Abstract
The ubiquitous cytochrome P450 hemoproteins play important functional roles in the metabolism and detoxification of foreign chemicals. However, other than established roles in cholesterol catabolism and steroid hormone biosynthesis, their cellular and/or organ physiological functions remain to be fully characterized. Here we show that the cytochrome P450 epoxygenase arachidonic acid metabolite 14,15-epoxyeicosatrienoic acid (14,15-EET) inhibits apoptosis induced by serum withdrawal, H2O2, etoposide, or excess free arachidonic acid (AA), as determined by DNA laddering, Hoechst staining, and fluorescein isothiocyanate-labeled annexin V binding. In the stable transfectants (BM3 cells) expressing a mutant bacterial P450 AA epoxygenase, F87V BM3, which was genetically engineered to metabolize arachidonic acid only to 14,15-EET, AA did not induce apoptosis and protected against agonist-induced apoptosis. Ceramide assays demonstrated increased AA-induced ceramide production within 1 h and elevated ceramide levels for up to 48 h, the longest time tested, in empty-vector-transfected cells (Vector cells) but not in BM3 cells. Inhibition of cytochrome P450 activity by 17-octadecynoic acid restored AA-induced ceramide production in BM3 cells. Exogenous C2-ceramide markedly increased apoptosis in quiescent Vector cells as well as BM3 cells, and apoptosis was prevented by pretreatment of Vector cells with exogenous 14,15-EET and by pretreatment of BM3 cells with AA. The ceramide synthase inhibitor fumonisin B1 did not affect AA-induced ceramide production and apoptosis; in contrast, these effects of AA were blocked by the neutral sphingomyelinase inhibitor scyphostatin. The pan-caspase inhibitor Z-VAD-fmk had no effect on AA-induced ceramide generation but abolished AA-induced apoptosis. The antiapoptotic effects of 14,15-EET were blocked by two mechanistically and structurally distinct phosphatidylinositol-3 (PI-3) kinase inhibitors, wortmannin and LY294002, but not by the specific mitogen-activated protein kinase kinase inhibitor PD98059. Immunoprecipitation followed by an in vitro kinase assay revealed activation of Akt kinase within 10 min after 14,15-EET addition, which was completely abolished by either wortmannin or LY294002 pretreatment. In summary, the present studies demonstrated that 14,15-EET inhibits apoptosis by activation of a PI-3 kinase–Akt signaling pathway. Furthermore, cytochrome P450 epoxygenase promotes cell survival both by production of 14,15-EET and by metabolism of unesterified AA, thereby preventing activation of the neutral sphingomyelinase pathway and proapoptotic ceramide formation.
Arachidonic acid is an important constituent of cellular membranes that is esterified to the sn-2 position of glycerophospholipids. Under normal conditions, the concentration of free, nonesterified arachidonic acid is nearly undetectable, and its release is under tight metabolic and physiologic control. As an important component of the signaling pathways of many receptor-mediated processes, specific phospholipases are activated, and arachidonic acid is released from selected lipid stores and metabolized by cyclooxygenases and/or lipoxygenases to potent bioactive lipid mediators such as prostanoids, thromboxanes, leukotrienes, lipoxins, or hydroxyeicosatetraenoic acids (HETEs) (38, 49). Numerous cellular responses have been attributed to cyclooxygenase- and lipoxygenase-dependent pathways, including regulation of cell growth and induction or inhibition of apoptosis (4, 26, 45, 54).
In addition to cyclooxygenase and lipoxygenase pathways, cytochrome P450 also catalyzes the in vivo metabolism of arachidonic acid to biologically active compounds by three types of NADPH-dependent oxidative reactions (12): (i) olefin epoxidation produces 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs), in a regio- and stereo-selective manner; (ii) allylic oxidation generates 5,8,9,11,12,15 HETEs; and (iii) ω- and ω-1-hydroxylation results in the formation of 19- and 20-HETEs. Endogenous EETs are biosynthesized in liver, kidney, and many other organs (12, 38) and are present in human plasma and urine (13, 56). Previous studies have demonstrated that EETs have potent biological activities, including modulation of vascular tone (31), glomerular hemodynamics (53), and regulation of mitogenesis (17, 32). EETs have been suggested to be an endothelium-derived hyperpolarizing factor (7). In addition, recent studies have also suggested that EETs serve as intracellular second messengers in vasculature (29) and in epithelia (6, 18). Cytochrome P450 is the predominant arachidonic acid metabolic pathway in cells such as the renal proximal tubule, in which cyclooxygenase and lipoxygenase are expressed at nearly undetectable levels (3, 21).
In certain cells, free arachidonic acid itself serves as a regulator of specific cellular processes, including the activation of intracellular kinases and lipases and modulation of Ca2+ transients (19, 33, 40). Intracellular concentrations of free arachidonic acid can be increased in response to oxidant stress and other stimuli that may induce apoptosis, and increasing evidence shows that high intracellular concentrations of free arachidonic acid may be proapoptotic in many cell types (9, 22, 52, 59, 60). Since cytochrome P450 epoxygenase is a major pathway for metabolism of arachidonic acid, the present studies were designed to explore the potential roles and mechanisms of P450-mediated arachidonic acid metabolism in cell survival.
MATERIALS AND METHODS
Reagents and antibodies.
(±)-14,15-Epoxyeicosatrienoic acid sulfonimide analog was synthesized as previously described (11, 17) and was shown to have biological activities and concentration responses identical to those of native 14,15-EET in renal epithelial cells (17). Arachidonic acid was obtained from NuCheck-Prep, Inc. (Elysian, Minn.). Epidermal growth factor (receptor grade) was purchased from Collaborative Research (Bedford, Mass.). Etoposide, PD98059, wortmannin, LY294002, C2-ceramide, Z-VAD-fmk, and Escherichia coli diacylglycerol kinase were obtained from Calbiochem (San Diego, Calif.). Fumonisin B1 was from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, Pa.). [γ-32P]ATP (specific activity, 3,000 Ci/mmol) was from NEN Life Science Products (Boston, Mass.). Polyclonal anti-Akt antibodies and protein A-agarose beads were from Santa Cruz Biotechnology (Santa Cruz, Calif.). The fluorescein isothiocyanate (FITC)-labeled annexin V binding kit was from Oncogene Research Products (Cambridge, Mass.). Histone H2B was from Boehringer Mannheim (Laval, Canada). Scyphostatin was a generous gift from Takeshi Ogita (Sankyo Co. Ltd., Tokyo, Japan) (42, 43). All other chemicals were from Sigma (St. Louis, Mo.).
Cell culture.
LLCPKcl4, an established adherent proximal tubule-like epithelial cell line derived from pig kidney (1), was routinely cultured in Dulbecco's modified Eagle medium–F-12 medium supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal bovine serum (Hyclone Laboratories, Logan, Utah) at 37°C in a 5% CO2 cell culture incubator. The medium was changed every 2 to 3 days.
Determination of apoptosis.
The following four markers of apoptosis were utilized.
(i) Cell survival assay.
LLCPKcl4 is an adherent epithelial cell line that will begin to undergo apoptosis if cultured in the absence of fetal bovine serum or growth factors for >4 days. For these studies, LLCPKcl4 cells grown to confluence were changed to serum-free media, and after 48 h, vehicle (dimethyl sulfoxide [Me2SO]) or 14,15-EET (10 μM) was added every 3 days. The cells were then observed and photographed using phase-contrast microscopy for up to 4 weeks.
(ii) DNA laddering by agarose gel electrophoresis.
Quiescent LLCPKcl4 cells were treated as indicated in each corresponding figure. Both adherent and floating cells were then collected, and genomic DNA was extracted and electrophoresed on a 1.5% agarose gel containing ethidium bromide and visualized under UV illumination.
(iii) Hoechst 33258 staining.
Morphological assessment of apoptosis was made after Hoechst 33258 dye staining as described elsewhere (20, 41). Briefly, quiescent cells were treated with the test agents and scraped into the culture medium. Both adherent and nonadherent cells were harvested, washed twice with Ca2+- and Mg2+-free phosphate-buffered saline and fixed with cold 100% methanol. Cells were stained with 1 μg of Hoechst 33258 per ml, mounted in 50% glycerol, and observed under a fluorescence microscope. Cells with fragmented nuclei were scored as apoptotic, and a minimum of 500 cells was scored for the incidence of apoptosis for each data point.
(iv) Detection of phosphatidylserine exposure.
Quiescent LLCPKcl4 cells grown on coverslips were pretreated with or without the inhibitors, treated with or without 14,15-EET for 1 h, and then exposed to H2O2 for 6 h. FITC-labeled annexin V binding was determined according to the manufacturer's instructions using a kit from Oncogene Research Products.
Stable transfection of mutant BM3.
We have previously reported stable transfection of LLCPKcl4 cells with the coding region of a mutant bacterial P450 from Bacillus megaterium (BM3) in which phenylalanine 87 was replaced with alanine, converting it to a stereo- and regioselective epoxygenase (F87V BM3) that generates only 14S,15R-EET from arachidonic acid (18, 28). We utilized these stable transfectants (BM3 cells) as well as empty vector-transfected LLCPKcl4 cells (Vector cells) to investigate further the cell survival effects of endogenously produced 14,15-EET in the present study.
EET production measurement and stereochemical analysis.
Quiescent BM3 cells and Vector cells were treated with or without arachidonic acid (30 μM), scraped with the culture medium containing 1 volume of CH3OH, and then mixed with 2 volumes of CHCl3 containing 1 mM triphenylphosphine and an equimolar mixture of synthetic 14C-labeled 8,9-, 11,12-, and 14,15-EET (55 to 56 mCi/mmol, 30 ng each). After acidification, the samples were extracted twice and the organic phases were evaporated under argon. To the resulting residue, 0.5 ml of 0.4 N KOH in 80% CH3OH was added and the mixture was incubated at 50°C for 60 min. Acidification was followed by extraction into ethylether and chromatography in SiO2 as described (10). The EETs were resolved into 14,15-EET and a mixture of 8,9- and 11,12-EET by reversed-phase high-pressure liquid chromatography and then derivatized to the corresponding pentafluorobenzyl (PFB) esters by reaction with PFB bromine. Aliquots of the purified EET-PFB regioisomers were individually dissolved in dodecane and analyzed by negative-ion, chemical ionization–gas chromatography–mass spectrometry, utilizing CH4 as the reagent gas, as described elsewhere (10).
For stereochemical analysis, samples of enzymatically derived [14C]14,15-EET (25 μg, 1 μCi/μmol) and of synthetic 14R,15S-EET and 14S,15R-EET were catalytically hydrogenated over PtO2 and esterified using excess PFB bromine, as described elsewhere (10). The resulting PFB esters were purified by reversed-phase high-pressure liquid chromatography, and after solvent evaporation, the optical antipodes of the purified PFB–14,15-EET were resolved by high-pressure liquid chromatography on a Chiralcel OD column (4.6 by 250 mm) (J. T. Baker Inc.) with an isocratic mixture of 0.11% isopropanol–99.89% n-hexane at 1 ml/min with UV monitoring at 210 nm. The retention times for the PFB esters of synthetic 14R,15S- and 14S,15R-EET were 70.6 and 78.9 min, respectively (28).
Akt kinase activity assay.
Quiescent LLCPKcl4 cells were treated with the agents indicated in Fig. 3C, cell lysates were prepared and subjected to immunoprecipitation with a polyclonal anti-Akt antibody. Immunoprecipitates were utilized for in vitro protein kinase reactions using histone H2B as a substrate in the presence of [γ-32P]ATP at 22°C for 25 min. The reactions were stopped by addition of 8 μl of Laemmli sample buffer, and 22-μl aliquots of the reaction mixtures were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After the gels were dried, the relative amounts of incorporated radioactivity were determined by autoradiography.
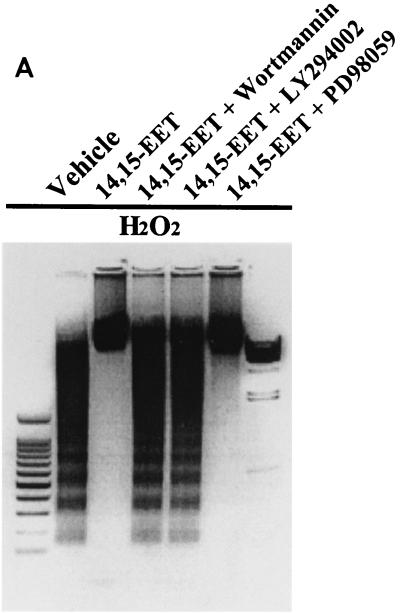
FIG. 3.
14,15-EET exerts its antiapoptotic effects in LLCPKcl4 cells by activation of a PI-3 kinase–Akt-signaling pathway. (A) 14,15-EET's antiapoptotic effects were abolished by inhibition of PI-3 kinase but not by inhibition of MAP kinase. After pretreatment with or without the specific PI-3 kinase inhibitor wortmannin (10 nM) or LY294002 (5 μM) or the MEK inhibitor PD98059 (10 μM) for 30 min, followed by 14,15-EET or vehicle for 1 h, LLCPKcl4 cells were exposed to H2O2 for 24 h, and DNA laddering was assessed. (B) Arachidonic acid's antiapoptotic effects in BM3 cells were abolished by inhibition of PI-3 kinase but not by inhibition of MAP kinase. Before induction of H2O2-mediated apoptosis, BM3 cells were pretreated with or without wortmannin, LY294002, or PD98059 and then treated with or without arachidonic acid. (A and B) Leftmost lane, 100-bp DNA ladder; rightmost lane, lambda DNA-HindIII size marker. (C) 14,15-EET increased Akt kinase activity, which was abolished by either wortmannin or LY294002. After treatment with or without the specific PI-3 kinase inhibitor wortmannin (10 nM) or LY294002 (5 μM), LLCPKcl4 cells were treated with or without 14,15-EET. The cells were then lysed and subjected to immunoprecipitation and Akt kinase activity assay with histone H2B as a substrate.
Ceramide assays.
Quiescent cells were treated with the indicated agents and lipids were then extracted as described by Van Veldhoven and Bell (57). Ceramide levels were measured using the E. coli diacylglycerol kinase assay (46). Briefly, after the diacylglycerol reaction and extraction of the labeled lipids, the lower chloroform phase was washed twice with 1% (wt/vol) HClO4 before drying under nitrogen. The extracts were dissolved in 5% methanol in chloroform and subjected to thin-layer chromatography. After development in a thin-layer chromatography mobile phase of chloroform-acetone-methanol-acetic acid-water (10:4:3:2:1), the plate was air dried and visualized by radioautography.
Statistics.
Data are presented as means ± standard errors for at least three separate experiments (each in triplicate or duplicate). An unpaired Student's t test was used for statistical analysis, and for multiple group comparisons, analysis of variance and Bonferroni t tests were used. A P value of <0.05 compared with control values was considered statistically significant.
RESULTS
Antiapoptotic effects of 14,15-EET.
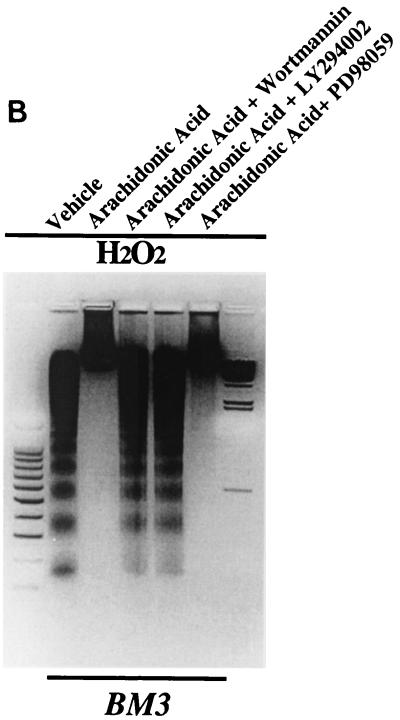
Initial experiments were designed to investigate the effects of long-term administration of 14,15-EET on the renal epithelial cell line LLCPKcl4. The cells were grown to confluence and changed to serum-free medium; after 48 h, vehicle (Me2SO) or 14,15-EET (10 μM) was added every 3 days. As shown in Fig. 1A, the number of viable cells in vehicle-treated cells diminished progressively, while the 14,15-EET-treated cells maintained a confluent monolayer with normal morphology. Virtually 100% of the cells not exposed to 14,15-EET died within 2 weeks; in contrast, with 14,15-EET treatment, there was no cell death after 2 weeks, and more than 80% of the cells survived for up to 4 weeks (n = 4).
FIG. 1.
Antiapoptotic effects of exogenously administered 14,15-EET in LLCPKcl4 cells. (A) 14,15-EET prevented the renal epithelial cell line LLCPKcl4 from progressive loss of cell viability caused by serum deprivation. LLCPKcl4 cells grown to confluence were changed to serum-free medium. After 48 h, vehicle (Me2SO) or 14,15-EET (10 μM) was added every 3 days. After 10 days, the majority of the vehicle-treated cells had died while the 14,15-EET treated cells still maintained a confluent monolayer with normal morphology. (B) 14,15-EET inhibited DNA laddering induced by H2O2 or etoposide. After pretreatment with vehicle (Me2SO) or 14,15-EET (10 μM) for 1 h, quiescent LLCPKcl4 cells were exposed to H2O2 (500 μM) or etoposide (100 μM) for 24 h, and genomic DNA was extracted and electrophoresed on a 1.5% agarose gel containing ethidium bromide. Leftmost lane, 100-bp DNA ladder; rightmost lane, lambda DNA-HindIII DNA size marker (Promega). (C) 14,15-EET decreased H2O2- or etoposide-induced apoptotic-cell number; cells were scored for morphological evidence of apoptosis as described in Materials and Methods.
Previous studies have indicated that in cultured cell systems the predominate mechanism of cell death following serum withdrawal is through apoptosis, not necrosis (39). In order to determine whether this antiapoptotic effect of 14,15-EET could be generalized to agents that acutely induced cell death, quiescent LLCPKcl4 cells were pretreated with vehicle or 14,15-EET (10 μM) for 1 h and then exposed to H2O2 (500 μM) or etoposide (100 μM). After 24 h, cells were scored for morphological evidence of apoptosis using Hoechst 33285 dye staining as described in Materials and Methods, and genomic DNA was extracted and electrophoresed. Both H2O2 and etoposide induced DNA laddering (Fig. 1B) and increased apoptotic-cell number (Fig. 1C), while the presence of 14,15-EET markedly reduced the number of apoptotic cells and eliminated DNA laddering induced by either maneuver.
Cytoprotective effects of endogenously produced 14,15-EET.
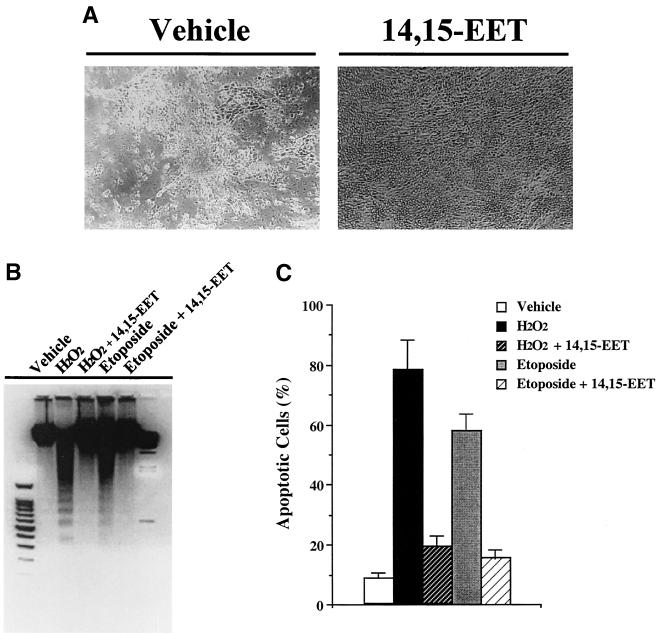
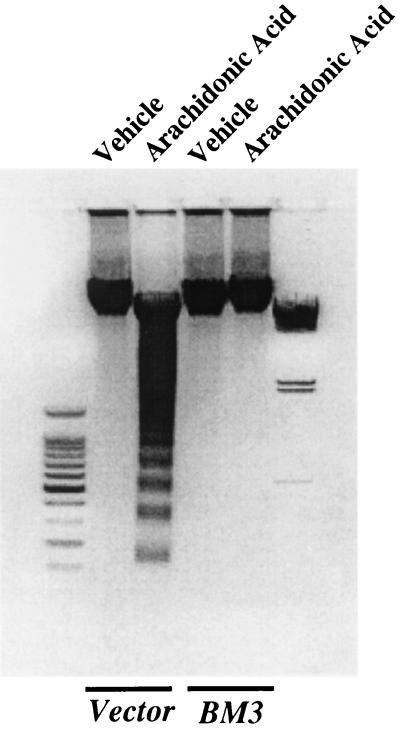
Synthetic eicosanoids have been widely utilized for the experimental analysis of their cellular and organ functions. However, in many cases, this approach provides only limited information with regard to their mechanisms of action and the enzymatic steps responsible for their biosynthesis from endogenous precursors, activation and disposition. This is of special relevance regarding P450-derived eicosanoids, since in most cultured cells, there is a rapid and progressive decrease in the expression of the P450 isoforms found in vivo. Therefore, to determine the effect of endogenously produced 14,15-EET on epithelial cell survival, we utilized a LLCPKcl4 cell line transfected with an engineered epoxygenase of bacterial origin, F87V BM3. This enzyme converts arachidonic acid selectively (>98% of total products) to 14S,15R-EET, the enantiomer that predominates in vivo in the kidney (18, 28, 36). Previous studies have shown that addition of exogenous arachidonic acid to BM3 cells increased endogenous 14,15-EET levels more than 100-fold, while there was no measurable increase in Vector cells (18). Accordingly, quiescent BM3 cells and Vector cells were pretreated with or without arachidonic acid (10 μM) for 1 h before exposure to H2O2 for 24 h. In BM3 cells, arachidonic acid significantly inhibited H2O2-induced DNA laddering; in contrast, in Vector cells, arachidonic acid not only did not prevent H2O2-induced DNA laddering but actually augmented DNA laddering and increased apoptotic-cell number, compared to treatment with H2O2 alone (Fig. 2).
FIG. 2.
Effects of endogenously produced 14,15-EET in LLCPKcl4 cells. Arachidonic acid blocked H2O2-induced apoptosis in BM3 cells but not in Vector cells. Quiescent BM3 cells and Vector cells were pretreated with or without arachidonic acid (10 μM) for 1 h before exposure to H2O2 for 24 h, and DNA laddering (A) and apoptotic cell number (B) were assessed as described in Materials and Methods. (A) Leftmost lane, 100-bp DNA ladder; rightmost lane, lambda DNA-HindIII DNA size marker.
14,15-EET activates a PI-3 kinase–Akt-signaling pathway.
We have previously reported that in quiescent nontransfected LLCPKcl4 cells, 14,15-EET administration activated the mitogen-activated protein (MAP) kinases p44/p42 extracellular signal-regulated kinases (ERKs) and phosphatidylinositol-3 kinase (PI-3 kinase) (17), while arachidonic acid addition activated ERKs and PI-3 kinase in BM3 cells but not in Vector cells (18), indicating that 14,15-EET can activate both MAP kinase and PI-3 kinase pathways. To determine if these pathways were involved in mediating the antiapoptotic effects of 14,15-EET, the specific MAP kinase kinase (MEK) inhibitor PD98059 and two mechanistically and structurally distinct PI-3 kinase inhibitors, wortmannin and LY294002, were employed. Inhibition of H2O2-induced DNA laddering (Fig. 3A) and apoptotic cell number (data not shown) by 14,15-EET was abolished by wortmannin and LY294002 but not by PD98059. In addition, H2O2-induced apoptotic body formation, detected by Hoechst 33258, was also abolished by wortmannin or LY294002 but not by PD98059 (data not shown). Determination of FITC-labeled annexin V binding further confirmed the involvement of PI-3 kinase, but not MAP kinase, in the antiapoptotic effects of 14,15-EET, because 14,15-EET inhibition of H2O2-induced annexin V binding was abolished by wortmannin but not by PD98059 (data not shown). In the BM3 cells, wortmannin and LY294002, but not PD98059, abolished arachidonic acid-mediated inhibition of DNA laddering (Fig. 3B) and apoptotic-cell number (data not shown) induced by hydrogen peroxide, indicating that endogenously produced 14,15-EET exerts antiapoptotic effects as well. As demonstrated in Fig. 3C, 14,15-EET significantly increased Akt kinase activity, which was completely blocked by wortmannin or LY294002.
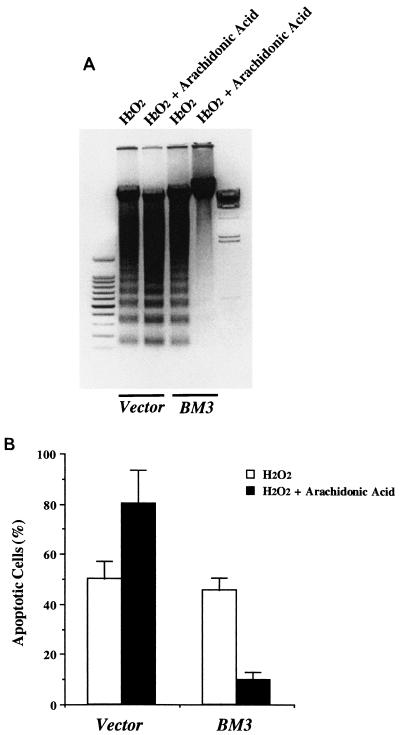
Arachidonic acid-induced apoptosis.
Hydrogen peroxide has been reported to activate phospholipase A2 (PLA2) and release arachidonic acid (8, 14). To investigate further the observation that arachidonic acid administration augmented H2O2-induced apoptosis in LLCPKcl4 cells (Fig. 2A), quiescent BM3 cells and Vector cells were treated with or without arachidonic acid (10 μM) for 48 h in the absence of other apoptosis-inducing stimuli. Arachidonic acid induced apoptotic cell death in Vector cells but not in BM3 cells, as indicated by DNA laddering (Fig. 4) and apoptotic cell counts (data not shown).
FIG. 4.
Arachidonic acid induced apoptosis in Vector cells but not in BM3 cells. Quiescent BM3 cells and Vector cells were treated with or without arachidonic acid (10 μM) for 48 h in the absence of other apoptosis-inducing stimuli and then subjected to assessment of DNA laddering. Leftmost lane, 100-bp DNA ladder; rightmost lane, lambda DNA-HindIII size marker.
Ceramide mediates arachidonic acid-induced apoptosis.
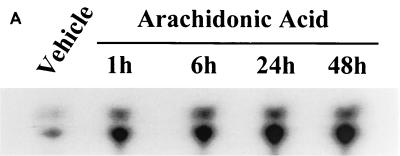
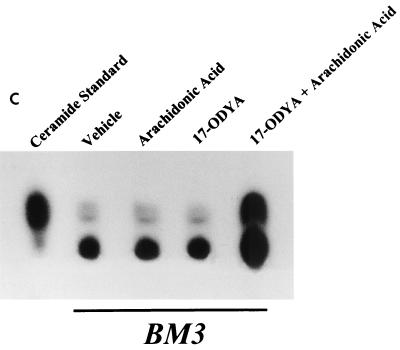
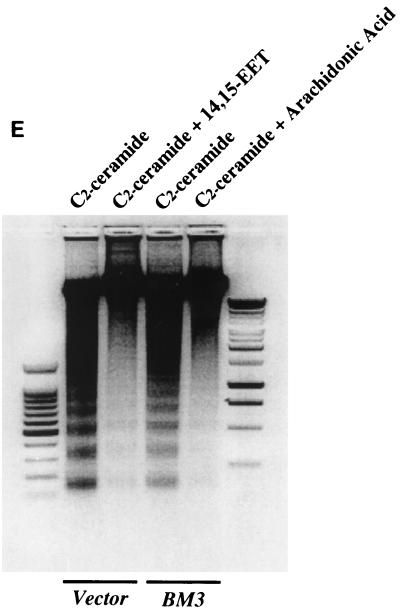
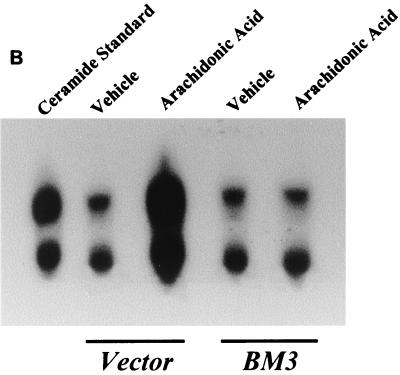
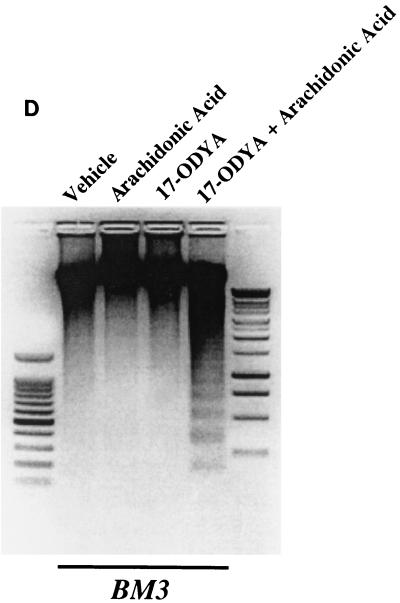
Administration of arachidonic acid to the nontransfected LLCPKcl4 cells induced ceramide production within 1 h that remained elevated for up to 48 h, the longest time tested (Fig. 5A). In contrast, arachidonic acid addition did not increase ceramide levels in BM3 cells, although it significantly increased ceramide levels in Vector cells (Fig. 5B), similar to its effect in the wild-type cells. In BM3 cells pretreated with the P450 inhibitor 17-octadecynoic acid, previously shown to inhibit BM3 epoxygenase activity (18, 51), administration of arachidonic acid increased ceramide levels (Fig. 5C) and induced apoptosis (Fig. 5D). Direct administration of the cell-permeable C2-ceramide (25 μM) induced apoptosis in quiescent Vector cells and BM3 cells, while pretreatment of Vector cells with 14,15-EET or BM3 cells with arachidonic acid decreased the extent of apoptosis (Fig. 5E).
FIG. 5.
Ceramide mediates arachidonic acid-induced apoptosis. (A) Time course of arachidonic acid induced-ceramide generation in LLCPKcl4 cells. Quiescent cells were treated with or without arachidonic acid (10 μM) for the indicated times, the cells were then harvested, and ceramide levels were quantitated as described in Materials and Methods. (B) Arachidonic acid increased ceramide generation in Vector cells but not in BM3 cells. Vector cells and BM3 cells were rendered quiescent and treated with or without arachidonic acid (10 μM) for 48 h. Ceramide levels were determined as described in Materials and Methods. (C and D) Arachidonic acid increased ceramide levels and induced apoptosis in BM3 cells pretreated with a P450 inhibitor. Quiescent BM3 cells were pretreated with or without the P450 inhibitor 17-ODYA (20 μM) and treated with or without arachidonic acid (10 μM). The cells were then harvested and ceramide levels were measured (C), and apoptosis was determined (D). (E) 14,15-EET and arachidonic acid inhibited apoptosis induced by administration of ceramide in Vector cells and BM3 cells, respectively. Vector cells and BM3 cells were made quiescent and pretreated with or without 14,15-EET (Vector cells) or arachidonic acid (BM3 cells); they were then exposed to the cell-permeable C2-ceramide (25 μM), and apoptosis was assessed. (D and E) Leftmost lane, 100-bp DNA ladder; rightmost lane, 1-kb DNA ladder.
Arachidonic acid induces apoptosis by activating the neutral sphingomyelinase pathway.
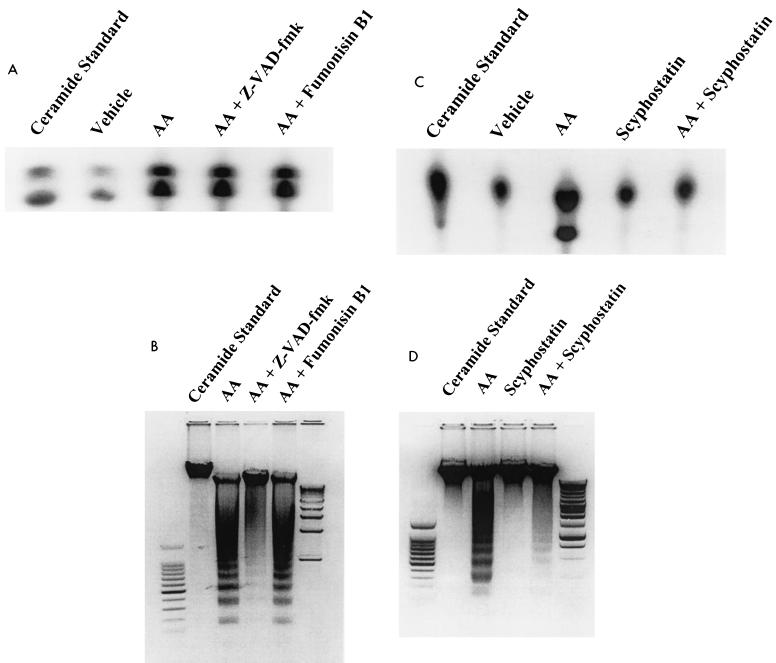
Pretreatment of LLCPKcl4 cells with the ceramide synthase inhibitor fumonisin B1 did not affect arachidonic acid-induced ceramide elevation (Fig. 6A) and had no effect on arachidonic acid-induced apoptosis (Fig. 6B); in contrast, the neutral sphingomyelinase inhibitor scyphostatin (42, 43) blocked arachidonic acid-induced ceramide production (Fig. 6C) and abolished arachidonic acid-induced apoptosis as well (Fig. 6D). Interestingly, the pan-caspase inhibitor Z-VAD-fmk had no effect on arachidonic acid-induced ceramide generation (Fig. 6A) but significantly inhibited arachidonic acid-induced apoptosis (Fig. 6B), indicating that caspases are downstream effectors of ceramide in the arachidonic acid-activated apoptotic signaling pathway.
FIG. 6.
Arachidonic acid induces apoptosis by activating neutral sphingomyelinase pathway. (A and B) Effects of ceramide synthase inhibitor and caspase inhibitor on arachidonic acid-induced ceramide production and apoptosis in LLCPKcl4 cells. Quiescent cells were pretreated with vehicle, fumonisin B1 (50 μM), or Z-VAD-fmk (50 μM) for 1 h, followed by treatment with or without arachidonic acid (10 μM). Cells were then harvested, ceramide levels were measured 1 h after administration of arachidonic acid (A), and apoptosis was determined 24 h after administration of arachidonic acid (B). (C and D) Effects of the neutral sphingomyelinase inhibitor scyphostatin on arachidonic acid-induced ceramide production and apoptosis in LLCPKcl4 cells. Quiescent cells were pretreated with or without scyphostatin (1 μM) for 1 h followed by treatment with or without arachidonic acid (10 μM). Cells were then harvested, ceramide levels were measured (C), and apoptosis was determined (D). (B and D) Leftmost lane, 100-bp DNA ladder; rightmost lane, 1-kb DNA ladder.
DISCUSSION
To date, three enzymatic pathways of arachidonic acid metabolism, cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes, have been identified in mammalian systems. Cyclooxygenase- and lipoxygenase-mediated arachidonic acid metabolic pathways have been extensively characterized, and their contributions to pathophysiology have been well established, including potential roles in epithelial cancers, such as colon, breast, and prostate carcinoma (2, 25, 44; R. A. Gupta and R. N. DuBois, Editorial, Gastroenterology 114:1095–1098, 1998). There is increasing interest in cyclooxygenase and lipoxygenase inhibitors as cancer-preventive agents, since these inhibitors are known to induce apoptosis (2, 35, 50). However, it remains to be determined which arachidonic acid metabolites produced by these pathways are involved in preventing apoptosis and/or how arachidonic acid itself induces apoptosis. In this regard, it has been suggested that cyclooxygenase and lipoxygenase inhibitors may be cancer preventive not only by inhibiting production of specific antiapoptotic arachidonic acid metabolites but also by preventing metabolism of free arachidonic acid, which promotes neutral sphingomyelinase activity and increases production of the proapoptotic lipid ceramide (15, 54). Utilizing human epithelial cell lines with stable, inducible expression of cyclooxygenase-2 and/or fatty acid-coenzyme A ligase 4, a recent study has shown that free arachidonic acid is a critical signal for apoptosis and that the induction of apoptosis by nonsteroidal anti-inflammatory drugs, the fatty acid-coenzyme A ligase inhibitor triacsin C, and other cyclooxygenase and lipoxygenase inhibitors of arachidonic acid metabolism is a consequence of an accumulation of intracellular free arachidonic acid (9). In contrast, much less attention has been paid to the cytochrome P450 monooxygenase pathway, the third major pathway of arachidonic acid metabolism.
The cytochrome P450 hemoproteins represent the most abundant and widely distributed group of eukaryotic monooxygenases. In mammals, these proteins are expressed in almost all cell types, but despite delineation of their biochemical and biophysical properties and extensive insight into roles in toxicology and pharmacology, little is known about their physiologic roles in nonsteroidogenic organs. The demonstration that arachidonic acid is an endogenous substrate for cytochrome P450 has stimulated investigations of biologic roles for these metabolites. EETs have been proposed to act as second messengers for hormones and growth factors. In this regard, we have demonstrated that EETs serve as second messengers for epidermal growth factor in renal epithelial cells (6, 18) and are potent mitogens via activation of a Src kinase-mediated tyrosine kinase cascade (16, 17). The present studies document an additional novel role for 14,15-EET to inhibit apoptosis induced by such diverse proapoptotic stimuli as H2O2, etoposide, and excess arachidonic acid. These antiapoptotic effects were observed either with exogenous addition of 14,15-EET or following transfection of the biosynthetic machinery necessary for its endogenous biosynthesis from arachidonic acid, thus establishing a clear cause-and-effect relationship between the cytochrome P450 epoxygenase pathway and its metabolite, 14,15-EET. Furthermore, we also show that a single chemical entity, 14,15-EET, can replace the serum requirements for LLCPKcl4 cells and support cell viability. An additional cytoprotective role of P450 epoxygenase is to metabolize and thereby prevent accumulation of toxic levels of free arachidonic acid.
PI-3 kinase, composed of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit, is activated by a wide range of cytokines, hormones, and growth factors (24, 55). PI-3 kinases and their phospholipid products, PI-3,4,5-trisphosphate [PI(3,4,5)P3] and PI(3,4)P2, have been demonstrated to promote growth factor-mediated cell survival and to prevent apoptosis in many cell types (47, 61). Our present studies show that although 14,15-EET activates both PI-3 kinase and ERKs, it is activation of PI-3 kinase that is essential for the antiapoptotic signaling pathway of 14,15-EET. It has previously been shown that the ubiquitously expressed serine/threonine protein kinase B, also called Akt, is the downstream target of PI-3 kinase in the growth factor-mediated cytoprotective signaling pathways (5, 23). In the present studies, 14,15-EET also significantly increased Akt activity, suggesting that 14,15-EET exerts its potent antiapoptotic effects through activation of a PI-3 kinase–Akt-signaling pathway.
The second lipid messenger, ceramide, regulates cellular differentiation, proliferation, and apoptosis in both stimulus- and cell-type-specific fashions. Very often, however, the outcome of ceramide-mediated signaling is apoptosis, and increasing evidence suggests that the sphingomyelin-signaling system is an upstream signaling mechanism that links cell surface receptors and environmental stresses to the apoptotic pathway (37). Although the mechanisms of ceramide-induced apoptosis are still incompletely understood, in the past years the c-Jun N-terminal kinase and p38 MAP kinase pathways have been linked to the ceramide-initiated apoptosis (30, 58). Interestingly, ceramide has recently been shown to induce apoptosis by dephosphorylation of Akt at residues Thr-308 and Ser-473 through a ceramide-activated protein phosphatase, thus inhibiting Akt activity (48). Akt has also been shown to prevent formation of ceramide in response to proapoptotic stimuli and protect cells against ceramide-induced apoptosis (27). The potent cytotoxic effect of tumor necrosis factor alpha has been shown to signal through activation of the cytosolic PLA2 (cPLA2) and release of free arachidonic acid, which acts as an essential precursor for tumor necrosis factor alpha to induce activation of the sphingomyelin-signaling cycle and, as a result, formation of the proapoptotic ceramide (34). In the present study, we showed that ceramide is a potent inducer of apoptosis for renal epithelial cells and that ceramide is an effector of arachidonic acid-mediated apoptosis. We demonstrate that cells that lack functional cytochrome P450 activity as well as cyclooxygenase and lipoxygenase are susceptible to apoptosis when exposed to free arachidonic acid. In contrast, introduction of enzymatically active P450 epoxygenase into these cells restored their arachidonic acid metabolic function and prevented arachidonic acid-induced ceramide production, and thereby provided these cells with resistance to free arachidonic acid-induced apoptosis.
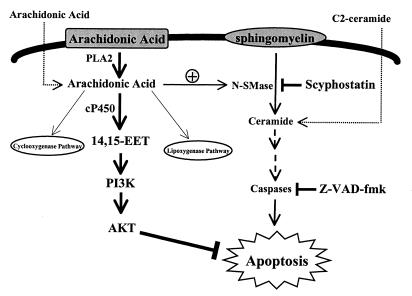
Since the ceramide synthase inhibitor had no inhibitory effect on ceramide elevation and apoptosis induced by arachidonic acid, these effects are likely not due to activation of ceramide synthase and initiation of de novo synthesis of ceramide. The blockade of these effects of arachidonic acid by a neutral sphingomyelinase inhibitor provides direct evidence that unesterified arachidonic acid induces apoptosis through activation of the neutral sphingomyelinase pathway, resulting in conversion of sphingomyelin to ceramide. The pan-caspase inhibitor had no effect on arachidonic acid-induced ceramide generation but did inhibit arachidonic acid-induced apoptosis, suggesting that ceramide is upstream of caspases in the arachidonic acid-activated apoptotic pathway, as schematized in Fig. 7.
FIG. 7.
Arachidonic acid-ceramide-apoptosis-signaling pathway is regulated by cytochrome P450 in LLCPKcl4 cells. Arachidonic acid, an important constituent of cell membrane, is released by activation of specific phospholipases (PLA2) and further metabolized by cyclooxygenases, lipoxygenases, and cytochrome P450 pathways. If unesterified arachidonic acid is not metabolized, it activates neutral sphingomyelinase (N-SMase), which converts sphingomyelin to the second messenger, ceramide. Ceramide induces caspase activation, which leads to apoptosis. In cells such as the renal proximal tubule, in which cyclooxygenase and lipoxygenase are expressed at nearly undetectable levels, arachidonic acid metabolism is shunted to the cytochrome P450 pathway. This metabolism not only metabolizes and detoxifies excess unesterified arachidonic acid to prevent proapoptotic ceramide formation but also produces a metabolite, 14,15-EET, which activates a PI-3 kinase–Akt-signaling pathway. Thus, cytochrome P450 mediates cell survival by two complementary mechanisms.
Moreover, the active P450 epoxygenase introduced into these cells had been engineered to metabolize arachidonic acid only to 14,15-EET, which renders the cells resistant to apoptosis-inducing agents such as hydrogen peroxide in addition to arachidonic acid per se. Thus, in addition to the production of the antiapoptotic lipid 14,15-EET, our present studies also show that metabolism of free arachidonic acid by P450 prevents free arachidonic acid-mediated ceramide formation. Therefore, P450 serves as an important regulator of cell survival by decreasing proapoptotic signals as well as by increasing antiapoptotic signals, as illustrated in Fig. 7.
In summary, the present results demonstrate that cytochrome P450 epoxygenase prevents apoptosis by two complementary mechanisms. This enzyme both metabolizes and detoxifies excess free arachidonic acid to prevent proapoptotic ceramide production and also produces a metabolite, 14,15-EET, that is a potent inhibitor of apoptosis by activation of a PI-3 kinase–Akt-signaling pathway. These studies elucidate a new functional role for the P450 epoxygenase pathway of arachidonic acid metabolism and shed new light on the mechanisms by which unesterified arachidonic acid signals apoptosis.
ACKNOWLEDGMENTS
We thank Takeshi Ogita (Sankyo Co. Ltd., Tokyo, Japan) for his generous provision of the newly described neutral sphingomyelinase inhibitor scyphostatin.
This work was supported by National Institutes of Health grant DK38226 (R.C.H., J.C., and J.R.F.) and funds from the Department of Veterans Affairs (R.C.H.).
REFERENCES
- 1.Amsler K, Cook J S. Development of Na+-dependent hexose transport in a cultured line of porcine kidney cells. Am J Physiol. 1982;242:C94–C101. doi: 10.1152/ajpcell.1982.242.1.C94. [DOI] [PubMed] [Google Scholar]
- 2.Ara G, Teicher B A. Cyclooxygenase and lipoxygenase inhibitors in cancer therapy. Prostaglandins Leukot Essent Fatty Acids. 1996;54:3–16. doi: 10.1016/s0952-3278(96)90075-7. [DOI] [PubMed] [Google Scholar]
- 3.Bonvalet J P, Pradelles P, Farman N. Segmental synthesis and actions of prostaglandins along the nephron. Am J Physiol. 1987;253:F377–F387. doi: 10.1152/ajprenal.1987.253.3.F377. [DOI] [PubMed] [Google Scholar]
- 4.Bortuzzo C, Hanif R, Kashfi K, Staiano-Coico L, Shiff S J, Rigas B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim Biophys Acta. 1996;1300:240–246. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 5.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 6.Burns K D, Capdevila J, Wei S, Breyer M D, Homma T, Harris R C. Role of cytochrome P-450 epoxygenase metabolites in EGF signaling in renal proximal tubule. Am J Physiol. 1995;269:C831–C840. doi: 10.1152/ajpcell.1995.269.4.C831. [DOI] [PubMed] [Google Scholar]
- 7.Campbell W B, Gebremedhin D, Pratt P F, Harder D R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 8.Cane A, Breton M, Koumanov K, Bereziat G, Colard O. Oxidant-induced arachidonic acid release and impairment of fatty acid acylation in vascular smooth muscle cells. Am J Physiol. 1998;274:C1040–C1046. doi: 10.1152/ajpcell.1998.274.4.C1040. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Pearman A T, Zimmerman G A, McIntyre T M, Prescott S M. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capdevila J H, Dishman E, Karara A, Falck J R. Cytochrome P450 arachidonic acid epoxygenase: stereochemical characterization of epoxyeicosatrienoic acids. Methods Enzymol. 1991;206:441–453. doi: 10.1016/0076-6879(91)06113-h. [DOI] [PubMed] [Google Scholar]
- 11.Capdevila J H, Falck J R, Dishman E, Karara A. Cytochrome P-450 arachidonate oxygenase. Methods Enzymol. 1990;187:385–394. doi: 10.1016/0076-6879(90)87045-5. [DOI] [PubMed] [Google Scholar]
- 12.Capdevila J H, Falck J R, Estabrook R W. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6:731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 13.Catella F, Lawson J A, Fitzgerald D J, FitzGerald G A. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci USA. 1990;87:5893–5897. doi: 10.1073/pnas.87.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborti S, Chakraborti T. Down-regulation of protein kinase C attenuates the oxidant hydrogen peroxide-mediated activation of phospholipase A2 in pulmonary vascular smooth muscle cells. Cell Signal. 1995;7:75–83. doi: 10.1016/0898-6568(94)00061-f. [DOI] [PubMed] [Google Scholar]
- 15.Chan T A, Morin P J, Vogelstein B, Kinzler K W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J K, Capdevila J, Harris R C. Overexpression of C-terminal Src kinase blocks 14,15-epoxyeicosatrienoic acid-induced tyrosine phosphorylation and mitogenesis. J Biol Chem. 2000;275:13789–13792. doi: 10.1074/jbc.275.18.13789. [DOI] [PubMed] [Google Scholar]
- 17.Chen J K, Falck J R, Reddy K M, Capdevila J, Harris R C. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 18.Chen J K, Wang D W, Falck J R, Capdevila J, Harris R C. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J Biol Chem. 1999;274:4764–4769. doi: 10.1074/jbc.274.8.4764. [DOI] [PubMed] [Google Scholar]
- 19.Damron D S, Van Wagoner D R, Moravec C S, Bond M. Arachidonic acid and endothelin potentiate Ca2+ transients in rat cardiac myocytes via inhibition of distinct K+ channels. J Biol Chem. 1993;268:27335–27344. [PubMed] [Google Scholar]
- 20.Edwards Y S, Sutherland L M, Power J H, Nicholas T E, Murray A W. Osmotic stress induces both secretion and apoptosis in rat alveolar type II cells. Am J Physiol. 1998;275:L670–L678. doi: 10.1152/ajplung.1998.275.4.L670. [DOI] [PubMed] [Google Scholar]
- 21.Endou H. Cytochrome P-450 monooxygenase system in the rabbit kidney: its intranephron localization and its induction. Jpn J Pharmacol. 1983;33:423–433. doi: 10.1254/jjp.33.423. [DOI] [PubMed] [Google Scholar]
- 22.Finstad H S, Kolset S O, Holme J A, Wiger R, Farrants A K, Blomhoff R, Drevon C A. Effect of n-3 and n-6 fatty acids on proliferation and differentiation of promyelocytic leukemic HL-60 cells. Blood. 1994;84:3799–3809. [PubMed] [Google Scholar]
- 23.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 24.Fry M J. Structure, regulation and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1994;1226:237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh J, Myers C E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetzl E J, An S, Smith W L. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995;9:1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- 27.Goswami R, Kilkus J, Dawson S A, Dawson G. Overexpression of Akt (protein kinase B) confers protection against apoptosis and prevents formation of ceramide in response to pro-apoptotic stimuli. J Neurosci Res. 1999;57:884–893. [PubMed] [Google Scholar]
- 28.Graham-Lorence S, Truan G, Peterson J A, Falck J R, Wei S, Helvig C, Capdevila J H. An active site substitution, F87V, converts cytochrome P450 BM-3 into a regio- and stereoselective (14S,15R)-arachidonic acid epoxygenase. J Biol Chem. 1997;272:1127–1135. doi: 10.1074/jbc.272.2.1127. [DOI] [PubMed] [Google Scholar]
- 29.Graier W F, Simecek S, Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol (London) 1995;482:259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannun Y A, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 31.Harder D R, Campbell W B, Roman R J. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- 32.Harris R C, Homma T, Jacobson H R, Capdevila J. Epoxyeicosatrienoic acids activate Na+/H+ exchange and are mitogenic in cultured rat glomerular mesangial cells. J Cell Physiol. 1990;144:429–437. doi: 10.1002/jcp.1041440310. [DOI] [PubMed] [Google Scholar]
- 33.Hwang S C, Jhon D Y, Bae Y S, Kim J H, Rhee S G. Activation of phospholipase C-gamma by the concerted action of tau proteins and arachidonic acid. J Biol Chem. 1996;271:18342–18349. doi: 10.1074/jbc.271.31.18342. [DOI] [PubMed] [Google Scholar]
- 34.Jayadev S, Hayter H L, Andrieu N, Gamard C J, Liu B, Balu R, Hayakawa M, Ito F, Hannun Y A. Phospholipase A2 is necessary for tumor necrosis factor alpha-induced ceramide generation in L929 cells. J Biol Chem. 1997;272:17196–17203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- 35.Kamitani H, Geller M, Eling T. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem. 1998;273:21569–21577. doi: 10.1074/jbc.273.34.21569. [DOI] [PubMed] [Google Scholar]
- 36.Karara A, Makita K, Jacobson H R, Falck J R, Guengerich F P, DuBois R N, Capdevila J H. Molecular cloning, expression, and enzymatic characterization of the rat kidney cytochrome P-450 arachidonic acid epoxygenase. J Biol Chem. 1993;268:13565–13570. [PubMed] [Google Scholar]
- 37.Kolesnick R N, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 38.Makita K, Falck J R, Capdevila J H. Cytochrome P450, the arachidonic acid cascade, and hypertension: new vistas for an old enzyme system. FASEB J. 1996;10:1456–1463. doi: 10.1096/fasebj.10.13.8940291. [DOI] [PubMed] [Google Scholar]
- 39.Mangan D F, Welch G R, Wahl S M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 40.McPhail L C, Clayton C C, Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984;224:622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- 41.Morin P J, Vogelstein B, Kinzler K W. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nara F, Tanaka M, Hosoya T, Suzuki-Konagai K, Ogita T. Scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima: taxonomy of the producing organism, fermentation, isolation, and physico-chemical properties. J Antibiot (Tokyo) 1999;52:525–530. doi: 10.7164/antibiotics.52.525. [DOI] [PubMed] [Google Scholar]
- 43.Nara F, Tanaka M, Masuda-Inoue S, Yamasato Y, Doi-Yoshioka H, Suzuki-Konagai K, Kumakura S, Ogita T. Biological activities of scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima. J Antibiot (Tokyo) 1999;52:531–535. doi: 10.7164/antibiotics.52.531. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi M, Rose D P, Earashi M, Miyazaki I. The role of fatty acids and eicosanoid synthesis inhibitors in breast carcinoma. Oncology. 1995;52:265–271. doi: 10.1159/000227471. [DOI] [PubMed] [Google Scholar]
- 45.Pica F, Franzese O, D'Onofrio C, Bonmassar E, Favalli C, Garaci E. Prostaglandin E2 induces apoptosis in resting immature and mature human lymphocytes: a c-Myc-dependent and Bcl-2-independent associated pathway. J Pharmacol Exp Ther. 1996;277:1793–1800. [PubMed] [Google Scholar]
- 46.Preiss J, Loomis C R, Bishop W R, Stein R, Niedel J E, Bell R M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 47.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 48.Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 49.Samuelsson B, Dahlen S E, Lindgren J A, Rouzer C A, Serhan C N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 50.Shiff S J, Rigas B. Nonsteroidal anti-inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology. 1997;113:1992–1998. doi: 10.1016/s0016-5085(97)99999-6. [DOI] [PubMed] [Google Scholar]
- 51.Shirane N, Sui Z, Peterson J A, Ortiz de Montellano P R. Cytochrome P450BM-3 (CYP102): regiospecificity of oxidation of omega-unsaturated fatty acids and mechanism-based inactivation. Biochemistry. 1993;32:13732–13741. doi: 10.1021/bi00212a044. [DOI] [PubMed] [Google Scholar]
- 52.Surette M E, Fonteh A N, Bernatchez C, Chilton F H. Perturbations in the control of cellular arachidonic acid levels block cell growth and induce apoptosis in HL-60 cells. Carcinogenesis. 1999;20:757–763. doi: 10.1093/carcin/20.5.757. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Capdevila J, Karara A, Falck J R, Jacobson H R, Badr K F. Cytochrome P-450 arachidonate metabolites in rat kidney: characterization and hemodynamic responses. Am J Physiol. 1990;258:F781–F789. doi: 10.1152/ajprenal.1990.258.4.F781. [DOI] [PubMed] [Google Scholar]
- 54.Tang D G, Chen Y Q, Honn K V. Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc Natl Acad Sci USA. 1996;93:5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 56.VanRollins M, Kochanek P M, Evans R W, Schiding J K, Nemoto E M. Optimization of epoxyeicosatrienoic acid syntheses to test their effects on cerebral blood flow in vivo. Biochim Biophys Acta. 1995;1256:263–274. doi: 10.1016/0005-2760(95)00029-c. [DOI] [PubMed] [Google Scholar]
- 57.Van Veldhoven P P, Bell R M. Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim Biophys Acta. 1988;959:185–196. doi: 10.1016/0005-2760(88)90030-6. [DOI] [PubMed] [Google Scholar]
- 58.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 59.Williams J R, Leaver H A, Ironside J W, Miller E P, Whittle I R, Gregor A. Apoptosis in human primary brain tumours: actions of arachidonic acid. Prostaglandins Leukot Essent Fatty Acids. 1998;58:193–200. doi: 10.1016/s0952-3278(98)90113-2. [DOI] [PubMed] [Google Scholar]
- 60.Wolf L A, Laster S M. Characterization of arachidonic acid-induced apoptosis. Cell Biochem Biophys. 1999;30:353–368. doi: 10.1007/BF02738119. [DOI] [PubMed] [Google Scholar]
- 61.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]