Abstract
Sigma-2 receptor/transmembrane protein 97 (TMEM97) is upregulated in cancer cells compared to normal cells. Traditional sigma-2 receptor agonists induce apoptosis and autophagy, making them of interest in cancer therapy. Recently, we reported a novel metabolically stimulative function of the sigma-2 receptor, showing increased 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction and stimulation of glycolytic hallmarks. 6-Substituted analogs of the canonical sigma-2 receptor antagonist, 6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (SN79), produce both metabolically stimulative and cytotoxic effects. Here, we compare the activities of two related compounds: 6-amino-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (CM571), the 6-amino derivative of SN79, which binds with high affinity to both sigma-1 and sigma-2 receptors, and 1,3-bis(3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-2-oxo-2,3-dihydrobenzo[d]oxazol-6-yl)thiourea (MAM03055A), a homo-bivalent dimer of CM571. MAM03055A resulted from the degradation of 3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-6-isothiocyanatobenzo[d]oxazol-2(3H)-one (CM572), the cytotoxic 6-isothiocyanato SN79 derivative. MAM03055A exhibited high affinity and strong preference for sigma-2 receptors (sigma-1 Ki = 3,371 nM; sigma-2 receptor Ki = 55.9 nM). Functionally, MAM03055A treatment potently induced cell death in SK-N-SH neuroblastoma, MDA-MB-231 breast, and both SW48 and SW480 colorectal cancer cell lines, causing proapoptotic BH3 interacting-domain death agonist (BID) cleavage in SK-N-SH cells. Conversely, CM571 induced metabolic stimulation. CM571 bound reversibly to both receptors, while MAM03055A bound pseudo-irreversibly to sigma-2 receptors and caused residual cytotoxic activity after acute exposure and removal of the compound from the media. Interestingly, MAM03055A induced a time-dependent loss of sigma-2 receptor/TMEM97 protein from cells, whereas monomer CM571 had no effect on receptor levels. These results suggest that monovalent and bivalent sigma-2 receptor ligands in this series interact differently with the receptor, thus resulting in divergent effects.
Keywords: TMEM97, sigma-2 receptor, progesterone receptor membrane component 1, neuroblastoma, cancer metabolism, apoptosis
1. Introduction
Sigma receptors are a pharmacologically distinct class of receptors, initially incorrectly associated with opioid receptor subtypes (Martin, 1984; Martin et al., 1976; Su, 1982; Tam and Cook, 1984). Sigma-1 and sigma-2 were delineated as receptor subtypes (Hellewell and Bowen, 1990; Hellewell et al., 1994). Sigma-1 receptors are chaperones that regulate calcium signaling and are involved in neuroprotection (Hayashi and Su, 2007; Rousseaux and Greene, 2016). Sigma-2 receptors were identified as transmembrane protein 97 (TMEM97), which forms a complex with progesterone receptor membrane component 1 (PGRMC1) (Alon et al., 2017; Abate et al., 2015; Riad et al., 2018; Xu et al., 2011). Sigma-2 receptors have been implicated as therapeutic targets in Alzheimer’s disease, neuropathic pain, alcohol addiction, cocaine toxicity, and other CNS disorders (Grundman et al., 2019; Intagliata et al., 2019; Izzo et al., 2014; Limegrover et al., 2021; Quadir et al., 2021; Rousseaux and Greene, 2016; Sahn et al., 2017; Vázquez-Rosa et al., 2019). Sigma-2 receptors have also been of interest in cancer due to their upregulation in cancer cell lines (Hellewell and Bowen, 1990; Vilner et al., 1995b). Some sigma-2 receptor ligands cause cell death in cancer cells via apoptotic and autophagic pathways (Crawford and Bowen, 2002; Ostenfeld et al., 2005; Zeng et al., 2014, 2012). However, knocking out TMEM97 did not impact the cytotoxic effect of a limited number of sigma-2 receptor ligands studied, suggesting that TMEM97 may not be mediating this effect (Zeng et al., 2019). Therefore, the cellular role and establishment of sigma-2 receptors as a pharmacological target in cancer needs further clarification.
6-Acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (SN79) is a putative sigma antagonist with only slight selectivity for sigma-2 receptors over sigma-1 receptors (Kaushal et al., 2011). Recently, development of a novel series of SN79 analogs with single-element variations at the 6-position of the benzoxazolone, N-methylbenzimidazolone, and benzothiazolone heterocyclic systems has yielded several noteworthy results (Nicholson et al., 2019). 3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-6-isothiocyanatobenzo[d]oxazol-2(3H)-one (CM572), the 6-isothiocyanato derivative of SN79, was characterized as an irreversibly binding, cytotoxic partial agonist that is highly selective for the sigma-2 receptor and therefore a useful tool for studies (Nicholson et al., 2015). Conversely, 6-acetyl-3-(4-(4-(2-amino-4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (CM764), with an amino substituent on the fluoro-phenyl ring, was the first sigma-2 receptor ligand found to have effects on cellular metabolism, including increased 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) reduction without increased proliferation and increased hypoxia inducing factor 1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF) levels (Nicholson et al., 2016). This could help explain the upregulation of sigma-2 receptors in cancer, as a potential pro-survival mechanism. 6-Amino-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (CM571), the 6-amino analog of SN79, also stimulated MTT reduction. Thus, SN79 analogs exhibited divergent effects, depending on the substituent at the 6-position (Nicholson et al., 2019).
Here, we characterize the novel bivalent sigma-2 receptor/TMEM97 ligand MAM03055A, a thiourea linked dimer of CM571, in the first study of a sigma-2-selective bivalent ligand. We demonstrate that MAM03055A has markedly different pharmacological properties than its monomer, and is more similar to CM572. The data further support the divergent cytotoxic and metabolic effects of the sigma-2 receptor.
2. Materials and Methods
2.1. Sigma-1 and sigma-2 receptor binding assays
Radioligand competition assays were performed using rat liver membrane preparations, as previously described (Hellewell et al., 1994). Sigma-1 receptors were labelled with 3 nM [3H]-(+)-pentazocine (PerkinElmer, Waltham, MA). Sigma-2 receptors were labelled with 5 nM [3H] 1,3-di-o-tolylguanidine ([3H]DTG) (PerkinElmer, Waltham, MA) with 100 nM (+)-pentazocine to block sigma-1 receptors. Nonspecific binding was determined in the presence of 10 μM haloperidol. Incubations including 150 μg rat liver membrane protein, radioligand, and the specified concentrations of MAM03055A were carried out in glass tubes in a total volume of 500 μl 20 mM HEPES, pH 7.4 for 2 h at 25°C. After the 2-h incubation, assays were terminated by dilution with 5 ml ice cold 10 mM Tris-HCl pH 7.4 and filtration through glass fiber filters using a Brandel Cell Harvester (Brandel, Gaithersburg, MD). Filters were then washed with two additional 5 ml aliquots of the same buffer. The wet filters were incubated overnight in Ecoscint H scintillation fluid (National Diagnostics, Atlanta, GA) and read on a scintillation counter (Beckman Coulter, Brea, CA). Filters were soaked in 0.5% polyethyleneimine for 30 min at 25°C prior to use. Sigma-1 and sigma-2 receptor inhibition constant (Ki) values were determined using GraphPad Prism 8 (GraphPad Software, San Diego, CA).
Irreversible binding assays were performed using rat liver membranes as follows. Membrane homogenate at a concentration of 0.3 mg protein/ml were incubated with the indicated concentrations of MAM03055A or CM571 for 60 min in 20 mM HEPES, pH 7.4 at 25°C. The incubation mixture was then diluted to 0.018 mg protein/ml with 20 mM HEPES at 4°C and centrifuged for 10 min at 37,000 × g at 4°C to remove and/or dissociate any free compound. This process was repeated twice, after which the pellet was resuspended in 50 mM Tris, pH 8.0 to a concentration of 0.6 mg protein/ml. Radioligand binding was then performed as described above using 150 μg of protein in order to determine recovery of sigma-1 and sigma-2 receptor binding activity.
2.2. Cell culture
Human SK-N-SH neuroblastoma, SW48 and SW480 colorectal adenocarcinoma, and MDA-MB-231 breast adenocarcinoma cells (ATCC, Manassas, VA) were cultured in Minimum Essential Medium (Thermo Fisher Scientific, Waltham, MA) with 10% fetal bovine serum (Bio-Techne, Flowery Branch, GA), 10 mg/L human insulin, 1 mM sodium pyruvate, 1X penicillin-streptomycin (0.5 mg/ml), 1X Non-essential Amino Acids (Thermo Fisher Scientific, Waltham, MA). Cells were maintained at 70% or less confluency at 37°C, 5% CO2 in a humidified incubator. For dissociation of cells, a 1.5 mM ethylenediaminetetraacetic acid in 1X phosphate-buffered saline solution was used.
2.3. MTT cell viability and metabolic stimulation assay
Cytotoxicity and metabolic stimulation were measured using the MTT assay (Trevigen, Gaithersberg, MD). Cells were plated at 15,000 cells per well (SK-N-SH), 5,000 cells per well (SW48, SW480), or 10,000 cells per well (MDA-MB-231) in a 96-well plate and allowed to attach overnight. Cells were subsequently treated with sigma-2 receptor ligands diluted in Minimum Essential Medium, to a total volume per well of 100 μl. SK-N-SH neuroblastoma cells have typically shown greater sensitivity to the cytotoxic effects of sigma-2 receptor ligands compared to non-neuronal cell lines (unpublished observation), so routinely a 24 h treatment time is used for SK-N-SH cells, while 48 h is used for the other cell lines. After 24 h (SK-N-SH) or 48 h (SW48, SW480, MDA-MB-231) of drug treatment, 10 μl of MTT reagent was added to each well, and cells were incubated at 37°C for 3 h. Then, 100 μl of MTT detergent reagent was added to each well, incubated at 37°C for 2 h. Absorbance was read at 570 nm. A decrease in absorbance indicated cytotoxicity, and was calculated as follows (O.D., optical density):
An increase in absorbance indicated metabolic stimulation and was presented as:
EC50 values were calculated using GraphPad Prism 8 (GraphPad Software, San Diego, CA). Since we have previously used SK-N-SH neuroblastoma cells to characterize other sigma receptor ligands, including SN79 analogs (Nicholson et al., 2015, 2016, 2019), all experiments except where specifically noted were carried out in human SK-N-SH neuroblastoma cells.
2.4. Western blot analysis
SK-N-SH cells were plated at 1,000,000 cells per 60 mm dish and allowed to attach overnight. Cells were treated with 30 μM MAM03055A for 0.5 – 6 h and subsequently lysed with radioimmunoprecipitation assay buffer and protease/phosphatase inhibitor (Thermo Fisher Scientific, Waltham, MA). Lysates were run on 4–15% Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad Laboratories, Hercules, CA). The proteins were transferred onto a polyvinylidene fluoride membrane at 40 V for 2 h at 4°C, then blocked in 5% non-fat powdered milk in 0.1% Tween-20/Tris-buffered saline for 1 h and incubated overnight at 4°C in primary antibody. Primary antibodies used were mouse anti-BH3 interacting-domain death agonist (BID, 1:200 dilution) (Catalog #11423, Santa Cruz Biotechnology, Dallas, TX), rabbit anti-sigma-2 receptor/TMEM97 (1:200 dilution) (Catalog #NBP1–30436, Novus Biologicals, Littleton, CO), or mouse anti-beta-tubulin (1:1000 dilution) (Catalog #E7, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). Blots were incubated in secondary antibodies for 1 h at room temperature (Cell Signaling Technology, Danvers, MA) and developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA). Imaging was performed on a Bio-Rad ChemiDoc XRS+ system using Image Lab Software (Bio-Rad Laboratories, Hercules, CA). Quantification of the optical density of bands was done using ImageJ software (NIH). Optical densities of proteins of interest were normalized to beta-tubulin.
2.5. Liquid chromatographic/mass spectrometric (LC/MS) analysis of degradation mixture
Mass spectrometric characterizations of fresh vs. stored 10 mM stock solutions of CM572 were carried out in the Brown University Mass Spectrometry Facility on the Agilent 6530 Accurate Mass Q-TOF LC-MS system (Agilent Technologies, Santa Clara, CA). The Agilent 1260 Infinity series LC is comprised of a HiP-ALS autosampler, Bin Pump, and TCC column oven. HPLC-MS analysis was carried out with an Agilent column Zorbax C18, 2.1 × 50 mm, with mobile phases A = 5% acetonitrile/95% H2O/0.1% formic acid and B = acetonitrile/0.1% formic acid and the following time program of the gradient: 0 min 5% B, linear to 65% B at 8 min, to 95% B at 9 min, back to 5% B at 11 min and equilibrate for 10 min. The Q-TOF MS instrument was operated with an electrospray ion source in the positive ionization mode, mass range 100 – 3200 m/z, and 1 spectra/sec acquisition rate. Data analysis was performed using MassHunter Workstation Software Qualitative Analysis Version B.06.00 (Agilent Technologies, Santa Clara, CA).
2.6. Chemistry
2.6.1. Material, instrumentation, and chromatography methods
Reagents and starting materials were obtained from commercial suppliers (Fisher Scientific or Millipore Sigma) and were used without purification. Precoated silica gel 60 F254 aluminum backed plates from EMD or GF Uniplates from Analtech were used for thin-layer chromatography (TLC). Column chromatography was performed on silica gel using an automated Teledyne Combiflash Flash column instrument. The low-resolution mass spectra (MS) were recorded on a Waters Aquity Ultra Performance LC coupled with a PDA detector and a QDA detector in ESI mode. Column (BEH C18, 1.7 μm, 2.1 × 50 mm). Mobile phases: A (water with 0.1% formic acid), B (acetonitrile with 0.1% formic acid). Gradient (0 min 10% B until 0.5 min, linear to 100% B at 3.5 min and then isocratic until 4.0 min, then linear back to 10% B at 4.5 min and isocratic until 6 min. 1H and 13C nuclear magnetic resonance (NMR) spectra were collected on a Bruker Avance Neo 600 MHz radio-frequency console operating with a Bruker Ascend 600/54 magnet equipped with a Prodigy Broad-Band Observe Z-gradient CryoProbe. Chemical names were generated using ChemDraw (CambridgeSoft, version 19.0). The precursor CM571 was synthesized according to previously published procedure (Nicholson et al., 2019).
2.6.2. Synthesis of MAM03055A (1,3-bis(3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-2-oxo-2,3-dihydrobenzo[d]oxazol-6-yl)thiourea)
Thiophosgene (21 μl, 0.234 mmol) was added to a stirring solution of CM571 (180 mg, 0.468 mmol) and Et3N (296 μl, 2.106 mmol) in DCM (10 ml) previously cooled to 0 °C and the mixture was stirred for 1 h allowing the temperature to reach rt. The mixture was stirred at rt overnight then thiophosgene (10 μl, 0.117 mmol) was added and the mixture was stirred for an additional 3 days. Silica was introduced before removing the solvent in vacuo and the crude was adsorbed on silica and purified by flash column chromatography (from 0% to 10% MeOH in DCM) to give the desired product as an off white solid (100 mg, 53%): 1H NMR (500 MHz, DMSO) δ 9.75 (s, 2H), 7.53 (s, 2H), 7.28 (d, J = 8.4 Hz, 2H), 7.18 (d, J = 8.4 Hz, 2H), 7.02 (t, JHH+HF = 8.6 Hz, 4H), 6.92 (dd, JHH = 9.0 Hz, JHF = 4.7 Hz, 4H), 3.84 (t, J = 7.0 Hz, 4H), 3.11 – 2.96 (m, 8H), 2.48 – 2.42 (m, 9H), 2.35 (t, J = 7.1 Hz, 4H), 1.80 – 1.67 (m, 4H), 1.57 – 1.42 (m, 4H). 13C NMR (126 MHz, DMSO) δ 181.00, 156.52 (d, JCF = 236.4 Hz), 154.52, 148.52, 142.03, 134.63, 128.78, 121.12, 117.59 (d, JCF = 7.2 Hz), 115.79 (d, JCF = 21.8 Hz), 109.26, 107.93, 57.51, 53.22, 49.54, 42.19, 25.61, 23.76. LCMS (Rt = 3.05 min, m/z [M+H]+ 811.69, [M+2H]2+ 406.61).
3. Results
3.1. Identification and synthesis of MAM03055A
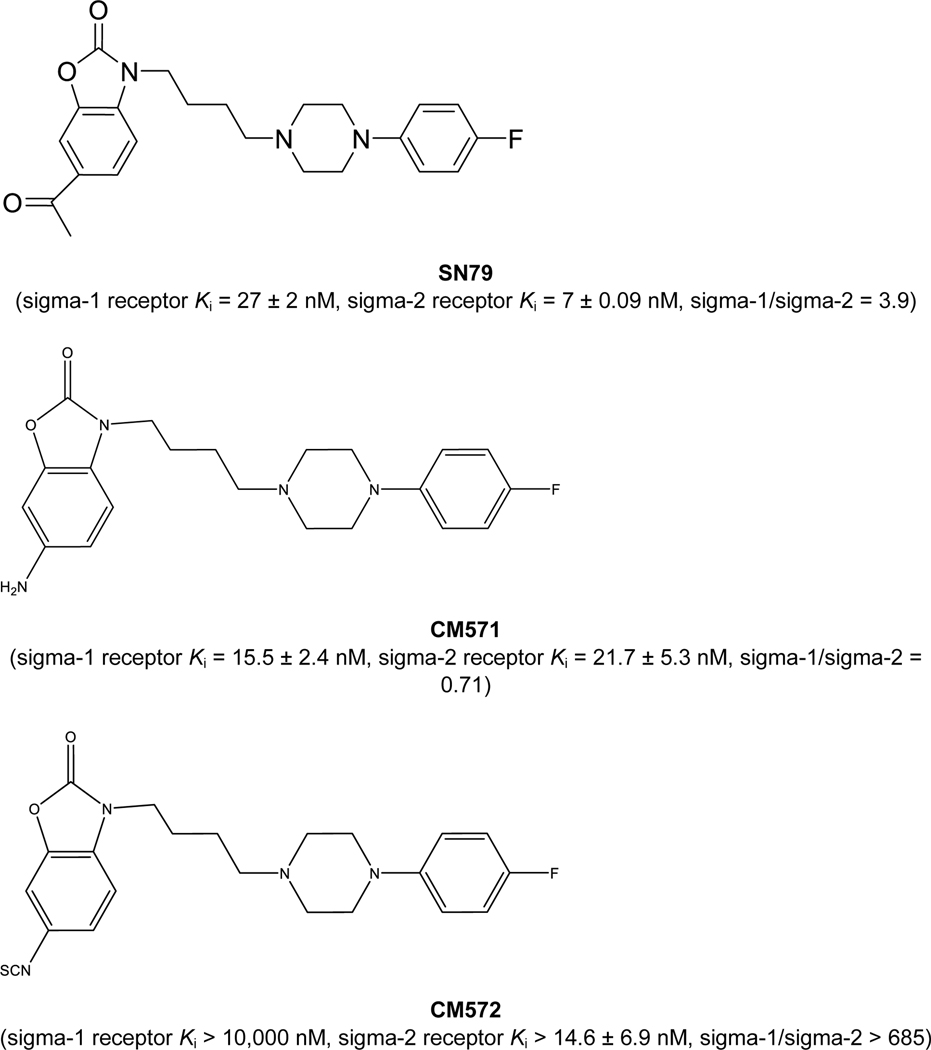
CM572 is the 6-isothiocyanato derivative of SN79 that was shown to be a potent, sigma-2 receptor irreversibly binding inducer of apoptotic cell death in human SK-N-SH neuroblastoma cells (Nicholson et al., 2015). SN79-derived reference compound structures, along with their binding affinities, are shown in Fig. 1. We subsequently observed that upon storage at −20°C as a 10 mM stock solution in DMSO, there was decomposition to an unknown mixture of compounds initially labelled as “CMHN”. Fig. 2A left panel shows the LC/MS trace of a fresh solution of CM572, stored at room temperature for 10 min. Fig. 2A right panel shows the LC/MS trace of a 10 mM stock solution of CM572 in DMSO, after storage for approximately six months at −20°C, labelled as CMHN. The LC/MS trace showed that CM572 (m/z 427.1605) had degraded into at least three unknown compounds with lower retention times and of m/z = 406.1892, 459.1856, and 473.2022, labeled a, b, and c, respectively, with a being the major degradation product.
Fig. 1. Chemical structures of SN79-derived compounds.
The chemical structures and sigma receptor Ki values are displayed for SN79 and its derivates, CM571 and CM572. Binding affinity values are from previously published articles (Kaushal et al., 2011; Nicholson et al., 2015, 2019).
Fig. 2. Comparison of freshly prepared and long-term stored CM572.
(A) LC/MS traces are shown for CM572 freshly solubilized at 10 mM in DMSO (left panel) compared to CM572 solubilized at 10 mM in DMSO and stored at −20°C for approximately 6 months (right panel). CM572 degraded into a mixture of compounds that we termed “CMHN” (peaks a – d, m/z values listed) shown in the right panel. The major degradation product was peak a, with lower retention time compared to CM572 (peak d). (B) SK-N-SH neuroblastoma cells were treated for 24 h with various concentrations of freshly prepared CM572 (left panel) or CMHN (right panel), and cell viability assessed using the MTT assay as described in Methods. Both preparations induced dose-dependent cell death. Data shown are from two independent experiments performed with five replicates and is expressed as percent % cytotoxicity relative to control; error bars show S.E.M. (C) The preparations were assessed for binding affinity at sigma-1 and sigma-2 receptors, using [3H]-(+)-pentazocine to label sigma-1 receptors and [3H]DTG in the presence of unlabeled (+)-pentazocine to label sigma-2 receptors, as described in Methods. These radioligand competition curves were used to calculate sigma-1 and sigma-2 receptor Ki values for CM572 and CMHN. Inhibition constant values are as follows: CM572 (sigma-1 receptor Ki ≥ 10,000 nM, sigma-2 receptor Ki = 14.6 ± 6.9 nM; data from Nicholson et al., 2015) and CMHN (sigma-1 receptor Ki ≥ 10,000 nM, sigma-2 receptor Ki = 60.15 ± 13.3 nM). Data shown are from two independent experiments performed in duplicate. Note: Because CMHN is a mixture of compounds, the concentrations on the X-axis and the CMHN EC50 and Ki values are approximations based on the molecular weight of native CM572 and are provided to demonstrate similar pharmacological trends to CM572.
Fig. 2B shows the effect of fresh vs. degraded CM572 (CMHN) on the viability of human SK-N-SH neuroblastoma cells, as measured by MTT assay. Both CM572 (left panel) and CMHN (right panel) induced cell death, with CMHN appearing to be somewhat more potent than CM572. Since CMHN retained cytotoxicity against SK-N-SH cells, we examined its sigma receptor binding profile in comparison to the previously published CM572. Data are shown in Fig. 2C. CMHN (right panel) exhibited sigma-1 receptor Ki ≥ 10,000 nM and sigma-2 receptor Ki = 60.2 nM. These binding parameters are comparable to those of CM572 shown in the left panel. The data show that CM572 nearly completely degrades over time to a mixture that has a pharmacological profile similar to native CM572.
To identify the degradation product, it was necessary to obtain further information on the degradation process and on the composition of the degradation mixture. Hence, an NMR degradation kinetic study was performed by analyzing a solution of CM572 in DMSO-d6 freshly prepared, after 6 days, and after 2 months of storage at room temperature to accelerate the degradation process. Fig. 3A shows the stacked 1H NMR spectra (from top to bottom: freshly prepared, after 6 days and after 2 months). In agreement with the previously obtained LC/MS data, 1H NMR spectra showed that CM572 quickly degraded into one single major compound after 6 days when stored at room temperature. The degradation product was stable and showed no further degradation after 2 months. Fig. 3B shows an expanded view of the region between 6 ppm and 8 ppm of the same NMR spectra. The signals corresponding to the fluoroaniline portion of CM572 did not show any significant change over time. In contrast, the signals corresponding to the three hydrogens of benzoxazolidinone ring maintained the same coupling pattern but showed drastic changes in chemical shifts. This strongly supports an initial hypothesis that the degradation occurred at the labile isothiocyante moiety. Analysis of the same degraded mixture by HRMS (Fig. 4) showed, not only the parent compound (CM572) but also, the presence of CM571, the synthetic precursor and 6-amino analog of CM572. In addition, two peaks of m/z 811.380 and 406.220 were identical to the single charge and double charge peak of a hypothetical dimeric structure.
Fig. 3. Controlled CM572 degradation study by 1H NMR.
(A) Stacked 1H NMR spectra of a freshly prepared solution of CM572 in DMSO-d6 (red) and of the same solution after 6 days (green) and after two months (blue). (B) Expansion of the range between 6.50 ppm and 8.00 ppm. Arrows indicate the conversion of the parent CM572 into the degradation product.
Fig. 4. High-resolution mass spectrum of the mixture from the controlled degradation study.
High relevance peaks are highlighted, and the corresponding suggested structures are shown.
Based on the results collected, the degradation sequence shown in Fig. 5 was proposed. It was hypothesized that the parent compound CM572 degraded by initial hydrolysis of the isothiocyanate group to the corresponding amino group (CM571). The amounts of CM571 formed competed with water in the nucleophilic attack on the isothiocyanate carbon to give the dimer MAM03055A.
Fig. 5. Proposed mechanism for the degradation of CM572.
CM572 undergoes an initial hydrolysis of the isothiocyanate moiety to the corresponding amino analog (CM571). The formed amine reacts with the remaining CM572 to give the proposed dimeric product of degradation.
The degradation hypothesis was corroborated by synthesizing compounds CM571 and CM572 according to the previously reported method (Nicholson et al., 2019), and stirring a solution of the two compounds in DMSO at room temperature. Initial comparison of the 1H NMR of the reaction mixture with the mixture originating from the degradation of CM572 showed great similarities (SI Fig. 1). Thus, the reaction conditions were adjusted for a larger scale reaction to allow the isolation of MAM03055A in amounts suitable for a full chemical and pharmacological characterization. The synthetic scheme is shown in Fig. 6. No further insight into the specifics of the degradation process were attempted because the only objective was to identify the decomposition product and reproduce such compound for more in-depth pharmacological characterization.
Fig. 6. Synthesis of MAM03055A.
The synthetic scheme for synthesis of MAM03055A from CM571 is shown. Conditions: a) thiophosgene (0.5 eq.), Et3N, DCM, 0 °C for 1 h, then room temperature for 18 h. Product is formed in a single step by the coupling of two CM571 molecules.
3.2. Sigma receptor binding affinities of MAM03055A
Since the degradation products of CM572 exhibited sigma-2 binding activity, radioligand competition assays were performed to determine the sigma-1 and sigma-2 receptor binding affinities for MAM03055A in rat liver membranes. This would help to support the degradation scheme. Sigma-2 receptors were labelled with [3H]DTG with 100 nM (+)-pentazocine to mask sigma-1 receptors, and sigma-1 receptors were labelled with [3H]-(+)-pentazocine, as described in section 2.1 of Materials and Methods. Ki values were calculated using the curves produced, as shown in Fig. 7A and 7B. The MAM03055A sigma-1 receptor Ki is 3,371 ± 245 nM and sigma-2 receptor Ki is 55.9 ± 4.2 nM, indicating a 60-fold sigma-2 receptor selectivity over sigma-1 receptor. This is comparable to the sigma receptor binding profile of CM572 (sigma-1R Ki ≥ 10,000 nM, sigma-2R Ki = 14.6 ± 6.9 nM; Nicholson et al., 2015). The data support the degradation leading to a sigma-2 receptor active species.
Fig. 7. Sigma-1 and sigma-2 receptor binding profiles of MAM03055A.
Competition radioligand binding assays were performed using [3H]-(+)-pentazocine to label sigma-1 receptors (left panel) and [3H]DTG in the presence of unlabeled (+)-pentazocine to label sigma-2 receptors (right panel). MAM03055A Ki values were calculated using the competition binding curves: sigma-1 receptor Ki = 3,371 ± 245 nM and sigma-2 receptor Ki = 55.9 ± 4.2 nM. As shown in the right panel, 10,000 nM MAM03055A was unable to completely displace [3H]DTG. Interestingly, a two-site fit of the [3H]DTG competition curves was not significantly better than the one-site fit. Data shown are from three independent experiments performed in triplicate; error bars represent S.E.M. The Ki values reported are an average of the individual Ki values from each independent experiment ± S.E.M., and were determined using GraphPad Prism.
As shown in Fig. 3 – 6, MAM03055A is a thiourea-linked dimer of the monovalent SN79 analog, CM571, which is also a compound along the degradation pathway. Interestingly, CM571 is non-selective, binding with high and equal affinity to both sigma-1 and sigma-2 receptors (sigma-1 receptor Ki = 15.5 ± 2.4 nM; sigma-2 receptor Ki = 21.7 ± 5.3 nM) (Nicholson et al., 2019). Many compounds have been shown to have good affinity for both receptor subtypes (for example, see Intagliata et al., 2020; Nicholson et al., 2019; Romeo et al., 2019; Zampieri et al., 2020). While developing sigma-2 receptor-selective ligands has been challenging, several such structurally diverse ligands have been recently reported (Intagliata et al., 2019; Mach et al., 2013; Sahn et al., 2017; Xie et al., 2021). MAM03055A, to our knowledge, is the first homo-bivalent sigma-2 receptor-selective ligand reported.
3.3. Effect of MAM03055A on cancer cell viability and comparison to CM571
Since the CM572 degradation mixture (CMHN) was found to have cytotoxic activity against human SK-N-SH neuroblastoma cells (Fig. 2B), we wanted to determine whether MAM03055A influenced cell viability by using the MTT assay, as described in section 2.3. Fig. 8 shows the cytotoxicity dose-response curves for MAM03055A in four human cancer cell lines, SK-N-SH neuroblastoma, colorectal adenocarcinoma SW48 with wild type KRAS and SW480 with mutant KRAS, and triple negative breast adenocarcinoma MDA-MB-231. MAM03055A induced dose-dependent cell death in all four cancer cell lines. In SK-N-SH neuroblastoma cells treated for 24 h with MAM03055A, the EC50 was determined to be 8.26 ± 0.30 μM. SW48 (KRAS wild type), SW480 (KRAS mutant), and MDA-MB-231 cells were all treated with MAM03055A for 48 h, and the EC50 values were 1.35 ± 0.32 μM, 2.02 ± 0.41 μM, and 3.26 ± 0.32 μM, respectively. Importantly, the more drug resistant SW480 cell line with the KRAS mutation had a comparable EC50 value to SW48, which is KRAS wild type.
Fig. 8. Effect of MAM03055A on cell viability.
Cells were treated for the indicated times with various concentrations of MAM03055A and cell viability measured using the MTT assay. MAM03055A caused a dose-dependent decrease in cell viability, plotted as % cytotoxicity. This effect occurs across multiple human cancer cell lines: SK-N-SH neuroblastoma, SW48 and SW480 colorectal adenocarcinoma, and MDA-MB-231 breast adenocarcinoma. MAM03055A EC50 values for these cell lines are 8.26 ± 0.30 μM (24 h), 1.35 ± 0.32 μM (48 h), 2.02 ± 0.41 μM (48 h), and 3.26 ± 0.32 μM (48 h), respectively, as calculated from the dose-response curves. Treatment times were chosen based on previous experience with these cell lines, and EC50 values are not meant to reflect relative potencies of MAM03055A across cell lines, except in the three cases where the treatment duration is the same. Data shown are from four (SK-N-SH and SW480) or six (SW48, MDA-MB-231) independent experiments performed with five replicates and is expressed as percent cytotoxicity relative to control, ± S.E.M.
We have previously shown that certain SN79 analogs induce a stimulation of metabolism instead of cell death in SK-N-SH cells (Nicholson et al., 2019, 2016). This is indicated by a stimulation of MTT reduction as opposed to the decrease induced by cytotoxic compounds. We refer to stimulative compounds as having increased MTT reduction and cytotoxic compounds as having decreased MTT reduction (see Section 2.3). Fig. 9 compares the effect of the bivalent MAM03055A to its monomer, CM571 on MTT reduction in SK-N-SH neuroblastoma cells, relative to controls. MAM03055A induced a dose-dependent decrease in MTT reduction, consistent with cell death (Fig. 9B). By contrast, CM571 induced a dose-dependent increase in MTT reduction (Fig. 9A). Maximal effect was observed at 30 μM, with an apparent decrease in the effect at higher concentrations. Thus, while structurally a dimer of the 6-amino compound CM571, MAM03055A behaved like the 6-isothiocyanato compound CM572, which exhibited a 24-h cell death EC50 of 7.6 ± 1.7 μM in SK-N-SH cells (Nicholson et al., 2015).
Fig. 9. Comparative effect of MAM03055A and CM571 on MTT reduction in SK-N-SH neuroblastoma.
SK-N-SH neuroblastoma cells were treated for 24 h with various concentrations of either MAM03055A or CM571 and changes in MTT reduction assessed as described in Methods. (A) Treatment of SK-N-SH cells with CM571 caused a dose-dependent increase in MTT reduction, with a bell-shaped curve. (B) Conversely, MAM03055A induced a dose-dependent decrease in MTT reduction in SK-N-SH cells, indicative of cell death. Note that data here are expressed simply as percent change in MTT reduction relative to control, without conversion to “% cytotoxicity” in order to directly reflect changes in metabolism. Data shown are the average from four independent experiments, each performed with five replicates; error bars show S.E.M.
An important component of the apoptotic mechanism of sigma-2 receptor ligands in SK-N-SH neuroblastoma cells is cleavage and activation of the pro-apoptotic Bcl family protein, BID. We previously showed that CM572 induced marked BID cleavage (Nicholson et al., 2015). Thus, we next explored whether MAM03055A activates the same cell death pathway in SK-N-SH cells as CM572. Fig. 10A and 10B demonstrate that treatment of SK-N-SH neuroblastoma cells with 30 μM MAM03055A resulted in a time-dependent decrease in full-length BID, which is significant as early as an 0.5-h treatment. This is similar to what occurred when SK-N-SH cells were treated with 30 μM CM572. However, significant BID cleavage was not observed until between 1 – 2 h of CM572 treatment in that case (Nicholson et al., 2015).
Fig. 10. Effect of MAM03055A on proapoptotic BID cleavage in SK-N-SH.
BID cleavage is a hallmark of sigma-2 receptor induced apoptosis in SK-N-SH neuroblastoma. (A) Cells were treated with 30 μM MAM03055A for 0.5, 1, 2, or 6 h and BID protein level determined by Western blot as described in Methods. Full-length BID protein levels decreased in a time-dependent manner showing cleavage. (B) Western blots were quantified using ImageJ software. Data shown are from 3 independent experiments; error bars show S.E.M.
3.4. Further characterization of MAM03055A binding characteristics
We previously carried out a study on a novel series of SN79 analogs with single-element variations at the 6-position of the benzoxazolone, N-methylbenzimidazolone, and benzothiazolone heterocyclic systems (Nicholson et al., 2019). The 6-substitutions included -isothiocyanato, -methylketo, -nitro, -amino, and -fluoro substituents. All these compounds bound with high affinity to sigma-2 receptors regardless of the heterocycle. In addition, the 6-isothiocyanato compounds are designed to bind irreversibly to the sigma-2 receptor via a putative nucleophilic attack on the isothiocyanate moiety by a receptor lysine or cysteine residue (Nicholson et al., 2019). Interestingly, within this series, only the irreversibly binding isothiocyanates were cytotoxic to SK-N-SH neuroblastoma cells, whereas the other compounds were either neutral or stimulated metabolism as indicated by a dose-dependent increase in MTT reduction (Nicholson et al., 2019).
Since MAM03055A induced cell death, we examined whether it was possible for MAM03055A to exhibit similar irreversible binding properties as the cytotoxic SN79 analogs. In order to examine irreversible binding, rat liver membranes were treated with 10, 100, or 1000 nM of MAM03055A for 1 h, the membranes extensively washed, followed by assay for recovery of sigma-1 and sigma-2 receptor binding activity as described in section 2.1 of Materials and Methods. The results in Fig. 11A show that 100% of sigma-1 receptor binding was recovered after treatment. However, there is a dose-dependent decrease in the recovery of sigma-2 receptor binding activity. This shows that MAM03055A is not removed from the sigma-2 receptor by the washing procedure, indicating either no dissociation or very slow dissociation from the receptor. By contrast, Fig. 11B shows that a saturating concentration of CM571 is completely removed from both sigma-1 and sigma-2 receptors by the wash procedure, consistent with reversible binding to both receptors. Since MAM03055A does not have a chemical moiety that could impart covalent binding, we will term its behavior at the sigma-2 receptor as “pseudo-irreversible” binding. This pseudo-irreversible characteristic is consistent with what has been observed with other members of the SN79-derived series, where only the irreversibly binding ligands were cytotoxic. Likewise, the reversible binding of CM571 is consistent with its metabolic stimulatory activity.
Fig. 11. Effect of ligand pretreatment on recovery of sigma receptor binding activity.
(A) Rat liver membranes were pretreated with 10, 100, or 1000 nM MAM03055A, followed by extensive washing as described in Methods to remove any unbound ligand. Treated membranes were then subjected to radioligand binding assays to determine recovery of sigma-1 and sigma-2 receptor binding activity. Radioligand binding analysis showed a complete recovery of [3H]-(+)-pentazocine binding and dose-dependent loss of [3H]DTG binding. (B) Membranes were pretreated with 1000 nM CM571 and washed extensively, then subjected to radioligand binding. There was full recovery of both [3H]-(+)-pentazocine binding and [3H]DTG binding. Data shown are from three independent experiments performed in duplicate; error bars show S.E.M.
The mechanism for the pseudo-irreversible binding of MAM03055A to sigma-2 receptors is not known. Unlike CM572, MAM03055A does not have a chemical moiety that can readily react with residues on the sigma-2 receptor protein. Also, MAM03055A was found to be chemically stable. However, we cannot rule out the possibility that under the conditions of the binding assay (20 mM HEPES, pH 7.4 at 25°C) there is decomposition to a reactive species such as isothiocyanate, although this is unlikely. Furthermore, we cannot rule out the possibility that in cells, cellular enzymes could catalyze conversion of the thiourea group to isothiocyanate or other active species, resulting in covalent binding to the receptor. But such enzymes are not expected to be present in the broken cell membrane preparation. The exact mechanism for pseudo-irreversible binding of MAM03055A to sigma-2 receptors will need further study.
3.5. Irreversible effect of MAM03055A on cell viability
We previously showed that CM572, by virtue of its irreversible sigma-2 receptor binding activity, produces persistent cytotoxicity after only a brief exposure of cells to the compound and subsequent removal from the system, which is a unique characteristic of CM572 not found in other typical sigma receptor ligands (Nicholson et al., 2015; Vilner et al., 1995a). Due to its similarity to CM572 in sigma-2 receptor binding affinity, sigma-2 selectivity, irreversible binding, and cytotoxicity, we next assessed whether MAM03055A is capable of causing cytotoxicity in an irreversible manner. To examine this, SK-N-SH cells were treated with various concentrations of MAM03055A for either a chronic 24-h exposure or an acute 60-min treatment, followed by removal by washing and replacement with fresh drug-free media for 24 h. Results are shown in Fig. 12A. At 10, 30, or 100 μM MAM03055A, compared to the continuous 24 h treatment, a significant fraction of the cytotoxic effect persisted 24 h after the 60-min acute treatment followed by washout, as measured by the MTT assay. For both 30 and 100 μM MAM03055A doses, there was no significant difference in cytotoxicity between the acute and chronic treatment conditions. By contrast, when the experiment was repeated with a single, maximally toxic dose of 1’-(4-(1-(4-fluorophenyl)-1H-indol-3-yl)butyl)-3H-spiro(2-benzofuran-1,4’-piperidine) (siramesine) (Fig. 12B), continuous treatment was required in order to produce a cytotoxic effect. While the continuous 24 h treatment with siramesine resulted in maximal cell death, only low levels of siramesine-induced cytotoxicity were observed 24 h later with the 60-min acute treatment followed by washout. This is similar to previous findings that cells require continuous treatment with sigma ligands to cause cell death and can recover with acute treatments, even in the presence of morphological changes such as cells rounding up (Vilner et al., 1995a). The results with siramesine and other sigma-2 ligands show that short exposures do not commit cells to apoptotic programs that cannot be reversed by removal of drug. Altogether, these results demonstrate that MAM03055A is unique in its ability to cause cell death with short, acute treatments and behaves similarly to CM572 in this regard. It is possible that MAM03055A enters cells and becomes pseudo-irreversibly bound to the sigma-2 receptor, and thus continues to activate cell death mechanisms after free ligand is removed from the system.
Fig. 12. Effect of acute vs. chronic treatment with sigma-2 ligands on cell viability.
The viability of SK-N-SH neuroblastoma cells was assessed at 24 h after either a 24 h continuous treatment (chronic) or an acute 60 min treatment, followed by wash out and placement in drug-free medium. Cell viability was measured by MTT assay. (A) Cells were treated with 10, 30, or 100 μM MAM03055A for 60 min (acute) or 24 h (chronic). MTT assays showed decreased cytotoxicity with acute exposure compared to chronic exposure at 10 μM. However, this difference was not significant at 30 or 100 μM, indicating residual cytotoxicity after the acute treatment. (B) SK-N-SH cells were incubated with siramesine (100 μM) acutely for 60 min or continuously for 24 h. A single high dose of siramesine was chosen that was previously known to ensure 100% cell kill. This is what was observed in the chronic treatment. However, there was relatively little residual cytotoxicity observed with the acute treatment. This demonstrates the need for continuous exposure to siramesine in order to induce cell death. It also suggests that the residual effect of MAM03055A is not due to an irreversible commitment to apoptosis during the 60 min exposure to drug. Data shown are from three independent experiments performed with five replicates; error bars show S.E.M. Two-tailed Student’s t-tests were performed (n.s.: not significant, **P < 0.01, **** P < 0.0001).
3.6. Effect of MAM03055A on sigma-2 receptor/TMEM97 protein level
Sigma-2 receptor ligands that induce cell death have traditionally been characterized as sigma-2 receptor agonists (Crawford et al., 2002; Nicholson et al., 2015; Zeng et al., 2014, 2012). Irreversible binding of an agonist to a receptor might be expected to induce some type of desensitization due to the continuous activation of the receptor. With some receptor systems, this may involve removal of the receptor from the surface and degradation of the receptor in the cell. While no such desensitization has been reported for the sigma-2 receptor, and it is largely an intracellular receptor, we nonetheless decided to investigate the effect of MAM03055A on sigma-2 receptor/TMEM97 protein levels over time. SK-N-SH neuroblastoma cells were treated for various times up to 6 h with 30 μM MAM03055A and cell extracts subjected to Western blot analysis for sigma-2 receptor/TMEM97. Fig. 13A and 13B show that 30 μM MAM03055A causes a time-dependent loss of TMEM97 that begins at 0.5 h of treatment and proceeds up to 6 h. Fig. 13C and 13D show the effect of the reversibly binding and metabolically stimulative, monomeric ligand CM571. Treating SK-N-SH cells with 30 μM CM571 had no effect on TMEM97 levels over the same time period. It is not known whether the MAM03055A-induced loss of TMEM97 is related to some type of desensitization phenomenon or to the cell death mechanism. However, the results collectively show that the monomeric and dimeric ligands have distinct binding modes to the sigma-2 receptor and induce vastly different cellular responses.
Fig. 13. Comparative effect of MAM03055A and CM571 on sigma-2 receptor/TMEM97 protein levels in SK-N-SH neuroblastoma cells.
(A) SK-N-SH cells were treated with 30 μM MAM03055A for 0.5, 1, 2, or 6 h, and TMEM97 levels determined by Western blot as described in Methods. TMEM97 protein levels decreased in a time-dependent manner, beginning at the 0.5-h time point (B) Western blots from the MAM03055A experiments were quantified using ImageJ software. (C) SK-N-SH cells were treated with 30 μM CM571 for 0.5, 1, 2, or 6 h. TMEM97 protein levels did not change significantly with CM571 treatment. (D) Western blots from the CM571 experiments were quantified using ImageJ software. Data shown are from three independent experiments; error bars show S.E.M.
An alternative explanation for these results is that if MAM03055A were covalently bound to the denatured sigma-2/TMEM97 receptor protein, it could potentially interfere with binding of the antibody during the Western blot. According to the manufacturer of the TMEM97 antibody (see Materials and Methods), there are two lysine residues and several serine and threonine residues in the TMEM97 peptide used for the antigen epitope (residues 105–155). Thus, there are indeed possible sites for covalent attachment of MAM03055A in the antigen epitope where, if MAM03055A were bound covalently, it could interfere with antibody binding. However, this is unlikely to be the case. MAM03055A binds rapidly to the receptor, and the pseudo-irreversible effect appears to be complete sometime within the 60 min of receptor interaction (Fig. 11). By contrast, the decrease in TMEM97 levels as shown in Fig. 13 is time-dependent, with levels steadily decreasing over a period of 6 h. If this were an effect of irreversibly bound MAM03055A blocking antibody binding, one would expect the effect to have no incremental time dependence as revealed by Western blotting, as maximal antibody blockade would be evident from the very first time point.
4. Discussion
Sigma-2 receptor ligands that induce cell death have traditionally been characterized as agonists. Historically, there have been few sigma-2 receptor selective antagonists. In an attempt to design sigma-2-selective antagonists to better characterize sigma-2 receptor pharmacology, we investigated analogs of the canonical sigma-1/2 antagonist, SN79, by examining various heterocycles and incorporation of substituents at the 6-position (Nicholson et al., 2015, 2019, 2016). Incorporation of the isothiocyanato group at the 6-position resulted in CM572. CM572 was found to be highly selective for sigma-2 receptors and to bind irreversibly to the receptor. Surprisingly, it showed partial agonist-like activity, blocking the receptor at low concentrations but inducing programmed cell death in the higher dose range (Nicholson et al., 2015). We show here that CM572 degraded upon storage to a product that has similar pharmacological and biological properties as the freshly prepared parent compound, with respect to sigma receptor binding affinity and ability to induce cell death (Fig. 2). The degradation process was investigated using LC/MS and NMR analyses to identify the active degradation product (Fig. 3–6). The hypothesis of an initial hydrolysis followed by coupling with yet undegraded CM572 was confirmed, and the degradation product, MAM03055A, was synthesized in order to perform chemical and pharmacological characterization. Due to the symmetry along the thiourea moiety, 1H NMR showed only a variation in the chemical shift but not in coupling pattern. However, 13C NMR proved the presence of an extra sp2 hybridized carbon corresponding to the thiourea group. In addition, through the modification of the reactive isothiocyante into the stable thiourea moiety, MAM03055A proved stable in solution at room temperature for at least two months. Concurrently, the chemical stability supposedly removed the alkylating properties present in the parent CM572 compound.
Since MAM03055A is a thiourea-linked dimer of the 6-amino SN79 derivative CM571, we compared the pharmacological profile of MAM03055A to that of CM571, in addition to that of the parent compound, CM572. MAM03055A was found to have good affinity for sigma-2 receptors, with 60-fold selectivity for the sigma-2 receptor over sigma-1 (sigma-1 receptor Ki = 3,371 nM, sigma-2 receptor Ki = 55.9 nM) (Fig. 7). This profile resembles that of parent CM572, which had over 600-fold selectivity for sigma-2 receptors over sigma-1 receptors (Nicholson et al., 2015). This contrasts with CM571 (sigma-1 receptor Ki = 15.5 nM, sigma-2 receptor Ki = 21.7 nM), which showed a lack of selectivity (Nicholson et al., 2019). Thus, dimerization of CM571 converted the non-selective ligand to a more selective sigma-2 receptor ligand. The high sigma-2 affinity of MAM03055A is an unexpected finding due to the size of the molecule. Interestingly, MAM03055A also shares some structural similarities with DTG, a symmetric diarylguanidine, the standard nonselective sigma-1 receptor and sigma-2 receptor ligand (Fig. 14).
Fig. 14. Structural similarities of MAM03055A and DTG.
The core of MAM03055A shows great structural similarities with DTG, a non-selective sigma-1R sigma-2R ligand. Despite the greater complexity of MAM03055A, both compounds are constituted by two phenyl rings joined by a urea-like linker (guanidine for DTG and thiourea for MAM03055A).
Like CM572, MAM03055A induced dose-dependent programmed cell death in several tumor cell lines (Fig. 8). MAM03055A potently induced cell death in human SK-N-SH neuroblastoma cells. BID cleavage is a hallmark of sigma-2 ligand-induced cell death in SK-N-SH neuroblastoma cells. MAM03055A induced rapid BID cleavage, as shown in Fig. 10, which has been observed with other sigma-2 agonists, including CM572 (Nicholson et al., 2015; Wang and Bowen, 2006). MAM03055A also induced cell death in colorectal cell lines. KRAS is an oncogene that is mutated in 90–95% of colorectal cancers, which makes it extremely difficult to treat, due to its resistance to cetuximab and gemcitabine, standard treatments for colon cancer (Menendez et al., 2016). MAM03055A exhibited equal potency and efficacy against SW480 (mutant KRAS) and SW48 (wild-type KRAS). Triple negative breast cancer is particularly aggressive and not amenable to targeted treatment due to the lack of estrogen receptors, progesterone receptors, and HER2 upregulation (Foulkes et al., 2010; Gluz et al., 2009; Lehmann et al., 2011). MAM03055A potently induced cell death in MDA-MB-231 breast cancer cells. The ability of MAM03055A to kill aggressive and drug-resistant cancer cells suggests potential as a therapeutic agent.
In contrast to MAM03055A, CM571 induced a stimulation of glycolytic activity, as indicated by the increase in MTT reduction. This was a dose-dependent effect with an apparent bell-shaped characteristic (Fig. 9). While not necessarily the case, it might be expected that MAM03055A and CM571 could behave similarly in their effects on cells, since CM571 is a monomeric unit of MAM03055A. However, this is not the case, suggesting vastly different modes of interaction with the receptor for the monomer and the dimer.
Our previous study had shown that in the series of SN79 analogs, only those with isothiocyanate moieties that bound irreversibly to the sigma-2 receptor were cytotoxic (Nicholson et al., 2019). MAM03055A does not possess a chemically reactive group that would impart covalent binding ability. However, the cytotoxic nature led us to investigate whether MAM03055A also exhibited irreversible binding activity. We found that MAM03055A fails to wash out of the sigma-2 receptor upon pre-exposure of membranes to compound. By contrast, CM571 washes completely out of both sigma-1 and sigma-2 receptors. The mechanism for the pseudo-irreversible binding of MAM03055A is not known and needs further investigation. Unlike the isothiocyanato moiety, the thiourea moiety is not expected to be susceptible to nucleophilic attack. We cannot eliminate the possibility that under the conditions of the irreversible binding assay or in cells that a reactive species is formed from the thiourea moiety that can covalently react with the receptor. It is also possible that the pseudo-irreversibility is due to the bivalent nature and/or size of the molecule. For example, if the sigma-2 receptor exists as a dimer or oligomer, a bivalent ligand could conceivably dissociate more slowly than a monomeric ligand.
Interestingly, the dose-dependent effect of MAM03055A on sigma-2 receptor recovery leveled off at about 20% of [3H]DTG binding remaining (Fig. 10). This same phenomenon was observed in membranes treated with CM572 and other irreversibly binding isothiocyanate SN79 analogs (Nicholson et al., 2015, 2019). This remaining [3H]DTG binding had a profile overlapping with, but distinct from either sigma receptor subtype (unpublished observation). Finally, low affinity, [3H]DTG binding remained after knock-out of TMEM97 and PGRMC1 (Zeng et al., 2019). The nature of this residual binding activity will require further study.
The pseudo-irreversible sigma-2 receptor binding activity was manifested in persistent cytotoxicity long after exposure of cells to MAM03055A and removal from the system (Fig. 12). When SK-N-SH neuroblastoma cells were treated acutely for 60 min compared to a continuous 24 h-treatment, significant residual cytotoxicity was evident in the acutely treated cells compared to the continuously treated samples. By contrast, when cells were treated acutely with siramesine and then washed, cells were completely viable 24 h later, which is typical of how sigma ligands besides CM572 behave (Vilner et al., 1995a). This is consistent with MAM03055A entering cells, binding to the sigma-2 receptor pseudo-irreversibly and by doing so, continuing to act on the receptor after free, unbound ligand is removed. A similar phenomenon was observed with the covalently binding CM572 (Nicholson et al., 2015). These results have interesting implications for therapeutic dosing. Though there is potentially more risk of toxicity, the drug would not have to be continually present in the system in order to be effective. Also, it is conceivable that drug efflux mechanisms would have less effect on drug action due to irreversible association with the sigma-2 receptors in the cell.
It is unclear why irreversible or pseudo-irreversible binding to the sigma-2 receptor by ligands within this class results in induction of cell death, while reversible binders cause metabolic stimulation. A clue may lie in the differential effect of these ligands on levels of TMEM97 protein. Fig. 13 shows that a toxic dose of MAM03055A produces a time dependent decrease in TMEM97 levels. On the other hand, CM571, a stimulative ligand, has no effect on the level of TMEM97. The effect of MAM03055A is evident within 30 min. Thus, this is unlikely to be due to an effect on gene expression but could represent internalization or increased turnover of the receptor protein. Riad et al. have shown that TMEM97 forms a complex with the low-density lipoprotein receptor and PGRMC1 that gets internalized to bring cholesterol into the cell (Riad et al., 2018). How this might relate to the current observation of MAM03055A-induced TMEM97 loss deserves more study with structurally diverse stimulative and toxic ligands
In summary, MAM03055A is a selective sigma-2 receptor ligand that is a degradation product of the 6-isothiocyanato SN79 analog, CM572. It represents a novel class of homo-bivalent sigma-2 receptor ligands. MAM03055A is a thiourea-linked dimer of CM571, a product along the degradation pathway. Compared to its monomeric unit, the bivalent nature of this ligand imparts novel characteristics. These include ability to bind pseudo-irreversibly to the sigma-2 receptor, ability to induce programmed cell death, and time-dependent decrease in the cellular level of TMEM97. Taken together, the data suggest that bivalent sigma-2 receptor ligands may have markedly different modes of interaction with the receptor compared to their monovalent counterparts and will be interesting tools for study of sigma-2 receptor structure and function, as well as potential therapeutic agents.
Supplementary Material
The 1H NMR of the reaction mixture between CM571 and CM572 in DMSO, despite the more convoluted appearance, shows strong similarities with the 1H NMR spectrum of the degraded CM572.
Chemical compounds (in order of appearance):
SN79: 6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one
CM571: 6-amino-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one
MAM03055A: 1,3-bis(3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-2-oxo-2,3-dihydrobenzo[d]oxazol-6-yl)thiourea
CM572: 3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-6-isothiocyanatobenzo[d]oxazol-2(3H)-one
Siramesine: 1’-(4-(1-(4-fluorophenyl)-1H-indol-3-yl)butyl)-3H-spiro(2-benzofuran-1,4’-piperidine)
CM764: 6-acetyl-3-(4-(4-(2-amino-4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one
DTG: 1,3-di-o-tolylguanidine
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Acknowledgments
Funding
This work was supported by NIH NIGMS 5T32GM077995 (CZL); Brown University Pharmacia Pre-doctoral Fellowship in Pharmacology (CZL); University of Florida College of Pharmacy start-up funds and the State of Florida (MM and CRM); and the Upjohn Professorship in Pharmacology, Brown University (WDB)
Footnotes
CRediT authorship contribution statement
Cheri Liu: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing –Original Draft, Writing – Review & Editing, Visualization. Marco Mottinelli: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – Original Draft, Writing – Review & Editing, Visualization. Hilary E. Nicholson: Conceptualization, Validation, Investigation, Visualization. Bridget M. McVeigh: Validation, Investigation, Visualization. Neelum K. Wong: Validation, Investigation. Christopher R. McCurdy: Conceptualization, Resources, Writing – Review & Editing, Supervision, Funding acquisition. Wayne D. Bowen: Conceptualization, Writing – Review & Editing, Supervision, Funding acquisition.
Declaration of competing interest
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- Bowen WD, Hellewell SB, McGarry KA, 1989. Evidence for a multi-site model of the rat brain sigma receptor. Eur. J. Pharmacol 163, 309–318. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Bowen WD, 2002. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 62, 313–22. [PubMed] [Google Scholar]
- Crawford KW, Coop A, Bowen WD, 2002. σ2 Receptors regulate changes in sphingolipid levels in breast tumor cells. Eur. J. Pharmacol 443, 207–209. 10.1016/S0014-2999(02)01581-9 [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS, 2010. Triple-Negative Breast Cancer. N. Engl. J. Med 363, 1938–1948. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N, 2009. Triple-negative breast cancer--current status and future directions. Ann. Oncol 20, 1913–27. 10.1093/annonc/mdp492 [DOI] [PubMed] [Google Scholar]
- Grundman M, Morgan R, Lickliter JD, Schneider LS, DeKosky S, Izzo NJ, Guttendorf R, Higgin M, Pribyl J, Mozzoni K, Safferstein H, Catalano SM, 2019. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv 5, 20–26. 10.1016/j.trci.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su T-P, 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131, 596–610. 10.1016/j.cell.2007.08.036 [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bowen WD, 1990. A sigma-like binding site in rat pheochromocytoma (PC12) cells: Decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 527, 244–253. 10.1016/0006-8993(90)91143-5 [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD, 1994. Rat liver and kidney contain high densities of σ1 and σ2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol 268, 9–18. 10.1016/0922-4106(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Intagliata S, Alsharif WF, Mesangeau C, Fazio N, Seminerio M, Xu YT, Matsumoto RR, McCurdy CR, 2019. Benzimidazolone-based selective σ2 receptor ligands: Synthesis and pharmacological evaluation. Eur. J. Med. Chem 165, 250–257. 10.1016/j.ejmech.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intagliata S, Sharma A, King TI, Mesangeau C, Seminerio M, Chin FT, Wilson LL, Matsumoto RR, McLaughlin JP, Avery BA, McCurdy CR, 2020. Discovery of a Highly Selective Sigma-2 Receptor Ligand, 1-(4-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-methyl-1H-benzo[d]imidazol-2(3H)-one (CM398), with Drug-Like Properties and Antinociceptive Effects In Vivo. AAPS J. 22, 1–11. 10.1208/s12248-020-00472-x [DOI] [PubMed] [Google Scholar]
- Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, Rehak C, Yurko R, Look G, Rishton G, Safferstein H, Cruchaga C, Goate A, Cahill MA, Arancio O, Mach RH, Craven R, Head E, LeVine H, Spires-Jones TL, Catalano SM, 2014. Alzheimer’s therapeutics targeting amyloid beta 1–42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One 9, 27–29. 10.1371/journal.pone.0111899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Robson MJ, Vinnakota H, Narayanan S, Avery BA, McCurdy CR, Matsumoto RR, 2011. Synthesis and pharmacological evaluation of 6-acetyl-3-(4-(4-(4fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (SN79), a cocaine antagonist, in rodents. AAPS J. 13, 336–346. 10.1208/s12248-011-9274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA, 2011. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest 121, 2750–2767. 10.1172/JCI45014DS1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limegrover CS, Yurko R, Izzo NJ, LaBarbera KM, Rehak C, Look G, Rishton G, Safferstein H, Catalano SM, 2021. Sigma-2 receptor antagonists rescue neuronal dysfunction induced by Parkinson’s patient brain-derived α-synuclein. J. Neurosci. Res 99, 1161–1176. 10.1002/jnr.24782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Zeng C, Hawkins WG, 2013. The σ 2 Receptor: A Novel Protein for the Imaging and Treatment of Cancer. J. Med. Chem 56, 7137–7160. 10.1021/jm301545c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, 1984. Pharmacology of Opioids. Pharmacol. Rev 35, 283–323. [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE, 1976. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther 197, 517–532. [PubMed] [Google Scholar]
- Menendez AG, Wanebo HJ, Curzake DJ, Luo L, 2016. C6-ceramide to induce sensitivity to cetuximab (Cet) in KRAS mutant colorectal cancer (CRC). J. Clin. Oncol 34, e14068–e14068. 10.1200/JCO.2016.34.15_suppl.e14068 [DOI] [Google Scholar]
- Nicholson H, Comeau A, Mesangeau C, McCurdy CR, Bowen WD, 2015. Characterization of CM572, a Selective Irreversible Partial Agonist of the Sigma-2 Receptor with Antitumor Activity. J. Pharmacol. Exp. Ther 354, 203–212. 10.1124/jpet.115.224105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson HE, Alsharif WF, Comeau AB, Mesangeau C, Intagliata S, Mottinelli M, McCurdy CR, Bowen WD, 2019. Divergent Cytotoxic and Metabolically Stimulative Functions of Sigma-2 Receptors: Structure-Activity Relationships of 6-Acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[ d ]oxazol-2(3 H )-one (SN79) Derivatives. J. Pharmacol. Exp. Ther 368, 272–281. 10.1124/jpet.118.253484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson HE, Mesangeau C, McCurdy CR, Bowen WD, 2016. Sigma-2 receptors play a role in cellular metabolism: Stimulation of glycolytic hallmarks by CM764 in human SK-N-SH neuroblastoma. J. Pharmacol. Exp. Ther 356, 434–445. 10.1124/jpet.115.228387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld MS, Fehrenbacher N, Høyer-Hansen M, Thomsen C, Farkas T, Jäättelä M, 2005. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 65, 8975–83. 10.1158/0008-5472.CAN-05-0269 [DOI] [PubMed] [Google Scholar]
- Quadir SG, Tanino SM, Rohl CD, Sahn JJ, Yao EJ, Cruz L. dos R, Cottone P, Martin SF, Sabino V, 2021. The Sigma-2 receptor / transmembrane protein 97 (σ2R/TMEM97) modulator JVW-1034 reduces heavy alcohol drinking and associated pain states in male mice. Neuropharmacology 184, 108409. 10.1016/j.neuropharm.2020.108409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad A, Zeng C, Weng CC, Winters H, Xu K, Makvandi M, Metz T, Carlin S, Mach RH, 2018. Sigma-2 Receptor/TMEM97 and PGRMC-1 Increase the Rate of Internalization of LDL by LDL Receptor through the Formation of a Ternary Complex. Sci. Rep 8, 1–12. 10.1038/s41598-018-35430-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G, Prezzavento O, Intagliata S, Pittalà V, Modica MN, Marrazzo A, Turnaturi R, Parenti C, Chiechio S, Arena E, Campisi A, Sposito G, Salerno L, 2019. Synthesis, in vitro and in vivo characterization of new benzoxazole and benzothiazole-based sigma receptor ligands. Eur. J. Med. Chem 174, 226–235. 10.1016/j.ejmech.2019.04.056 [DOI] [PubMed] [Google Scholar]
- Rousseaux CG, Greene SF, 2016. Sigma receptors [σRs]: Biology in normal and diseased states. J. Recept. Signal Transduct 36, 327–388. 10.3109/10799893.2015.1015737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn JJ, Hodges TR, Chan JZ, Martin SF, 2017. Norbenzomorphan Scaffold: Chemical Tool for Modulating Sigma Receptor-Subtype Selectivity. ACS Med. Chem. Lett 8, 455–460. 10.1021/acsmedchemlett.7b00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, 1982. Evidence for sigma opioid receptor: Binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther 223, 284–290. [PubMed] [Google Scholar]
- Tam SW, Cook L, 1984. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H]SKF-10, and [3H]haloperidol binding in guinea pig brain membranes. Proc. Natl. Acad. Sci 81, 5618–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Rosa E, Watson MR, Sahn JJ, Hodges TR, Schroeder RE, Cintrón-Pérez CJ, Shin MK, Yin TC, Emery JL, Martin SF, Liebl DJ, Pieper AA, 2019. Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury. ACS Chem. Neurosci. 10, 1595–1602. 10.1021/acschemneuro.8b00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilner BJ, de Costa BR, Bowen WD, 1995a. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J. Neurosci 15, 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD, 1995b. Sigma-1 and Sigma-2 Receptors Are Expressed in a Wide Variety of Human and Rodent Tumor Cell Lines. Cancer Res. 55, 408–413. [PubMed] [Google Scholar]
- Wang X. and Bowen WD Sigma-2 receptors mediate apoptosis in SK-N-SH neuroblastoma cells via caspase-10-dependent Bid cleavage and mitochondrial release of endonuclease G and apoptosis-inducing factor. Program No. 90.1. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience, 2006. Online. [Google Scholar]
- Xie XY, Li YY, Ma WH, Chen AF, Sun YT, Lee JY, Riad A, Xu DH, Mach RH, Huang YS, 2021. Synthesis, binding, and functional properties of tetrahydroisoquinolino-2-alkyl phenones as selective σ2R/TMEM97 ligands. Eur. J. Med. Chem 209, 112906. 10.1016/j.ejmech.2020.112906 [DOI] [PubMed] [Google Scholar]
- Zampieri D, Fortuna S, Calabretti A, Romano M, Menegazzi R, Schepmann D, Wünsch B, Mamolo MG, 2020. Synthesis, Cytotoxicity Evaluation, and Computational Insights of Novel 1,4-Diazepane-Based Sigma Ligands. ACS Med. Chem. Lett 11, 651–656. 10.1021/acsmedchemlett.9b00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Rothfuss J, Zhang J, Chu W, Vangveravong S, Tu Z, Pan F, Chang KC, Hotchkiss R, Mach RH, 2012. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br. J. Cancer 106, 693–701. 10.1038/bjc.2011.602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Rothfuss JM, Zhang J, Vangveravong S, Chu W, Li S, Tu Z, Xu J, Mach RH, 2014. Functional assays to define agonists and antagonists of the sigma-2 receptor. Anal. Biochem 448, 68–74. 10.1016/j.ab.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Weng C-C, Schneider ME, Puentes L, Riad A, Xu K, Makvandi M, Jin L, Hawkins WG, Mach RH, 2019. TMEM97 and PGRMC1 do not mediate sigma-2 ligand-induced cell death. Cell Death Discov. 5. 10.1038/s41420-019-0141-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 1H NMR of the reaction mixture between CM571 and CM572 in DMSO, despite the more convoluted appearance, shows strong similarities with the 1H NMR spectrum of the degraded CM572.