Abstract
Background:
Hepatitis B vaccine birth dose (HepB-BD) was introduced in Lao People’s Democratic Republic to prevent perinatal hepatitis B virus transmission in 2008; high coverage is challenging since only 38% of births occur in a health facility. Healthcare workers report being unaware of home births and thus unable to conduct timely postnatal care (PNC) home visits. A quasi-experimental pilot study was conducted wherein mobile phones and phone credits were provided to village health volunteers (VHV) and healthcare workers (HCWs) to assess whether this could improve HepB-BD administration, as well as birth notification and increase home visits.
Methods:
From April to September 2014, VHVs and HCWs in four selected intervention districts were trained, supervised, received outreach per diem for conducting home visits, and received mobile phones and phone credits. In three comparison districts, VHVs and HCWs were trained, supervised, and received outreach per diem for conducting home visits. A post-study survey compared HepB-BD coverage among children born during the study and children born one year before. HCWs and VHVs were interviewed about the study.
Findings:
Among intervention districts, 463 study children and 406 pre-study children were enrolled in the survey; in comparison districts, 347 study children and 309 pre-study children were enrolled. In both arms, there was a significant improvement in the proportion of children reportedly receiving a PNC home visit (intervention p < 0.0001, comparison p = 0.04). The median difference in village level HepB-BD coverage (study cohort minus pre-study cohort), was 57% (interquartile range [IQR] 32–88%, p < 0.0001) in intervention districts, compared with 20% (IQR 0–50%, p < 0.0001) in comparison districts. The improvement in the intervention districts was greater than in the comparison districts (p = 0.0009).
Conclusion:
Our findings suggest that the provision of phones and phone credits might be one important factor for increasing coverage. However, reasons for improvement in both arms are multifactorial and discussed.
Keywords: Postnatal visits, Lao PDR, Hepatitis B, Lay health worker, Mobile phones
1. Introduction
Globally, approximately 240 million people have chronic hepatitis B virus (HBV) infection; an estimated 600,000 die from its complications yearly [1,2]. Perinatal transmission plays a significant role in the development of chronic infection; infants infected during the first year of life have a 90% chance of developing chronic infection [3]. Hepatitis B vaccine birth dose (HepB-BD), recommended for all newborns within 24 h of birth, with at least 2 additional doses of hepatitis B vaccine, are 85–90% effective in preventing perinatal transmission [4,5]. It is challenging to achieve high HepB-BD coverage in many countries, since many newborns are born at home and lack access to timely vaccination.
An estimated 8% of the Lao People’s Democratic Republic (Lao-PDR) population has chronic HBV infection [6]. Since 2008, Lao-PDR’s national policy is to provide HepB-BD to all newborns within 7 days of birth, preferably providing it within 24 h of birth. In 2014, 50% of newborns received HepB-BD [7]. One challenge to achieving high coverage is that only 38% of births occur in a health facility; furthermore, 2011–2012 data show that only 38% of mothers and newborns received a health check at delivery or a postnatal care (PNC) check within 2 days of birth [8].
One way to improve HepB-BD coverage is to improve access to healthcare services, specifically PNC outreach services, whereby a mother and newborn receive life-saving interventions such as a medical examination; education on early and exclusive breastfeeding and clean cord care; Vitamin A, folate, and iron administration for the mother; and vaccination of the newborn, including HepB-BD. In Lao-PDR, one reason anecdotally reported by healthcare workers (HCWs) for suboptimal PNC outreach is lack of awareness of imminent deliveries or recent births. Some village health volunteers (VHVs) use their personal phones to notify HCWs of births. However, reporting of births is sporadic, dependent on VHVs’ awareness of a birth, access to a functional phone, ability to afford making a call, and awareness about what events should be communicated to HCWs. Mobile phones have been used in multiple settings to improve access to care, but few evaluations have been done to assess their health impact [9–15]. Despite limited evidence, a policy paper recommends providing mobile phones to community health workers to improve access to care [16].
To understand if mobile phones could improve access to care, a pilot study was conducted in Lao-PDR, in which mobile phones and phone credits were provided to VHVs and HCWs in intervention districts during April-September 2014, to assess the impact on HepB-BD coverage; secondary impact on timely birth notification and PNC home visits was also evaluated.
2. Methods
2.1. Pilot study overview
The study design was a quasi-experimental design where the intervention was district-level non-random assignment, similar to a previous pilot where HepB-BD was stored outside the cold chain [17]. The design included a control group, and an evaluation of a cohort of children born before the study. Because of low reported HepB-BD coverage (reported administrative coverage in 2011: 2–15%), three districts (Ngoy, Nambark, and Phoukhoun) in Luang Prabang province and one district (Parklai) in neighboring Xayabuly province were selected to participate in the 6-month study. Three other districts (Phonxay, Chomphet, and Viengkham) in Luang Prabang and one district (Xienghone) in Xayabuly were chosen as comparison districts because of similar birth rates and reported HepB-BD coverage (reported administrative coverage in 2011: 2–13%). These districts are rural, located in the northern part of the country. In 2011, the intervention districts had 5083 births, while the comparison districts had 4236 births. Only health centers (HCs) with a functional cold chain (needed for vaccine storage) were enrolled. All intervention and comparison villages in the catchment area of the enrolled HCs were enrolled unless there was no VHV; in the intervention arm, villages were excluded if the VHV could not charge a phone or the village lacked network coverage.
The catchment area of the enrolled HCs consisted of 4–16 villages. Villages were classified as fixed, outreach, or mobile, depending on travel time and distance between the HC and village. Fixed villages had a HC within the village or one located a few kilometers away. Outreach villages were further from a HC, but HCWs could make a roundtrip visit in one day. Mobile villages were far from HCs, requiring HCWs to stay overnight during visits. [16]
2.1.1. Intervention districts
VHVs and HCWs received a one-day training on when VHVs should call HCWs (imminent delivery, mother/baby with danger signs, birth notification), what PNC services HCWs should provide, how HCWs should administer HepB-BD, and how to use the phones provided. One trained VHV from every enrolled village and every HC received a phone and US $6 of phone credit monthly to facilitate communication between the VHV and HC. To cover the cost of conducting outreach, standard government per diem (US $5) was provided to HCWs for each home birth they attended and PNC home visit conducted.
2.1.2. Comparison districts
VHVs and HCWs received identical training provided in the intervention districts except training on phones. These staff were not provided with mobile phones or credits, but they were given identical per diem for each home visit.
2.2. Household evaluation survey
From October to December 2014, an evaluation survey was conducted, using similar methods to a pilot to improve HepB-BD coverage among health facility births, where data on children born before the study (12–21 months of age, considered the baseline) and on children born during the intervention (0–9 months of age) were collected [17]. The survey was designed to evaluate the difference in the change in HepB-BD coverage between intervention and comparison districts. A sample size of 14 villages per arm had a 90% power to detect a difference of 10% in HepB-BD coverage between the intervention and comparison arms, with an estimated standard deviation of 0.10 and a significance level (alpha) of 0.05, using a two-sided Wilcoxon test assuming that the actual distribution was normal. This was inflated to 18 villages per access strata (fixed, outreach, mobile) to account for attrition due to poor access or lack of births.
In this stratified cluster survey, we defined three access strata because we expected access to affect improvement. Intervention HCs served 16 fixed, 52 outreach, and 71 mobile villages; comparison HCs served 13 fixed, 36 outreach, and 29 mobile villages. Three mobile intervention villages were ⩾360 min away from their respective HCs and were excluded. In the first stage of sampling, 18 villages (or all villages if <18) were chosen per stratum by systematic random sampling. In each village, all children born between April 1, 2013-September 30, 2013 (pre-study cohort) and between April 1, 2014-September 30, 2014 (study cohort) were eligible. (Supplemental Fig. 1)
In each village, enumerators conducted a census of all age-eligible children born during the study and born one year prior to the study, including age-eligible deceased children. For each eligible child, consenting caregivers were administered a questionnaire, which collected demographic data, birth and PNC details, and documented vaccination history. After completing the interviews, teams reviewed vaccination registers at the villages’ primary HCs to obtain vaccination data on all children identified, as well as age-eligible children who were missed. Children without any documented vaccination data were assumed unvaccinated.
2.3. VHV and HCW evaluation survey
VHVs and HCWs servicing the selected villages were interviewed with a structured questionnaire at the end of the study to understand practices and challenges. Interviewers asked the respondent open-ended questions and classified answers into pre-defined categories; if their answer was unable to be categorized, the full answer was noted.
2.4. Data analysis
Data were entered and stored in a CSPro v5.0 database (Washington, DC) and analyzed in SAS v9·3 (Cary, NC). During data cleaning, if vaccination dates by register and card were discrepant, registers were considered more accurate. Weights accounting for the selection probability of villages within each stratum were calculated and applied to all results except for basic demographics. Proportions were calculated for various demographic and population characteristics by cohort and arm. HepB-BD was defined as any dose given within 30 days of life. Children who died on day of life 0 or 1 were excluded. Change in village-level HepB-BD coverage was estimated by subtracting coverage in children born one year before the study from children born during the study by village. For the village-level analysis, to assess change in HepB-BD coverage, only villages with ⩾1 child in each age cohort were analyzed. Within arms, non-parametric Wilcoxon sign-rank test was used to test whether change in village BD coverage was significantly different from zero. At the village-level, to test the difference in the median change in HepB-BD coverage between intervention and control arms, non-parametric Wilcoxon two-sample test was used. At the individual child-level, secondary analyses were performed (e.g. factors related to HepB-BD receipt); we accounted for village-level clustering using survey methods and report the second-order Rao-Scott χ2 p-values. For analysis of the VHV and HCW surveys, proportions were calculated based on the pre-defined categorization described above; Fisher p-values were calculated because of small sample size.
2.5. Human subjects’ rights and ethics
We obtained informed consent from caregivers. Study protocol was approved by the Lao-PDR Ministry of Health and the Ethics Review Committee at the WHO Regional Office for the Western Pacific. CDC determined this activity was not human subjects’ research.
3. Results
3.1. Pilot study implementation
In the intervention arm, 17 of 21 HCs had a functional cold chain; HCWs from these HCs and VHVs from 139 villages were trained as described. In the comparison districts, 13 of 25 HCs had a functional cold chain; HCWs from these HCs and VHVs from 78 villages were trained as described. No HCs in Chomphet District had a functional cold chain; thus, only 3 comparison districts were included. Six enrolled HCs (3 intervention, 3 comparison) reported HepB-BD vaccine shortages during the study.
3.2. Household evaluation survey
In the intervention arm, 51 villages (16 fixed, 18 outreach, and 17 mobile) were visited; one selected mobile village was excluded because there were no eligible children in either age cohort. In this arm, 465 children aged 0–9 months and 406 children aged 12–21 months were identified. In the comparison arm, 49 villages (13 fixed, 18 outreach, and 18 mobile) were visited; 349 children aged 0–9 months and 309 children aged 12–21 months were identified. There were a median of 12 (IQR 7–21) age-eligible children in the intervention villages; there were a median of 12 (IQR 7–16) age-eligible children in the comparison villages. There were two refusals, one (0.3%) in each age cohort, in the comparison arm; vaccination data were available in the EPI register, so these two children were included in the vaccination analysis.
At baseline, intervention and comparison pre-study children were different with respect to religion, ethnicity, maternal education, and HepB-BD receipt (Table 1). Among them, 95 (15%) of 406 in intervention villages had received HepB-BD compared with 135 (39%) of 309 in comparison villages (p = 0.001).
Table 1.
Characteristics of children participating in the final survey — Mobile Phone Pilot Study, Lao-PDR, 2014.a
| Intervention | Comparison | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-studyb | During the studyc | Pre-studyb | During the studyc | |||||||||
| Total | n | % | Total | n | % | Total | n | % | Total | n | % | |
| Male | 354 | 191 | 54 | 420 | 203 | 48 | 263 | 131 | 50 | 309 | 153 | 50 |
| Ethnic group | ||||||||||||
| Lao | 354 | 175 | 49 | 420 | 211 | 50 | 263 | 21 | 8 | 309 | 21 | 7 |
| Khmu | 354 | 100 | 28 | 420 | 106 | 25 | 263 | 208 | 79 | 309 | 237 | 77 |
| Hmong | 354 | 71 | 20 | 420 | 96 | 23 | 263 | 28 | 11 | 309 | 38 | 12 |
| Other | 354 | 8 | 2 | 420 | 7 | 2 | 263 | 6 | 2 | 309 | 13 | 4 |
| Village classification | ||||||||||||
| Fixed | 406 | 188 | 46 | 465 | 220 | 47 | 309 | 117 | 38 | 349 | 106 | 30 |
| Outreach | 406 | 150 | 37 | 465 | 153 | 33 | 309 | 80 | 26 | 349 | 112 | 32 |
| Mobile | 406 | 68 | 17 | 465 | 92 | 20 | 309 | 112 | 36 | 349 | 131 | 38 |
| Deceased at time of survey | 354 | 4 | 1 | 422 | 10 | 2 | 263 | 3 | 1 | 309 | 4 | 1 |
| Highest level of maternal education completed | ||||||||||||
| None | 351 | 65 | 19 | 419 | 55 | 13 | 262 | 52 | 20 | 309 | 62 | 20 |
| Primary school | 351 | 186 | 53 | 419 | 209 | 50 | 262 | 166 | 63 | 309 | 199 | 64 |
| Secondary school or higher | 351 | 100 | 28 | 419 | 155 | 37 | 262 | 44 | 17 | 309 | 48 | 16 |
| Religion | ||||||||||||
| Buddhist | 354 | 193 | 55 | 420 | 233 | 55 | 263 | 26 | 10 | 309 | 33 | 11 |
| Animist | 354 | 161 | 45 | 420 | 186 | 44 | 263 | 237 | 90 | 309 | 276 | 89 |
| Christian | 354 | 0 | - | 420 | 1 | 0 | 263 | - | - | 309 | - | - |
| Birth detailsd | ||||||||||||
| Born at health facility | 350 | 169 | 39 | 413 | 241 | 52 | 260 | 123 | 42 | 308 | 156 | 47 |
| Born at home with SBA | 350 | 20 | 4 | 413 | 28 | 6 | 260 | 18 | 5 | 308 | 20 | 7 |
| Born at home without SBA | 350 | 161 | 57 | 413 | 144 | 42 | 260 | 119 | 53 | 308 | 132 | 46 |
| HCW made postnatal care visit to homed | 353 | 62 | 11 | 418 | 132 | 30 | 260 | 57 | 19 | 308 | 88 | 28 |
| Children with written vaccination data (card or register)d | 406 | 393 | 96 | 463 | 439 | 93 | 309 | 306 | 99 | 347 | 342 | 99 |
| Hepatitis B birth dose (⩽30 days)d | 406 | 95 | 15 | 463 | 348 | 70 | 309 | 135 | 39 | 347 | 257 | 71 |
| HepB BD timingd | ||||||||||||
| 0–1 day | 95 | 63 | 74 | 348 | 287 | 81 | 135 | 114 | 83 | 257 | 232 | 90 |
| 2–7 days | 95 | 29 | 24 | 348 | 55 | 17 | 135 | 14 | 11 | 257 | 22 | 9 |
| 8–30 days | 95 | 3 | 2 | 348 | 6 | 2 | 135 | 7 | 6 | 257 | 3 | 1 |
| Bacillus Calmette-Guerin (BCG)4 | 406 | 381 | 94 | 463 | 400 | 84 | 309 | 295 | 96 | 347 | 314 | 90 |
2 children born during the study in the intervention arm and 2 child born during the study in the comparison arm died on day of life 0 or 1 and are excluded as they were not eligible for vaccination. In each arm, denominators change as children were identified during the health facility vaccination record review who were not identified during the household visits. These age-eligible children are included as they were eligible for services from the health facility during the defined time periods.
Pre-study = born one year prior to study implementation (12–21 month olds).
During study = born during study implementation (0–9 month olds).
Weighted percentages shown.
Improvement was seen in heath facility delivery rates during the study. In the intervention arm, children born during the study were more likely to be born in a health facility than pre-study children (p = 0.003); the comparison had no change in health facility birth rates (p = 0.26) (Table 1). In both arms, there was a significant improvement in the proportion of children reportedly receiving a PNC home visit during the study versus pre-study (intervention p < 0.0001, comparison p = 0.04) (Table 1).
To compare change in village HepB-BD coverage, two intervention villages (1 fixed, 1 mobile) and five comparison villages (1 fixed, 4 outreach) were excluded as children were only identified in one cohort precluding change calculation. In the intervention arm, pre-study median village HepB-BD coverage was 0% (IQR 0–20%); study median village HepB-BD was 75% (IQR 57–100%) (Fig. 1). In the comparison arm, pre-study median village HepB-BD coverage was 37% (IQR 0–87%); study median village HepB-BD was 82% (IQR 50–100%) (Fig. 1). In the intervention arm, median change in village HepB-BD coverage was 57% (IQR 32–88%, Signed Rank p < 0.0001) compared with median change of 20% (IQR 0–50%, Signed Rank p < 0.0001) in the comparison (Wilcoxon two-sample test p-value = 0.0009) (Fig. 1). There was no significant difference in median change in Bacillus Calmette-Guérin vaccination between arms (data not shown, Wilcoxon p = 0.79).
Fig. 1.
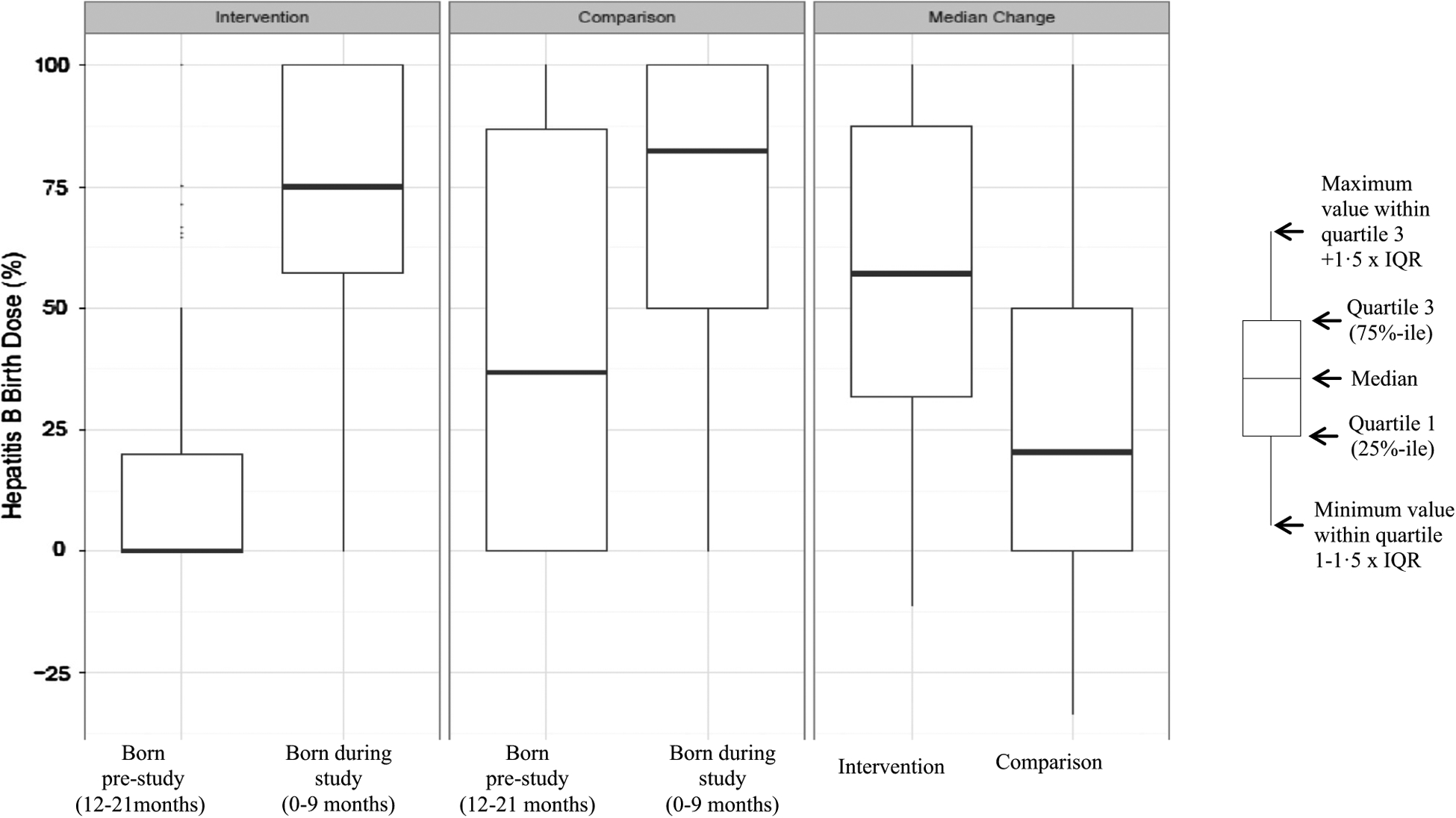
Comparison of hepatitis B vaccine birth dose (HepB-BD) coverage by village among children born before and during the study in the intervention and comparison arms — Mobile Phone Pilot Study, Lao-PDR, 2014.
At the child-level, HepB-BD coverage improved in both arms over time (Table 1); factors related to HepB-BD receipt were analyzed. In both arms, HepB-BD coverage improved significantly among both home and health facility births during the study, with higher coverage among children born in health facilities than at home in all four groups (Table 2). HepB-BD coverage also improved among children who reportedly received and did not receive a PNC home visit. Significantly more intervention study children received a HepB-BD if they had a PNC home visit compared to those that did not report a visit (p = 0.003). Compared with pre-study children, study children had a higher HepB-BD coverage in all access strata (Table 2, Supplemental Fig. 2). A sub-analysis was done among home births (where PNC outreach was assumed to have its greatest impact); similar improvements were seen regardless of access strata and receipt of a PNC home visit (Table 3).
Table 2.
Selected factors associated with receipt of hepatitis B vaccine birth dose (HepB-BD) among children born before and during the pilot study in the intervention and comparison arms — Mobile Phone Pilot Study, Lao-PDR, 2014.
| Intervention | Comparison | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Born pre-study | Born during the study | p Value comparing HepB-BD between those born pre-study to those born during the study | Born pre-study | Born during the study | p Value comparing HepB-BD between those born pre-study to those born during the study | |||||||||
| Total | Received HepB-BD | % | Total | Received HepB-BD | % | Total | Received HepB-BD | % | Total | Received HepB-BD | % | |||
| Birth location | ||||||||||||||
| Health facility | 169 | 60 | 29 | 241 | 208 | 82 | <0.0001 | 123 | 88 | 71 | 156 | 147 | 95 | 0.0002 |
| Home | 181 | 26 | 8 | 170 | 111 | 59 | <0.0001 | 137 | 25 | 16 | 150 | 81 | 50 | <0.0001 |
| p-Value comparing HepB-BD between health facility and home births | 0.001 | 0.001 | <0.0001 | <0.0001 | ||||||||||
| PNC home visit | ||||||||||||||
| Yes | 62 | 26 | 34 | 132 | 118 | 86 | <0.0001 | 57 | 24 | 38 | 86 | 64 | 70 | <0.0001 |
| None | 291 | 62 | 14 | 284 | 203 | 64 | <0.0001 | 203 | 89 | 39 | 220 | 164 | 72 | 0.009 |
| p-Value comparing HepB-BD between those receiving and those not receiving a PNC visit | 0.06 | 0.003 | 0.89 | 0.92 | ||||||||||
| Village classification | ||||||||||||||
| Fixed | 188 | 74 | 39 | 220 | 181 | 82 | <0.0001 | 117 | 60 | 51 | 106 | 97 | 92 | <0.0001 |
| Outreach | 150 | 15 | 10 | 151 | 109 | 72 | <0.0001 | 80 | 15 | 19 | 110 | 53 | 48 | 0.0003 |
| Mobile | 68 | 6 | 9 | 92 | 58 | 63 | <0.0001 | 112 | 60 | 54 | 131 | 107 | 82 | 0.0001 |
| p-value comparing HepB-BD between the three village classifications | 0.02 | 0.13 | 0.01 | 0.0003 | ||||||||||
Bold values indicate a statistically significant p value (⩽0.05).
Table 3.
Selected factors associated with receipt of hepatitis B vaccine birth dose (HepB-BD) among children born at home before and during the pilot study in the intervention and comparison arms — Mobile Phone Pilot Study, Lao-PDR, 2014.
| Intervention | Comparison | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Born pre-study | Born during the study | p Value comparing HepB-BD between those born pre-study to those born during the study | Born pre-study | Born during the study | p Value comparing HepB-BD between those born pre-study to those born during the study | |||||||||
| Total | Received HepB-BD | % | Total | Received HepB-BD | % | Total | Received HepB-BD | % | Total | Received HepB-BD | % | |||
| PNC home visit | ||||||||||||||
| Yes | 24 | 9 | 36 | 65 | 57 | 84 | 0.0002 | 37 | 9 | 22 | 62 | 43 | 65 | 0.002 |
| None | 156 | 17 | 6 | 104 | 53 | 41 | 0.0002 | 98 | 16 | 14 | 88 | 38 | 40 | 0.009 |
| p-Value comparing HepB-BD between those receiving and those not receiving a PNC visit | 0.09 | 0.001 | 0.44 | 0.11 | ||||||||||
| Village classification | ||||||||||||||
| Fixed | 68 | 19 | 28 | 65 | 50 | 77 | <0.0001 | 40 | 12 | 30 | 26 | 24 | 92 | <0.0001 |
| Outreach | 68 | 5 | 7 | 59 | 37 | 63 | <0.0001 | 50 | 6 | 12 | 77 | 26 | 34 | 0.005 |
| Mobile | 45 | 2 | 4 | 46 | 24 | 52 | <0.0001 | 47 | 7 | 15 | 47 | 31 | 66 | <0.0001 |
| p-Value comparing HepB-BD between the three village classifications | 0.05 | 0.22 | 0.27 | 0.005 | ||||||||||
Bold values indicate a statistically significant p value (⩽0.05).
3.3. HCW survey
Seventeen intervention HCWs and 13 comparison HCWs were interviewed. All HCWs reported it was important to visit a newborn within 24 h of birth. Only 10 (59%) intervention HCWs and 9 (69%) comparison HCWs reportedly provided HepB-BD to all newborns. All intervention HCWs and 11 (85%) comparison HCWs reportedly were notified by VHVs of imminent deliveries or births. In the intervention arm, all reported notifications were by mobile phone; in the comparison, 8 (73%) of the 11 reporting notification stated they were notified by mobile phone (Fisher p = 0.05). In both arms, there were challenges to visiting a home within 24 h of notification, with village inaccessibility being reported most frequently. (Table 4)
Table 4.
Healthcare worker (HCW) survey assessing the study implementation and postnatal care outreach provided — Mobile Phone Pilot Study, Lao-PDR, 2014.
| Intervention (n = 17) | Comparison (n = 13) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Knowledge | ||||
| Reported it was important to visit a baby within 24 h of birth | 17 | 100 | 13 | 100 |
| Knew optimal timing of HepB-BD is ⩽24 h | 15 | 88 | 10 | 77 |
| Notification | ||||
| Village health volunteers (VHVs) notify HCW of imminent deliveries/births | 17 | 100 | 11 | 85 |
| VHV notified HCW by mobile phone | 17 | 100 | 8 | 73 |
| Postnatalhomevisit | ||||
| % of the home births that HCW visited within 24 h of notification | ||||
| Never | 0 | 0 | 0 | 0 |
| 25% of the time | 7 | 41 | 3 | 23 |
| 50% of the time | 1 | 6 | 3 | 23 |
| 75% of the time | 1 | 6 | 1 | 8 |
| Most of the time | 8 | 47 | 4 | 31 |
| All of the time | 0 | 0 | 2 | 15 |
| Problems preventing visiting home births within 24 h of notification | ||||
| Village too far | 4 | 24 | 2 | 15 |
| Village inaccessible | 10 | 59 | 6 | 46 |
| No outreach money | 4 | 24 | 3 | 23 |
| Too busy | 0 | 0 | 2 | 15 |
| Reportedly provide HepB-BD for all newborns | 10 | 59 | 9 | 69 |
| Services provided at a postnatal home visit | ||||
| BCGa vaccine for neonate | 11 | 65 | 10 | 77 |
| HepB-BD vaccine for neonate | 17 | 100 | 11 | 85 |
| Education on clean cord care | 15 | 88 | 12 | 92 |
| Education on exclusive breast feeding | 17 | 100 | 12 | 92 |
| Education on vaccine schedule | 11 | 65 | 8 | 62 |
| Vitamin A for mother | 12 | 71 | 12 | 92 |
| Iron/folate for mother | 12 | 71 | 11 | 85 |
| Birth notification form | 14 | 82 | 10 | 77 |
| Examined baby | 16 | 94 | 12 | 92 |
| Examined mother | 16 | 94 | 12 | 92 |
Bacillus Calmette-Guérin.
3.4. VHV survey
Fifty-two intervention VHVs and 49 comparison VHVs were interviewed. In both arms, 76–83% reportedly informed HCWs about imminent deliveries/births most or all of the time. In the intervention arm, 43 (83%) VHVs reported informing HCWs either during labor or within 1 day of birth compared to 32 (65%) comparison VHVs. In the intervention arm, 43 (96%) used the study-provided mobile phone; in the comparison, 37 (84%) used their own mobile phone. Forty (89%) intervention and 23 (62%) VHVs reported no phone problems (Fisher p = 0.008). Lack of phone credit was reported by 4 (9%) intervention VHVs and 13 (35%) comparison VHVs.
4. Discussion
The purpose of this study was to ascertain if the provision of a mobile phone and phone credits, along with training and outreach per diem, could improve timely birth notification by VHVs to HCWs, as an intermediate step to increasing PNC home visits and HepB-BD administration. A significant improvement in HepB-BD coverage was seen in both arms, with a greater improvement seen in the intervention arm; our interpretation of this is that the provision of phones and credits was an important factor to increase coverage, along with training and regular outreach per diem. This was unexpected, as mobile phones are common in Lao-PDR; 84% of comparison VHVs used their own mobile phone to notify HCW. Some insight can be gained from the VHV survey where comparison VHVs reported more problems with mobile phones, specifically a lack of phone credits, that might have contributed to fewer notifications; lack of credits has been identified as a challenge elsewhere [18]. Furthermore, providing a phone to intervention VHVs might have been an incentive for them to actively participate in this study; increased motivation, increased community credibility, and increased empowerment from receiving a phone have been seen previously [10,19–21].
Our intervention was targeted at improving PNC home visits and HepB-BD coverage among children born at home. However, an unexpected increase in HepB-BD coverage among health facility births was seen, probably because of training and increased monitoring; neither outreach per diem nor phones should improve health facility vaccination [22–24]. However, not all health facility-born children received HepB-BD; possible reasons could include vaccine stockout or HCW apathy, competing workload, forgetfulness, or misperceptions of false contraindications. Further improvements are needed to ensure all health facility-born children receive HepB-BD.
HepB-BD coverage improvement in this study among home births was probably multifactorial, a combination of increased notification because of VHV education in both arms, mobile phone provision in the intervention arm, improved HCW knowledge, increased PNC visits due to increased notification and per diem, and the Hawthorne effect. However, we could not separate out the relative role of these different factors, since data were not collected on the number of notifications, the HCW response, and what was provided at the PNC visit. As expected, among home births, access affected a child’s HepB-BD status, as has been seen previously in Lao-PDR [17]. Unfortunately, health service access cannot be quickly changed; however, a dramatic improvement in HepB-BD coverage was seen in both arms in all access strata. Further improvements are still needed, most easily accomplished in the fixed villages, as these newborns are easy for HCWs to visit and vaccinate.
We expected that increased birth notifications would lead to increased PNC home visits and, in turn, to increased HepB-BD coverage. There was a statistically significant improvement in reported PNC visits in both arms, but among home births, a PNC visit was associated with HepB-BD receipt only in the intervention arm during the study. A significant association in the comparison was not seen, but this could have been due to insufficient power.
This study has several limitations. Findings might not be generalizable. The quasi-experimental design relied on administrative data to select comparable control districts; however, the survey found that the intervention and control populations were different at baseline. This makes inference regarding observed differences challenging. Some villages had 100% coverage at baseline and therefore no further improvement could be expected; this was more frequent in the comparison and could have skewed the findings. Several selected villages only had children from one age group, making comparison impossible. Some villages only had a small number of eligible children; small changes in the number of children born and vaccinated could lead to large changes in coverage. Use of the non-parametric statistical tests ameliorated this issue’s impact. The survey identified more children in the younger cohort which might mean older children were missed, since there was no evidence of a changing birth rate; these missed children could not be accounted for. Additionally, some secondary analyses had small sample sizes and were potentially under-powered to detect a difference. We cannot account for the impact of the Hawthorne effect. Recall bias could affect responses. Documented vaccination data were not available for 3% of children.
Scaling up this pilot could improve HepB-BD coverage in Lao-PDR. However, while provision of phones improved coverage, this could be costly. Comparison VHVs had phones, but insufficient phone credits; a more cost-effective approach might be to provide VHVs with credits. This intervention provided a platform to integrate other mother and child health service interventions, so an integrated program with cost-sharing could be considered. Scale-up of some or all of the interventions implemented in this study could lead to decreased chronic HBV infection. Furthermore, more home visits will enable HCWs to provide additional PNC services that could improve other health indicators. Future research is needed to understand driving factors in the provision of PNC home visits and HepB-BD, especially among home births.
Supplementary Material
Acknowledgements
We are thankful to all the village health volunteers, healthcare workers, district, provincial, and national public health staff who participated in this work and the parents and children of the surveyed villages who agreed to be part of this survey, without which this important activity would not have been a reality. We would also like to thank Alicia Ruiz, Eric Wiesen, Keith Feldon, and Alejandro Ramirez-Gonzalez for their support. We would like to thank the World Health Organization for funding this work.
Role of the funding source
Funding for this survey was provided by WHO; WHO Lao-PDR office staff supported the Ministry of Health in implementation and interpretation of results.
Footnotes
Conflicts of interest
Authors declare that they have no conflicts of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.09.056.
References
- [1].Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212–9. [DOI] [PubMed] [Google Scholar]
- [2].Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329–39. [DOI] [PubMed] [Google Scholar]
- [3].Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 1993;253:197–201. [DOI] [PubMed] [Google Scholar]
- [4].Hepatitis B vaccines: WHO position paper–recommendations. Vaccine 2010;28:589–90. [DOI] [PubMed] [Google Scholar]
- [5].Van Damme P, Ward J, Shouval D, Wiersma S, Zanetti A. Hepatitis B vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. China: Elsevier Saunders; 2013. [Google Scholar]
- [6].World Health Organization Regional Office for the Western Pacific. Western Pacific Regional Plan for Hepatitis B control through immunization. Manila (Philippines); 2007. http://www.wpro.who.int/immunization/documents/docs/POA_HepB.pdf [March 17, 2016]. [Google Scholar]
- [7].Lao People’s Democratic Republic Ministry of Health. WHO-Unicef joint reporting form; 2014. http://hiip.wpro.who.int/portal/Dashboards/Immunization/Immunizationdashboards/TabId/169/ArtMID/905/ArticleID/76/Default [March 17, 2016].
- [8].Lao People’s Democratic Republic Ministry of Health, Lao Statistics Bureau. Lao social indicator survey 2011–12. Vientiane (Lao PDR); 2012. http://dhsprogram.com/pubs/pdf/FR268/FR268.pdf [March 17, 2016]. [Google Scholar]
- [9].Noordam AC, Kuepper BM, Stekelenburg J, Milen A. Improvement of maternal health services through the use of mobile phones. Trop Med Int Health 2011;16:622–6. [DOI] [PubMed] [Google Scholar]
- [10].Agarwal S, Perry HB, Long LA, Labrique AB. Evidence on feasibility and effective use of mHealth strategies by frontline health workers in developing countries: systematic review. Trop Med Int Health 2015;20:1003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ngabo F, Nguimfack J, Nwaigwe F, Mugeni C, Muhoza D, Wilson DR, et al. Designing and implementing an innovative SMS-based alert system (RapidSMS-MCH) to monitor pregnancy and reduce maternal and child deaths in Rwanda. Pan Afr Med J 2012;13:31. [PMC free article] [PubMed] [Google Scholar]
- [12].Braun R, Catalani C, Wimbush J, Israelski D. Community health workers and mobile technology: a systematic review of the literature. PLoS ONE 2013;8: e65772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hall CS, Fottrell E, Wilkinson S, Byass P. Assessing the impact of mHealth interventions in low- and middle-income countries–what has been shown to work? Glob Health Action 2014;7:25606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee SH, Nurmatov UB, Nwaru BI, Mukherjee M, Grant L, Pagliari C. Effectiveness of mHealth interventions for maternal, newborn and child health in low- and middle-income countries: systematic review and meta-analysis. J Glob Health 2016;6:010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Watterson JL, Walsh J, Madeka I. Using mHealth to improve usage of antenatal care, postnatal care, and immunization: a systematic review of the literature. Biomed Res Int 2015;2015:153402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Africa Progress Panel. Maternal health: investing in the lifeline of healthy societies and economies; 2010. http://www.who.int/pmnch/topics/maternal/app_maternal_health_english.pdf [March 17, 2016].
- [17].Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, Wannemuehle K, Vongxay V, Vilayvone V, et al. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccine 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jennings L, Ong’ech J, Simiyu R, Sirengo M, Kassaye S. Exploring the use of mobile phone technology for the enhancement of the prevention of mother-to-child transmission of HIV program in Nyanza, Kenya: a qualitative study. BMC Public Health 2013;13:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Medhi I, Jain M, Tewari A, Bhavsar M, Matheke-Fischer M, Cutrell E. Combating rural child malnutrition through inexpensive mobile phones. In: Proceedings of the 7th Nordic conference on human-computer interaction: making sense through design. Copenhagen (Denmark): ACM; 2012. , http://research.microsoft.com/pubs/170446/Medhi-NordiCHI2012-CommCareCasestudy.pdf [March 17, 2016]. [Google Scholar]
- [20].Chib A The Aceh Besar midwives with mobile phones project: design and evaluation perspectives using the information and communication technologies for healthcare development model. J Comput-Med Commun 2010;15:500–25. [Google Scholar]
- [21].Little A, Medhanyie A, Yebyo H, Spigt M, Dinant GJ, Blanco R. Meeting community health worker needs for maternal health care service delivery using appropriate mobile technologies in Ethiopia. PLoS ONE 2013;8:e77563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].World Health Organization. Practices to improve coverage of the hepatitis B birth dose vaccine. Geneva (Switzerland): World Health Organization; 2012. , http://apps.who.int/iris/bitstream/10665/78616/1/WHO_IVB_12.11_eng.pdf [March 17, 2016]. [Google Scholar]
- [23].Djibuti M, Gotsadze G, Zoidze A, Mataradze G, Esmail LC, Kohler JC. The role of supportive supervision on immunization program outcome – a randomized field trial from Georgia. BMC Int Health Hum Rights 2009;9(Suppl 1):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Uskun E, Uskun SB, Uysalgenc M, Yagiz M. Effectiveness of a training intervention on immunization to increase knowledge of primary healthcare workers and vaccination coverage rates. Public Health 2008;122:949–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


