Summary
Information concerning the longevity of immunity to SARS-CoV-2 following natural infection may have considerable implications for durability of immunity induced by vaccines. Here, we monitored the SARS-CoV-2 specific immune response in COVID-19 patients followed up to 15 months after symptoms onset. Following a peak at day 15–28 postinfection, the IgG antibody response and plasma neutralizing titers gradually decreased over time but stabilized after 6 months. Compared to G614, plasma neutralizing titers were more than 8-fold lower against variants Beta, Gamma, and Delta. SARS-CoV-2-specific memory B and T cells persisted in the majority of patients up to 15 months although a significant decrease in specific T cells, but not B cells, was observed between 6 and 15 months. Antiviral specific immunity, especially memory B cells in COVID-19 convalescent patients, is long-lasting, but some variants of concern may at least partially escape the neutralizing activity of plasma antibodies.
Subject areas: Immunology, Immune response, Virology
Graphical abstract
Highlights
-
•
Plasma neutralizing antibodies persist in the majority of patients up to 15 months
-
•
Neutralizing activity is lower against variants of concern Delta, Beta, and Gamma
-
•
Specific memory B and T cells were present in 95% of patients up to 15 months
-
•
Specific T cells, but not B cells, were decreased between 6 and 15 months
Immunology; Immune response; Virology
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rapidly resulted in a pandemic constituting a global health emergency. The COVID-19 pathological process exhibits a wide spectrum of clinical manifestations, ranging from asymptomatic to mild, moderate, severe, and critical disease. The genome of SARS-CoV-2 encodes four major structural proteins that occur in all coronavirus species: spike protein (S), nucleoprotein (N), membrane protein (M), and envelope protein (E) (Naqvi et al., 2020). The S protein binds to the host receptor (ACE 2 [ACE2]) through the receptor-binding domain (RBD) in the S1 subunit, followed by the S2 subunit-mediated cell membrane fusion (Zhou et al., 2020).
The adaptive immune response is likely to be critical for the development of protective immunity to SARS-CoV-2 including viral clearance and the persistence of antiviral immunity (Poland et al., 2020). Generation of neutralizing antibodies that specifically target the receptor-binding domain (RBD) of the S protein is considered to be essential in controlling SARS-CoV-2 infection (Addetia et al., 2020; Khoury et al., 2021). A robust adaptive immune response with presence of RBD and S-specific neutralizing antibodies, memory B cells, and T cell response have been found in patients who have recovered from infection (Dan et al., 2021; Sherina et al., 2021; Wang et al., 2021c). Although circulating antibodies derived from plasma cells wane over time, long-lived immunological memory can persist in expanded clones of memory B cells (Wang et al., 2021c).
Although SARS-CoV-2 started spreading to Europe and the Americas in March 2020, the B.1 lineage carrying the D614G mutation (G614) quickly became the dominant lineage (Tao et al., 2021). The SARS-CoV-2 variant of concern (VOC) B.1.1.7 (Alpha) first identified in late September 2020 and rapidly growing in the United Kingdom in December 2020 then became the dominant lineage across much of Europe and America. During the same period, two additional VOCs rose in prevalence in South Africa (B.1.351, Beta) and Brazil (P.1, Gamma) but without causing as much damage in other continents as it caused in their place of emergence. Subsequently, a novel variant (B.1.617.2) increased in prevalence in India in winter 2021 and has since spread widely in multiple countries. Mutations in the S1 subunit in VOCs may lead to changes in the structure of the S protein and RBD (Davies et al., 2021a) and result in higher binding of the virus to the receptor (Ramanathan et al., 2021), increased risk of transmission, and severity of illness (Davies et al., 2021b), as well as a reduction in neutralization susceptibility by antibodies (Jangra et al., 2021; Liu et al., 2021; Wang et al., 2021b). The Delta variant is associated with an estimated 60% higher risk of household transmission than the Alpha variant and is becoming dominant worldwide (Mahase, 2021).
We have previously reported the longevity of the SARS-CoV-2 adaptive immune response (up to 6–8 months) in cohorts of Swedish and Italian patients infected with the SARS-CoV-2 G614 strain (Sherina et al., 2021). The antibody and neutralizing titers were sustained at a relatively high level for at least 6 months after the onset of symptoms, whereas specific memory B and T cells were maintained for at least 6–8 months. In this study, the adaptive immune response in convalescent patients from the same cohorts was followed for up to 15 months. In addition, the specific antibody levels and neutralizing antibody titers were tested against VOCs.
Results
Longevity of anti-SARS-CoV-2 antibody response
The majority of patients (99) were recruited between February 28 and December 3, 2020 during the first and second wave in Europe when the G614 variant (B.1 lineage) was the predominant circulating strain in both Sweden and Italy. The rest of the patients (37) were recruited between January 28 and June 16, 2021 during the third wave in which Alpha became a dominant variant and accounted for more than 50–60% of the cases at the beginning of March in Italy and Sweden (Carlsson and Söderberg-Nauclér, 2021; Di Giallonardo et al., 2021).
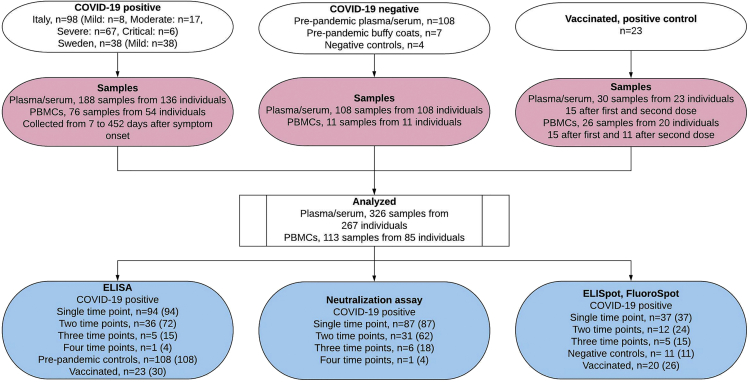
For the entire 15 months follow-up, a total of 188 serum or plasma samples were collected from 136 COVID-19 patients (98 from Italy and 38 from Sweden) experiencing mild symptoms of critical disease (Figure 1, Table S1 and Table S2). Plasma from 108 historical negative controls collected before the SARS-CoV-2 pandemic were also analyzed. Plasma anti-RBD and anti-S antibody titers were measured by an in-house ELISA (ELISA) (Sherina et al., 2021).
Figure 1.
Flowchart illustrating the study design and analysis
For ELISA, Neutralization and ELISpot/Fluorospot, the number of individuals (n = ), and the number of analyzed samples (in parentheses) are indicated. See also Tables S1, S2, S3 and S4
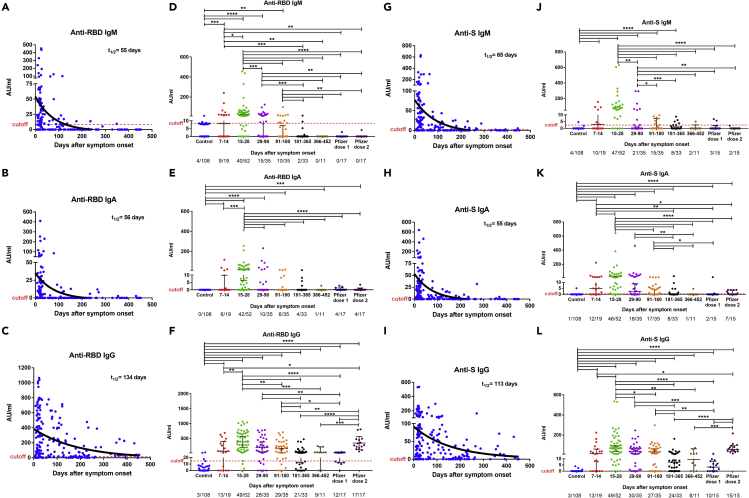
For the cross-sectional analysis, we further divided samples into six groups based on the sample time points; collected at 7–14 days, 14–30 days, 31–90 days, 91–180 days, 181–365 days, and 366–452 days, post-onset of symptoms (Table S3). At the peak of the antibody response, 15–28 days after symptoms onset, anti-RBD IgM and IgA were increased in 77% (40/52) and 85% (44/52) of convalescent patients, respectively, but rapidly decreased between 1 and 3 months and were detected in less than 4.5% (2/44) and 11% (5/44) of patients tested between 6 and 15 months when assessing all COVID-19 subjects by cross-sectional analysis (Figures 2A, 2B, 2D, and 2E). Similarly, IgM and IgA anti-S proteins were detected in 88% (46/52) and 90% (47/52) of convalescent patients at 15–28 days, respectively, but less than 23% (10/44) of patients for both immunoglobulins from 6 to 15 months (Figures 2G, 2H, 2J, and 2K). Using a one-phase exponential decay model, we estimated the half-lives (t1/2) of the RBD- and S-specific IgM antibodies to be 55 and 65 days, respectively (Figures 2A, 2G), and that of RBD-specific and S-specific IgA antibodies to be 56 and 55 days, respectively (Figures 2B and 2H).
Figure 2.
Cross-sectional analysis of plasma anti-SARS-CoV-2 antibody titers patients over time
Levels of anti-RBD (A–F) and anti-S (G–L) IgM, IgA, and IgG antibodies in plasma of COVID-19 patients, historical controls, and vaccinated individuals. Antibodies were measured in 185 samples from 136 COVID-19 patients, 108 historical controls (before the SARS-CoV-2 pandemic), and 23 vaccinated individuals. The RBD (A–C) and S (G–I) specific IgM, IgG, IgA antibody decay curves (in black) and half-lives (t1/2) were estimated by a one-phase exponential decay model. Samples from patients were further divided in six study periods: 7–14 days (n = 19), 15–28 days (n = 52), 29–90 days (n = 35), 91–180 days (n = 35), 181–365 days (n = 33), and 366–452 (n = 11) after symptom onset (D–F and J–L) for comparison. Vaccinated individuals were sampled 14–35 days after the first dose and 14–36 days after the second dose. For each time interval, the proportion of positive samples is indicated below the X axis. Symbols represent individual subjects; horizontal black lines indicate the median and 95% CI. The dashed red line indicates the cutoff value for elevated anti-S and anti-RBD antibody titers (2.5 and 8.4 AU/mL for IgM, 0.5 and 0.08 AU/mL for IgA, and 0.03 and 14.81 AU/mL for IgG, respectively, giving a specificity of 96% for IgM, 99% for IgA, and 97% for IgG). The cutoff-value is not visible in some graphs because it is very close to the X axis. Mann-Whitney U test. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ∗∗∗∗p < 0.0001. See also Figures S1 and S2.
Plasma IgG antibodies binding to SARS-CoV-2 RBD and S protein increased in 94% (49/52) of COVID-19 convalescent participants tested 15–28 days after symptoms onset. The median RBD and S IgG antibody titers gradually decreased by less than 4-fold from the peak of the antibody response until 6 months (15–28 vs 181–365 days, p < 0.0001 for both antibodies), but thereafter, remained at a relatively steady level up to 15 months (181–365 vs 366–452 days, p = 0.3866, and p = 0.7105 for anti-RBD and anti-S antibody titers, respectively; Figures 2C, 2F, 2I, and 2L). Both anti-RBD and anti-S IgG antibodies were still detected in 68% (30/44) and 73% (32/44) of patient samples tested 6 to 15 months after symptoms onset (Figures 2F, 2L). The half-lives of anti-RBD and anti-S IgG antibody response estimated by a one-phase decay model were 134 and 113 days, respectively (Figures 2C and 2I), and were shorter in patients with mild/moderate (t1/2 = 52 days and t1/2 = 40 days) than severe/critical (t1/2 = 372 days and t1/2 = 239 days) disease (Figures S1A and S1B).
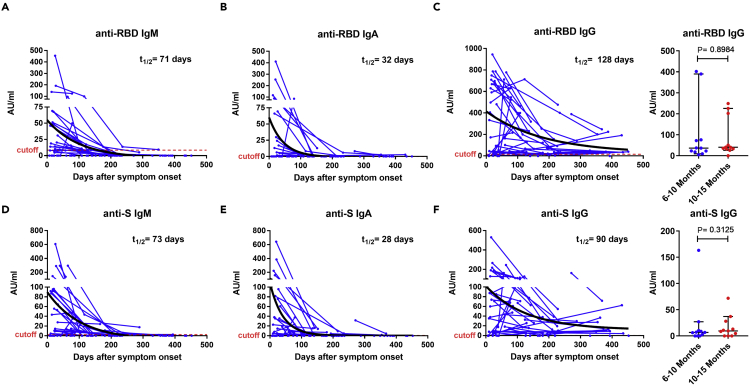
As a complementary approach, we analyzed the antibody titers from 42 patients who donated blood at two or more time points and estimated the half-lives of the RBD-specific and S-specific antibody response in IgM (t1/2 = 71 and 73 days), IgA (t1/2 = 32 and 28 days), and IgG (t1/2 = 128 and 90 days) (longitudinal analysis; Figure 3). No significant difference in the anti-S and anti-RBD IgG titers were observed between the paired samples (n = 11) containing a sampling time point between 181 and 300 days (6–10 months) and a second one between 301 and 452 days (10–15 months), confirming that specific IgG antibody titers reached a plateau phase after 6 months (p = 0.8984 and p= 0.3125, respectively; Figures 3C and 3F).
Figure 3.
Longitudinal analysis of plasma antibody titers
Anti-RBD (A–C) and anti-S (D–F) IgM, IgA, and IgG antibodies in paired samples from 42 COVID-19 patients over 15 months. For anti-RBD (C) and anti-S (F) IgG antibodies, further comparisons were made between paired samples (n = 11) at two time points ranging from 6 to 15 months (TP1: 181–300 and TP2: 301–452 days after symptoms onset; right panel). Symbols represent individual subjects; horizontal black lines indicate the median and 95% CI. The antibody decay curves (in black) and half-lives (t1/2) were estimated by a one-phase exponential decay model. The dashed red line indicates the cutoff value for elevated anti-S and anti-RBD antibody titers (2.5 and 8.4 AU/mL for IgM, 0.5 and 0.08 AU/mL for IgA, and 0.03 and 14.81 AU/mL for IgG, respectively, giving a specificity of 96% for IgM, 99% for IgA, and 97% for IgG). The cutoff-value is not visible in some graphs because it is very close to the X axis. Wilcoxon signed-rank test. See also Figure S1.
We further compared the antibody response induced from natural infection to that induced by one or two doses of the Comirnaty (Pfizer-BioNTech) vaccine (Table S4). The vaccine induced no or low level of RBD-specific, S-specific IgM (Figures 2D and 2J), and IgA (Figures 2E and 2K) antibody titers compared to those detected during natural infections. The plasma RBD and S specific IgG antibody titers measured 14–35 days after one dose of vaccine were similar to those measured in patients more than six months after infection when the antibody titers have decreased (vs 181–365 days, p = 0.6959, and p = 0.1169, respectively, vs 366–452 days, p = 0.0685, and p = 0.1121, respectively; Figures 2F and 2L). The RBD IgG and S antibody titers measured 14–36 days after the second dose of vaccine corresponded to those observed at the peak of antibody response in convalescent patients (15–28 days) (p = 0.7489 and p = 0.8435, respectively; Figures 2F and 2L).
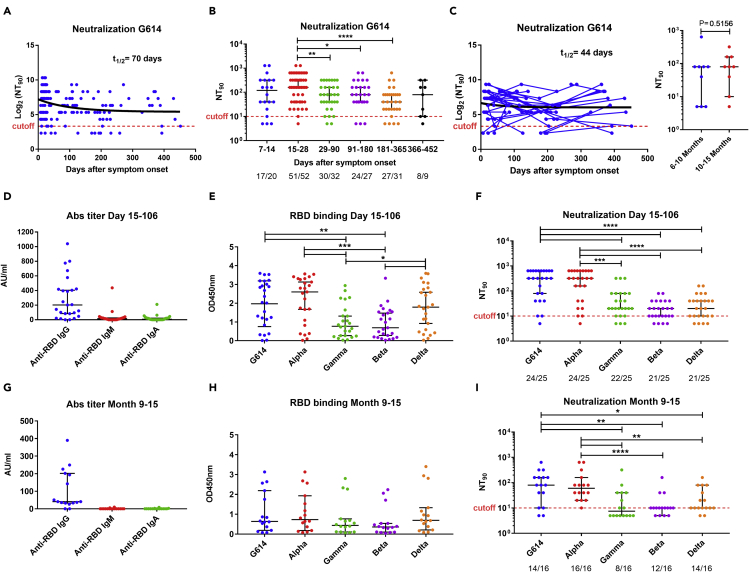
The neutralizing activity against the G614 variant was measured by a microneutralization assay and was expressed as the neutralizing titers (≥1:10) which inhibit 90% of the virus infectivity (NT90). Similar to the dynamic of the anti-RBD and anti-S antibodies, the median NT90 reached a peak of 1:160 between 15 and 28 days with 98% (51/52) of convalescent patients exhibiting plasma neutralizing activity (Figures 4A and 4B). The plasma NT90 gradually decreased by about 2-fold up to 6 months (15–28 vs 91–180 days, p = 0.0493) but was still observed in more than 87% (35/40) of the patients sampled at 181–365 (27/31) and 366–452 days (8/9) (Figure 4B). Furthermore, no significant difference was observed in the median NT90 measured at 181–365 (1:40) and 366–452 days (1:80) (p = 0.6674; Figure 4B) or between paired samples at two time points between 6 and 15 months (n = 9, TP1: 181–300 days vs TP2: 301–452 days, p = 0.5156; Figure 4C). A one-phase decay model showed a rapid initial decay using both cross-sectional (t1/2 = 70 days, Figure 4A) or longitudinal analysis (t1/2 = 44 days, Figure 4C) which slow down to a plateau phase extending from around four up to 15 months. A decrease of NT90 followed by a plateau phase was observed in both patients with mild/moderate and severe/critical diseases using cross-sectional analysis (Figure S1C), whereas no decline was observed for the severe/critical patients group using longitudinal analysis (Figure S1F) suggesting that neutralization activity may persist longer in this group. The NT90 directly correlated (p < 0.0001) with the levels of RBD-specific IgM (r = 0.45), IgA (r = 0.37), and IgG (r = 0.49) as well as with the level of S-specific IgM (r = 0.44), IgA (r = 0.44), and IgG (r = 0.55) antibodies (Figure S2).
Figure 4.
Cross-sectional and longitudinal analysis of plasma neutralization activity against SARS-CoV-2 and variants of concern
(A) Dynamics of plasma neutralizing activity against G614 variant in COVID-19 patient samples over time.
(B) Samples from patients were taken at seven study periods: 7–14 days (n = 20), 15–28 days (n = 52), 29–90 days (n = 32), 91–180 days (n = 27), 181–365 days (n = 31), and 366–452 (n = 9) after symptom onset. For each time interval, the proportion of positive samples is indicated below the X axis.
(C) For longitudinal analysis, samples were taken at two (n = 31) or more (n = 7) time points and further comparisons were made between paired samples (n = 9) at two time points ranging from 6 to 15 months (TP1: 181–300 and TP2: 301–452 days after symptoms onset; right panel). The NT90 decay curves (in black) and corresponding half-lives (t1/2) were estimated by a one-phase decay model (A, C). To test cross-neutralization, the level of anti-RBD IgM, IgA, and IgG titers (D, G), binding activity of IgG antibody to RBD from SARS-CoV-2 variants (E, H), and plasma neutralizing activity against variants (F, I) were tested in plasma collected from COVID-19 patients at 15–106 days (median day of 24) and 9–15 months (241–452 days, median day of 370). The data in D and G represent a subset of data presented in Figure 2. The dashed red line indicates the titer cutoff value (≥1:10). The cutoff-value is not visible in some graphs because it is very close to the X axis. Symbols represent individual subjects; horizontal black lines indicate the median and 95% CI. Mann-Whitney U test. ∗p≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ∗∗∗∗p < 0.0001. See also Figures S1 and S2.
To evaluate if the convalescent patients could be protected from the circulating VOCs, we determined the cross-binding and cross-neutralizing activity against Alpha, Gamma, Beta, and Delta variants using plasma collected at the early (15–106 days, median day of 24) and late (259–452 days, median day of 370) phase of convalescence from February to May 2021. As described earlier, the IgG anti-RBD titers against wild-type RBD were higher in early (Figure 4D) compared to late convalescence (Figure 4G). Furthermore, a reduction in the level of IgG antibodies binding to Gamma and Beta RBD was observed using both plasma samples collected at 15–106 days and 259–452 days (9–15 months) although it was only significant in early convalescence (15–106 days, p = 0.0039, and p = 0.0045 for Gamma and Beta, respectively; Figures 4E and 4H). The majority of samples (96%, 24/25) collected at earlier time points (15–106 days) showed neutralizing activity against G614 and Alpha, and 84–88% of them against the other VOCs. The median NT90 was similar (1:320) against G614 and Alpha but 16-fold lower (1:20, p < 0.0002) for Gamma, Beta, and Delta variants (Figure 4F). Between 9 and 15 months after infection, the majority of patient samples still showed neutralizing activity against G614 (14/16, 88%), Alpha (16/16, 100%), Beta (12/16, 75%), and Delta (14/16, 88%) variants, but a lower proportion of patient samples had neutralizing activity against Gamma (8/16, 50%) (Fisher exact test, p = 0.002 compared to Alpha; Figure 4I). The NT90 were six- to 8-fold lower against Gamma (<1:10, p = 0.0039, and p = 0.0010), Beta (1:10, p = 0.0025, and p < 0.001), and Delta (1:10, p = 0.0237, and p = 0.0050) compared to G614 (1:80) and Alpha (1:60), respectively (Figure 4I).
These data suggest that the plasma anti-SARS-CoV-2 antibody response and neutralizing activity decrease up to around 6 months but neutralizing activity is maintained in the majority of patients up to 15 months. Furthermore, plasma neutralizing activity was lower against Beta, Gamma, and Delta variants, particularly 9–15 months after infection.
SARS-CoV-2-specific memory B cells
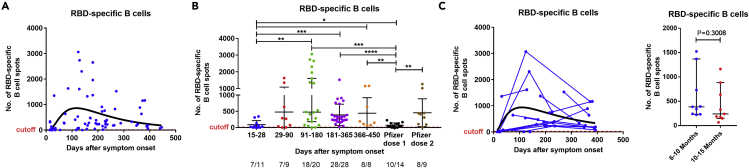
We analyzed 76 peripheral blood mononuclear cell (PBMC) samples collected from 54 patients (mild = 34, moderate = 4, severe = 15, critical = 1) for the presence of SARS-CoV-2 specific B and T cells. Using the highest value observed from all of the COVID-19 negative controls as a cutoff, RBD-specific IgG-producing B cells were detected by ELISpot assay in 89% (68/76) of the patient samples tested (Figure 5A). We further divided samples into five groups based on the sample time points; collected at 14–30 days, 31–90 days, 91–180 days, 181–365 days, and 366–452 days, post-onset of symptoms, i.e., ∼2–4 weeks, 1–3 months, 3–6 months, 6–12 months, and 12–15 months, respectively. RBD-specific IgG-producing B cells were detected in 64% (7/11), 78% (7/9), and 90% (18/20) of the samples collected at 2–4 weeks, 1–3 months, and 3–6 months, respectively, and in all samples collected at 6–12 (n = 28) and 12–15 (n = 8) months after the onset of symptoms (Figure 5B). After an expansion over the first 3 months, the RBD-specific IgG-producing cells reached a maximum at 3–6 months (91–180 days) postinfection followed by a gradual but nonsignificant decrease after 6 months using cross-sectional analysis (91–180 vs 181–365 days, p = 0.1518; vs 366–452 days, p = 0.3039; Figure 5B) or by comparing the paired samples at two time points ranging between 6 and 15 months (n = 9, TP1: 180–300 vs TP2: 301–452 days, p = 0.3008; Figure 5C). RBD-specific IgG-producing B cells were still maintained in relatively high numbers in all patients (n = 36) followed up between 6 and 15 months.
Figure 5.
Cross-sectional and longitudinal analysis of SARS-CoV-2-specific memory B cell responses in COVID-19 patients
(A) Dynamics of RBD-specific memory B cells levels in COVID-19 patient samples over time with the corresponding log-normal fitting curve (in black).
(B) B cells were measured in control (n = 11), COVID-19 samples at five study periods: 14–30 days (n = 11), 31–90 days (n = 9), 91–180 (n = 20), 181–365 (n = 28), and 366–452 (n = 8) days after symptom onset, as well as vaccine samples after first (n = 14) and second (n = 9) dose. For each time interval, the proportion of positive samples is indicated below the X axis.
(C) For longitudinal analysis (C), samples were taken at two (n = 12) or more (n = 5) time points, and further comparisons were made between paired samples (n = 9) at two time points ranging from 6 to 15 months (TP1: 181–300 and TP2: 301–452 days after symptoms onset) (right panel). The results were expressed as the number of spots per 300,000 seeded cells after subtracting the background spots of the negative control. The horizontal black lines indicate the median value and 95% CI of the group. The cutoff value (dashed red line) was set at the highest number of specific B cell spots for the negative controls (>12 spots/300,000 cells). The cutoff-value is not visible in some graphs because it is very close to the X axis. Mann-Whitney U test. ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ∗∗∗∗p ≤ 0.0001. See also Figures S3 and S6.
No statistically significant differences were observed in the number of memory B cells between mild/moderate and severe/critical COVID-19 patients over the period ranging from 6 to 15 months suggesting that the intensity and duration of the B cell response are not dependent on the disease severity (p = 0.5835; Figures S3 and S6).
Noninfected individuals sampled 14–35 days after the first vaccine dose showed a B cell response significantly lower than that observed in convalescent patients between 3 and 15 months (vs 91–180 days, p = 0.0002; vs 181–365 days, p < 0.0001; vs 366–452 days, p = 0.0077 after onset of symptoms; Figure 5B). Subjects sampled 14–36 days after the second vaccine dose showed a median number of circulating RBD-specific memory B cells similar to that observed in convalescent patients sampled during the same period (vs 91–180 days, p = 0.3222; vs 181–365 days, p = 0.6337; vs 366–452 days, p = 0.8884; Figure 5B).
SARS-CoV-2-specific memory T cells
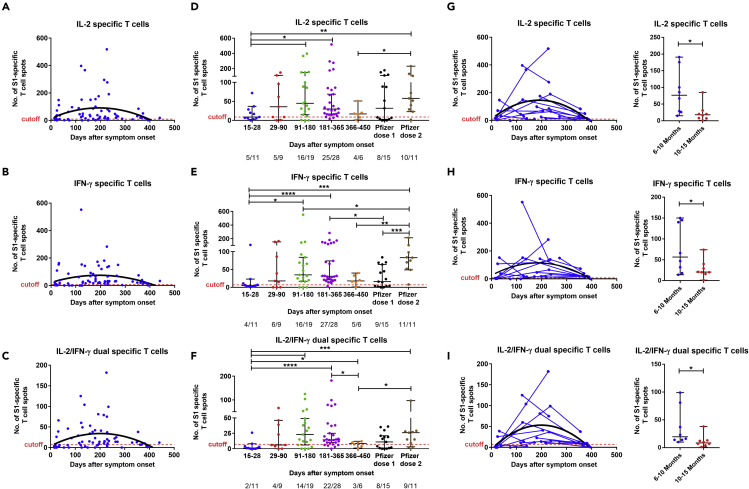
The S1 and S N M O (Orf3a and Orf7a) peptide pool-specific T cells expressing interleukin-2 (IL-2) and/or interferon-gamma (IFN-γ) were measured by FluoroSpot assay. No or a negligible number of IL-2, IFN-γ, or IL-2/IFN-γ -producing T cells against the two peptide pools were detected in the negative controls. Overall, a T cell response against at least one of the SARS-CoV-2 peptide pools (S1, or S N M O protein derived) was detectable at a level above the cutoff in 95% (69/73) of the patient samples tested over the study period (Figures 6A–6C and S4A–S4C). When divided by groups based on the sample time points, specific T cells were detected in the majority of patient samples at 2–4 weeks (10/11, 91%) and 1–3 months (7/9, 78%), and more than 98% (52/53) of patient samples tested between 3 and 15 months.
Figure 6.
Cross-sectional and longitudinal analysis of S1-specific memory T cell responses in COVID-19 patients
(A–C) Dynamics of S1-specific memory IL-2, IFN-γ, and IL-2/IFN-γ-producing T cells with the corresponding second order polynomial fitting curve (in black).
(D–F) T cells were measured in control (n = 11), COVID-19 samples at five study periods: 14–30 days (n = 11), 31–90 (n = 9), 91–180 days (n = 19), 181–365 (n = 28), and 366–452 (n = 6) days after symptom onset, as well as vaccine samples after first (n = 15) and second (n = 11) dose. For each time interval, the proportion of positive samples is indicated below the X axis.
(G–I) For longitudinal analysis, samples were taken at two (n = 10) or more (n = 5) time points and further comparisons were made between paired samples (n = 8) at two time points ranging from 6 to 15 months (TP1: 181–300 and TP2: 301–452 days after symptoms onset; right panel). The results were expressed as the number of spots per 300,000 seeded cells after subtracting the background spots of the negative control. The horizontal black lines indicate the median value and 95% CI of the group. The cutoff value (dashed red line) was set at the highest number of specific T cell spots for the negative controls (>7 to nine spots/300,000 seeded cells depending on the T cell population). The cutoff-value is not visible in some graphs because it is very close to the X axis. Mann-Whitney U test. ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ∗∗∗∗p ≤ 0.0001. See also Figures S4, S5 and S6.
Using cross-sectional analysis, the number of S1-specific IL-2, IFN-γ, and IL-2/IFN-γ-producing T cells reached a peak between 3 and 6 months and was maintained up to 15 months except for IL-2/IFN-γ-producing T cells which significantly decreased at 366–452 days (vs 181–365 days, p = 0.0215; Figures 6D–6F). We further analyzed the results using longitudinal data and observed a significant decrease of S1-specific T cells producing IL-2, IFN-γ, and IL-2/IFN-γ between TP1 (180–300 days) and TP2 (301–452 days) (n = 8; p= 0.0156 for each of the three cell populations; Figures 6G–6I). We similarly detected a peak of S N M O pool-specific IL-2, IFN-γ, and IL-2/IFN-γ-producing T cells between 3 and 6 months and a significant decrease of those T cell populations at 12–15 months using both cross-sectional (366–450 vs 181–365 days; p = 0.0082, p = 0.0004, and p= 0.0026 respectively; Figures S4D–S4F) and paired samples analysis (n = 8, TP1: 181–300 days vs TP2: 301–452 days, p = 0.0078 for the three T cell populations; Figures S4G–S4I).
The numbers of S1 and S N M O peptide pool-specific IL2 and IL-2/IFN-γ -producing T cells were higher in severe/critical COVID-19 than mild/moderate patients between 6 and 15 months (p = 0.0044 and p = 0.0202 for S1 specific T cells, p = 0.0302, and p = 0.0427 for S N M O specific T cells), whereas no significant difference was observed in S1 and S N M O specific IFN-γ -producing T cells (p = 0.0610 and p = 0.1012, respectively) (Figures S5 and S6).
The S1-specific T cell response measured in samples collected after one vaccine dose was equivalent to that observed in convalescent patients at 12–15 months when the specific T cell number decrease (p = 0.6333, p = 0.8352, p = 0.6893; Figures 6D–6F). Compared to the peak of T cell response in patients (3–6 months), individuals with two doses of vaccine present a similar level of S1-specific IL-2 and IL-2/IFN-γ-producing T cells but a 2-fold higher number of IFN-γ-producing T cells (p = 0.5177, p = 0.9915, and p = 0.0475, respectively; Figures 6D–6F).
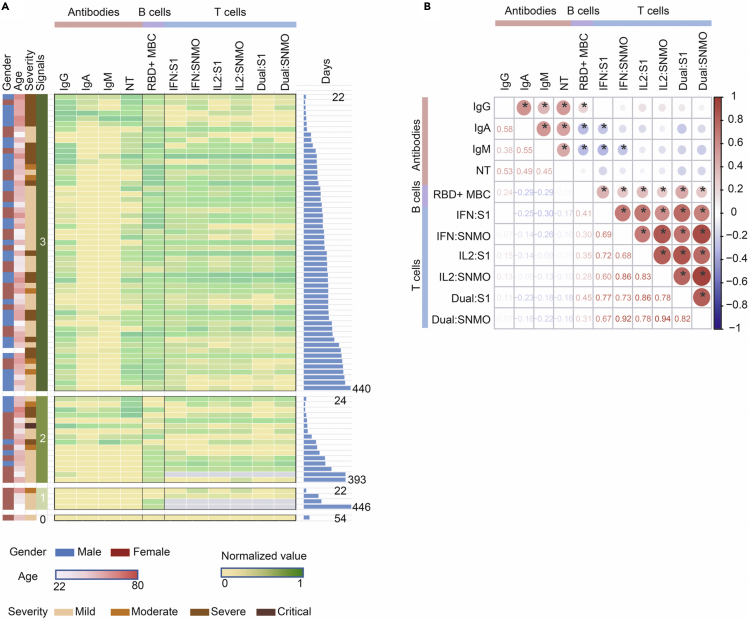
Finally, we have investigated the presence of all three arms of immunity for each individual who has been evaluated for SARS-CoV-2-specific adaptive immunity in all assays i.e., 1) IgM, IgG, and IgA antibody response and/or neutralization activity, 2) RBD-specific IgG-producing B cells, and 3) S1 peptide or S N M O pool-specific IL-2 and/or IFN-γ-producing T cells (Figure 7A). The majority of individuals (72%, 55/76) had three arms of immunity active against SAR-CoV-2, particularly from 2 to 15 months (80%, 48/60), highlighting the increase of memory B and T cells between 0 and 2 months and the long duration of the adaptive immune response.
Figure 7.
Correlations between SARS-CoV-2-specific immune responses and assessment of covariates (A) Heatmap representation of the SARS-CoV-2 specific adaptive immune responses
For subjects with available data on all three “arms” of adaptive immunity (serum anti-RBD IgM, IgA, IgG, and neutralization titers (NT90), the number of RBD-specific memory B cells, and the number of T cells specific for the virus protein-derived peptides pools producing IFN-γ, IL-2, or IFN-γ and IL-2 (Dual), are indicated. For each arm of immunity, the relative intensity of signals varies from no (gray), low (yellow) to high (dark green) signals. The gender, age (from 22 to 86) years), severity (from mild to critical), number of signals (1, 2, or 3), and the number of days after symptoms onset (from 22 to 446 days) are shown. (B) Heatmap showing Spearman correlations between immune parameters at each sample point with significance levels: ∗p < 0.05. The circle size and color intensity correspond to the absolute value of the Spearman rank correlation coefficient, with red or blue indicating a positive or negative correlation, respectively. NT: neutralization titers. MBC: IgG + memory B cell.
The interrelationship between the components of the immune system was then examined by a multiparameter analysis (Figure 7B). We observed a positive correlation between the level of IgG, IgM, and IgA anti-RBD antibodies and the neutralization titers. The level of RBD-specific IgG-producing memory B cells was also positively associated with the IgG anti-RBD antibody and the S1 and S N M O pool-specific T cell response. However, no correlation was observed between the T cell response and the anti-RBD antibody response.
Taken together, SARS-CoV-2-specific memory B and T cells remained present in the majority (>95%) of patients followed up between 6 and 15 months. A reduction of the specific T cell response, but not B cell memory, was observed at 12–15 months.
Discussion
The magnitude, duration, and quality of immunological memory are crucial for preventing reinfection. In this study, we extended our assessment of the longevity of the SARS-CoV-2-specific antibody, B and T cell immune response in cohorts of convalescent patients in Italy and Sweden that experienced mild to critical symptoms of COVID-19. In many viral infections, such as those caused by Dengue and Zika virus, serum IgM responses precede the appearance of IgG and IgA antibodies (Roltgen and Boyd, 2021). In contrast, IgG antibodies to SARS-CoV-2 S and RBD appear at approximately the same time as serum IgM and IgA antibodies, usually within the first 2 weeks after symptom onset which might be because of a longer asymptomatic period. In accordance with previous studies, although the RBD- and S-specific IgG titers peaked 14–30 days after infection and gradually decreased over time, the IgG antibody response stabilized after 6 months and was still detected in the majority of convalescent plasma donors at 6–15 months (Addetia et al., 2020; Jangra et al., 2021; Khoury et al., 2021; Liu et al., 2021). The sustained persistence of RBD-IgG titer over time suggests the generation of long-lived bone marrow plasma cells. Anti-S antibody titers were previously shown to correlate with the frequency of S-specific bone-marrow plasma cells of SARS-CoV-2 convalescent patients 7 to 8 months after infection (Turner et al., 2021). Only a low proportion of individuals had anti-S- and anti-RBD IgM or IgA antibodies more than 6 months after infection confirming the faster decrease of the specific IgM and IgA antibody response observed in other studies (Anand et al., 2021; Dan et al., 2021; Roltgen et al., 2020; Xiang et al., 2021) although a few studies reported a high prevalence of specific IgA up to one year (Cohen et al., 2021; Wang et al., 2021c). In accordance with a previous study, the persistence of IgG antibody level was associated with disease severity and patients with milder disease appeared to have more rapid IgG anti-RBD antibody waning (Chia et al., 2021). It has been reported that antibodies against SARS-CoV and Middle Eastern respiratory syndrome (MERS)-CoV, could still be detected 1–3 years after infection onset despite lack of reexposure to this virus (Huang et al., 2020). After a rapid decline of antibodies against SARS-CoV in the first two years, specific IgG have been detected in some patients up to 12 years after infection (Guo et al., 2020). A longer follow up will be necessary to evaluate if it is also the case for SARS-CoV-2-specific IgG antibodies as both viruses present different etiology.
Functional neutralizing antibodies specific to SARS-CoV-2 (anti-S and anti-RBD) that are produced following infection or vaccination are considered important for viral neutralization and viral clearance (Bergwerk et al., 2021; Poland et al., 2020). In the absence of definitive correlates of protective immunity, the presence of neutralizing antibodies against SARS-CoV-2 provides the best current indication for protection against reinfection (Addetia et al., 2020; Bergwerk et al., 2021; Khoury et al., 2021). As previously reported, the neutralizing ability of polyclonal plasma correlated positively with anti-S IgG or anti-RBD IgG (Roltgen et al., 2020; Sherina et al., 2021; Wajnberg et al., 2020). Plasma neutralizing activity reached a plateau after 4–6 months and was maintained in the majority of patients up to 15 months which was consistent with a previous study showing no significant difference in anti-RBD IgG antibody level and plasma neutralizing activities against Wuhan strain between 6 and 12 months (Wang et al., 2021c). In addition, the two-phase pattern with an initial rapid decay of neutralizing antibodies which slowed down to a flat slope (Pradenas et al., 2021) might explain why early studies with shorter follow-up (2–4 months) reported a fast decline of the antibody response (Ibarrondo et al., 2020). The long-lasting neutralizing activity might be caused by the accumulation of somatic mutations in IgG antibody genes and the production of antibodies with increased neutralizing potency (Pradenas et al., 2021; Wang et al., 2021c). Although no significant difference in neutralization activity was observed beyond 6 months, it might be important to predict how the long-term neutralizing antibody decay will affect the clinical outcomes following both natural infection and vaccination. However, a recent study in fully vaccinated health care workers showed that the occurrence of breakthrough infection was more associated with the peak of antibody and neutralization titers induced by the vaccine rather than on the subsequent antibody decay suggesting that the degree of protection might depend more on the initial immune response and the generation of memory B and T cells (Bergwerk et al., 2021).
Although there is evidence that SARS-CoV-2 seropositivity is associated with protection against the same strain (Harvey et al., 2021; Lumley et al., 2021), reinfection has been observed in some patients, which may represent nondurable protective immunity or infection with different variants (Breathnach et al., 2021; Hansen et al., 2021; Sabino et al., 2021). Notable mutations identified in the S1 subunit of the Alpha (Del69–70, N501Y, and P681H), Beta (K417N, E484K, and N501Y), Gamma (K417T, E484K, and N501Y), and Delta (L452R, T478K, P681R, and occasionally E484Q) variants may lead to small changes in the structure of the S protein and RBD (Davies et al., 2021a) leading to a higher infection rate and reduction of acquired immunity by neutralizing antibodies. As previously reported, we observed a substantial reduction of neutralization activity in the plasma of convalescent patients collected at early and more particularly, late phase of convalescence against the Beta, Gamma, and to a lower extent Delta (Wang et al., 2021a). The N501Y mutation in the Alpha variant RBD does not alter the neutralizing ability of plasma antibodies from naturally infected individuals (Edara et al., 2021), whereas the Beta (B.1.351) and Gamma (P.1) carrying the N501Y and E484 mutations are more resistant to the neutralizing activity from convalescent and vaccine immune sera as well as neutralizing antibodies (Chen et al., 2021; Planas et al., 2021; Wang et al., 2021b, 2021c). The substitution at position E484 in the RBD of pseudovirus or recombinant virus is known to confer resistance to neutralization by convalescent human sera (Jangra et al., 2021; Liu et al., 2021). The L452R mutation found in Delta was shown to impair neutralization by antibodies (Starr et al., 2021), whereas the T478K mutation in the RBD is unique to Delta and close to the E484K mutation. Recent studies suggest that immunity from infection with prior lineage of the SARS-CoV-2 virus provides a lower protection against reinfection with Delta (Dhar et al., 2021), although it could still reduce disease severity and hospitalization (Sheikh et al., 2021).
Memory B and T cell immune responses with SARS-CoV-2 have been detected in convalescent individuals but clear correlates for protective immunity have yet to be defined. RBD and S-specific memory B cells are very rare in unexposed individuals but start to appear within 2 weeks after SARS-CoV-2 infection (Dan et al., 2021; Rodda et al., 2021; Sherina et al., 2021). RBD-specific and S-specific memory B cells steadily increased over the following months and were still present more than 6 months after initial infection (Dan et al., 2021; Sherina et al., 2021). To date, only one study has examined the RBD-specific memory B cell response up to 12 months after onset of symptoms (Wang et al., 2021c). Using ELISpot, we measured the number of B cells secreting IgG antibodies specific for SARS-CoV-2 RBD which are the most numerous and more likely to produce neutralizing antibodies. In accordance with our previous results (Wang et al., 2021c), we observed that while serum anti-RBD antibodies peaked 15–30 days postinfection and gradually decrease, RBD-specific memory B cells increased with time, reaching a maximum at 3–6 months postinfection, to a similar level induced in individuals receiving two doses of Pfizer vaccines. Previous studies also showed that RBD specific IgM+ memory B cells formed the largest fraction of total memory B cells in the first month after symptom onset, but declined in frequency at later time points, whereas RBD specific IgG+ memory B cells predominate at later time points with a peak at 3–4 months (Hartley et al., 2020). More importantly, the memory B cell response persists without further significant decrease for up to 15 months post-symptom onset, irrespective of disease severity. This study highlights that a decline in serum antibodies in convalescent patients may not reflect waning immunity, but rather a contraction of the immune response, with the development and persistence of virus-specific, long-lived memory B cells in the bone marrow. It has been shown that previous infection with SARS-CoV-2 increases both the number of RBD binding memory cells and neutralizing antibody titers after a single dose of vaccine (Goel et al., 2021; Planas et al., 2021; Wang et al., 2021c) suggesting the induction of plasma cells differentiation from the memory B cell compartment (Turner et al., 2021).
Early T cell responses during COVID-19 have been correlated with rapid viral clearance and reduced disease severity (Tan et al., 2021). In accordance with previous results, memory IL-2 and/or IFN-γ−producing T cells specific to M, N nonstructural proteins, as well as S protein, were generated in the majority of convalescent patients following SARS-CoV-2 infection (Grifoni et al., 2020; Rydyznski Moderbacher et al., 2020; Sekine et al., 2020; Tan et al., 2021). Functional SARS-CoV-2 specific T cells were detected at low levels within 2–4 weeks from onset of symptoms and reached a peak between 3 and 6 months at levels similar to that of fully vaccinated individuals (Addetia et al., 2020). Specific IFN-γ producing Th1 CD4+ and cytotoxic CD8+ effector cells are considered important in supporting immunity against SARS-CoV-2 (Sattler et al., 2020). Polyfunctional T cells, secreting more than one cytokine, are also typically associated with superior control of pathogens and may be important to prevent reinfection by SARS-CoV-2 (Boyd et al., 2015; Owen et al., 2010). The presence of dual positive IFN-γ and IL-2 S1-specific T cells that preferentially retain characteristics of both effector function and proliferative potential in vivo is indicative of strong and sustained S-specific T cell immunity. Similar to previous results (Dan et al., 2021), a decrease in specific T cell response, and more particularly in dual positive IFN-γ and IL-2 S1-specific and S N M O-specific T cells, was observed after 6 months. Nevertheless, memory T cells were observed in the majority of patients between 12 and 15 months and a longer follow-up period with more participants will be necessary to evaluate the sustainability of the T cell response beyond 15 months postinfection.
Lasting immunity following acute viral infection and vaccination requires maintenance of both serum antibody and antigen-specific memory B and T lymphocytes, ranging from lifelong for smallpox or measles (Crotty et al., 2003), to more transient for common cold coronaviruses (Edridge et al., 2020). The majority of individuals in our study had three arms of immunity active against SAR-CoV-2, particularly from 2 to 15 months. We also found that the ability to mount high levels of RBD-specific IgG+ memory B cells was associated with RBD-specific IgG antibodies and S1-specific and S N M O-specific T cells. The half-life of memory CD4+ T cells to smallpox vaccination was estimated to be about 8–15 years (Hammarlund et al., 2003), whereas SARS-CoV T cells were detected 17 years after the initial infection (Le Bert et al., 2020). Our data suggest that T and B cell memory following SARS-CoV-2 infection might reach a more stable plateau, or slower decay phase, beyond 15 months postinfection. Nevertheless, long-term follow up study will be necessary to evaluate if the immunity to SARS-CoV-2 will be lifelong or will be more similar to that against endemic common cold coronavirus, where serum antibody responses decline and susceptibility to homologous virus reinfection occurs within 1–2 years (Edridge et al., 2020).
In conclusion, we observed that circulating memory B and T cells, and neutralizing antibodies are present in the majority of convalescent patients around 15 months after SARS-CoV-2 infection, demonstrative of a long-lasting immune response. Recent studies have shown that although the Pfizer-BioNTech and AstraZeneca vaccines were effective in reducing the risk of infection and COVID-19 hospitalization caused by Delta, these effects on infection appeared to be diminished when compared to those with the Alpha variant (Sheikh et al., 2021). Our neutralization data suggest that immunity develops during earlier waves of infection may not be fully protective against reinfection with Delta and other VOCs (and most likely against the recently isolated Omicron), indicating that convalescent patients may still benefit from vaccination. Furthermore, although two doses of vaccines could trigger rapid and robust immune responses as observed in naturally infected individuals, the longevity of immunity in vaccinated individuals should be followed up in future studies.
Limitations of the study
We are reporting the simplest statistical model that better fits our data. The magnitude of the antibody response over time reflects antibodies produced first by short-lived plasma cells and then long-lived plasma cells. More complex kinetics such as the power law model, which models a scenario in which the rate of antibody decay slows down over time could have been used. In addition, a higher number of individuals with more sampling time points over the 15 months period would have provided a more precise understanding of the kinetics of durability of SARS-CoV-2 antibodies. Other potential limitations to our study include the limited number of samples at the late time points (12–15 months) and a higher proportion of samples from severe patients at earlier time points (0–3 months) compared to later time points (12–15 months) (Table S3). This might have influenced the estimation of half-life of both antibody and neutralization responses although we also made a comparison between mild/moderate and severe/critical patients (Figure S2). Furthermore, although based on available clinical data, no evidence suggests reinfection in any of the donors tested, the long duration of the immune response, including the increase in neutralization activity in some recovered participants, could theoretically be related to a natural boost after a reexposure to the virus.
Cell phenotyping using flow cytometry was not performed, and it was not possible to distinguish whether the T cells measured at early time points were effector or memory cells. Although Fluorospot cannot differentiate phenotype in a mixture of cell population, it is a more sensitive method than flow cytometry, partly because it can detect antibody or cytokine production from cells in 24–48 h. Furthermore, our findings are consistent with other studies predominantly based on mild to moderate disease cohorts, and where flow cytometry assays were used to measure the RBD-specific memory B cells and which similarly observed a reduction in T cell but not B cell SARS-CoV-2 specific response over a period of 8 months (Dan et al., 2021).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| (HRP)-conjugated goat anti-human IgM | Invitrogen | Cat# A18835; RRID: AB_2535612 |
| (HRP)-conjugated goat anti-human IgA | Jackson | Cat# 109-036-011; RRID: AB_2337592 |
| (HRP)-conjugated goat anti-human IgG | Invitrogen | Cat# A18805; RRID: AB_2535582 |
| Bacterial and virus strains | ||
| SARS-CoV-2 variants | Patient isolates (Italy) | N/A |
| Biological samples | ||
| Blood samples (convalescent) | Italy, this paper | Table S1 |
| Blood samples (convalescent, healthy) | Sweden, this paper | Table S1 |
| Serum (convalescent, healthy) | Sweden, this paper | Table S1 |
| Buffy coat (healthy donor) | Sweden, this paper | Table S1 |
| Chemicals, peptides, and recombinant proteins | ||
| Ni-NTA resin | Thermofisher | 88221 |
| 16/600 Superdex 200 kDa | Cytiva | 28989335 |
| Lymphoprep | Axis-Shield | 1114547 |
| RNeasy mini kit | Qiagen | 74106 |
| imidazoquinoline resiquimod | Mabtech AB | R848 |
| Tetramethylbenzidine substrate | Sigma | T0440 |
| Tween 20 | Sigma | P9416 |
| PBS | Karolinska University Hospital | MIK3125-1000 |
| BSA | Sigma | A7906 |
| RPMI1640 | ThermoFisher Scientific | 61870010 |
| FBS | ThermoFisher Scientific | 10270106 |
| Penicillin-Streptomycin | ThermoFisher Scientific | 15140122 |
| SARS-CoV-2 S1 peptide pool | Mabtech AB | #36291 |
| SARS-CoV-2 SNMO peptide pool | Mabtech AB | #3622-1 |
| SARS-CoV-2 S2N peptide pool | Mabtech AB | #3620-1 |
| RBD-His recombinant protein | In house, this paper | N/A |
| S1-S2-His recombinant protein | In house, this paper | N/A |
| Critical commercial assays | ||
| RBD ELISpotPLUS (ALP) kit | Mabtech AB | 3850-4APW-R1-1 |
| Human IFN-γ/IL-2 FluoroSpot PLUS kit | Mabtech AB | FSP-0102-2 |
| Experimental models: Cell lines | ||
| Expi293 | Thermo Fisher Scientific | A14527 |
| High Five insect cells | Thermo Fisher Scientific | BTI-TN-5B1-4 |
| Vero-E6 | ATCC | CRL-1586 |
| Software and algorithms | ||
| GraphPad Prism 7 | GraphPad Software | https://www.graphpad.com/ |
| R version 3.6.1 | RStudio | https://rstudio.com/ |
| Other | ||
| High-binding half area flat bottom plates | Corning | #3690 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Harold Marcotte (harold.marcotte@ki.se).
Materials availability
RBD and S1-S2 proteins can be generated and shared on a collaborative basis.
Experimental model and subject details
Study design and participants
Previously enrolled study participants were asked to return for a follow-up visit at the Fondazione IRCCS Policlinico San Matteo in Pavia, Italy and Karolinska Institutet, Stockholm, Sweden. Eligible participants were 18 years of age with a history of participation in prior study visit(s) of our longitudinal cohort study of COVID-19 recovered individuals (Sherina et al., 2021). All participants had a confirmed history of SARS-CoV-2 infection, who had tested PCR- or serology-positive for SARS-CoV-2 (Sherina et al., 2021). Of those patients, 7 Italian and 4 Swedish patients returned for a follow-up sample between February 1 and June 10, 2021. In addition, 21 Italian and 28 Swedish convalescent patients were recruited between November 18 and June 16, 2021. Study inclusion criteria included subjects over 18 years of age, who were willing and able to provide informed consent, confirmed positivity of SARS-CoV-2 by real-time RT-PCR targeting the E and RdRp genes according to Corman et al. (2020) protocols and monitored until two subsequent samples with negative results.
Blood sample was taken from 53 patients at one single time point between day 15 and 452 after symptoms onset while 7 patients had blood taken at two time points for a total of 67 samples including 20 between 241 to 452 days (9–15 months). Results obtained from the new recruited and new follow-up samples were merged with previously published data assessing the immune response to SARS-CoV-2 up to 6–8 months (Sherina et al., 2021). Following merging, a total of 188 blood samples were collected from 136 patients, 98 Italians and 38 Swedish, for the entire 15-months follow-up (Figure 1). Ninety-four donors had blood drawn at one single time point ranging from 7 to 452 days after symptom onset while 35, 5, and 1 donors had blood taken at two, three and four time points, respectively. For detailed participant characteristics see Tables S1).
Disease severity was defined as mild (non-hospitalized), moderate (hospitalized, with lower respiratory tract infection, with dyspnea or not, but without oxygen support), severe (infectious disease/sub intensive ward with a need for oxygen and/or positive chest computed tomography scan, severe lower tract infections, with any oxygen support) and critical (intensive care unit (ICU) patients, intubated or with extracorporeal membrane oxygenation procedures) (Sherina et al., 2021).
The demographic and clinical characteristics of the patients for the entire 15 months follow-up are detailed in Table S1 and summarized in Table S2. The Italian patients, 58 (59.2%) males and 40 (40.8%) females, had a median age of 66.0 years (range 22–89). The degree of clinical severity of COVID-19 in the cohort was mild (n = 8), moderate (n = 17), severe (n = 67) and critical (n = 6). The Swedish patients had a median age of 44 years (range 18–75) with 16 (42.1%) males and 22 (57.9%) females and all 38 of them had mild symptoms.
In addition, serum samples from 108 anonymized individuals (16 to 80 years of age), collected before the SARS-CoV-2 pandemic (1995 to 2005) were used as historical negative controls for the ELISA. PBMCs from four healthy controls (median age 41 years, range 39–50) and seven additional buffy coats collected in Sweden before the SARS-CoV-2 pandemic (2011- January 2020) were included as negative controls for the B and T cell assays. Patients and samples tested in different assays are summarized in a flow chart (Figure 1).
Furthermore, 23 individuals (9 males and 14 females) with a median age of 40 years (range 23–64) who received the mRNA Comirnaty vaccine (Pfizer-BioNTech) were included for comparison. Vaccinated individuals were sampled 14–35 days after the first dose and 14–36 days after the second dose. Seven individuals were samples after the first and second doses and 16 were sampled either after the first (n = 8) or second (n = 8) dose for a total of 15 samples after each dose (Figure 1, Table S4).
The study in Italy was performed under the approval of the Institutional Review Board of Policlinico San Matteo (protocol number P_20200029440). The study in Sweden was approved by the ethics committee in Stockholm (Dnr 2020-02646). All participants provided written informed consent before participation in the study.
Method details
Production of SARS-CoV-2 RBD and S proteins
RBD-His protein was expressed in Expi293 cells and purified on Ni-NTA resin (#88221, Thermo Fisher) followed by size-exclusion chromatography on a Superdex 200 gel filtration column in PBS (Amanat et al., 2020). S1-S2-His (referred as S) protein from Wuhan-Hu-1 strain and RBD from variants (Alpha, Beta, Gamma, and Delta) were expressed baculovirus-free in High Five insect cells (Bleckmann et al., 2019) and purified on HisTrap excel column (Cytiva) followed by preparative size exclusion chromatography on 16/600 Superdex 200 pg column (Cytiva) (Bertoglio et al., 2021; Korn et al., 2020).
Detection of antibodies specific to SARS-CoV-2
Levels of anti-S and anti-RBD IgM, IgA and IgG antibodies were determined by ELISA (Sherina et al., 2021). High-binding Corning Half area plates (Corning #3690) were coated overnight at 4°C with purified S or RBD protein derived from wild-type virus (1.7 μg/mL for IgM and IgG; 2.0 μg/mL for IgA) in PBS. Serum or plasma diluted 1:3200 (S IgM), 1:6400 (S IgG), 1:1600 (S IgA; RBD IgM, IgA, IgG) in 0.1% BSA in PBS, was incubated for 1.5 h at room temperature. Plates were then washed and incubated for 1h at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-human IgM (Invitrogen #A18835), goat anti-human IgA (Jackson #109-036-011), or goat anti-human IgG (Invitrogen #A18805), (all diluted 1:15 000 in 0.1% BSA-PBS). Bound antibodies were detected using tetramethylbenzidine substrate (Sigma #T0440). The color reaction was stopped with 0.5M H2SO4 and absorbance was measured at 450nm. Antibody levels were presented as arbitrary units (AU/ml), based on a standard curve made from a serially diluted highly positive serum pool. In-house standards made by pooled highly positive serum were calibrated by using the WHO International Standard for anti-SARS-CoV-2 immunoglobulin (NIBSC, 20/136). 1 AU/ml of in-house serum standard equal to 30.20 BAU/ml IgM, 185.59 BAU/ml IgA and 4.10 BAU/ml IgG of the WHO international standards, respectively. Cutoff values for antibody positivity were determined based on receiver operating characteristic curves with data from convalescent COVID-19 patients and negative historical control samples. The cutoff value for positivity was set at > 2.5 AU/ml for anti-S IgM, > 0.5 AU/ml for anti-S IgA, > 0.03 AU/ml for anti-S IgG, > 8.4 AU/ml for anti-RBD IgM, > 0.08 AU/ml for anti-RBD IgA, and > 14.8 AU/ml for anti-RBD IgG, giving a specificity of 96% for IgM, 99% for IgA and 97% IgG.
For assessing the anti-RBD IgG binding activity against VOCs, plates were coated with RBD derived from Alpha, Beta, Gamma, and Delta (1.7 μg/mL) and 1:1600 dilution of selected serum or plasma collected from February to May 2021 at early (15–106 days, median day of 24) and late (259–452 days, median day of 370) phase of convalescence were added. Horseradish peroxidase (HRP)-conjugated goat anti-human IgG and tetramethylbenzidine substrate were added as described above and the color reaction was stopped with 0.5M H2SO4 after 10 min incubation. All samples were run in the same experiment and the results expressed as OD450. As an internal control, a diluted in-house developed monoclonal antibody cross-binding all RBD variants was used.
Neutralization
SARS-CoV-2 strain G614 and VOCs (Alpha, Beta, Gamma, and Delta) were isolated from patients in Pavia, Italy and identified through next-generation sequencing method. A microneutralization assay was used to determine the titers of neutralizing antibodies against SARS-CoV-2 strains in 171 samples (Bonelli et al., 2020; Percivalle et al., 2020). Briefly, 50 μL of sample, starting from 1:10 in a serial two-fold dilution series (up to 1:640), was added to two wells of a flat-bottom tissue-culture microtitre plate (COSTAR, Corning Incorporated), mixed with an equal volume of 100 TCID50 of a SARS-CoV-2 strain, previously titrated and incubated at 33°C in 5% CO2. All dilutions were made in Eagle's minimum essential medium with addition of 1% penicillin, streptomycin and glutamine and 5 μg/mL of trypsin. After 1 h of incubation at 33 °C in 5% CO2, VERO E6 cells (VERO C1008 (Vero 76, cloneE6, Vero E6); ATCC® CRL-1586™) were added to each well. After 48 h of incubation at 33°C in 5% CO2, wells were stained with Gram's crystal violet solution (Merck) plus 5% formaldehyde 40% m/v (Carlo ErbaSpA) for 30 min. Microtitre plates were then washed in running water. Wells were scored to evaluate the degree of cytopathic effect compared with the virus control. Blue staining of wells indicated the presence of neutralizing antibodies. The neutralizing titer (NT90) was the maximum dilution giving a reduction of 90% of the cytopathic effect. The cutoff for positivity was ≥1:10. Positive and negative controls were included in all test runs.
Isolation of PBMCs
PBMCs were isolated from blood or buffy coat samples by standard density gradient centrifugation using Lymphoprep (Axis-Shield) and were cryopreserved and stored in liquid nitrogen until analysis.
ELISPOT and FluoroSpot
After thawing and washing, the cells were counted with trypan blue. PBMCs were incubated for four days in RPMI-1640 medium with 10% FCS, supplemented with the TLR7 and TLR8 agonist imidazoquinoline resiquimod (R848, 1 μg/mL; Mabtech AB, Nacka, Sweden), and recombinant human IL-2 (10 ng/mL) for stimulation of memory B cells. The ELISpot plates pre-coated with capturing monoclonal anti-human IgG antibodies were incubated with a total of 300 000 or 30 000 viable pre-stimulated cells per well for detection of RBD-specific IgG and total IgG (positive control) secreting cells, respectively. The number of B cells secreting SARS-CoV-2 RBD-specific IgG and total IgG were measured using the Human IgG SARS-CoV-2 RBD ELISpotPLUS kit (Mabtech AB) (Sherina et al., 2021).
S1 and S N M O specific IFN-γ and IL-2-secreting T cells were detected using the Human IFN-γ/IL-2 SARS-CoV-2 FluoroSpotPLUS kit (Mabtech AB) (Sherina et al., 2021). The plates pre-coated with capturing monoclonal anti-IFN-γ and anti-IL-2 were incubated overnight in RPMI-1640 medium containing 10% FCS supplemented with a mixture containing the SARS-CoV-2 peptide pool (scanning or defined pools), anti-CD28 (100 ng/mL) and 300 000 viable cells per well in humidified incubators (5% CO2, 37°C). A polyclonal activator for human T cells (anti-human CD3 monoclonal antibody CD3-2, #3605-1, Mabtech) was used as a positive control for cytokine secretion. The SARS-CoV-2 S1 scanning pool contains 166 peptides from the human SARS-CoV-2 virus (#3629-1, Mabtech AB). The peptides are 15-mers overlapping with 11 amino acids, covering the S1 domain of the S protein (amino acid 13-685). The SARS-CoV-2 S N M O defined peptide pool contains 47 synthetic peptides binding to human HLA, derived from the S, N, M ORF3a and ORF7a proteins (#3622-1, Mabtech AB) (Peng et al., 2020). The SARS-CoV-2 S2 N defined peptide pool contains 41 synthetic peptides binding to human HLA derived from the S and N proteins of the SARS-CoV-2 virus (#3620-1, Mabtech AB) (Ahmed et al., 2020).
Results of ELISpot and FluoroSpot assays were evaluated using an IRIS-reader and analyzed by the IRIS software version 1.1.9 (Mabtech AB). The results were expressed as the number of spots per 300 000 seeded cells after subtracting the background spots of the negative control. The cutoff value was set at the highest number of specific B- and T cell spots from the negative controls. The number of SARS-CoV-2 specific T cells (per 300 000 cells) producing either IL-2, IFN-γ or both IL-2 and IFN-γ (IL-2/IFN-γ) were plotted.
Quantification and statistical analysis
Mann-Whitney U test was used for comparisons between groups in anti-SARS-CoV-2 antibody levels, neutralization titers and numbers of specific memory B and T cells. Correlation analysis was performed using Spearman’s rank correlation. The number of patients showing neutralization activity against variants was compared by Fisher’s exact test. A Wilcoxon signed-rank test was used for comparison of paired samples. Continuous decay (linear regression), one-phase decay, two-phase decay, polynomial second order or log-normal of non-transformed and log2 transformed data were assessed with the best fitting statistical model chosen based on the F test. The half-lives of antibody and neutralization titers were estimated by a one-phase exponential decay model using non-transformed and log2 transformed data, respectively. All analyses and data plotting were performed using GraphPad or R version 3.6.1. Statistical significance are presented in the figures as asterisks (∗p≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 and ∗∗∗∗p < 0.0001).
Acknowledgments
We thank all the patients, blood donors, clinicians, and nurses for their contributions. This project was supported by the European Union’s Horizon 2020 Research and Innovation Programme (ATAC, No. 101003650), the Italian Ministry of Health (Ricerca Finalizzata grant no. GR-2013-02358399), the Center for Innovative Medicine, Knut and Alice Wallenberg Foundation, and the Swedish Research Council. J.A. was supported by the SciLifeLab/Knut and Alice Wallenberg national COVID-19 research program project grant 2020.
Author contributions
F.Z. performed the ELISA experiment. A.P., L.D., I.C., E.P., J.C.S., J. Attevall, F. Bergami, M.C., and F. Baldanti contributed to patient data curation, sample preparation, and the neutralization assay. L.D., Y.W., and S.V. designed and/or performed the ELISpot and FluoroSpot experiments. M.V., M. Sambo, V.Z., E.A., R.B., T.O., and F.M. contributed to the enrollment of patients, patient management, and collection of clinical data. J. Andréll, F. Bertoglio, M. Schubert, and M.H. performed the production and purification of proteins from SARS-CoV-2 and variants. H.M., F.Z., L.D., I.C., H.W., M.K.-B., E.P., H.A., L.C., L.V, L.H., and Q.P.-H. contributed to the analysis and interpretation of data. H.M. and Q.P.-H. drafted the manuscript. H.M., A.P., Y.X., L.H., F.Baldanti, and Q.P.-H. conceived and supervised the study. All authors reviewed the manuscript.
Declaration of interests
The other authors declare no competing interests.
Published: February 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.103743.
Supplemental information
Data and code availability
The article includes all datasets generated or analyzed during this study.
-
•
Data reported in this paper will be shared by the lead contact upon reasonable request.
References
- Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S.P., Prevost J., Nayrac M., Beaudoin-Bussieres G., Benlarbi M., Gasser R., Brassard N., Laumaea A., Gong S.Y., Bourassa C., et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021;2:100290. doi: 10.1016/j.xcrm.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoglio F., Fuhner V., Ruschig M., Heine P.A., Abassi L., Klunemann T., Rand U., Meier D., Langreder N., Steinke S., et al. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021;36:109433. doi: 10.1016/j.celrep.2021.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann M., Schurig M., Endres M., Samuels A., Gebauer D., Konisch N., van den Heuvel J. Identifying parameters to improve the reproducibility of transient gene expression in high five cells. PLoS One. 2019;14:e0217878. doi: 10.1371/journal.pone.0217878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli F., Sarasini A., Zierold C., Calleri M., Bonetti A., Vismara C., Blocki F.A., Pallavicini L., Chinali A., Campisi D., et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J. Clin. Microbiol. 2020;58:e01224-20. doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Almeida J.R., Darrah P.A., Sauce D., Seder R.A., Appay V., Gorochov G., Larsen M. Pathogen-specific T Cell polyfunctionality is a correlate of T Cell efficacy and immune protection. PLoS One. 2015;10:e0128714. doi: 10.1371/journal.pone.0128714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach A.S., Riley P.A., Cotter M.P., Houston A.C., Habibi M.S., Planche T.D. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J. Infect. 2021;82:e11–e12. doi: 10.1016/j.jinf.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M., Söderberg-Nauclér C. Indications that Stockholm has reached herd immunity, given limited restrictions, against several variants of SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.07.07.21260167. [DOI] [Google Scholar]
- Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., Liu J., Errico J.M., Xie X., Suryadevara N., et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021;27:717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.W., Le Bert N., Tan C.W., Tiu C., Zhang J., Tan S.Y., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K.W., Linderman S.L., Moodie Z., Czartoski J., Lai L., Mantus G., Norwood C., Nyhoff L.E., Edara V.V., Floyd K., et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021;2:100354. doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S., Felgner P., Davies H., Glidewell J., Villarreal L., Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Jarvis C.I., Group C.C.-W., Edmunds W.J., Jewell N.P., Diaz-Ordaz K., Keogh R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar M.S., Marwal R., Vs R., Ponnusamy K., Jolly B., Bhoyar R.C., Sardana V., Naushin S., Rophina M., Mellan T.A., et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science. 2021;374:995–999. doi: 10.1126/science.abj9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giallonardo F., Puglia I., Curini V., Camma C., Mangone I., Calistri P., Cobbin J.C.A., Holmes E.C., Lorusso A. Emergence and spread of SARS-CoV-2 lineages B.1.1.7 and P.1 in Italy. Viruses. 2021;13:794. doi: 10.3390/v13050794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edara V.V., Norwood C., Floyd K., Lai L., Davis-Gardner M.E., Hudson W.H., Mantus G., Nyhoff L.E., Adelman M.W., Fineman R., et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe. 2021;29:516–521.e513. doi: 10.1016/j.chom.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e1415. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Guo Z., Duan C., Chen Z., Wang G., Lu Y., Li M., Lu J. Long-term persistence of IgG antibodies in SARS-CoV infected healthcare workers. medRxiv. 2020 2020.02.12.20021386. [Google Scholar]
- Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hansen C.H., Michlmayr D., Gubbels S.M., Molbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley G.E., Edwards E.S.J., Aui P.M., Varese N., Stojanovic S., McMahon J., Peleg A.Y., Boo I., Drummer H.E., Hogarth P.M., et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci. Immunol. 2020;5:eabf8891. doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R.A., Rassen J.A., Kabelac C.A., Turenne W., Leonard S., Klesh R., Meyer W.A., 3rd, Kaufman H.W., Anderson S., Cohen O., et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern. Med. 2021;181:672–679. doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra S., Ye C., Rathnasinghe R., Stadlbauer D., Personalized Virology Initiative Study, Group. Krammer F., Simon V., Martinez-Sobrido L., Garcia-Sastre A., Schotsaert M. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021;2:e283–e284. doi: 10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Korn J., Schackermann D., Kirmann T., Bertoglio F., Steinke S., Heisig J., Ruschig M., Rojas G., Langreder N., Wenzel E.V., et al. Baculovirus-free insect cell expression system for high yield antibody and antigen production. Sci. Rep. 2020;10:21393. doi: 10.1038/s41598-020-78425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., Zhao H., Errico J.M., Theel E.S., Liebeskind M.J., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29:477–488.e474. doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley S.F., O'Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., Marsden B.D., Cox S., James T., Warren F., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Delta variant: what is happening with transmission, hospital admissions, and restrictions? BMJ. 2021;373:n1513. doi: 10.1136/bmj.n1513. [DOI] [PubMed] [Google Scholar]
- Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R.E., Heitman J.W., Hirschkorn D.F., Lanteri M.C., Biswas H.H., Martin J.N., Krone M.R., Deeks S.G., Norris P.J., Immunology, N.C.f.H.A.V. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS. 2010;24:1095–1105. doi: 10.1097/QAD.0b013e3283377a1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percivalle E., Cambie G., Cassaniti I., Nepita E.V., Maserati R., Ferrari A., Di Martino R., Isernia P., Mojoli F., Bruno R., et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the lodi red zone in lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25:2001031. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradenas E., Trinite B., Urrea V., Marfil S., Avila-Nieto C., Rodriguez de la Concepcion M.L., Tarres-Freixas F., Perez-Yanes S., Rovirosa C., Ainsua-Enrich E., et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med (N Y) 2021;2:313–320.e314. doi: 10.1016/j.medj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Ferguson I.D., Miao W., Khavari P.A. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect. Dis. 2021;21:1070. doi: 10.1016/S1473-3099(21)00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda L.B., Netland J., Shehata L., Pruner K.B., Morawski P.A., Thouvenel C.D., Takehara K.K., Eggenberger J., Hemann E.A., Waterman H.R., et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e117. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltgen K., Boyd S.D. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe. 2021;29:1063–1075. doi: 10.1016/j.chom.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., Hunter M., Wang H., Sahoo M.K., Huang C., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020;5:eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012 e1019. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino E.C., Buss L.F., Carvalho M.P.S., Prete C.A., Jr., Crispim M.A.E., Fraiji N.A., Pereira R.H.M., Parag K.V., da Silva Peixoto P., Kraemer M.U.G., et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler A., Angermair S., Stockmann H., Heim K.M., Khadzhynov D., Treskatsch S., Halleck F., Kreis M.E., Kotsch K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Invest. 2020;130:6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T Cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e114. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A., McMenamin J., Taylor B., Robertson C., Public Health S., the E.I.I.C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesch M., Andrell J., Braesch-Andersen S., Cassaniti I., Percivalle E., Sarasini A., et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med. (N Y) 2021;2:281–295.e284. doi: 10.1016/j.medj.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Dingens A.S., Bloom J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep. Med. 2021;2:100255. doi: 10.1016/j.xcrm.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.T., Linster M., Tan C.W., Le Bert N., Chia W.N., Kunasegaran K., Zhuang Y., Tham C.Y.L., Chia A., Smith G.J.D., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K., Tzou P.L., Nouhin J., Gupta R.K., de Oliveira T., Kosakovsky Pond S.L., Fera D., Shafer R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021;22:757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., Hansen L., Haile A., Klebert M.K., Pusic I., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.L., Wang Z.Y., Duan L.J., Meng Q.C., Jiang M.D., Cao J., Yao L., Zhu K.L., Cao W.C., Ma M.J. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N. Engl. J. Med. 2021;384:2354–2356. doi: 10.1056/NEJMc2103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., Ho D.D. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751.e744. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T., Liang B., Fang Y., Lu S., Li S., Wang H., Li H., Yang X., Shen S., Zhu B., et al. Declining levels of neutralizing antibodies against SARS-CoV-2 in convalescent COVID-19 patients one year post symptom onset. Front. Immunol. 2021;12:708523. doi: 10.3389/fimmu.2021.708523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The article includes all datasets generated or analyzed during this study.
-
•
Data reported in this paper will be shared by the lead contact upon reasonable request.