (Fig. 3A–C). This revealed significantly elevated levels of Syndecan-1, Galectin-1 and p-selectin in septic patients when compared to both healthy subjects and non-septic burn patients. Furthermore, an AUROC was generated that included the sum of galectin-1, syndecan-1, and P-selectin concentrations, which resulted in an AUROC of 0.81 (Fig. 3D).
TABLE 2.
Area Under the Receiver Operating Characteristic Curve, 95% CIs, and p Values for Area Under the Receiver Operating Characteristic Curve Analysis of Top-Performing Protein Biomarkers
| Marker | Area Under the Receiver Operating Characteristic Curve (95% CI) | p |
|---|---|---|
| Galectin-1 | 0.797 (0.691–0.902) | < 0.001 |
| P-selectin | 0.766 (0.684–0.849) | < 0.001 |
| Syndecan-1 | 0.724 (0.539–0.888) | < 0.001 |
| MMP-1 | 0.682 (0.545–0.820) | 0.002 |
| MMP-7 | 0.735 (0.608–0.862) | < 0.001 |
| Complement component 5a | 0.732 (0.586–0.860) | < 0.001 |
| Tenascin | 0.729 (0.537–0.922) | < 0.001 |
MMP = matrix metalloproteinase.
Figure 3.
Circulating proteins involved with endotheliopathy. Concentrations of syndecan-1 (A), galectin-1 (B), and p-selectin (C) were significantly higher in septic samples than both healthy and nonseptic samples (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, healthy n = 4 samples, four patients, nonseptic n = 33 samples, 15 patients, septic n = 69 samples, 19 patients). D, When the sum of these endotheliopathy biomarkers was subjected to area under the receiver operating characteristic curve (AUROC) analysis, the highest AUROC (0.808) was achieved. AUC = area under the curve, ROC = receiver operating characteristic.
Test characteristics were examined using standard definitions of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). At the initial peak sensitivity which preserved the highest specificity (sensitivity 98.6% using cutoff value of 53,320 pg/mL), the specificity was 11.8%, PPV was 69.7%, and NPV was 80.0% for detecting clinical sepsis in burn patients. At the initial peak specificity which preserved the highest sensitivity (specificity 100% using a cutoff value of 131,452 pg/mL), the sensitivity was 28.6%, PPV was 100%, and NPV was 40.5% for detecting clinical sepsis in burn patients. The approximate cutoff value providing near-equivalence for sensitivity (72.9%) and specificity (73.5%) was 85,000 pg/mL. Clinical utility of the assay was expressed using the number NNM one patient, defined as 1/(false positives + false negatives). The cutoff value associated with the optimal NNM (seven patients) was 65,000 pg/mL, at which sensitivity was 92.7% and specificity was 35.3%. The associated PPV and NPV were 74.7% and 70.6%, respectively.
DISCUSSION
The destruction of the cutaneous barrier function caused by thermal injury renders burn patients susceptible to the development of sepsis via transcutaneous invasion (16). Diagnostic criteria for sepsis in other patient populations are relatively unhelpful after burn injury because some, or all, of the criteria are often met in the absence of infection due to the underlying pathophysiology of burns as well as its surgical management. While several algorithms using clinical criteria have been examined, the prospect of biomarker identification for aiding sepsis diagnosis in burns is alluring. The salient findings in this report are that: 1) SEPSIS-2 criteria applied to the burn patient leads to similar sensitivity and specificity as in the general population and that 2) a number of inflammatory and endotheliopathy proteins display promising prognostic value for the diagnosis of sepsis in the severely burned patient.
It is widely accepted that early recognition and treatment of sepsis can improve outcomes (17, 18). Clinical criteria for sepsis have evolved over the last several decades. In 1991, the American College of Chest Physicians and the Society of Critical Care Medicine defined sepsis based on SIRS criteria in addition to a documented or suspected infection (19). While this was modified in the 2001 International Sepsis Definition Conference, the resulting definition still relied heavily on SIRS criteria (20). Although sepsis screening with qSOFA scoring and SEPSIS-2 criteria have been compared in other patient populations, severe burn patients present with unique pathophysiology that satisfies some of these criteria in the absence of sepsis, and the qSOFA score has previously been shown to be ineffective at predicting bacteremia in burn patients (6).
Our current study was designed to discover attractive protein biomarkers as opposed to rigorously assess clinical scoring criteria. A recent study with a larger sample size revealed diagnostic accuracy of Sepsis-3 criteria in burn patients (21). Our study did not enforce collection of the all of the variables necessary for Sepsis 3, but we were able to report qSOFA scores. However, the study by Yan et al (21) concluded that no criteria could be considered a diagnostic standard and recommended that sepsis be clinically assessed. As such, we employed a pragmatic clinical definition of sepsis, despite the potential for introducing bias. Surprisingly, in our current pilot study, both the qSOFA and SEPSIS-2 scoring systems led to AUROC values, which are comparable with the general population. Criteria developed for burns by the American Burn Association and Mann-Salinas et al (10) did not prove to be as accurate in our study. Given the inadequacy of clinical scoring criteria, biomarkers for sepsis in the burn patient could aid in prompt diagnosis, potentially improving outcomes by reducing the time to intervention.
Inflammatory cytokines have received great interest for diagnostic potential, including in burn patients. To our surprise, our results indicate that many of these proinflammatory cytokines may have limited value in burns. The inflammatory proteins that revealed diagnostic value were those related to complement (C5a), CCL8, or MMPs, which have also shown immunomodulatory properties (22). Mechanistically tied to inflammation, endothelial dysfunction has recently emerged as an important contributor in the pathophysiology of severe trauma (23, 24). This endotheliopathy is mediated in large part by shedding of the endothelial glycocalyx, which drastically alters leukocyte adhesion and vascular permeability. Syndecan-1 is a constituent of the glycocalyx and is widely accepted as a reliable marker of endotheliopathy in trauma (25), which is also associated with mortality in trauma patients (26). Consistent with our results, syndecan-1 levels are increased after burn trauma in both animal models (27, 28) and clinical studies (29, 30). Elevated syndecan-1 levels within 4 hours have also been shown to be associated with the development of sepsis in patients who survived the initial insult (31). Similarly, we found that circulating syndecan-1 levels were higher in burn patients who met our definition of sepsis compared with nonseptic patients and healthy individuals. While logistically challenging due to relative limitations in burn patient sample size, prospective validation of this marker in larger cohort merits further investigation.
Besides syndecan-1 (32), the glycocalyx components that have been most intensively studied in trauma are hyaluronan and heparin sulfate (33). However, another potential biomarker is p-selectin (34), which participates in the interaction between leukocytes and endothelial cells, and has been shown to be elevated after burn injury (35). As with syndecan-1, we found that p-selectin levels were significantly elevated after burns but only in those patients who were classified as septic. Whether or not there is a mechanistic basis of p-selectin in the development of sepsis in burn patients remains unclear.
While galectins, which also mediate cell-cell interactions, have been infrequently studied as biomarkers in burns and sepsis, they are also located within the glycocalyx (36). Galectin-1 expression from the extracellular matrix has been shown to mediate T cell apoptosis (37, 38) and may contribute to the reduction in lymphocytes in burns. This is known to occur after radiation, for example, where release of galectin-1 from activated tumors then results in global lymphopenia (39, 40). Preclinical models are needed to examine the role of galectin-1 after burns and have recently been used to show elevated galectin-1 levels in hypertrophic scarring (41). Herein, we found that galectin-1 as a single biomarker outperformed all clinical criteria for diagnosing sepsis in our study. It is possible that the unique pathophysiology of burn sepsis makes some constituents of the glycocalyx (e.g., galectin-1) more valuable or specific than others.
There are several limitations of this study. We used a pragmatic definition of sepsis that was based upon the actions of the treatment team indicating concerns over sepsis. This definition is not objective and could be biased by variations in the clinical practice of individual clinicians. Because sepsis could not be unequivocally confirmed in several patients, there exists a risk of misclassification bias. Furthermore, as a single-center study, validation across other burn centers and in a larger cohort is needed. Indeed, the limited sample size precluded analyses such as multivariate controlling and algorithm development. Additionally, the clinical utility of this observational study would be aided by the implementation of decision criteria to see if incorporating these biomarkers improves outcomes.
CONCLUSIONS
We report evidence of single biomarkers with superior diagnostic capacity compared with clinical sepsis criteria in burn patients. While a number of the 26 proteins examined indicated potential value in burn sepsis diagnosis, the highest specificity and sensitivity among the markers we examined were glycocalyx components associated with endothelial dysfunction. If validated in a larger cohort, these findings, as well as clinical observations, could support the development of diagnostic algorithms to improve sepsis diagnosis in severely burned patients.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
This study was conducted under a protocol reviewed and approved by the U.S. Army Medical Research and Development Command Institutional Review Board and in accordance with the approved protocol.
The authors have disclosed that they do not have any potential conflicts of interest.
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, Uniformed Services University of the Health Sciences, the DoD, or the U.S. Government.
REFERENCES
- 1.Cumming J, Purdue GF, Hunt JL, et al. : Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. J Trauma 2001; 50:510–515 [DOI] [PubMed] [Google Scholar]
- 2.Fitzwater J, Purdue GF, Hunt JL, et al. : The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma 2003; 54:959–966 [DOI] [PubMed] [Google Scholar]
- 3.D’Avignon LC, Hogan BK, Murray CK, et al. : Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: An autopsy series. Burns 2010; 36:773–779 [DOI] [PubMed] [Google Scholar]
- 4.Sharma BR, Harish D, Singh VP, et al. : Septicemia as a cause of death in burns: An autopsy study. Burns 2006; 32:545–549 [DOI] [PubMed] [Google Scholar]
- 5.Greenhalgh DG: Sepsis in the burn patient: A different problem than sepsis in the general population. Burns Trauma 2017; 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladhani HA, Sajankila N, Zosa BM, et al. : Utility of sequential organ failure assessment score in predicting bacteremia in critically ill burn patients. Am J Surg 2018; 215:478–481 [DOI] [PubMed] [Google Scholar]
- 7.Yoon J, Kym D, Hur J, et al. : Comparative usefulness of sepsis-3, burn sepsis, and conventional sepsis criteria in patients with major burns. Crit Care Med 2018; 46:e656–e662 [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Sakr Y, Sprung CL, et al. ; Sepsis Occurrence in Acutely Ill Patients Investigators: Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med 2006; 34:344–353 [DOI] [PubMed] [Google Scholar]
- 9.Greenhalgh DG, Saffle JR, Holmes JH, 4th, et al. ; American Burn Association Consensus Conference on Burn Sepsis and Infection Group: American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007; 28:776–790 [DOI] [PubMed] [Google Scholar]
- 10.Mann-Salinas EA, Baun MM, Meininger JC, et al. : Novel predictors of sepsis outperform the American Burn Association sepsis criteria in the burn intensive care unit patient. J Burn Care Res 2013; 34:31–43 [DOI] [PubMed] [Google Scholar]
- 11.Torres MJM, Peterson JM, Wolf SE: Detection of infection and sepsis in burns. Surg Infect (Larchmt) 2021; 22:20–27 [DOI] [PubMed] [Google Scholar]
- 12.Tan J, Li N, Gong Y, et al. : Procalcitonin kinetics early after severe burn injury and its value in diagnosis of sepsis. Burns 2021; 47:1802–1809 [DOI] [PubMed] [Google Scholar]
- 13.Burmeister DM, Smith SL, Muthumalaiappan K, et al. : An assessment of research priorities to dampen the pendulum swing of burn resuscitation. J Burn Care Res 2021; 42:113–125 [DOI] [PubMed] [Google Scholar]
- 14.StataCorp LLC: Mata Reference Manual. College Station, TX, StataCorp LLC, 2017 [Google Scholar]
- 15.Habibzadeh F, Habibzadeh P, Yadollahie M: On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem Med (Zagreb) 2016; 26:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker HL, McLeod CG, Jr, Leppla SH, et al. : Evaluation of Pseudomonas aeruginosa toxin A in experimental rat burn wound sepsis. Infect Immun 1979; 25:828–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 18.Yealy DM, Kellum JA, Huang DT, et al. : A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bone RC, Balk RA, Cerra FB, et al. : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. ; International Sepsis Definitions Conference: 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med 2003; 29:530–538 [DOI] [PubMed] [Google Scholar]
- 21.Yan J, Hill WF, Rehou S, et al. : Sepsis criteria versus clinical diagnosis of sepsis in burn patients: A validation of current sepsis scores. Surgery 2018; 164:1241–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manicone AM, McGuire JK: Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 2008; 19:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chignalia AZ, Yetimakman F, Christiaans SC, et al. : The glycocalyx and trauma: A review. Shock 2016; 45:338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuma M, Canestrini S, Alwahab Z, et al. : Trauma and endothelial glycocalyx: The microcirculation helmet? Shock 2016; 46:352–357 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, et al. : Syndecan-1: A quantitative marker for the endotheliopathy of trauma. J Am Coll Surg 2017; 225:419–427 [DOI] [PubMed] [Google Scholar]
- 26.Johansson PI, Stensballe J, Rasmussen LS, et al. : A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg 2011; 254:194–200 [DOI] [PubMed] [Google Scholar]
- 27.Gómez BI, Dubick MA, Schmidt EP, et al. : Plasma and urinary glycosaminoglycans as evidence for endotheliopathy in a swine burn model. J Surg Res 2020; 248:28–37 [DOI] [PubMed] [Google Scholar]
- 28.Vigiola Cruz M, Carney BC, Luker JN, et al. : Plasma ameliorates endothelial dysfunction in burn injury. J Surg Res 2019; 233:459–466 [DOI] [PubMed] [Google Scholar]
- 29.Osuka A, Kusuki H, Yoneda K, et al. : Glycocalyx shedding is enhanced by age and correlates with increased fluid requirement in patients with major burns. Shock 2018; 50:60–65 [DOI] [PubMed] [Google Scholar]
- 30.Welling H, Henriksen HH, Gonzalez-Rodriguez ER, et al. : Endothelial glycocalyx shedding in patients with burns. Burns 2020; 46:386–393 [DOI] [PubMed] [Google Scholar]
- 31.Wei S, Gonzalez Rodriguez E, Chang R, et al. ; PROPPR Study Group: Elevated syndecan-1 after trauma and risk of sepsis: A secondary analysis of patients from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial. J Am Coll Surg 2018; 227:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitsma S, Slaaf DW, Vink H, et al. : The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch 2007; 454:345–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchimido R, Schmidt EP, Shapiro NI: The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit Care 2019; 23:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperandio M: Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J 2006; 273:4377–4389 [DOI] [PubMed] [Google Scholar]
- 35.Kaufman T, Magosevich D, Moreno MC, et al. : Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clin Immunol 2017; 183:254–262 [DOI] [PubMed] [Google Scholar]
- 36.Rapoport EM, Matveeva VK, Vokhmyanina OA, et al. : Localization of galectins within glycocalyx. Biochemistry (Mosc) 2018; 83:727–737 [DOI] [PubMed] [Google Scholar]
- 37.He J, Baum LG: Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem 2004; 279:4705–4712 [DOI] [PubMed] [Google Scholar]
- 38.Stillman BN, Hsu DK, Pang M, et al. : Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 2006; 176:778–789 [DOI] [PubMed] [Google Scholar]
- 39.Kuo P, Bratman SV, Shultz DB, et al. : Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res 2014; 20:5558–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welsh JW, Seyedin SN, Cortez MA, et al. : Galectin-1 and immune suppression during radiotherapy. Clin Cancer Res 2014; 20:6230–6232 [DOI] [PubMed] [Google Scholar]
- 41.Kirkpatrick LD, Shupp JW, Smith RD, et al. : Galectin-1 production is elevated in hypertrophic scar. Wound Repair Regen 2021; 29:117–128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.