Abstract
We have introduced a time-resolved fluorometry (TRF)-based microwell hybridization assay for PCR products in detection of herpes simplex virus (HSV) in cerebrospinal fluid (CSF) specimens. TRF is a sensitive nonradioactive detection technique which involves the use of lanthanide chelates as fluorescent labels. We used PCR primers from the glycoprotein D genes of HSV type 1 (HSV-1) and HSV-2. The biotinylated PCR products were collected on streptavidin-coated microtitration wells and hybridized with short oligonucleotide probes, europium labeled for HSV-1 and samarium labeled for HSV-2. The TRF results were obtained as counts per second and as signal-to-noise (S/N) ratios. The sensitivity of the assay was 0.1 infectious units (PFU) of HSV in CSF specimens, and the S/N values increased with the virus amount, up to 68.5 for 103 PFU of HSV-1 and to 58.5 for 103 PFU of HSV-2, allowing semiquantitation of HSV in CSF. The primers and probes recognized all the studied 48 HSV wild-type samples, with S/N ratios of 12.4 to 190 (HSV-1) and 5.1 to 248 (HSV-2). We tested CSF specimens, 100 for each HSV type, which were HSV PCR negative by Southern blot and 22 CSF specimens which were HSV-1 or -2 PCR blot positive. In the TRF test, the mean S/N ratio for the HSV-1-negative CSF was 1.37 (standard deviation [SD] = 0.513) and for the HSV-2-negative CSF it was 1.03 (SD = 0.098). The HSV-1 blot-positive CSF yielded S/N ratios of 3.6 to 85.9, and the HSV-2 blot-positive CSF yielded ratios from 1.9 to 13. Using the mean S/N ratio for negative CSF specimens + 3 SD as the cutoff yielded all the previously HSV-positive specimens as TRF positive. The TRF PCR assay for HSV in CSF specimens is a rapid and sensitive method, improves interpretation of PCR results, and is well suited for automation.
PCR has been successfully used in laboratory diagnosis of the central nervous system infections caused by herpes simplex virus (HSV) (1, 12, 18, 19). The availability of effective agents against HSV infections emphasizes the need for sensitive and rapid diagnostic methods. As an alternative to the conventional laboratory techniques, PCR has been considered as the new standard for the diagnosis of the HSV infections of the central nervous system (13, 16, 24). Its applicability as a diagnostic method is not affected by antiviral therapy, since it is shown that the viral DNA remains well detectable by PCR also during the first days of acyclovir treatment (17).
The detection of PCR products has been based on Southern hybridization using radiolabeled (13) or nonradioactive probes (7). Several microwell assays have been applied to the detection of PCR products, many of these utilizing the biotin-streptavidin interaction for capture of the PCR product and digoxigenin (DIG)-based systems for detection (24, 25). Recently, fluorescent dye-based quantitative techniques have been introduced for the detection and typing of HSV in clinical samples (20).
Time-resolved fluorometry (TRF) technology provides highly sensitive, nonradioactive detection methods, utilizing the long-lived fluorescence of lanthanide ions, such as europium (Eu3+) and samarium (Sm3+) (10, 15). The detection of the lanthanide fluorescence after a time delay, leading to decay of background fluorescence, highly increases the sensitivity of the assays (22). TRF technology has been applied to direct detection of viral DNA (2) and to detection of PCR products in assays for human immunodeficiency virus type 1 (3), human T-cell lymphoma virus types 1 and 2 (11), adenoviruses (8), and picornaviruses (6, 14). The advantages of the TRF methods include high sensitivity, use of microwell formats, and potential for quantitation as well as for use of multiple labels in the same assay (21).
In the present study we describe a TRF technology-based PCR assay for detection of herpes simplex virus type 1 (HSV-1) and HSV-2 in cerebrospinal fluid (CSF) specimens. In this assay, the biotinylated PCR products are collected onto streptavidin-coated microtitration wells and the hybridization is carried out by use of probes labeled with europium (for HSV-1) and samarium (for HSV-2).
MATERIALS AND METHODS
Samples.
The CSF specimens were obtained from patients with suspected viral meningitis or encephalitis, mainly from hospitals in southwestern Finland. The samples were submitted to the Department of Virology, University of Turku, for PCR testing for HSV in the CSF. Samples with visible blood contamination were not included in the study. For each PCR test, 100 μl of CSF was boiled for 10 min, the DNA was recovered by ethanol precipitation (1), dissolved in 20 μl of PCR-grade water, and boiled again for 10 min immediately before testing. The samples were stored at −70°C in ethanol, when necessary.
The non-CSF clinical specimens, used for validation of the HSV primers and probes, consisted of cervix and vesicle swabs. Twenty-four of them were positive for HSV-1 and 24 were positive for HSV-2 in a rapid culture detection assay (26). All these specimens were derived from the Department of Virology, University of Turku, where they were originally tested and stored at −20°C. For PCR analysis, 10 μl of the clinical specimen, diluted with 90 μl of PCR-grade water (total volume, 100 μl) was boiled for 10 min, and then the DNA was precipitated by ethanol (1). The pellet was dissolved in 20 μl of PCR-grade water and boiled for 10 min before being added to the PCR mixture.
As positive controls, dilutions of HSV-1 virions, strain F (made from a high-titer stock [1.38 × 109 PFU/ml), and HSV-2 virions, strain G (106 PFU/ml), were used. The virus strains were from the American Type Culture Collection.
PCR primers and probes.
Primer sequences for HSV-1 and -2 were from the glycoprotein D (gD) gene (Table 1). The HSV-1 primer pair was the same as the outer primer pair described by Aurelius et al. (1). The HSV-2 primer pair was selected from the corresponding region of the gD-2 gene, so that the same oligonucleotide probe (HS1D-5 [reference 1]) could be used for Southern hybridization. The short probes for the TRF hybridization were selected from the gD region, with an aim to differentiate the HSV types. The short probes had equal melting temperatures. Biotinylated oligonucleotide targets, representing the probe-binding sequences of the PCR products, were used in adjustment of the hybridization conditions. The primers and probes as well as the target sequences are presented in Table 1.
TABLE 1.
PCR primers and probes used in this study
| Code | Sequencec | Location |
|---|---|---|
| HS1D-1 Primer | 5′-ATCACGGTAGCCCGGCCGTGTGACA | gD-1 23–47a |
| Bio-HS1D-4 Primer | 5′-Bio-CATACCGGAACGCACCACACAA | gD-1 243–222 |
| HS2D-1 Primer | 5′-GCGGACCCACCGCACCACCATACTC | gD-2 69–93 |
| Bio-HS2D-4 Primer | 5′-Bio-ACGGCGACTAGTGGTTCGCAATGCA | gD-2 241–217 |
| Eu-647 TRF probe | 5′-TTATCCTTAAGGT-Eu | gD-1 204–216 |
| Sm-644 TRF probe | 5′-GAGTATAATAGAG-Sm | gD-2 158–170 |
| HS1D-5 Southern hybridization probe | 5′-TACGAGGAGGAGGGGTATAACAAAGTCTGT | gD-1 100–129, gD-2 146–177b |
| Target for HSV-1 probe | 5′-Bio-AGAGACCTTAAGGATAACTG | gD-1 220–201 |
| Target for HSV-2 probe | 5′-Bio-AAGACTCTATTATACTCCTC | gD-2 174–155 |
Nucleotide numbers as in the GenBank files Hs1gd.Gb_vi and Hs2gd.Gb_vi, respectively.
Incompletely matching sequence.
Bio, biotinylation; Eu, europium label; Sm, samarium label.
Oligonucleotide synthesis.
Oligonucleotide probes, primers, and targets were synthesized using phosphoramidite chemistry in an ABI 392 DNA/RNA synthesizer. For labeling purposes, 20 diaminohexane-modified deoxycytidine phosphoramidites (23) were coupled to the 5′ end of probes at the end of synthesis and 1 was coupled to the 5′ end of primers as well as to the targets. Oligonucleotides were purified by polyacrylamide gel electrophoresis using urea as a denaturing agent.
Labeling of oligonucleotides.
For labeling, 50 μg of oligonucleotide was dried down and dissolved in 1 mM EDTA–50 mM Na2CO3 buffer, pH 9.8. Labeling was carried out in an overnight reaction at room temperature using a 50-fold molar excess of active biotin (Sigma, St. Louis, Mo.) and a 12-fold molar excess of active Eu or Sm chelate (10) with regard to the amino groups for biotinylation and lanthanide labeling, respectively. The biotinylated oligonucleotides were purified by high-performance liquid chromatography in a reverse-phase column (PepRPC HR 5/5; Pharmacia, Uppsala, Sweden). Purification of probes from the labeling mixture was done by ethanol precipitation followed by gel filtration in a Sephadex G-50 DNA grade column (Pharmacia).
PCR protocol.
The total PCR volume was 100 μl. The PCR mixture contained a 200 μM concentration (each) of the deoxyribonucleoside triphosphates (Pharmacia, Sweden), 20 pmol (each) of the appropriate primers, and 2 U of the DyNAzyme thermostable DNA polymerase (Finnzymes, Espoo, Finland). The buffer was the commercial DyNAzyme II buffer. Each sample was boiled in a volume of 20 μl before adding to the PCR.
The reaction mixture was covered with 100 μl of mineral oil. After an initial PCR cycle (95°C for 5 min, 55°C for 30 s, and 72°C for 60 s), the remaining 39 cycles were run similarly except that the 95°C incubation was reduced to 30 s and during the last cycle the 72°C step was extended to 5 min. The Perkin-Elmer DNA thermal cycler was used. The PCR products were subjected to TRF hybridization (see below), but a 25-μl fraction was electrophoresed in 2% agarose gels, and this was followed by Southern hybridization. Blotting was done on a GeneScreen Plus (NEN DuPont) nylon filter, and hybridization was performed using a DIG-labeled oligonucleotide probe (10 pmol/ml; HS1D-5) (Table 1) at temperature of 55°C. The hybridization buffer contained 25% deionized formamide, 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate, pH 7.0), 1% sodium dodecyl sulfate, 10% dextran sulfate, 5× Denhardt's solution, and 200 μg of single stranded DNA (DNA from herring testes; Sigma) per ml. After hybridization, the membranes were washed three times for 30 min at 52°C in 2× SSC–1% sodium dodecyl sulfate, subjected to DIG detection using CSPD reagent (Roche Molecular Biochemicals), and exposed to X-ray film at room temperature for 4 to 30 min.
Time-resolved hybridization assay.
TRF hybridization was carried out in solution using two short oligonucleotide probes, the HSV-1 probe (Eu-647) and the HSV-2 probe (Sm-644). For detection, 10 μl of each PCR product was added to streptavidin-coated wells (three parallel wells per specimen) together with 50 μl of DELFIA assay buffer (Wallac Oy, Turku, Finland). The amplified, biotinylated DNA was collected during 30 min of shaking on a Wallac DELFIA plate shaker. The wells were washed four times in an automated washer (Wallac plate washer) using 1× DELFIA wash concentrate. The bound DNA was denaturated by adding 150 μl of 50 mM NaOH per well and incubating on a shaker for 5 min at room temperature (25°C). Finally, wells were washed again four times, and 100 μl of hybridization solution was added per well. Hybridization was carried out for 2 h at room temperature in assay buffer adjusted to 1 M NaCl and 0.1% Tween 20, containing 3 ng of Eu-647 (HSV-1 probe) per well or 5 ng of Sm-644 (HSV-2 probe) per well. The wells were washed six times, and 150 μl of DELFIA enhancement solution was added to enhance the Eu and Sm fluorescence signal. Measurement of fluorescence signal was carried out in a time-resolved fluorometer (VICTOR; Wallac) after 25 min of shaking at 25°C. Hybridization signals were expressed as numerical fluorescence counts per second (cps) and as signal-to-noise (S/N) ratios, determined by comparison with specimens containing only distilled water.
RESULTS
The hybridization conditions were established using short oligonucleotide targets for the lanthanide-labeled probes (Table 1). Using 1010 to 1011 target molecules per well, the optimal probe amounts were found to be 3 ng of the HSV-1 probe (Eu) per well and 5 ng of the HSV-2 probe (Sm) per well. The hybridization temperature was set to a melting temperature of −9°C (+25°C), which was the same for both probes. The washing temperature was 25°C as well. Under these conditions the use of 1010 target molecules/well yielded an S/N ratio of 3.6 for the HSV-1 probe and 4.3 for the HSV-2 probe. Higher amounts, for example 1011 target molecules per well (HSV-2), yielded an S/N ratio of 56. Using these target amounts, the probes did not show cross-reactivity with the target representing the heterologous HSV type. The hybridization time was kept at 2 h throughout the experiments.
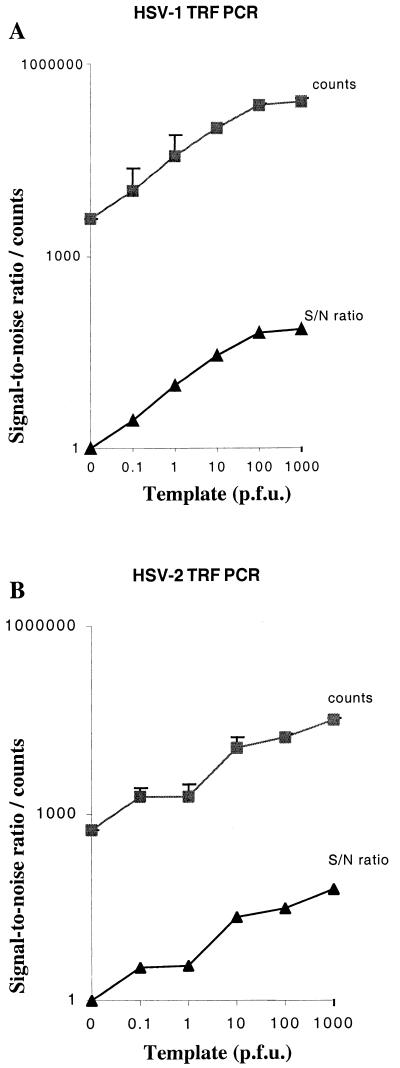
We assessed the sensitivity of our TRF PCR assay by testing various amounts of the standard HSV-1 and -2 virus strains, which were diluted in HSV-negative CSF. The purpose of testing the sensitivity in detection of infectious virus particles instead of dilutions of viral DNA was to obtain information on the performance of the whole PCR procedure, including the sample preparation. Dilutions of HSV-1 (F) and HSV-2 (G) virions, from 0.1 to 103 infectious units (PFU) per specimen, were tested by using TRF hybridization combined with PCR (Fig. 1). The S/N ratios for HSV-1 ranged from 2.8 (0.1 PFU per specimen) to 68.5 (103 PFU per specimen). For HSV-2 the S/N ratios were from 3.3 (0.1 PFU) to 58.5 (103 PFU) per specimen. The samarium counts were typically lower than those of europium, so that 10 PFU of HSV-1 template yielded 105 europium counts whereas 10 PFU of HSV-2 yielded 1.1 × 104 samarium counts. For both HSV types, the sensitivity of the assay was at the level of 0.1 PFU of virus per specimen.
FIG. 1.
Detection of HSV-1 (A) and HSV-2 (B) using PCR and TRF hybridization. Various amounts of the virus, shown as infectious units (PFU), were tested by PCR in duplicate, and the PCR products were assayed in triplicate by the TRF hybridization using the type-specific lanthanide-labeled probes. The results (+ 1 SD [error bars]) are expressed as cps and as S/N ratios, on a logarithmic scale.
The probes did not cross-react with products of PCR tests for human cytomegalovirus IE gene (4) or the DNA polymerase gene of varicella-zoster virus (5). There was also no cross-reaction with the PCR products from the other HSV type, with template amounts of 100 to 1,000 PFU of the heterologous HSV type per specimen.
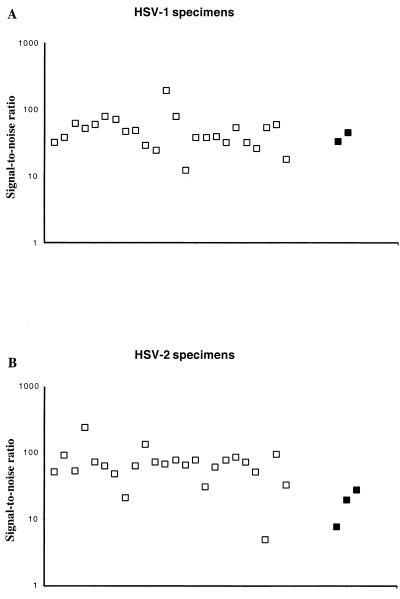
The primers and probes were tested for their ability to recognize the wild-type HSV strains existing in southwestern Finland. This was carried out by study of 24 individual HSV-1 and HSV-2 samples cultured from vesicle and cervix swabs (Fig. 2). For HSV-1 isolates the S/N ratios varied from 12.4 to 190, depending on the amount of virions in the sample. For HSV-2 the S/N ratios varied from 5.1 to 248. All of the isolates were thus recognized by the primers and the probes. Most of the clinical specimens yielded S/N values equal to or higher than the positive controls (1 and 10 PFU of HSV) (Fig. 2).
FIG. 2.
Reactivity of 24 HSV-1 (A) and 24 HSV-2 (B) culture-positive specimens, from clinical cervix and vesicle swabs, in the TRF HSV PCR assay. The S/N ratios are shown on a logarithmic scale. Open squares, clinical HSV specimens; black squares, positive HSV controls (1 and 10 PFU for HSV-1; 1, 10, and 100 PFU for HSV-2).
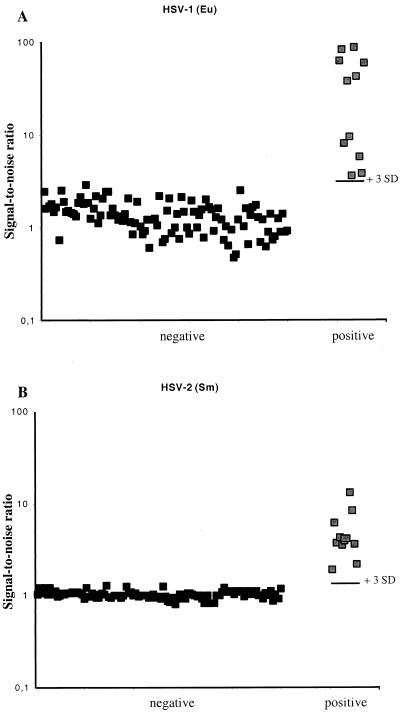
For estimation of the cut-off values for positive specimens, we tested CSF specimens which were previously negative by Southern blot analysis of the PCR products (n = 100 for each virus type) and 22 CSF specimens which had been considered HSV-1 or HSV-2 PCR positive by Southern hybridization (Fig. 3). The results were expressed as signal-to-noise ratios, in comparison with samples containg only sterile water instead of the CSF specimen. The mean S/N ratio for the HSV-1-negative specimens was 1.37 (standard deviation [SD] = 0.513), and for the HSV-2-negative CSF it was 1.03 (SD = 0.098). The previously blot-positive HSV-1 CSF specimens yielded S/N ratios ranging from 3.6 to 85.9, and those for the HSV-2-positive CSF ranged from 1.9 to 13. The mean + 3 SDs of the negative samples was 2.91 for HSV-1 and 1.32 for HSV-2. The use of the mean + 3 SDs as a cutoff divided the material in a way that all the previously blot-positive specimens remained positive.
FIG. 3.
TRF PCR of CSF specimens, negative (n = 100) or positive (n = 11 for each HSV type) for HSV in Southern blot analysis of the PCR products. The S/N ratios are shown on a logarithmic scale, as well as the +3 SD level of the 100 negative samples. (A) HSV-1; (B) HSV-2.
DISCUSSION
We have developed a rapid PCR assay for HSVs based on TRF technology. The method involves the use of biotinylated primers for capture of the PCR products on streptavidin-coated microtiter wells and solution hybridization using lanthanide-labeled oligonucleotide probes. The results are obtained as fluorescence counts, which can be converted to S/N ratios, improving the interpretation of the results.
We have applied the HSV-1 gD primers, which have been validated for HSV-1 PCR but were suboptimal for HSV-2 (1). A new HSV-2 primer set that bracketed the corresponding region of gD-2 was selected so that in Southern blot testing a common DIG-labeled oligonucleotide probe could be used (7). The short lanthanide-labeled probes were also designed for this study, and they were found to be type specific.
We analyzed the applicability of the new primer-probe sets by testing wild-type HSV samples, which were from genital or oral lesions, representing local HSV strains. All the culture-positive samples were strongly positive in our TRF PCR test (Fig. 2). The HSV strains F and G were used as the positive HSV-1 and -2 controls in the test, respectively.
We have expressed the virus amounts as infectious units (PFU) instead of numbers of DNA molecules, since measuring infectious virus is more like testing for HSV in clinical CSF specimens, unlike studying dilutions of purified DNA molecules. Studying the performance of the test in terms of viral infectious units also reveals the characteristics of the specimen handling. In this study the specimens and the positive controls were treated by the boiling-ethanol precipitation method (1), which has also been found favorable by Lakeman et al. (13). However, testing and applying new methods for specimen handling will be important in developing PCR assays for HSV in CSF (9, 16).
The sensitivity of our test in detection of HSV-1 and -2 in CSF is comparable to that of other PCR methods, both in hybridization detection of the target molecules (25) and in detection of the viral DNA in CSF (20). Moreover, our sensitivity testing was performed using CSF spiked with known amounts of infectious virus, thus covering all the steps of the assay.
One of the advantages of the TRF methodology over Southern blotting is that TRF yields numerical results, allowing definition of the cutoff values. It is favorable to establish a sensitive cutoff value in order not to miss borderline-positive cases of herpes simplex encephalitis (HSE), especially considering that the disease can be treated safely with antiviral chemotherapy. In tests for other virus groups, such as enteroviruses, where it is likely to encounter higher virus amounts in CSF during CNS infections, cutoff values such as five times the negative reference have been applied (14). In HSE in adults, the virus amounts in the CSF are regularly low, very rarely, if ever, allowing a positive virus culture. Thus, we wanted to establish as effective a cutoff limit as possible. This was carried out by analysis of clinical CSF specimens in parallel by TRF PCR and by Southern hybridization of the PCR products. The study of 100 Southern blot-negative CSF specimens for each HSV type allowed us to calculate the means and SDs of the negative samples, analyzed as S/N ratios (in comparison with negative reference samples containing water). During the study we obtained 22 HSV-positive CSF samples by Southern hybridization of the PCR products and calculated their S/N ratios by the TRF test. All the HSV-1 and HSV-2 blot-positive cases also remained positive in the TRF assay, when the cutoff limit was set at the mean + 3 SDs of the negative samples (Fig. 3). There were five samples, defined as HSV negative by Southern blotting, which were borderline positive or equivocal in the TRF assay upon the first testing. Two of these had one aberrantly high cps value among the triplicates, and when the CSF samples were retested, all the borderline cases turned out to be TRF PCR negative. We recommend retesting all borderline-positive CSF samples with results at or below the mean of the negative samples +10 SDs.
There is usually a tendency for higher fluorescence count values with europium than with samarium, both in the positive samples and in the negative references. This is normalized by the use of S/N ratios in the interpretation. The use of the mean of the negative material + 3 SDs as a cutoff limit enables recognition of the borderline HSV-positive CSF samples, which can then be rapidly reported to the clinic, with a request for a new CSF sample for further testing. Bringing the borderline-positive result to the clinician's attention increases opportunities for early chemotherapy of the HSE cases.
The advantages of the TRF techniques include the possibility of using multiple labels in the same assay (21). Work is in progress to establish the TRF PCR for HSV-1 and -2 as a one-tube reaction, followed by detection of HSV-1 using europium label and HSV-2 using samarium label in the same microwell hybridization reactions. The use of microwell-based hybridization and fluorometry also enables further automation of the test. We have developed a nonradioactive, fluorescence-based, sensitive PCR detection method for diagnosis of HSV infections of the CNS, and the method has proven to be well-suited for our regular neurovirological diagnostic service.
REFERENCES
- 1.Aurelius E, Johansson B, Sköldenberg B, Staland Å, Forsgrén M. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet. 1991;337:189–192. doi: 10.1016/0140-6736(91)92155-u. [DOI] [PubMed] [Google Scholar]
- 2.Dahlén P, Hurskainen P, Lövgren T, Hyypiä T. Time-resolved fluorometry for the identification of viral DNA in clinical specimens. J Clin Microbiol. 1988;26:2434–2436. doi: 10.1128/jcm.26.11.2434-2436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlén P, Iitiä A, Skagius G, Frostell Å, Nunn M, Kwiatkowski M. Detection of human immunodeficiency virus type 1 by using the polymerase chain reaction and a time-resolved fluorescence-based hybridization assay. J Clin Microbiol. 1991;29:798–804. doi: 10.1128/jcm.29.4.798-804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demmler G, Buffone G, Schimbor C, May R. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis. 1988;158:1177–1184. doi: 10.1093/infdis/158.6.1177. [DOI] [PubMed] [Google Scholar]
- 5.Echevarria J, Casas I, Tenorio A, de Ory F, Martinez-Martin P. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J Med Virol. 1994;43:331–335. doi: 10.1002/jmv.1890430403. [DOI] [PubMed] [Google Scholar]
- 6.Halonen P, Rocha E, Hierholzer J, Holloway B, Hyypiä T, Hurskainen P, Pallansch M. Detection of enteroviruses and rhinoviruses in clinical specimens by polymerase chain reaction and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–653. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heino P, Hukkanen V, Arstila P. Digoxigenin labeled probes and their use in the laboratory diagnosis of virus infections. In: Kurstak E, Marusyk R, Murphy F A, Van Regenmortel M H V, editors. Applied virology research. New diagnostic procedures. Vol. 3. New York, N.Y: Plenum Medical Book Co.; 1994. pp. 101–112. [Google Scholar]
- 8.Hierholzer J, Halonen P, Dahlén P, Bingham P, McDonough M. Detection of adenovirus in clinical specimens by polymerase chain reaction and liquid-phase hybridization quantitated by time-resolved fluorometry. J Clin Microbiol. 1993;31:1886–1891. doi: 10.1128/jcm.31.7.1886-1891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch H, Bossart W. Two-centre study comparing DNA preparation and PCR amplification protocols for herpes simplex virus detection in cerebrospinal fluids of patients with suspected herpes simplex encephalitis. J Med Virol. 1999;57:31–35. doi: 10.1002/(sici)1096-9071(199901)57:1<31::aid-jmv5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Hurskainen P, Dahlén P, Ylikoski J, Kwiatkowski M, Siitari H, Lövgren T. Preparation of europium-labelled DNA probes and their properties. Nucleic Acids Res. 1991;19:1057–1061. doi: 10.1093/nar/19.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iitiä A, Dahlén P, Nunn M, Mukkala V-M, Siitari H. Detection of amplified HTLV-I/-II viral sequences using time-resolved fluorometry. Anal Biochem. 1992;202:76–81. doi: 10.1016/0003-2697(92)90209-p. [DOI] [PubMed] [Google Scholar]
- 12.Klapper P, Cleator G, Dennett C, Lewis A. Diagnosis of herpes encephalitis via Southern blotting of cerebrospinal fluid DNA amplified by polymerase chain reaction. J Med Virol. 1990;32:261–264. doi: 10.1002/jmv.1890320413. [DOI] [PubMed] [Google Scholar]
- 13.Lakeman F, Whitley R the NIAID CA Study Group. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 14.Lönnrot M, Sjöroos M, Salminen K, Maaronen M, Hyypiä T, Hyöty H. Diagnosis of enterovirus and rhinovirus infections by RT-PCR and time-resolved fluorometry with lanthanide chelate labeled probes. J Med Virol. 1999;59:378–384. [PubMed] [Google Scholar]
- 15.Lövgren T, Hurskainen P, Dahlén P, Iitiä A. Detection of lanthanide chelates by time-resolved fluorescence. In: Kricka L, editor. Nonisotopic probing, blotting and sequencing. San Diego, Calif: Academic Press; 1995. pp. 331–376. [Google Scholar]
- 16.Mitchell P, Espy M, Smith T, Toal D, Rys P, Berbari E, Osmon D, Persing D. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J Clin Microbiol. 1997;35:2873–2877. doi: 10.1128/jcm.35.11.2873-2877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puchhammer-Stöckl E, Heinz F X, Kundi M, Popow-Kraupp T, Grimm G, Millner M, Kunz C. Evaluation of the polymerase chain reaction for diagnosis of herpes simplex virus encephalitis. J Clin Microbiol. 1993;31:146–148. doi: 10.1128/jcm.31.1.146-148.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puchhammer-Stöckl E, Popow-Kraupp T, Heinz F X, Mandl C, Kunz C. Establishment of PCR for the early diagnosis of herpes simplex encephalitis. J Med Virol. 1990;32:77–82. doi: 10.1002/jmv.1890320202. [DOI] [PubMed] [Google Scholar]
- 19.Rowley A, Whitley R, Lakeman F, Wolinsky S. Rapid detection of herpes simplex virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis. Lancet. 1990;335:440–441. doi: 10.1016/0140-6736(90)90667-t. [DOI] [PubMed] [Google Scholar]
- 20.Ryncarz A, Goddard J, Wald A, Huang M-L, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37:1941–1947. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjöroos M, Ilonen J, Reijonen H, Lövgren T. Time-resolved fluorometry based sandwich hybridisation assay for HLA-DQA1 typing. Dis Markers. 1998;14:9–19. doi: 10.1155/1998/350145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soini E, Lövgren T. Time-resolved fluorescence of lanthanide probes and applications in biotechnology. Crit Rev Anal Chem. 1987;18:105–154. [Google Scholar]
- 23.Sund C, Ylikoski J, Hurskainen P, Kwiatkowski M. Construction of europium (Eu 3+)-labelled oligo DNA hybridization probes. Nucleosides Nucleotides. 1988;7:655–659. [Google Scholar]
- 24.Tang Y-W, Mitchell P S, Espy M, Smith T, Persing D. Molecular diagnosis of herpes simplex virus infections in the central nervous system. J Clin Microbiol. 1999;37:2127–2136. doi: 10.1128/jcm.37.7.2127-2136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vesanen M, Piiparinen H, Kallio A, Vaheri A. Detection of herpes simplex virus DNA in cerebrospinal fluid samples using the polymerase chain reaction and microplate hybridization. J Virol Methods. 1996;59:1–11. doi: 10.1016/0166-0934(95)01991-x. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler T, Waris M, Rautiainen M, Arstila P. Herpes simplex virus detection by macroscopic reading after overnight incubation and immunoperoxidase staining. J Clin Microbiol. 1988;26:2013–2017. doi: 10.1128/jcm.26.10.2013-2017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]