Summary
Background
The dynamic trends of pulmonary function in coronavirus disease 2019 (COVID-19) survivors since discharge have been rarely described. We aimed to describe the changes of lung function and identify risk factors for impaired diffusion capacity.
Methods
Non-critical COVID-19 patients admitted to the Guangzhou Eighth People's Hospital, China, were enrolled from March to June 2020. Subjects were prospectively followed up with pulmonary function tests at discharge, three and six months after discharge.
Findings
Eighty-six patients completed diffusion capacity tests at three timepoints. The mean diffusion capacity for carbon monoxide (DLCO)% pred was 79.8% at discharge and significantly improved to 84.9% at Month-3. The transfer coefficient of the lung for carbon monoxide (KCO)% pred significantly increased from 91.7% at discharge to 95.7% at Month-3. Both of them showed no further improvement at Month-6. The change rates of DLCO% pred and KCO% pred were significantly higher in 0–3 months than in 3–6 months. The alveolar ventilation (VA) improved continuously during the follow-ups. At Month-6, impaired DLCO% pred was associated with being female (OR 5.2 [1.7–15.8]; p = 0.004) and peak total lesion score (TLS) of chest CT > 8.5 (OR 6.6 [1.7–26.5]; p = 0.007). DLCO% pred and KCO% pred were worse in females at discharge. And in patients with impaired diffusion capacity, females’ DLCO% pred recovered slower than males.
Interpretation
The first three months is the critical recovery period for diffusion capacity. The impaired diffusion capacity was more severe and recovered slower in females than in males. Early pulmonary rehabilitation and individualized interventions for recovery are worthy of further investigations.
Keyword: COVID-19, Pulmonary diffusion capacity, Dynamic changes
Research in context.
Evidence before this study
The survivors are still suffering from the sequelae of the COVID-19. Long-term follow-up studies have also shown that a significant proportion of survivors have impaired lung function. We searched PubMed without language restriction for studies published up to September 10, 2021, using search terms ("COVID-19 OR "SARS-CoV-2″) AND ("discharge" OR "survivors") AND ("lung function" OR "pulmonary function" OR "diffusion capacity") and their synonyms. However, the longitudinally dynamic changes of lung function in survivors of non-critical COVID-19 remain unclear and risk factors associated with pulmonary function impairment are not fully understood.
Added value of this study
We have conducted a prospective study with pulmonary function tests (PFT) and questionnaires at discharge, three and six months post-discharge. Carbon monoxide (DLCO)% pred improved in the first 3 months after discharge. At Month-6, impaired DLCO% pred was associated with being female (OR 5.2 [1.7-15.8]). Further analysis showed that in patients with impaired diffusion capacity, DLCO% pred of females recovered slower than that in males.
Implications of all the available evidence
Our study suggested that the first three months is the critical recovery period for diffusion capacity and that the recovery of diffusion capacity is slower in females than that in males. Early pulmonary rehabilitation and individualized interventions for recovery are worthy of further investigations.
Alt-text: Unlabelled box
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) has brought a heavy burden on health expenditure. As of August 30, 2021, more than 214 million laboratory-confirmed cases have been documented globally, and 4.4 million have died from it.1 The survivors are still suffering from the sequelae of the COVID-19. A single-center longitudinal study demonstrated that clinical sequelae during early COVID-19 convalescence were common, including general symptoms, respiratory symptoms, cardiovascular-related symptoms, psychosocial symptoms and alopecia.2 And 33–39% of patients complained of respiratory symptoms such as dyspnea (mainly exertional dyspnea), chest distress, cough after discharge.2, 3, 4 Besides, some survivors were identified with impaired exercise tolerance.3,5 These residual symptoms and functional sequelae may correlate to impaired lung function.6 Therefore, the changes of lung function in COVID-19 patients after discharge need to be paid attention to.
In our previous study, the percentage of COVID-19 patients with impaired ventilatory function at discharge was 13.64%, and as high as 47.22% in impaired diffusion capacity.7 Long-term follow-up studies of COVID-19 have also shown that a significant proportion of patients have impaired lung function.8, 9, 10 Pulmonary diffusion dysfunction was the main feature, followed by the restrictive or obstructive ventilatory defect.8,11 In González et al., the proportion of impaired diffusion function was 82% among critical COVID-19 patients at three months after discharge.5 Huang et al. showed that 34% of COVID-19 patients had diffusion dysfunction at six months after discharge.12 In patients with severe COVID-19, there were 54%, 33% of patients still presenting diffusion dysfunction at six, twelve months after discharge, respectively.13 Additionally, in patients recovered from other coronavirus pneumonia, follow-up studies found that a long period would be taken for recovery of diffusion capacity. At one or even two years after discharge, there were 21–52.7% of SARS patients had diffusion dysfunction.14, 15, 16 For MERS, 37% of survivors remained impaired diffusion capacity one year after discharge.17 Above of these indicated that diffusion dysfunction may be a long-term sequela in COVID-19. The long-term follow-up of lung function, particularly diffusion capacity, may need to be performed for COVID-19 survivors.
The longitudinally dynamic changes of lung function in patients with COVID-19 since discharge from the hospital have been rarely described. We have conducted a prospective study with pulmonary function tests (PFT) at discharge, three and six months post-discharge. The current study aimed to comprehensively describe the trends of pulmonary rehabilitation in COVID-19 survivors, and identify risk factors associated with pulmonary function impairment.
Methods
Study design, subjects and data collection
The prospective study was performed at the Guangzhou Eighth People's Hospital from March to December 2020. Non-critical COVID-19 patients aged 18–75 years were consecutively included between March and June 2020. All patients had subsequent laboratory confirmation of SARS-CoV-2 using real-time RT-PCR. Patients were excluded if they (1) could not return to the hospital or cooperate to complete medical tests due to any reason, (2) had chronic respiratory diseases or severe pulmonary comorbidities(such as pulmonary embolism, pulmonary resection). The severity of COVID-19 was assessed by the guideline from China18 as follows: (1) Mild cases (the clinical symptoms were mild, and there was no sign of pneumonia on imaging); (2) Moderate cases (showing fever and respiratory symptoms with radiological findings of pneumonia); (3) Severe cases (respiratory distress (≧ 30 breaths/ min); oxygen saturation≤ 93% at rest; arterial partial pressure of oxygen (PaO2)/ fraction of inspired oxygen (FiO2) ≦ 300 mmHg (l mmHg = 0.133 kPa); cases with chest imaging that shows obvious lesion progression within 24–48 h > 50%; 4) critical cases (respiratory failure and requiring mechanical ventilation; Shock; With other organ failure that requires ICU care).We included patients with non-critical COVID-19 in the study.
Demographic, chest CT during hospitalization, routine blood tests as well as inflammatory biomarkers at admission were collected from medical records. The eligible patients underwent three times of follow-up including the discharge, three months and six months after discharge, respectively. All subjects were interviewed with modified Medical Research Council dyspnea scale (mMRC)19 and sequela symptoms, and underwent pulmonary function tests (PFT) at discharge, three months and six months after discharge. The sequela symptoms consisted of cough, chest tightness, chest pain, sleep difficulties, fatigue or weakness, shortness of breath, hair loss, spitting, palpitation, diarrhea or vomiting, dizziness, bone or joint pain, dysosmia. The above follow-up data constituted a complete case dataset. Chest CT were performed again at 6 months after discharged.
Written informed consents were obtained from all patients. This study was approved by the Ethics Commission of Guangzhou Eighth People's Hospital (No. 2020–92).
Pulmonary function tests
Spirometry, carbon monoxide (CO) diffusing tests and lung volume measurements were performed on Quark PFT (Cosmed, Rome, Italy) according to American Thoracic Society guidelines.20 Technicians from the pulmonary function laboratory performed these tests. DLCO was measured by the single-breath method. The parameters were recorded as follows: forced expiratory volume in the first second (FEV1)% pred, forced vital capacity (FVC)% pred, FEV1/FVC ratio, diffusing capacity of the lung for carbon monoxide (DLCO)% pred, transfer coefficient of the lung for carbon monoxide (KCO)% pred, alveolar ventilation (VA), residual volume (RV)% pred, total lung volume (TLC), RV/TLC ratio. The DLCO% pred had been adjusted for hemoglobin. The change rates of diffusion capacity parameters were calculated as below: the change rate of variable X = [(value _after – value _before) / value _before] * 100%.
Chest CT acquisition and image analysis
Chest CT was conducted by an Optima CT680 scanner (GE Medical Systems, Milwaukee, WI), with a minimum slice thickness of 1 mm. All images were analyzed in a consistent manner by an experienced radiologist and a pulmonologist. Ground glass opacity (GGO), crazy-paving pattern, and consolidation defined by the Fleischner Society glossary were recorded.21 A semi-quantitative scoring system was used to quantitatively estimate the pulmonary involvement of these abnormalities on the basis of the area involved.22 Specifically, each of the five lung lobes was visually scored from 0 to 5 as: 0, no involvement; (1) < 5% of a lobe (minimal but not normal), (2) 5–25% of a lobe, (3) 26–49% of a lobe; (4) 50–75% of a lobe; (5) > 75% of a lobe. Total lesion score (TLS) is calculated by adding up the lung lesion score of five lobes, with a total score 0–25.23 The peak TLS was calculated by the most severely impaired CT image during hospitalization.
Statistical analysis
Continuous variables were described as mean (standard deviation, SD) or median (interquartile range, IQR). Categorical variables were presented as numbers (percentage). A two-sample independent T-test or Mann-Whitney U test or χ² test was used between two groups. One-way ANOVA or Kruskal–Wallis H test or χ² test was performed among three different groups. Generalized estimating equations were used for repeated measurement in different periods. Univariate and multivariate logistic regression analyses were conducted to explore risk factors associated with impaired pulmonary function at different stages of convalescence. All tests were two-sided and a p value less than 0.05 was defined as statistically significant and significance values have been adjusted by the Bonferroni correction for multiple comparisons. Statistical analyses were performed using SPSS (version 26.0), violin figures were done by OriginPro (version 9.1.0) and the smoothed curve was done by R language (version 4.0.3).
Role of funding source
Funders had no role in the study design, study participant selection and recruitment, data collection and analysis, data interpretation, decision to publish, or preparation of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Demographic and clinical characteristics
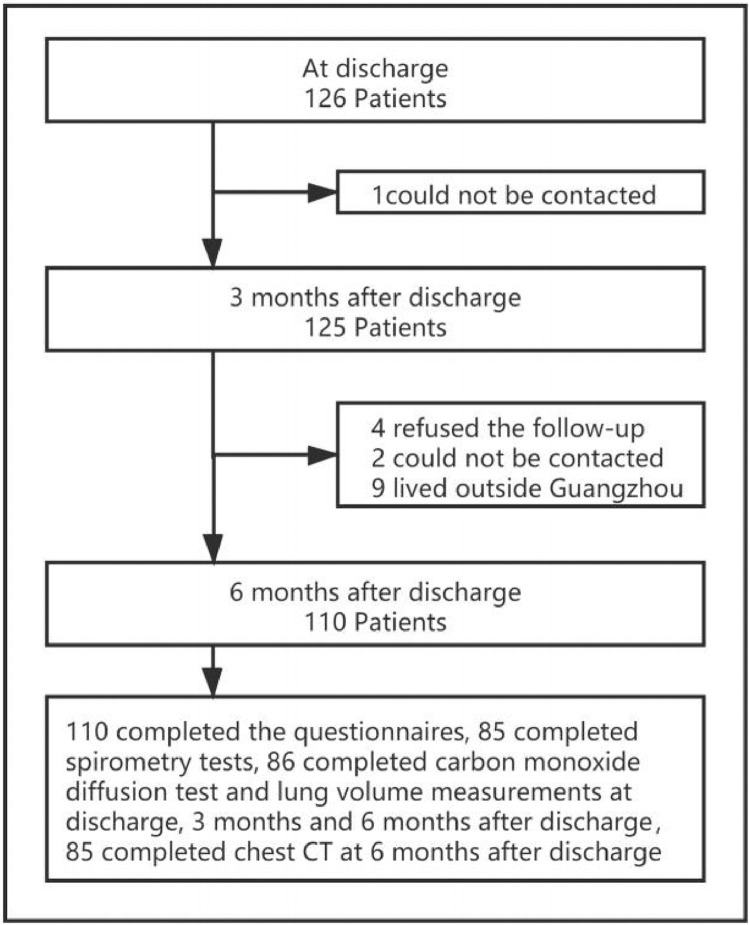
Among 126 cases, 16 patients lost follow-up and 110 patients finishing three times of follow-up were included in the study. There were 85 patients completing three times of spirometry, and 86 patients completing three times of DLCO and lung volume measurements (Fig. 1). Of the 86 patients who completed three times of DLCO and lung volume measurements, 85 completed chest CT at the 6th month after discharge. The demographic and clinical characteristics of participants are shown in Table 1. The median age of the enrolled patients was 45 years (34–56), and 51.8% (57/110) being female. The median body-max index (BMI) was 23.6 kg/m2 (21.9–25.6). There were 25.5% (28/110) of patients with at least one comorbidity. Common comorbidities included hypertension (13.6%, 15/110), diabetes (8.2%, 9/110). During hospitalization, the main radiographic abnormalities were ground-glass opacities (90.0%), consolidation (48.2%) and crazy paving pattern (40.9%). The median peak TLS was 7.0 (3.0–12.0), increasing with the severity of the disease. Compared with the mild or moderate group, the proportion of abnormal CRP, SAA and d-dimer were higher in patients with severe COVID-19 (all p < 0.01) (Table 1).
Figure 1.
Flow chart of the enrollment of patients with COVID-19 discharged from Guangzhou Eighth People's Hospital between March and June 2020.
Table 1.
Demographic and clinical characteristics during hospitalization.
| Total | Mild | Moderate | Severe | |
|---|---|---|---|---|
| Number of patients | 110 | 11 | 83 | 16 |
| Demographics | ||||
| Age, years | 45.0 (33.8, 56.3) | 35.0 (21.0, 45.0) | 45.0 (35.0, 56.0) | 52.5 (45.3, 57.0)* |
| Female | 57 (51.8%) | 5 (45.5%) | 47 (56.6%) | 5 (31.3%) |
| BMI, kg•m−2 | 23.6 (21.9, 25.6) | 23.7 (20.7, 24.5) | 23.4 (21.4, 25.4) | 24.7 (23.6, 26.7) |
| Smoking status | ||||
| Never smoke | 96 (87.3%) | 10 (91.0%) | 73 (87.9%) | 13 (81.3%) |
| Current smokers | 8 (7.3%) | 1 (9.1%) | 5 (6.0%) | 2 (12.5%) |
| Ever-smokers | 6 (5.5%) | 0 (0%) | 5 (6.0%) | 1 (6.3%) |
| Comorbidities | 28 (25.5%) | 1 (9.1%) | 23 (27.7%) | 4 (25.0%) |
| Hypertension | 15 (13.6%) | 1 (9.1%) | 9 (10.8%) | 5 (31.3%) |
| Diabetes | 9 (8.2%) | 1 (9.1%) | 6 (7.2%) | 2 (12.5%) |
| Liver disease | 6 (5.5%) | 0 (0.0%) | 5 (6.0%) | 1 (6.3%) |
| Cerebrovascular disease | 2 (1.8%) | 0 (0.0%) | 2 (2.4%) | 0 (0.0%) |
| Solid tumor | 2 (1.8%) | 0 (0.0%) | 1 (1.2%) | 1 (6.3%) |
| Laboratory | ||||
| CRP > 10 mg/L | 57/110 (51.8%) | 0/11 (0.0%) | 42/83 (50.6%)⁎⁎ | 15/16 (93.8%)**,## |
| SAA > 10 mg/L | 56/91 (61.5%) | 0/9 (0.0%) | 42/68 (61.8%)⁎⁎ | 14/14 (100.0%)**,## |
| D-dimer > 1000 μg/mL | 79/109 (70.9%) | 6/11 (54.5%) | 56/82 (68.3%) | 16/16 (100.0%)**,## |
| Blood cell count† | ||||
| White blood cell, × 109/L | 5.5 ± 2.2 | 6.9 ± 3.0 | 5.2 ± 1.7* | 6.0 ± 3.2 |
| Neutrophils, × 109/L | 3.6 ± 1.9 | 4.4 ± 2.6 | 3.3 ± 1.5 | 4.5 ± 3.0 |
| Lymphocytes, × 109/L | 1.4 ± 0.6 | 1.9 ± 0.8 | 1.4 ± 0.6* | 1.1 ± 0.6## |
| Chest CT | ||||
| GGO | 99 (90.0%) | 0 (0.0%) | 83 (100.0%)⁎⁎ | 16 (100.0%)⁎⁎ |
| Consolidation | 53 (48.2%) | 0 (0.0%) | 42 (50.6%)⁎⁎ | 11 (68.8%)⁎⁎ |
| Crazy paving pattern | 45 (40.9%) | 0 (0.0%) | 33 (39.8%)⁎⁎ | 12 (75.0%)**,# |
| Total lesion score | 7.0 (3.0–12.0) | 0.0 (0.0–0.0) | 7.0 (4.0–11.0)⁎⁎ | 15.5 (11.3–19.8)**,## |
| Peak mMRC score ≥ 1 | 38 (34.5%) | 3 (27.3%) | 30 (36.1%) | 5 (31.2%) |
| Inpatient days | 22.5 (16.0, 28.0) | 17.0 (8.0, 21.0) | 23.0 (16.0, 29.0) | 23.0 (17.5, 27.5) |
| Oxygen therapy | ||||
| Nasal cannula or face mask | 68/110 (61.8%) | 2/11 (18.2%) | 63/83 (75.9%)⁎⁎ | 3/16 (18.8%)## |
| HFNC | 12/110 (10.9%) | 0 (0.0%) | 0 (0.0%) | 12/16 (75.0%)**,## |
| NIV | 1/110 (0.9%) | 0 (0.0%) | 0 (0.0%) | 1/16 (6.3%) |
1. Data are n (%), n/N (%) or median (IQR). N is the total number of patients with available data.
2. Abbreviations: BMI, body mass index; CRP, C-reactive protein; SAA, serum amyloid A; GGO, ground-glass opacities; HFNC, high-flow nasal cannula. NIV, noninvasive ventilation.
3. *p < 0.05 and ⁎⁎p < 0.01, compared with Mild.
#p < 0.05 and ##p < 0.01, compared with Moderate. Significance values have been adjusted by the Bonferroni correction for multiple comparisons.
4. †Peripheral blood cell count was obtained at the day of admission.
67.2%, 54.5% and 45.5% of patients reported at least one sequela symptom at discharge, three months and six months after discharge, respectively. The number of participants with sequela symptoms decreased significantly at three months (p < 0.01), but not further decreased at six months (Table 2). The number of patients with an mMRC score of at least 1 point was 10 (9.1%), 5 (4.5%) and 4 (3.6%) in the three visits. It was significantly decreased at six months (p < 0.01) (Table 2).
Table 2.
Sequela symptoms and pulmonary function findings of COVID-19 patients in different follow-up periods.
| At discharge | Month 3 | Month 6 | |
|---|---|---|---|
| Spirometry (N = 85) | |||
| FVC% predicted | 95.1 ± 10.9 | 96.6 ± 11.8 | 95.6 ± 11.7 |
| FVC < 80% predicted | 7 (8.2%) | 6 (7.1%) | 6 (7.1%) |
| FEV1% predicted | 93.6 ± 10.7 | 93.6 ± 11.0 | 92.3 ± 11.5 |
| FEV1 < 80% predicted | 8 (9.4%) | 7 (8.2%) | 8 (9.4%) |
| FEV1/FVC% | 81.7 ± 6.2 | 80.7 ± 5.7 | 81.1 ± 6.1 |
| FEV1/FVC< 70% | 3 (3.5%) | 3 (3.5%) | 3 (3.5%) |
| Lung volume (N = 86) | |||
| TLC% predicted | 88.4 ± 9.0 | 90.6 ± 9.0⁎⁎ | 91.5 ± 9.5⁎⁎ |
| TLC < 80% predicted | 11 (12.8%) | 9 (10.5%) | 7 (8.1%) |
| RV% predicted | 88.4 ± 16.8 | 90.6 ± 16.3 | 98.7 ± 18.3**,## |
| RV < 65% predicted | 3 (3.5%) | 2 (2.3%) | 2 (2.3%) |
| Diffusion capacity (N = 86) | |||
| DLCO% predicted | 79.8 ± 12.0 | 84.9 ± 12.0⁎⁎ | 85.7 ± 12.8⁎⁎ |
| DLCO < 80% predicted | 35 (40.7%) | 30 (34.9%) | 28 (32.6%) |
| KCO% predicted | 91.7 ± 14.3 | 95.7 ± 16.3⁎⁎ | 95.0 ± 17.0⁎⁎ |
| KCO < 80% predicted | 21 (24.4%) | 12 (14.0%)⁎⁎ | 17 (19.8%) |
| VA L/min | 4.9 ± 0.9 | 5.0 ± 0.9⁎⁎ | 5.1 ± 0.9**,# |
| Sequela symptoms† (N = 110) | 74 (67.2%) | 60 (54.5%)⁎⁎ | 50 (45.5%)⁎⁎ |
| mMRC score≥ 1 (N = 110) | 10 (9.1%) | 5 (4.5%) | 4 (3.6%)* |
1. Data are n (%), mean ± SD or median (IQR).
2. Abbreviations: mMRC, modified Medical Research Council dyspnea scale; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; TLC, total lung volume; RV, residual volume; DLCO, diffusing capacity of the lung for carbon monoxide; KCO, transfer coefficient of the lung for carbon monoxide.
3. *p < 0.05 and ⁎⁎p < 0.01, compared with at discharge.
#p < 0.05 and ##p < 0.01, compared with Month 3. Significance values have been adjusted by the Bonferroni correction for multiple comparisons.
4. †: The proportion of patients with at least one sequela symptom.
Progress of pulmonary function at discharge, 3-month, 6-month
As shown in Table 2, FVC% pred, FEV1% pred and FEV1/FVC% showed no significant difference among the three times of follow-up. 8.2%, 9.4% and 3.5% of patients presented abnormal FVC, FEV1 and FEV1/FVC at discharge, respectively. A minority of patients still remained abnormal spirometry at three and six months after recovery. Compared with that at discharge, TLC% pred significantly increased at three months (90.6% vs 88.4%, p < 0.01) and 6 months (91.5% vs 88.4%, p < 0.01) after recovery. The mean RV% pred at six months after discharge was higher than those at discharge (98.7% vs 88.4%, p < 0.01) and three months (98.7% vs 90.6%, p < 0.01). The percentage of TLC < 80% pred at discharge was 12.8%, and the RV < 65% pred was accounted for 3.5% (Table 2).
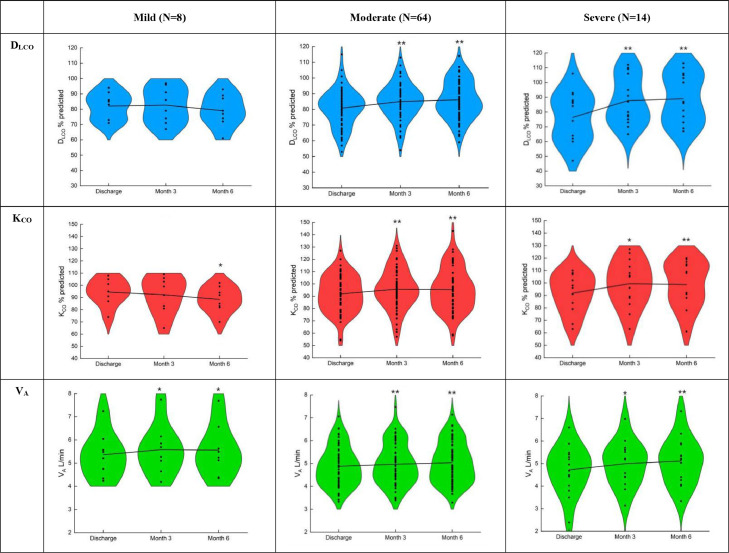
In terms of diffusion capacity, 40.7%, 34.9%, 32.6% of patients remained abnormal DLCO at discharge, three months and six months, respectively. Compared with that at discharge, DLCO% pred significantly improved at three months (84.9% vs. 79.8%, p < 0.01) and six months (85.7% vs. 79.8%, p < 0.01) after recovery. But there was no significant difference in DLCO% pred between the 3-month and 6-month follow-up. The change trend of KCO% pred during the period of follow-up were similar to DLCO% pred. (Table 2). These results were particularly shown in moderate and severe cases (Fig. 2 and appendix pp 1–2). VA at three months (5.0 vs 4.9, p < 0.01) and six months (5.1 vs 4.9, p < 0.05) were higher than that at discharge. And compared with three months after discharge, VA also significantly increased at six months (5.1 vs 5.0, p < 0.05). DLCO% pred and KCO% pred showed significant improvement at month 3, but no further improvement at the month 6. VA significantly improved over time during the follow-up (Table 2 and Fig. 3)
Figure 2.
The changes of pulmonary diffusion capacity in COVID-19 patients with different severity during the follow-up.
DLCO, diffusing capacity of the lung for carbon monoxide; KCO, transfer coefficient of the lung for carbon monoxide; VA, alveolar ventilation. Data is expressed as mean ± SD. *p < 0.05 and ⁎⁎p < 0.01, compared with At discharge; #p < 0.05 and ##p < 0.01, compared with Month 3.
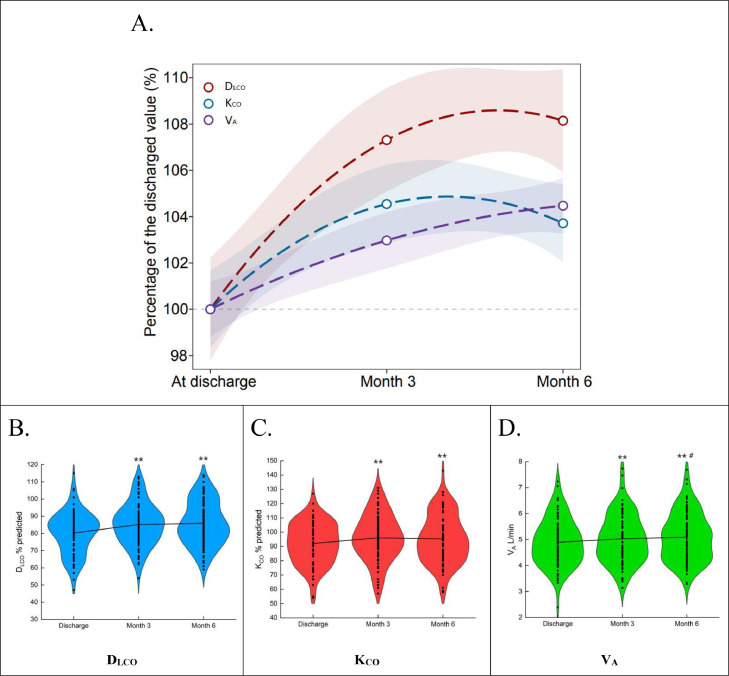
Figure 3.
The dynamic changes of the diffusion capacity in patients with COVID-19 during the follow-up (N = 86).
(A) The trends of DLCO, KCO and VA at the follow-ups. Data is expressed as a percentage of the discharged value, the shaded areas are 95% confidence intervals (CI). The Generalized Linear Models function in R package 'stats' (version 4.0.3) is used to fit a generalized linear model using a formula 'y ∼ poly(x, 2). The Violin plots show the mean and standard deviation of (B) DLCO, (C) KCO, and (D) VA. DLCO, diffusing capacity of the lung for carbon monoxide; KCO, transfer coefficient of the lung for carbon monoxide; VA, alveolar ventilation. *p < 0.05 and ⁎⁎p < 0.01, compared with At discharge; #p < 0.05 and ##p < 0.01, compared with Month 3.
After discharge, the change rate of DLCO% pred (4.7% and 1.2%, p < 0.01) and KCO% pred (4.2% vs. −1.1%, p < 0.01) in 0–3 months was significantly higher than that in 3–6 months. And the VA change rate in 0–3 months was not significantly different from that in 3–6 months (2.4% vs. 0.8%, p = 0.086) (Appendix p 2).
Risk factors of abnormal DLCO
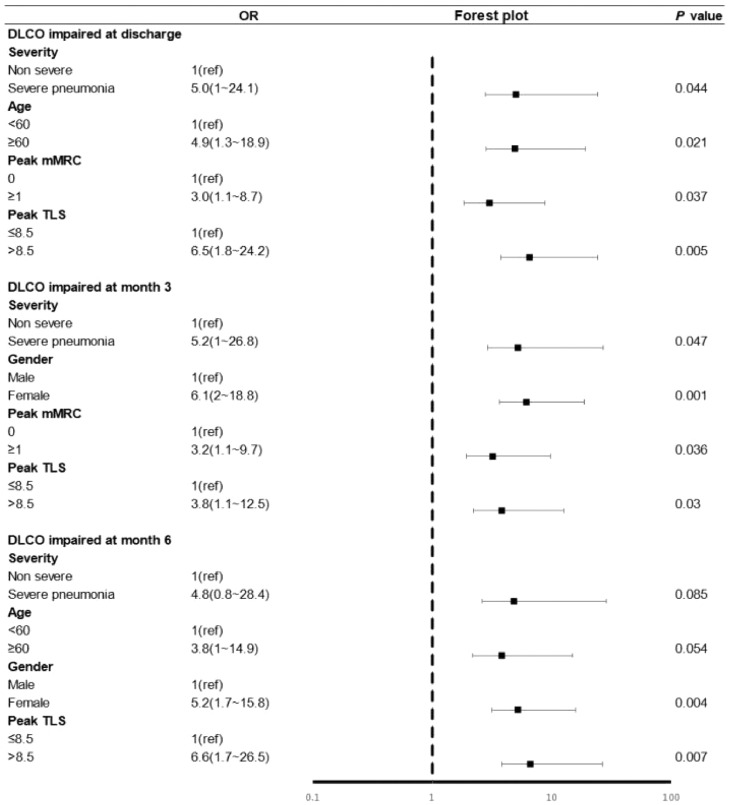
To explore the potential risk factors of abnormal DLCO in different stages of follow-up, the univariate (Appendix p 3) and multivariate logistic regression models were conducted (Fig. 4). As shown in Fig. 4, at discharge, severe (OR 5.0 [95% CI 1.0-24.1]; p = 0.044), ≥ 60 years old (OR 4.9 [95% CI 1.3–18.9]; p = 0.021), peak mMRC (OR 3.0 [95% CI 1.1–8.76.5-]; p = 0.037), peak TLS (OR 6.5 [95% CI 1.8–24.2]; p = 0.005) were risk factors of DLCO impairment. At six months, peak TLS > 8.5 (OR 6.6 [95% CI 1.7–26.5]; p = 0.007), and female (OR 5.2 [95% CI 1.7–15.8]; p = 0.004) were risk factors of abnormal DLCO.
Figure 4.
Risk factors associated with impaired DLCO during the follow-up.
Impaired DLCO is defined as DLCO < 80% at discharged. DLCO, diffusing capacity of the lung for carbon monoxide; Peak mMRC, peak modified British Medical Research Council dyspnea scale during hospitalization; Peak TLS, peak chest CT total lesion score during hospitalization; OR, odds ratio. The group of Non severe included mild and moderate patients.
In comparisons of gender, the DLCO% pred, KCO% pred of females were significantly lower than those of males throughout all visits. The change rates of these diffusion indexes showed no significant difference according to gender (Table 3). But in patients with impaired diffusion capacity, DLCO% pred and KCO% pred had no significant difference between males and females at discharge. The change rate of DLCO% pred and KCO% pred in females in 0–3 months after discharge was significantly lower than that in males (both p < 0.01). There was no significant difference in the change rate of DLCO% pred and KCO% pred in 3–6 months between gender. While the VA change rate between males and females had no significant difference in 0–3 months and 3–6 months. The female's proportion with impaired DLCO and KCO were significantly higher than male's after Month 3 (Appendix p 6).
Table 3.
Sequela symptoms and pulmonary function findings in COVID-19 patients categorized by gender in different follow-up periods.
| At discharge |
Month 3 |
Month 6 |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Spirometry | ||||||
| FVC% predicted,% | 94.2 ± 9.0 | 96.0 ± 12.4 | 95.7 ± 10.0 | 97.4 ± 13.3 | 93.2 ± 9.4 | 97.8 ± 13.2 |
| FVC < 80% predicted | 1/40 (2.5%) | 6/45 (13.3%) | 1/40 (2.5%) | 5/45 (11.1%) | 3/40 (7.5%) | 3/45 (6.7%) |
| FEV1% predicted,% | 92.0 ± 9.2 | 95.1 ± 11.8 | 92.3 ± 9.0 | 94.6 ± 12.5 | 90.4 ± 8.9 | 94.0 ± 13.2 |
| FEV1 < 80% predicted | 4/40 (10.0%) | 4/45 (8.9%) | 3/40 (7.5%) | 4/45 (8.9%) | 4/40 (10.0%) | 4/45 (8.9%) |
| FEV1/FVC% | 80.1 ± 6.4 | 83.0 ± 5.8* | 79.4 ± 6.3 | 81.9 ± 4.8* | 79.8 ± 6.7 | 82.3 ± 5.4 |
| FEV1/FVC< 70% | 2/40 (5.0%) | 1/45 (2.2%) | 2/40 (5.0%) | 1/45 (2.2%) | 2/40 (5.0%) | 1/45 (2.2%) |
| Diffusion capacity | ||||||
| DLCO% predicted,% | 82.7 ± 12.0 | 77.1 ± 11.4* | 89.4 ± 11.3 | 80.8 ± 11.2⁎⁎ | 89.5 ± 11.8 | 82.1 ± 12.9⁎⁎ |
| DLCO < 80% predicted | 14/41 (34.1%) | 21/45 (46.7%) | 7/41 (17.1%) | 23/45 (51.1%)⁎⁎ | 7/41 (17.1%) | 21/45 (46.7%)⁎⁎ |
| KCO% predicted,% | 98.0 ± 12.8 | 85.9 ± 13.2⁎⁎ | 104.2 ± 15.0 | 87.9 ± 13.5⁎⁎ | 103.0 ± 15.3 | 87.7 ± 15.1⁎⁎ |
| KCO< 80% predicted | 5/41 (12.2%) | 16/45 (35.6%) | 2/41 (4.9%) | 10/45 (22.2%)* | 2/41 (4.9%) | 15/45 (33.3%)⁎⁎ |
| VA L/min | 5.6 ± 0.7 | 4.3 ± 0.6⁎⁎ | 5.8 ± 0.7 | 4.4 ± 0.6⁎⁎ | 5.8 ± 0.8 | 4.8 ± 0.6⁎⁎ |
| Recovery rate | Discharge to Month 3 | Month 3 to Month 6 | ||||
| DLCO% predicted,% | 9.2 ± 14.0 | 4.5 ± 9.2 | 0.5 ± 8.7 | 1.9 ± 9.9 | ||
| KCO% predicted,% | 6.6 ± 11.3 | 2.7 ± 8.0 | −1.5 ± 7.2 | −0.2 ± 8.9 | ||
| VA L/min,% | 4.1 (−0.6, 6.9) | 1.90 (−1.6, 5.4) | 0.5 (−1.8, 4.2) | 1.1(−2.4, 5.2) | ||
| Lung volume | ||||||
| TLC% predicted,% | 84.9 ± 7.5 | 91.6 ± 9.1⁎⁎ | 87.9 ± 6.9 | 93.1 ± 9.9⁎⁎ | 88.2 ± 7.2 | 94.5 ± 10.3⁎⁎ |
| TLC < 80% predicted | 9/41 (22.0%) | 2/45 (4.4%)* | 5/41 (12.2%) | 4/45 (8.9%) | 4/41 (9.8%) | 3/45 (6.7%) |
| RV% predicted,% | 86.2 ± 16.2 | 90.4 ± 17.3 | 88.7 ± 14.9 | 92.2 ± 17.4 | 97.4 ± 18.0 | 99.9 ± 18.8 |
| RV < 65% predicted | 2/41 (4.9%) | 1/45 (2.2%) | 1/41 (2.4%) | 1/45 (2.2%) | 1/41 (2.4%) | 1/45 (2.2%) |
| Sequela symptoms† | 34/53 (64.2%) | 40/57 (70.2%) | 26/53 (49.1%) | 34/57 (59.6%) | 21/53 (39.6%) | 29/57 (50.9%) |
| mMRC score ≥1 | 5/53 (9.4%) | 5/57 (8.8%) | 2/53 (3.8%) | 3/57 (5.3%) | 1/53 (1.9%) | 3/57 (5.3%) |
1. Data are n/N (%), mean ± SD or median (IQR). N is the total number of patients with available data.
2. Abbreviations: FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; KCO, transfer coefficient of the lung for carbon monoxide; RV, residual volume; TLC, total lung volume; mMRC, modified Medical Research Council dyspnea scale.
3. *p < 0.05 and ⁎⁎p < 0.01, compared with Males.
4. †: The proportion of patients with at least one sequela symptom.
Discussion
To our knowledge, the dynamic changes of pulmonary function in patients with COVID-19 since discharge have not been described in previous follow-up studies. The current study was firstly discussed longitudinally dynamic trends of lung function since discharge. The results demonstrated that the first three months after discharge may be a critical period for recovery of DLCO for COVID-19 patients. It was possibly associated with the improvement of KCO and VA in different periods. KCO and VA might improve slowly after the first three months. In addition, compared with males, female patients had more severe diffusion impairment and lower recovery rate.
DLCO mainly depends on two factors, including the rate constant for carbon monoxide uptake from alveolar gas and the “accessible” alveolar volume. Decreased KCO occurs in alveolar–capillary damage, microvascular pathology, or anemia. Decreased VA occurs in reduced alveolar expansion, alveolar damage or loss, or maldistribution of inspired gasses with airflow obstruction.24,25 Compared to discharge, DLCO improved at three months and six months after discharge. But the recovery from discharge to three months was faster than that from three to six months. KCO improved mainly within three months after discharge, and the subsequent improvement rate was very low. Besides, VA had sustained improvement within 6 months after discharged, but the improvement had a decreased trend over time. It suggested that the capillary component damage and accessible alveolar volume reduction presenting in COVID-19 patients may mainly recover within the first three months after discharge. Above of these demonstrate that the first three months post-discharge may be an essential period for DLCO recovery.
In a longitudinal cohort study on COVID-19 recently published, researchers completed pulmonary tests at 6-month and 12-month follow-up visit and discovered that the lung diffusion impairment did not significantly improve from six months to twelve months.26 This suggests that the improvement of diffusion function in patients with COVID-19 may mainly occur within the first 6 months after discharge. There are 33%−34.8% of patients with COVID-19 still had diffusion dysfunction at twelve months after discharge.13,26 As previous studies reported, impaired diffusion capacity was also present in patients with SARS or MERS. 21–52.7% of SARS patients had diffusion dysfunction at one or even two years after discharge.14, 15, 16 And 37% of MERS patients had diffusion dysfunction one year after discharge.17 These studies indicate that diffusion dysfunction may be the long-term sequela in coronavirus pneumonia. In Liu et al., lung rehabilitation training within six weeks after discharge could significantly improve DLCO% pred in COVID-19 patients.27 Therefore, early lung rehabilitation training may be helpful in recovering the lung function of COVID-19 survivors. In Wu et al., the DLCO% pred of patients with severe COVID-19 also showed some degree of recovery from six to twelve months after discharge.13 Since the longest follow-up period was six months in our study, it was difficult to clarify the improvement of diffusion capacity more than six months after discharge.
In the results of multivariate logistic analysis, at six months after discharge, female and peak TLS > 8.5 points were risk factors for diffusion capacity impairment. Peak TLS was associated with the severity of COVID-19 patients.12,28 Previous studies have reported that female was the risk factor for the impairment of diffusion capacity in COVID-19 patients at both six months and twelve months post-discharge.12,13,26 The same result was also shown in our study.
Further analysis shows that female patients had lower DLCO% pred and KCO% pred than males at discharge. It suggested that female patients may suffer from more severe damage of alveolar capillary and microvascular in COVID-19. In patients with impaired diffusion function, women had lower change rates of DLCO% pred and KCO% pred in 0–3 months than men. So the DLCO% pred and KCO% pred in females less than males after 0–3 months. It indicated that the improvement of alveolar capillary and microvascular damage in females might be poorer than males. Takahashi et al. proved that male patients had higher plasma levels of innate immune cytokines and more robust induction of non-classical monocytes, while female patients had more robust T cell activation than male patients during SARS-CoV-2 infection.29 Another study showed that female patients can express more ZNF proteins and stronger transcriptional activities than male patients in response to SARS-CoV-2 infection. The ZNF protein activity might promote the abundance and the antiviral activity of multiple immune cells to effectively suppress SARS-CoV-2 infection.30 These studies suggest that different immune response mechanisms exist between men and women. However, gender differences in these mechanisms may cause the more severe damage and difficult recovery of alveolar capillary and microvascular in women, which in turn affects their diffusion capacity.
Some limitations should be noted in our study. Firstly, we have only carried out the follow-up for the first six months. The long-term prognosis of diffusion capacity in patients with COVID-19 remains unclear. Secondly, the data of pulmonary function before infection with SARS-CoV-2 are lacking. It is difficult to directly clarify the impact of COVID-19 on diffusion capacity. Thirdly, the exercise capacity (e.g. 6 min walk distance test) were not assessed during the follow-up. So that the changes of cardiopulmonary function and exercise endurance in COVID-19 patients could not be observed. Finally, our findings reflect the “natural history” of the COVID pneumonia. The subsequent recovery of the lung function may be modified in patients who receive anti-inflammation therapy.
Overall, we have firstly described the dynamic changes of pulmonary function in COVID-19 patients since discharge to six months post-discharge. Diffusion dysfunction is a common pulmonary sequela in COVID-19. The first three months is the critical period for diffusion capacity recovery, which may be associated with the recovery of the capillary component damage and accessible alveolar volume reduction. The impaired diffusion capacity in females was more severe than males at discharge. And in patients with impaired diffusion capacity, females recovered slower than males. Further investigation are needed to address the effects of early pulmonary rehabilitation and gender-specific interventions on the recovery of lung function in COVID-19 survivors.
Author contributions
RC, XM, NZ and SL had the idea for and designed the study. All authors had full access to the data and had final responsibility for the decision to submit for publication. MC, JL, PP, SZ, WJ, YG, LF drafted the paper. MC, JL, LF, YY, SZ, SD did the analysis and all authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. JL, YY, HP, LF, XD, YZ, SD collected the data. LF, SZ and JL had verified the underlying data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Guangdong Provincial Department of Science and Technology (2020B1111340014), Zhong Nanshan Medical Foundation of Guangdong Province (ZNSA-2020012, 2020013), Guangzhou Medical University (GMU2020-207), China Evergrande Group (2020GIRHHMS17), Emergency Key Program of Guangzhou Laboratory (EKPG21-29, EKPG21-31).
Data sharing
The data that support the findings of this study are available from the corresponding author (SL, NZ, XM, RC) upon reasonable request and with permission of Guangzhou Institute of Respiratory Health and Guangzhou Eighth People's Hospital, Guangzhou Medical University, Guangzhou, China.
Declaration of interests
We declare no competing interests.
Acknowledgments
We acknowledge all the physicians and nurses who collected the samples and cared for these patients during they hospitalization. We thank Mei Jiang and Zhaowei Yang in The First Affiliated Hospital of Guangzhou Medical University for their help in statistic data. We also thank Zhongping Wu in The First Affiliated Hospital of Guangzhou Medical University for his assistance in pulmonary function tests quality control. We also thank Chen Zhan in The First Affiliated Hospital of Guangzhou Medical University, Difei Chen, Liqin Lin, Junfeng Huang and Wanwan Li in Guangzhou Medical University for their assistance in data preparation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101255.
Contributor Information
Ruchong Chen, Email: chen_rch@163.com.
Xiaoneng Mo, Email: moxiaoneng@126.com.
Nanshan Zhong, Email: nanshan@vip.163.com.
Shiyue Li, Email: lishiyue@188.com.
Appendix. Supplementary materials
References
- 1.WHO. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Date last accessed: August 30, 2021.
- 2.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellan M., Soddu D., Balbo P.E., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daher A., Balfanz P., Cornelissen C., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174 doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González J., Benítez I.D., Carmona P., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160:187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortini A., Torrigiani A., Sbaragli S., et al. COVID-19: persistence of symptoms and lung alterations after 3-6 months from hospital discharge. Infection. 2021:1–9. doi: 10.1007/s15010-021-01638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55 doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin W., Chen S., Zhang Y., et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58 doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah A.S., Wong A.W., Hague C.J., et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76:402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 10.Yan X., Huang H., Wang C., et al. Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J Infect. 2021;83:381–412. doi: 10.1016/j.jinf.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Sar-van der Brugge S., Talman S., Boonman-de Winter L., et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X., Liu X., Zhou Y., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui D.S., Wong K.T., Ko F.W., et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngai J.C., Ko F.W., Ng S.S., To K.W., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong K.C., Ng A.W.K., Lee L.S.U., et al. 1-year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest. 2005;128:1393–1400. doi: 10.1378/chest.128.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park W.B., Jun K.I., Kim G., et al. Correlation between pneumonia severity and pulmonary complications in middle east respiratory syndrome. J Korean Med Sci. 2018;33:e169. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia. https://www.who.int/docs/default-source/wpro-documents/countries/china/covid-19-briefing-nhc/1-clinical-protocols-for-the-diagnosis-and-treatment-of-covid-19-v7.pdf?sfvrsn=c6cbfba4_2. Date last updated: March 3, 2020. Date last assessed: August 17, 2021.
- 19.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham B.L., Steenbruggen I., Miller M.R., et al. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 22.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest ct during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y., Han X., Gu J., et al. Prognostic value of baseline clinical and HRCT findings in 101 patients with severe COVID-19 in Wuhan, China. Sci Rep. 2020;10:17543. doi: 10.1038/s41598-020-74497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes J.M.B., Pride N.B. Examination of the carbon monoxide diffusing capacity (DL(CO)) in relation to its KCO and VA components. Am J Respir Crit Care Med. 2012;186:132–139. doi: 10.1164/rccm.201112-2160CI. [DOI] [PubMed] [Google Scholar]
- 25.Chen R., Gao Y., Chen M., et al. Impaired pulmonary function in discharged patients with COVID-19: more work ahead. Eur Respir J. 2020;56 doi: 10.1183/13993003.02194-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L., Yao Q., Gu X., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39 doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J.J., Cao Y.Y., Tan G., et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021;76:533–550. doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi T., Ellingson M.K., Wong P., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin S., Xu W., Wang C., et al. Analyzing master regulators and scRNA-seq of COVID-19 patients reveals an underlying anti-SARS-CoV-2 mechanism of ZNF proteins. Brief Bioinform. 2021 doi: 10.1093/bib/bbab118. Apr 27;bbab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.