Abstract
Background
Supervised high-intensity interval training (HIIT) has been proposed to be more effective than moderate-intensity continuous training (MICT) for improving exercise capacity, but there are not sufficient information effects of home-based HIIT and MICT in patients with myocardial infarction (MI).
Aims
To compare the effects of home-based HIIT and MICT in patients with MI.
Methods
Twenty-one patients with MI were randomly assigned to one of two home-based exercise modes: HIIT group and MICT group. Home-based HIIT and MICT were performed twice a week for 12 weeks with an exercise intensity of 85–95% of heart rate (HR) reserve and 70–75% HR reserve, respectively. The primary outcome measure was functional capacity. Secondary outcomes included resting blood pressure and HR, peripheral oxygen saturation, pulmonary function and respiratory muscle strength, dyspnea severity, body composition (body fat%, body mass ındex (BMI), fat free muscle), peripheral muscle strength, and health-related quality of life (HRQoL).
Results
Functional capacity, measured by 6-minute walk test, increased in HIIT and MICT group (p < 0.05). Resting BP and HR, body fat%, and BMI were significantly decreased, and pulmonary functions, respiratory-peripheral muscle strength, and HRQoL were significantly increased in the both groups (p < 0.05). Home-based HIIT was more effective than MICT in improving pulmonary functions and lower extremity muscle strength (p < 0.05).
Conclusions
This study suggests that HIIT and MICT can be applied at home-based in patients with MI and play an important role in improving functional capacity, health outcomes, and HRQoL.
Trial registration
Clinical Trials Number: NCT04407624.
Keywords: Functional capacity, Health-related quality of life, High-intensity interval training, Home-based exercise, Myocardial infarction
Background
Myocardial infarction (MI) continues to be the leading cause of death and disability worldwide, with significant physical, psychological, and social consequences for society. However, in addition to pharmacological treatments that have shown significant development in the last two decades, exercise training has become a widely used adjuvant therapy for patients with acute MI [1]. Recently evidences demonstrate that exercise training after acute MI may significantly improve endothelial function, cardiovascular efficiency, exercise capacity, quality of life, and survival rates, as well as reduce the incidence of re-infarction [2].
Aerobic exercise training aiming to maintain and improve aerobic capacity has become an important part of cardiac rehabilitation [3, 4]. Intensity of aerobic exercise training is an important factor in treatment effectiveness, and accumulating evidences have shown that high-intensity interval training (HIIT) is more effective than low-to-moderate-intensity continuous training (MICT) in improving cardiac output, aerobic capacity, and cardiovascular risk profile [5–7]. It is known that the risks of a well-designed supervised exercise program are very low, as previous studies have mostly conducted with strictly supervised of exercise intensity in patients with MI [8, 9]. On the other hand, home-based exercise training protocols were previously considered valid options in patients who have undergone cardiac rehabilitation. A recent systematic review found that home-based and center-based cardiac rehabilitation were equally effective in improving health-related quality of life (HRQoL) and functional capacity [10, 11].
There is limited knowledge about the risks, feasibility, and effects of home-based HIIT and MICT exercises. Owing to lack of home-based exercise recommendations for patients with MI, the aim of this randomized controlled trial (RCT) was to evaluate and compare the effects of HIIT and MICT in patients with MI.
Methods
Study design
The study was a RCT where the patients were randomly allocated to one of two modes of exercise programs; HIIT group and MICT group. The allocation ratio was 1:1 and randomization was stratified by age and gender. Randomization was performed block design by a computer-generated list of random numbers (the Random Number Generator Pro v2.00 software (Segobit, Issaquah, WA, USA)) after the baseline measurements. Participants were assigned a depersonalized identification number to provide blinding during data analyses. Measurements were made by unblinded evaluators at baseline and immediately after the end of the exercise programs (after 12 weeks).
Participants
This RCT was carried out at the outpatients’ cardiopulmonary rehabilitation clinic of Dokuz Eylül University Hospital. Inclusion criteria were diagnosed MI, being between the ages of 35 and 65 years, left ventricular ejection fraction > 50%, being between 3 months and 1 year after the MI, clinically stable on sinus rhythm, able to walk independently, and being volunteer to participate in the study. Exclusion criteria were having severe heart failure, severe arrhythmias, atrial fibrillation, uncontrolled hypertension, hemodynamic instability, severe kidney disease, severe peripheral artery disease, musculoskeletal, and neurological problems interfering with exercise, medical condition contraindicative to high-intensity training and participating in any exercise program in the past 6 months. All patients were stable in terms of disease severity and medical treatment.
Interventions
All patients in two groups performed exercise programs twice a week (non-consecutive days) for 12 weeks. The home-based exercise programs started with two initial sessions with personal instruction of two physiotherapists where they learned how to perform HIIT and MICT and how to calculate and monitor heart rate (HR). All participants were individually instructed in use of the HR monitor (Polar H10 HR Sensor, UK), and how to reach target HR. HR reserve and the rate of perceived exertion (RPE) using the Borg’s original scale (6–20 points) were used to determine aerobic training intensity in HIIT and MICT. In both groups, each session started with a 10-min warm-up and finished with a 10-min cool-down period. Warm-up and cool-down period included stretching, flexibility exercises (i.e., the neck, shoulders, upper back, hips, and ankles), and low-to-moderate intensity (50–70% of HR reserve) walking.
HIIT sessions included four intervals lasting 4 min each at an exercise intensity of 85–95% of HR reserve and 15–18 intensity according to the RPE. Each interval was separated by active recovery with 3 min of walking at 70% HR reserve (RPE < 14). HIIT was performed in the preferred exercise mode in the home environment: walking uphill, brisk walking, jogging, crouching, going up and down the front-side steps. MICT was performed with walking at an RPE of 12–14 and HR reserve of 70–75% for 20–45 min. The patients exercised at the lower intensity and time limit for the first 2 weeks of the training period before increasing the intensity towards the upper limit. The exercise programs were progressed by increasing the intensity and duration of the exercises every 2 weeks. The patients were given a brochure containing the exercises in written and visual form and an exercise diary for exercise program follow-up. In addition, the patients were provided weekly phone counsel to inquire and assess any problems related to exercise and provide positive reinforcement. Weekly text messages were delivered to check whether patients completed their daily exercise.
Outcome measures
Demographic and clinical characteristics, and primary and secondary outcomes were measured on the same day, respectively. The primary outcome was functional capacity. Secondary outcomes were resting blood pressure, HR and peripheral oxygen saturation, Charlson comorbidity index, pulmonary functions, respiratory muscle strength, dyspnea severity, body composition, peripheral muscle strength, and HRQoL. The patients rested for 5 min between the each assessment to avoid test-related fatigue. All measurements were made at the beginning and at the end of the 12-week exercise programs.
Primary outcome
Functional capacity
The primary outcome was functional capacity measured by the 6 minute walk test (6MWT). All 6MWTs were conducted according to American Thoracic Society/ATS guidelines [12].
Secondary outcomes
Resting blood pressure, heart rate, and peripheral oxygen saturation
Resting BP and HR were evaluated using an automated blood pressure monitor (OSZ5). Pulse oximetry was used to measure peripheral oxygen saturation (SpO2) using a Nellcor N-200 co-oximeter finger probe in a sitting position.
Charlson comorbidity index
The Charlson comorbidity index is scored according to the severity of comorbid diseases. Comorbidities are scored as 1, 2, 3, and 4, respectively, from mild to severe disease [13].
Pulmonary function
Pulmonary function test was measured with a portable digital spirometer (Pony FX, COSMED). Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, and peak expiratory flow (PEF) were measured in accordance with the methods recommended by the ATS/European Respiratory Society (ERS) [14].
Respiratory muscle strength
Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were measured with a digital manometer (Pony FX, COSMED) as indicators of inspiratory and expiratory muscle strength, respectively, in accordance with the ATS/ERS [15].
Dyspnea severity
The severity of dyspnea perceived by the patients in activities of daily living was evaluated using the Modified Medical Research Council (mMRC) scale. The mMRC is a 0–4 point categorical scale range of dyspnea from nothing (grade 0) to almost complete disability (grade 4). In addition, ratings of the overall perception of effort (RPE) were made using the original Borg scale (6–20 points) [16, 17].
Body composition
Body weight, body mass index, body fat %, and fat free mass (FFM) were measured without shoes and in light clothing using Tanita electronic (Body Composition Analyzer, TBF—300).
Peripheral muscle strength
Dominant side knee extensors (M. quadriceps femoris) were evaluated with a hand-held dynamometer (HHD, MicroFET2®). Handgrip strength of the dominant hand was assessed using the Jamar Hydraulic Hand Dynamometer, using the average of three measurements [18, 19].
Health-related quality of life
MacNew Heart Disease Health-Related Quality of Life Questionnaire was used for assessment of HRQoL. MacNew Heart Disease HRQoL consists of 27 items assessing the perceived physical, emotional, and social functioning status of heart patients in the previous 2 week period. Higher scores indicate better HRQoL [20].
Statistical analysis
Data were analyzed on a per-protocol basis, using the statistical software package “Statistical Package for Social Sciences” (SPSS) (Version 23.0, IBM Corp., Armonk, NY, USA). The normal distribution of the data was analyzed by examining the Shapiro–Wilk test. Continuous variables were presented by giving mean and standard deviation following normal distribution and the remaining variables were summarized as median (interquartile range (IQR)). Categorical variables were expressed as numbers and percentages. Differences in proportions were tested using chi-squared tests or Fisher’s exact test. The baseline comparisons between the two groups were performed using independent Student’s t-tests and Mann–Whitney U tests. Paired t-tests and Wilcoxon rank-sum test were performed to test the significance of changes from baseline at 12 weeks. To compare any change in the outcome measures among the two groups, independent Student’s t-tests and Mann–Whitney U tests were performed. The significance level was set at p < 0.05. Based on the results of previous studies, the sample size was calculated using the G*Power program for detecting at least a 15% difference in the 6MWT distance between the initial and final test with an effect size of 1.17, power of 85%, and a 0.05 α value [21]. Thus, the minimum sample size required to detect a significant difference should be at least a total of 24 subjects (with 9 subjects in each group + 3 subjects per group, considering dropouts 25%).
Results
Participants
Forty patients with MI recruited from Dokuz Eylül University Hospital. Ten patients were not included in the study because they did not meet the inclusion criteria. Moreover, 6 patients refused to participate in the study due to reasons such as family issues, limited time, the thought of unable to exercise, and fear of COVID-19 pandemic. The 24 eligible patients were divided into randomly and equally the HIIT (n = 12) and MICT (n = 12) groups. One patient in HIIT and two patients in MICT did not complete the study due to the COVID-19 pandemic and lack of motivation. Finally, the data of 21 patients who completed the exercise programs were analyzed. A flow diagram of the progress of the study is presented in Fig. 1. No adverse events, defined as cardiac arrests, arrhythmias, acute MI, or ankle sprain, were registered during the exercise interventions.
Fig. 1.
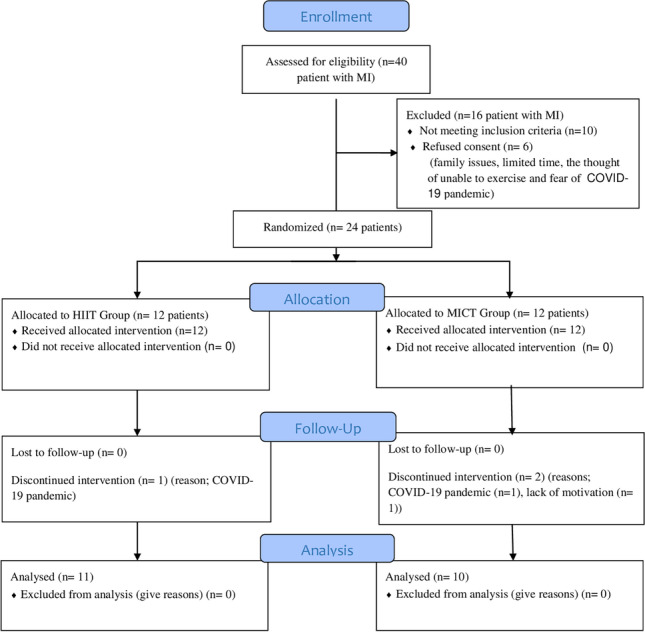
Flow diagram of the study. MI myocardial infarction, HIIT high-intensity interval training, MICT moderate-intensity continuous training
The demographic and clinical features of patients are shown in Table 1. The groups did not differ significantly in any baseline demographic and clinical features (p > 0.05). There was no significant difference between HIIT and MICT groups for the baseline primary and secondary outcomes (p > 0.05, Table 2).
Table 1.
Demographic and clinical parameters of groups before the intervention
| HIIT | MICT | p | |
|---|---|---|---|
| Gender, male/female (%) | 10(90)/1(10) | 8 (80)/2(20) | 0.566§ |
| Age (yrs) | 59.6 ± 4.5 | 58.5 ± 5.6 | 0.652* |
| Height (cm) | 168.8 ± 9.1 | 169.1 ± 5.7 | 0.934* |
| Weight (kg) | 82.1 ± 13.2 | 82.3 ± 16.7 | 0.979* |
| Month between EI and MI | 5.5 ± 0.6 | 5.0 ± 1.2 | 0.305* |
| Comorbidity | |||
| Hypertension, n (%) | 6 (54) | 7 (70) | 0.474* |
| Diabetes mellitus, n (%) | 4 (36) | 6 (60) | 0.370* |
| Chronic pulmonary disease, n (%) | 3 (27) | 4 (40) | 0.642* |
| Charlson comorbidity index score | 3.0 (2.0, 3.0) | 3.0 (2.5, 5.0) | 0.311† |
| Intervention | |||
| PCI, n (%) | 6 (54) | 8 (80) | 0.457* |
| CABG, n (%) | 3 (27) | 4 (40) | 0.642* |
| Smoking habits | |||
| Smoker, n (%) | 5 (45) | 4 (40) | 0.850§ |
| Ex-smoker, n (%) | 4 (36) | 5 (50) | 0.850§ |
| Non-smoker, n (%) | 2 (19) | 1 (10) | 0.769§ |
| Cigarette consumption (pack-yrs) | 27.4 ± 14.7 | 29.2 ± 18.1 | 0.861 |
| Regular exercise, yes/no, n (%) | 4(36)/ 7(64) | 2 (20)/ 8(80) | 0.329§ |
Data are expressed as mean ± SD and median (interquartile range) or n (%), § chi-squared test or Fisher’s exact test,
HIIT high-intensity interval training, MICT moderate-intensity continuous training, MI myocardial infarction, EI exercise intervention, PCI percutaneous coronary intervention, CABG coronary artery bypass graft
*independent Student’s t-tests,
† Mann–Whitney U tests,
Table 2.
High-intensity interval training group versus moderate-intensity continuous training group: baseline outcome variables
| Variables | HIIT | MICT | p |
|---|---|---|---|
| Primary outcome | |||
| Functional capacity | |||
| 6MWT walking distance (m) | 509.0 ± 59.8 | 491.8 ± 79.7 | 0.654* |
| Secondary outcomes | |||
| Resting blood pressure and heart rate | |||
| Systolic BP (mmHg) | 141.6 ± 10.1 | 145.5 ± 12.6 | 0.363* |
| Diastolic BP (mmHg) | 79.1 ± 12.2 | 85.0 ± 9.3 | 0.248* |
| Heart rate (beats/min) | 77.1 ± 13.6 | 85.3 ± 18.7 | 0.269* |
| Peripheral oxygen saturation | |||
| SpO2, % | 95.1 ± 2.1 | 95.1 ± 0.8 | 0.978 |
| Pulmonary function | |||
| FEV1, % pred | 82.9 ± 9.2 | 77.0 ± 16.7 | 0.330* |
| FVC, % pred | 83.4 ± 7.8 | 79.8 ± 10.8 | 0.388* |
| FEV1/FVC, % | 79.1 ± 5.1 | 74.3 ± 11.3 | 0.228* |
| PEF, % pred | 69.3 ± 16.2 | 69.7 ± 16.1 | 0.945* |
| Respiratory muscle strength | |||
| MIP, cmH2O % pred | 92.4 ± 12.7 | 91.3 ± 16.0 | 0.892* |
| MEP, cmH2O % pred | 63.5 ± 12.8 | 64.3 ± 12.5 | 0.842* |
| Dyspnea severity | |||
| mMRC score | 0.5 (0.0, 1.0) | 1.0 (0.5, 2.0) | 0.201† |
| Body composition | |||
| BMI (kg/m2) | 29.3 ± 5.2 | 29.4 ± 5.3 | 0.965* |
| Body fat % | 29.1 ± 9.6 | 29.8 ± 7.3 | 0.883* |
| FFM (kg) | 57.4 ± 7.7 | 57.68 ± 9.1 | 950* |
| Peripheral muscle strength | |||
| KES (kg) | 10.5 ± 2.7 | 9.3 ± 2.5 | 0.850* |
| HGS (kg) | 36.9 ± 7.4 | 33.9 ± 10.0 | 0.456* |
| Health-related quality of life | |||
| MacNew HRQoL | |||
| Physical | 5.5 ± 1.0 | 5.6 ± 1.2 | 0.706* |
| Emotional | 5.6 ± 0.9 | 5.5 ± 1.1 | 0.307* |
| Social | 5.7 ± 1.1 | 5.2 ± 1.0 | 0.619* |
| Global | 5.5 ± 0.6 | 5.5 ± 1.1 | 0.267* |
Data are expressed as mean ± SD and median (interquartile range),
HIIT high-intensity interval training, MICT moderate-intensity continuous training, 6MWT 6-min walk test, BP blood pressure, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, PEF peak expiratory flow, MIP maximal inspiratory pressure, MEP maximal expiratory pressure, mMRC Modified Medical Research Council, BMI body mass index, FFM fat free muscle, KES knee extensor strength, HGS handgrip strength, HRQoL health-related quality of life
* independent Student’s t-tests,
† Mann–Whitney U tests,
Primary outcome
There were statistically significant increases in 6MWT distance in both groups (Table 3). However, there was no statistically significant difference between the groups in changes from baseline to 12 weeks (HIIT: 40.3 ± 34.4 and MICT: 25.5 ± 20.7, p = 0.386). The number of patients in each group reaching the minimum clinical difference of 60 m indicated for 6MWT was similar, 27% in the HIIT group and 20% in the MICT group (p > 0.05).
Table 3.
Comparison of changes in clinical parameters of groups after the intervention
| Variables | HIIT | MICT | |||||
|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | p | |
| 6MWT (m) | 509.0 ± 59.8 | 549.4 ± 62.3 | 0.013§ | 491.9 ± 79.7 | 517.4 ± 82.1 | 0.015§ | |
| ∆ 6MWT (m) | 40.3 ± 34.4 | 25.5 ± 20.7 | 0.386* | ||||
| SBP (mmHg) | 141.6 ± 10.1 | 129.0 ± 9.8 | 0.002§ | 145.5 ± 12.6 | 135.1 ± 16.1 | 0.011§ | |
| ∆SBP(mmHg) | − 11.6 ± 9.4 | − 10.4 ± 9.5 | 0.782* | ||||
| DBP (mmHg) | 79.1 ± 12.2 | 73.8 ± 11.2 | 0.005§ | 85.0 ± 9.3 | 77.6 ± 6.9 | 0.015§ | |
| ∆DBP(mmHg) | − 5.3 ± 4.9 | − 7.4 ± 6.2 | 0.435* | ||||
| HR(beats/min) | 77.1 ± 13.6 | 68.2 ± 10.2 | 0.005§ | 85.3 ± 9.3 | 74.9 ± 10.9 | 0.026§ | |
| ∆HR(beats/min) | − 8.9 ± 7.1 | − 10.4 ± 10.3 | 0.731* | ||||
| SpO2, % | 95.1 ± 2.1 | 96.1 ± 2.1 | 0.008§ | 95.1 ± 0.8 | 96.3 ± 1.1 | 0.023§ | |
| ∆SpO2, % | 1.0 ± 1.0 | 1.2 ± 1.2 | 0.671* | ||||
| FEV1, %pred | 82.9 ± 9.2 | 87.0 ± 7.5 | 0.002§ | 77.0 ± 16.8 | 78.3 ± 17.3 | 0.022§ | |
| ∆ FEV1, %pred | 4.1 ± 1.6 | 1.3 ± 1.1 | 0.034* | ||||
| FVC, %pred | 83.4 ± 7.8 | 87.5 ± 7.8 | < 0.001§ | 79.8 ± 10.8 | 81.2 ± 9.6 | 0.032§ | |
| ∆ FVC, %pred | 4.1 ± 1.6 | 1.4 ± 1.2 | 0.002* | ||||
| FEV1/FVC, % | 79.1 ± 5.1 | 82.4 ± 5.8 | < 0.001§ | 74.3 ± 11.3 | 76.9 ± 9.8 | 0.004§ | |
| ∆FEV1/FVC, % | 3.3 ± 1.6 | 2.5 ± 1.8 | 0.379* | ||||
| PEF, %pred | 69.3 ± 16.2 | 73.4 ± 15.2 | 0.014§ | 69.8 ± 16.1 | 70.7 ± 16.6 | 0.009§ | |
| ∆ PEF, %pred | 4.2 ± 3.6 | 0.9 ± 0.7 | 0.043* | ||||
| MIP, %pred | 92.4 ± 12.7 | 107.7 ± 20.1 | 0.006 § | 91.3 ± 16.0 | 92.5 ± 15.4 | 0.249§ | |
| ∆ MIP, %pred | 15.4 ± 13.7 | 1.9 ± 1.2 | 0.110* | ||||
| MEP, %pred | 63.5 ± 12.8 | 78.4 ± 13.4 | 0.001§ | 64.3 ± 12.5 | 80.1 ± 25.1 | 0.006§ | |
| ∆ MEP, %pred | 14.9 ± 9.9 | 16.0 ± 11.1 | 0.835* | ||||
| mMRC score |
0.5 (0.0, 1.0) |
0.0 (0.0, 0.0) |
0.049** |
1.0 (0.5, 2.0) |
1.0 (0.0, 1.5) |
0.157** | |
| ∆ mMRC score |
0.0 (− 1.0, 0.0) |
0.0 (− 0.5, 0.0) |
0.552† | ||||
Data are expressed as mean ± SD and median (interquartile range),
HIIT high-intensity interval training, MICT moderate-intensity continuous training, ∆ after-before difference, 6MWT 6-min walk test, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, SpO2 peripheral oxygen saturation, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, PEF peak expiratory flow, MIP maximal inspiratory pressure, MEP maximal expiratory pressure, mMRC Modified Medical Research Council
* independent Student’s t-tests,
** Wilcoxon test,
† Mann–Whitney U tests, § paired Student t tests,
Secondary outcomes
After exercise interventions, both groups had a significant decrease in rest systolic-diastolic blood pressure, HR, and increase in SpO2 (p < 0.05). There was no significant difference between the two groups in terms of changes in these parameters (p > 0.05). Both groups showed a statistically significant increase in FEV1%, FVC%, FEV1/FVC%, and PEF% (p < 0.05). The HIIT group demonstrated greater improvements in FEV1%, FVC%, and PEF% (p = 0.034, p = 0.002, and p = 0.043, respectively). HIIT group had a significant increase in MIP% and MEP% and decrease in mMRC score. Significant increases were observed in the MEP% (p = 0.006), while no significant changes were in the MIP% and mMRC score in MICT group (p > 0.05) (Table 3).
While BMI and body fat % decreased significantly in both groups (p < 0.05), there was no significant change in FFM (p > 0.05). The changes in BMI and body fat % were not different across the groups after the completion exercise programs (p = 0.776 and p = 0.287, respectively). Knee extensor and handgrip strength significantly increased in the both groups (p < 0.001). Compared to MICT, the HIIT group demonstrated greater improvements in knee extensor strength and there was no significant difference between the two groups in terms of handgrip strength (p > 0.05). Significant increase was observed in physical, emotional, social, and global score of MacNew HRQoL in HIIT group (p < 0.05). MCIT group only had a significant increase in global score of MacNew HRQoL (p = 0.012). There was no difference between the two groups in terms of changes in subscores and global score of MacNew HRQoL (p > 0.05) (Table 4).
Table 4.
Comparison of changes in clinical parameters of groups after the intervention
| Variables | HIIT | MICT | |||||
|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | p | |
| BMI, (kg/m2) | 29.3 ± 5.2 | 28.1 ± 5.1 | 0.001§ | 29.4 ± 5.3 | 28.3 ± 4.9 | 0.007§ | |
| ∆ BMI, (kg/m2) | -1.2 ± 0.8 | -1.1 ± 0.9 | 0.776* | ||||
| Body fat % | 29.1 ± 9.6 | 26.3 ± 10.3 | 0.012§ | 29.8 ± 7.3 | 27.5 ± 7.9 | 0.008§ | |
| ∆ Body fat % | -2.7 ± 2.6 | -1.6 ± 1.8 | 0.287* | ||||
| FFM (kg) | 57.4 ± 7.7 | 58.5 ± 7.9 | 0.214§ | 57.7 ± 9.1 | 59.1 ± 9.8 | 0.321§ | |
| ∆ FFM (kg) | 1.1 ± 1.7 | 1.4 ± 1.6 | 0.800* | ||||
| KES (kg) | 10.5 ± 2.7 | 14.9 ± 3.1 | < 0.001§ | 9.3 ± 2.5 | 10.3 ± 2.5 | 0.001§ | |
| ∆ KES (kg) | 4.4 ± 2.1 | 1.0 ± 0.6 | < 0.001* | ||||
| HGS (kg) | 36.9 ± 7.4 | 40.6 ± 7.8 | 0.001§ | 33.9 ± 10.0 | 35.8 ± 8.8 | 0.003§ | |
| ∆ HGS (kg) | 3.7 ± 2.7 | 1.9 ± 1.4 | 0.070* | ||||
| MacNew HRQoL | |||||||
| Physical | 5.5 ± 1.0 | 6.0 ± 0.5 | 0.008§ | 5.3 ± 1.0 | 5.6 ± 1.2 | 0.241§ | |
| ∆ Physical | 0.5 ± 0.3 | 0.3 ± 0.1 | 0.362* | ||||
| Emotional | 5.7 ± 0.9 | 6.1 ± 0.7 | 0.005§ | 5.1 ± 1.5 | 5.5 ± 1.1 | 0.062§ | |
| ∆ Emotional | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.862* | ||||
| Social | 5.7 ± 1.1 | 5.8 ± 0.9 | 0.048§ | 5.41 ± 1.43 | 5.2 ± 1.0 | 0.677§ | |
| ∆ Social | 0.1 ± 0.3 | 0.1 ± 0.0 | 0.632* | ||||
| Global | 5.5 ± 0.6 | 5.8 ± 0.5 | 0.008§ | 5.04 ± 1.16 | 5.5 ± 1.1 | < 0.012§ | |
| ∆ Global | 0.3 ± 0.3 | 0.4 ± 0.2 | 0.660* | ||||
Data are expressed as mean ± SD and median (interquartile range),
HIIT high-intensity interval training, MICT moderate-intensity continuous training, ∆ after-before difference, BMI body mass index, FFM fat free muscle, KES knee extensor strength, HGS handgrip strength, HRQoL health-related quality of life
* independent Student’s t-tests,
** Wilcoxon test,
† Mann–Whitney U tests, § paired Student t tests,
Discussion
The current study revealed that home-based HIIT and MICT were effective in improving functional capacity, resting systolic and diastolic BP, resting HR, peripheral oxygen saturation, pulmonary function and respiratory muscle strength, body composition, peripheral muscle strength and HRQoL. Importantly, HIIT was a more potent stimulus in improving pulmonary function and lower limb muscle strength than MICT. Furthermore, no adverse events occurred during the exercise intervention in both groups. Therefore, 12-week home-based HIIT and MICT were feasible and effective in patients with MI.
Despite strong proof of reduced mortality and morbidity from cardiac rehabilitation in patients with cardiovascular disease (CVD), the use of cardiac rehabilitation is reported to be less than 20% in many countries. This has mainly been attributed to patient- and system-related problems such as dislike for group-based classrooms, demands for return to work, lack of CR programs, transportation, poor motivation, and lack of time [22, 23]. Alternative methods have been used to contribute to improving functionality and quality of life in various patients with CVD, including post-MI. Recently a study demonstrated that unsupervised walking-based home cardiac rehabilitation led to improvements in functional capacity and health-related quality of life in post-MI patients [24]. Similarly, an interval and resistive home-based cardiac rehabilitation program increased aerobic capacity and HRQoL in patients with heart failure [25]. Consistent with the results of previous studies, functional capacity increased in the HIIT and MICT group after 12 weeks of home-based exercise programs in our study. However, functional capacity did not differ significantly between groups after intervention. These results indicate that a home-based exercise program including HIIT and MICT can improve exercise capacity in patient with MI. In previous studies, it was stated that a significant clinical improvement in functional capacity is at least 60.4 m as a result of the CR program in patients with CAD [24]. However, in our study, the change in both groups was lower than this clinical improvement value. These differences in results may be due to differences in the type, duration and intensity of exercise training, lack of motivation due to without supervision, and high initial walking distance in our study.
Increasing evidence suggests that HIIT can improve post infarction left ventricular remodeling, and optimize HR recovery, blood pressure, and body composition in patients with MI [26]. Aamot et al. [27] showed that hospital-based and home-based HIIT programs have similar effect on blood pressure, but home-based HIIT is more effective on resting HR. Our study corroborates these findings, showing that home-based HIIT and MICT protocol could lower resting HR and blood pressure. With regard to the respiratory system, derangement of pulmonary function and respiratory muscle dysfunction is a common manifestation in older patients with CVD, including MI [28]. The present study demonstrated that patients with MI in both groups had impairment of FEV1/FVC%, PEF%, and MEP% (< 80%). The MICT group also had impairment of FVC%, FEV1% (< 80%). Tasoulis et al. stated that 12 weeks of HIIT significantly improved respiratory muscle function in older adults with heart failure [29]. Matos-Garcia et al. reported that a home-based walking exercise is effective in improving inspiratory strength and endurance in patients with MI [30]. However, to our knowledge, there are no studies investigating the effects of home-based HIIT and MICT on pulmonary functions and respiratory muscle strength in patients with MI. We found a statistically significant increase in pulmonary function and respiratory muscle strength (especially in expiratory muscle) for both groups, but an extra benefit in pulmonary function for the HIIT exercise component. Previous studies indicated that exercise programs can positively support the inflammatory process and cardiac remodeling, increase lung and chest wall compliance, and increase the endurance of type II fibers [25, 31, 32]. It is possible that these gains contributed to the improvement observed in pulmonary function and respiratory muscle strength in our study. One study suggests that HIIT supplemented with peripheral and inspiratory resistance training might be more beneficial because of the higher impact on the peripheral and inspiratory muscles, with less symptoms of dyspnea and lower amount of dropouts in patients with chronic heart failure [33]. Likewise, in our study, the perceived dyspnea severity decreased in the HIIT group, but did not change in the MICT group. In particular, inspiratory muscle weakness has shown to contribute to dyspnea severity [34]. Thus, an improvement in dyspnea severity can occur when an increase in MIP% is gained. These relationships may explain our results of HIIT and MICT on dyspnea severity in MI patients.
Overweight, increased body fat percentage and obesity are associated with an increased likelihood of developing cardiovascular disease and all-cause mortality [35]. In our study, patients in both groups were similarly overweight and had an increased body fat percentage. Although it is known that HIIT has a greater effect on fat loss, the underlying mechanisms remain unclear [35]. Contrary to previous studies, in our study, there was a decrease in BMI and body fat percentage in both groups, but there was no difference between the groups after intervention. These findings are clinically significant because most patients with MI have an increased body fat percentage and BMI. Therefore, our results suggest that HIIT and MICT should be considered important adjunct treatment strategy to decrease body fat percentage and BMI in patients with MI, especially for those who are overweight or obese.
A recent study reported that home-based resistance training improves muscle strength, exercise endurance and lower extremity functional status in respiratory diseases [36]. Intermittent type aerobic and strength training is safe, does not cause any side effects, and leads to significant improvements in physical fitness and muscle strength in patients with stable CAD, including MI [37]. After the 12-week home-based exercise programs, we observed significant improvements in hand grip strength and knee extensor strength in the both groups. In addition, the greater increase in knee extension strength in the HIIT group is consistent with the results of studies indicating greater cardiometabolic benefits from HIIT [35]. Previous studies have shown that aerobic exercise can enhance skeletal muscle mitochondrial biogenesis and function, peripheral angiogenesis, and cardiac remodeling. Similar to previous studies, we think that the increase in peripheral muscle strength can be explained by numerous improvements on skeletal muscle structure and physiology of aerobic exercise [25, 32, 38]. Furthermore, skeletal muscle strength is closely related to exercise capacity in patients with MI. Therefore, in addition to traditional cardiac rehabilitation, home-based HIIT and MICT can be recommended to increase peripheral muscle strength, which is an important factor in maintaining functional independence in patients with MI.
Previous systematic review reported that home-based and center-based interventions were equally effective on MacNew HRQoL in CVD [39]. Total MacNew HRQoL score increased in both groups, but change of total MacNew HRQoL score did not differ significantly between groups after intervention. While the physical, emotional, and social sub-scores of MacNew HRQoL improved in the HIIT group, no significant change was observed in the MICT group. This result suggests that HIIT may be more effective on HRQoL, even if there was no difference between total HRQoL scores. This may be due to greater gains of HIIT on parameters such as functional capacity, pulmonary function, and lower extremity muscle strength. In addition to physical-functional benefits, exercise training can lead to a positive impact on social participation, resulting in a good level of emotional and psychosocial health, factors that contribute to increase total HRQoL.
Limitations and implications for future research
This study has several limitations. An important limitation of this study was the small sample size. Although powered for completion rate outcome, it may be small to show difference in outcomes between the two groups. Due to ethical concerns, we could not include a third control group that did not receive any exercise type intervention. Despite these limitations, to our knowledge, the present study compared for the first time the effects of home-based HIIT and MICT in patients with MI. Future studies that can monitor the execution of a home-based exercise protocol in real time, have larger sample sizes, and include a control group without any intervention are needed.
Conclusion
The 12-week home-based HIIT and MICT exercise program was reliable and effective in improving and maintaining functional capacity, resting systolic and diastolic BP, resting HR, peripheral oxygen saturation, pulmonary function and respiratory muscle strength, body composition, peripheral muscle strength, and HRQoL.
This study demonstrated that home-based HIIT prescribed by HR reserve and RPE can achieve greater improvements in pulmonary function and lower limb muscle strength compared to home-based MICT prescribed by HR reserve and RPE for patients with MI.
Home-based HIIT and MICT are safety and effective alternative intervention for patients with MI unable to access supervised or hospital-based cardiac rehabilitation.
The results of this trial will provide good evidence for home-based exercise programs in patients with MI and what type of intervention produces better health outcomes and HRQoL.
Author contribution
Hazal Yakut, PT, PhD: the conception and design of the study, acquisition of data, drafting the article, revising it critically for important intellectual content, final approval of the version to be submitted. Hüseyin Dursun, Assoc. Prof., MD: the conception and design of the study analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, final approval of the version to be submitted. Elvan Felekoğlu, PT, MsC: the conception and design of the study, drafting the article, revising it critically for important intellectual content, final approval of the version to be submitted. Ahmet Anıl Başkurt, MD: the conception and design of the study, analysis and interpretation of data, final approval of the version to be submitted. Aylin Özgen Alpaydın, Assoc. Prof., MD: the conception and design of the study, final approval of the version to be submitted. Sevgi Özalevli, Prof., PT: the conception and design of the study, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, final approval of the version to be submitted.
Data availability
Data available on request. The data underlying this article will be shared on reasonable request to the corresponding author. Participants only gave informed consent for anonymized patient-level data sharing with the research team and publications to include aggregate data.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Noninvasive Research Ethics Board of Dokuz Eylül University and conducted according to the second Helsinki Declaration. All participants were outpatients and gave their written informed consent to participate before entering the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aylin Özgen Alpaydın, Email: aylin.alpaydin@deu.edu.tr.
Sevgi Özalevli, Email: sevgi.ozalevli@deu.edu.tr.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 2.Otsuka Y, Takaki H, Okano Y, et al. Exercise training without ventricular remodeling in patients with moderate to severe left ventricular dysfunction early after acute myocardial infarction. Int J Cardiol. 2003;87(2–3):237–244. doi: 10.1016/s0167-5273(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 3.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 4.Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156(2):292–300. doi: 10.1016/j.ahj.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 6.Wisløff U, Ellingsen Ø, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37(3):139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 7.Rognmo Ø, Hetland E, Helgerud J, et al. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 8.Franklin BA, Bonzheim K, Gordon S, et al. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest. 1998;114(3):902–906. doi: 10.1378/chest.114.3.902. [DOI] [PubMed] [Google Scholar]
- 9.Joyce SR, Lance CD, Arnt ET, et al. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45(5):679–692. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 10.Varnfield M, Karunanithi M, Lee CK, et al. Smartphone-based home care model improved use of cardiac rehabilitation in post myocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100(22):1770–1779. doi: 10.1136/heartjnl-2014-305783. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RS, Dalal H, Jolly K et al (2015) Home based versus Center based cardiac rehabilitation. Cochrane Database Syst Rev 18(8):CD007130. 10.1002/14651858.CD007130.pub3 [DOI] [PubMed]
- 12.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 16.Richards JB. Calculated decisions: mMRC (Modified Medical Research Council) Dyspnea Scale. Emerg Med Pract. 2017;19(Suppl 10):1–2. [PubMed] [Google Scholar]
- 17.Borg GA. Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–153. doi: 10.1249/00003677-197400020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Deones VL, Wiley SC, Worrell T. Assessment of quadriceps muscle performance by a hand-held dynamometer and an isokinetic dynamometer. J Orthop Sports Phys Ther. 1994;20(6):296–301. doi: 10.2519/jospt.1994.20.6.296. [DOI] [PubMed] [Google Scholar]
- 19.Haidar SG, Kumar D, Bassi RS, et al. (2004) Average versus maximum grip strength: Which is more consistent? J Hand Surg Br. 2004;29:82–84. doi: 10.1016/j.jhsb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Daskapan A, Höfer S, Oldridge N, et al. The validity and reliability of the Turkish version of the MacNew Heart Disease Questionnaire in patients with angina. J Eval Clin Pract. 2008;14(2):209–213. doi: 10.1111/j.1365-2753.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 21.Verril DE, Barton C, Beasley W, et al. Six minute walk performance and quality of life comparisons in North Carolina cardiac rehabilitation programs. Heart Lung. 2003;32(1):41–51. doi: 10.1067/mhl.2003.7. [DOI] [PubMed] [Google Scholar]
- 22.Suaya JA, Shepard DS, Normand SL, et al. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 23.Scott IA, Lindsay KA, Harden HE. Utilisation of outpatient cardiac rehabilitation in Queensland. Med J Aust. 2003;179(7):341–345. doi: 10.5694/j.1326-5377.2003.tb05588.x. [DOI] [PubMed] [Google Scholar]
- 24.Peixoto TC, Begot I, Bolzan DW, et al. Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: a randomized controlled trial. Can J Cardiol. 2015;31(3):308–313. doi: 10.1016/j.cjca.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Piotrowicz R, Wolszakiewicz J. Cardiac rehabilitation following myocardial infarction. Cardiol J. 2008;15(5):481–487. [PubMed] [Google Scholar]
- 26.Campbell WW, Kraus WE, Powell KE, et al. High-intensity interval training for cardiometabolic disease prevention. Med Sci Sports Exerc. 2019;51(6):1220–1226. doi: 10.1249/MSS.0000000000001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aamot IL, Forbord SH, Gustad K, et al. Home-based versus hospital-based high-intensity interval training in cardiac rehabilitation: a randomized study. Eur J Prev Cardiol. 2014;21(9):1070–1080. doi: 10.1177/2047487313488299. [DOI] [PubMed] [Google Scholar]
- 28.Verissimo P, Casalaspo TJ, Goncalves LH et al (2015) High prevalence of respiratory muscle weakness in hospitalized acute heart failure elderly patients. PLoS One 10(2) [DOI] [PMC free article] [PubMed]
- 29.Tasoulis A, Papazachou O, Dimopoulos S, et al. Effects of interval exercise training on respiratory drive in patients with chronic heart failure. Respir Med. 2010;104(10):1557–1565. doi: 10.1016/j.rmed.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Matos-Garcia BC, Rocco IS, Maiorano LD, et al. Home-based walking program improves respiratory endurance in patients suffering an acute myocardial infarction: A randomized controlled trial. Can J Cardiol. 2017;33(6):785–791. doi: 10.1016/j.cjca.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Geddes EL, O'Brien K, Reid WD, et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med. 2008;102(12):1715–1729. doi: 10.1016/j.rmed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Tamburús NY, Kunz VC, Salviati MR, et al. Interval training based on ventilatory anaerobic threshold improves aerobic functional capacity and metabolic profile: a randomized controlled trial in coronary artery disease patients. Eur J Phys Rehabil Med. 2016;52(1):1–11. [PubMed] [Google Scholar]
- 33.Hornikx M, Buys R, Cornelissen V, et al. Effectiveness of high intensity interval training supplemented with peripheral and inspiratory resistance training in chronic heart failure: a pilot study. Acta Cardiol. 2020;75(4):339–347. doi: 10.1080/00015385.2019.1591676. [DOI] [PubMed] [Google Scholar]
- 34.Montemezzo D, Fregonezi GA, Pereira DA, et al. Influence of inspiratory muscle weakness on inspiratory muscle training responses in chronic heart failure patients: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(7):1398–1407. doi: 10.1016/j.apmr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Dun Y, Thomas RJ, Medina-Inojosa JR, et al. High-intensity interval training in cardiac rehabilitation: impact on fat mass in patients with myocardial infarction. Mayo Clin Proc. 2019;94(9):1718–1730. doi: 10.1016/j.mayocp.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Niu M, Zhang X, et al. Effects of home-based lower limb resistance training on muscle strength and functional status in stable Chronic obstructive pulmonary disease patients. J Clin Nurs. 2018;27(5–6):e1022–e1037. doi: 10.1111/jocn.14131. [DOI] [PubMed] [Google Scholar]
- 37.Lehti M, Valkeinen H, Sipilä S, et al. Effects of aerobic and strength training on aerobic capacity, muscle strength, and gene expression of lymphomonocytes in patients with stable CAD. Am J Transl Res. 2020;12(8):4582–4593. [PMC free article] [PubMed] [Google Scholar]
- 38.Dun Y, Liu S, Zhang W, et al. Exercise combined with Rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev. 2017;2017:8024857. doi: 10.1155/2017/8024857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepherd CW, While AE. Cardiac rehabilitation and quality of life: a systematic review. Int J Nurs Stud. 2012;49(6):755–771. doi: 10.1016/j.ijnurstu.2011.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request. The data underlying this article will be shared on reasonable request to the corresponding author. Participants only gave informed consent for anonymized patient-level data sharing with the research team and publications to include aggregate data.


