Abstract
The distribution and prevalence of strains of Mycobacterium avium subsp. paratuberculosis were determined among sheep, cattle, and other species with Johne's disease in Australia. A total of 328 isolates were evaluated from numerous farms in New South Wales, Victoria, Tasmania, and South Australia, Australia. Restriction fragment length polymorphism (RFLP) analysis of genomic DNA using BstEII and an IS900 probe and IS1311 polymorphism analysis using PCR and restriction endonuclease analysis (PCR-REA) was used to classify isolates as cattle (C) or sheep (S) strains. IS1311 PCR-REA provided similar information to IS900 RFLP analysis but was more useful than RFLP analysis where DNA was degraded or scarce. Twelve IS900 RFLP types were found. Johne's disease in sheep was always due to S strains, while cattle were infected only with C strains. RFLP type S1 was the dominant strain in sheep in New South Wales (97% of isolates) and was the only strain found in sheep from Victoria. Seven RFLP types were present in cattle. RFLP types C3 and C1 were most common (collectively, 85% of isolates), but C1 was not found in New South Wales and C3 was present in dairy cattle but not in beef cattle in Victoria. These differences may be explained by restricted livestock trading patterns between different segments of the cattle industry. Up to five RFLP types were present in some geographic regions in Victoria, while up to three RFLP types were found among cattle on some farms. Individual cattle usually were infected with only one RFLP type, but one animal was infected with both C5 and CU4. Two isolates from goats were C type as were three from alpacas, one from a rhinoceros, and two from a human with Crohn's disease. The prevalences of specific RFLP types in Australia differ from those reported in Europe and elsewhere. Given the existence of geographical and farm enterprise differences in IS900 RFLP type, this technique may be applied selectively to trace the spread of Johne's disease, at least in the cattle industries. As these observations reflect past exposure of livestock to M. avium subsp. paratuberculosis, the monitoring of strains present in animals in Australia is continuing.
Paratuberculosis, or Johne's disease, is a chronic granulomatous enteropathy of animals caused by infection with Mycobacterium avium subsp. paratuberculosis. The infection is transmitted most commonly to herbivores by the fecal-oral route, i.e., via contaminated pasture, teats, soil, and water, although milk-borne and intrauterine infection are known. In Australia, the disease has been identified in cattle, sheep, goats, deer, alpacas, llamas, and a rhinoceros. In other countries, rabbits, bison, and other herbivores have been diagnosed with paratuberculosis, and the organism has been isolated from primates, including humans. Experimentally, many species are susceptible to infection with M. avium subsp. paratuberculosis, and transmission between different species of farmed livestock and/or free-living wildlife species and their predators may occur (4, 17, 18, 31).
Paratuberculosis can be an economically significant problem, and control programs have been developed or are planned in many countries. Antibiotic treatment of paratuberculosis cases is impractical in commercial livestock, and options for control are limited to reducing prevalence by an extended test-and-cull operation or by changing farm management practices. The alternative is depopulation of herds or flocks containing infected livestock, waiting through a lengthy decontamination period, and then restocking with uninfected individuals. The success of depopulation and decontamination on farms with multiple grazing species depends on the segregation of infection within individual species and may also depend on the absence of wildlife reservoirs. In Australia, where sheep and cattle are the predominant grazing species and where paratuberculosis is believed not to be spread commonly between these species, a management option has been to use cattle to graze pastures during the decontamination period after depopulation of paratuberculous sheep. There is limited epidemiological and microbiological evidence to support these ideas.
It has been known for many decades that there are at least two types of M. avium subsp. paratuberculosis. A less readily cultivated type is the common but not invariable cause of paratuberculosis in sheep, while another readily cultivated type is the most common cause of the disease in cattle. The latter type is frequently associated with infection in other species that graze in contact with cattle but may also occur in sheep (23, 31, 32, 33). Evidence of the difficulty of culture of M. avium subsp. paratuberculosis from sheep with paratuberculosis comes from a variety of countries and is so consistent that there can be no doubt about phenotypic strain differences (reviewed in reference 45).
There is somewhat less certainty regarding the true degree of host adaptation or preference of these phenotypically distinct strains. In Scotland, ovine paratuberculosis was most often attributed to pigmented, poorly growing mycobacteria. Nonpigmented and readily cultivated strains similar to those commonly obtained from cattle were also isolated from sheep in Scotland but only from a minority of farms (37, 38). The pigmented ovine strains appeared to be more pathogenic for sheep than the classical nonpigmented bovine strains (35). In Iceland, ovine paratuberculosis was caused by essentially noncultivable mycobacteria and prior to a compulsory vaccination program was associated with significant mortality rates, though the infection in cattle due to these strains tended to be subclinical (15, 19). There are reports from England, Australia, and New Zealand that paratuberculosis was not diagnosed in cattle in contact with paratuberculous sheep (2, 23, 34) and a report of apparent failure of natural transmission of the disease to sheep that were exposed to paratuberculous cattle (2). Conversely, a sheep on a cattle farm was found to be infected with a cattle strain (28), rotational grazing of sheep with paratuberculous cattle resulted in infection in four of six sheep in a study in New Zealand (32), and infection was probably transmitted to a sheep by close contact with paratuberculous cattle in Australia (22).
These observations appear to have led to a common belief in Australia that sheep may succumb to infection with either strain and that cattle are not particularly susceptible to the strains that cause the predominant form of ovine paratuberculosis. However, there is one report of natural paratuberculosis in a cow in Scotland due to a pigmented ovine strain (41) which was then transmitted experimentally back to sheep, and there are several reports in which young cattle succumbed to paratuberculosis following experimental dosing with either pigmented or so-called Icelandic organisms derived from sheep (23, 39). In Iceland, epidemiological observations are consistent with M. avium subsp. paratuberculosis having been introduced to the local sheep population with imported European sheep, its spread to the local cattle population in which it became endemic, and its subsequent transmission from cattle back to sheep after an ovine depopulation and restocking program (15, 27).
In summary, solid evidence of host specificity for the phenotypically defined sheep and cattle strains of M. avium subsp. paratuberculosis is lacking. Massive doses of the organism sufficient to overwhelm normal host defences have been used in experimental infections so that the conclusions may not be applicable in the field, while epidemiological evidence for the segregation of strains within ruminant hosts can be contrasted with epidemiological evidence of transmission between these hosts.
In addition to phenotypic data and epidemiological observations, there is clear evidence at the molecular level of heterogeneity among isolates of M. avium subsp. paratuberculosis. Collins et al. (9) identified a repetitive element, IS900 (16, 26), which was used as a probe to generate restriction fragment length polymorphism (RFLP) patterns from isolates of M. avium subsp. paratuberculosis. Examination of 46 isolates by this method and restriction endonuclease analysis (REA) led to the definition of two groups of isolates with different animal host distributions, broadly defined as cattle (C) and sheep (S) strains (10). There were also phenotypic differences between S and C strains, the former being very difficult to cultivate in vitro. Strains with an intermediate (I) RFLP pattern most similar to S strains were identified in a subsequent study (14). This simple classification of M. avium subsp. paratuberculosis was confirmed in an independent study (3). RFLP typing data are now available for many hundreds of isolates from widely separated geographic regions but principally from the northern hemisphere (30) (Table 1). Almost without exception and regardless of geographic location, isolates from cattle have been of the C type, as have most isolates from goats and deer. In complete contrast, isolates from sheep have been of C, S, or I type, with most countries tending to have only one type in their sheep population. The underrepresentation of S strains in cattle, deer, and goats might reflect the difficulty of laboratory culture of such strains, but their identification in sheep from a similar range of countries suggests that culturability alone does not explain the apparent segregation within host species. Indeed, a C strain was recovered from a cow and an S strain was recovered from seven sheep on the same farm in one study (3). That isolation of M. avium subsp. paratuberculosis from cattle with paratuberculosis is rarely reported to be difficult is indirect evidence that S strains are uncommon in cattle. Although a considerable amount of information is being compiled in Europe (30), little information is available to assess whether there have been patterns of transmission of paratuberculosis within and between species and regions. A recent report suggests that there may be significant differences in the prevalence of particular RFLP types in cattle with paratuberculosis in Europe compared to South America (25).
TABLE 1.
Reported occurrence in ruminants of strains of M. avium subsp. paratuberculosis in which the presence of IS900 was confirmed
| Origin of isolates | No. of isolates | Restriction enzymec | No. of isolates of RFLP type:
|
Reference(s) | ||
|---|---|---|---|---|---|---|
| C | S | I | ||||
| Cattle | ||||||
| New Zealand | 13, 2 | BstEII, others | 13, 2 | 10, 12 | ||
| Australia | 6, 5, 47 | BstEII, PstI, others | 6, 5, 47 | 6, 10, 12a | ||
| South America | 50 | BstEII, PstI | 50 | 25 | ||
| North America | 29 | BstEII, others | 29 | 42 | ||
| United States | 1 | PvuII | 1 | 40 | ||
| Denmark | 5, 3 | PvuII, PstI | 5, 3 | 28, 40 | ||
| France | 7 | PstI | 7 | 28 | ||
| Germany | 9, 2 | PvuII, PstI | 9, 2 | 28, 40 | ||
| Hungary | 1 | PstI | 1 | 28 | ||
| Czech Republic | 16 | PstI | 16 | 28 | ||
| Slovakia | 39 | PstI | 39 | 28 | ||
| Morocco | 1 | PvuII | 1 | 40 | ||
| Various | 381 | BstEII | 380 | 1 | 29b | |
| Sheep | ||||||
| New Zealand | 7, 2 | BstEII, others | 7, 2 | 10, 12 | ||
| Australia | 5, 11 | BstEII | 5, 11 | 6, 12 | ||
| Canada | 7 | BstEII | 6 | 1 | 10 | |
| Faroe | 1, 1 | BstEII, PvuII | 1, 1 | 10, 40 | ||
| North America | 2 | BstEII, others | 2 | 42 | ||
| France | 1 | PstI | 1 | 28 | ||
| Czech Republic | 14 | PstI | 14 | 28 | ||
| Greece | 1 | PvuII | 1 | 40 | ||
| Morocco | 26 | PvuII | 26 | 3 | ||
| South Africa | 5, 2, 2 | PvuII, BstEII, others | 2 | 5, 2 | 3, 12, 14 | |
| Various | 28 | BstEII | 24 | 2 | 2 | 29 |
| Goats | ||||||
| New Zealand | 9 | BstEII | 8 | 1 | 10 | |
| Australia | 3 | BstEII, others | 3 | 12 | ||
| North America | 2 | BstEII, others | 2 | 42 | ||
| Norway | 1, 16 | BstEII, PvuII | 1, 16 | 10, 40 | ||
| Germany | 7 | PvuII | 7 | 3 | ||
| Czech Republic | 1 | PstI | 1 | 28 | ||
| Various | 17 | BstEII | 16 | 1 | 29 | |
| Deer | ||||||
| New Zealand | 20 | BstEII | 17 | 3 | 13 | |
| South America | 11 | BstEII, PstI | 11 | 25 | ||
| Denmark | 2 | PvuII | 2 | 40 | ||
All isolates were of BstEII cattle type based on a match between PstI and BstEII genotypic category.
Countries represented were Europe (91.6% of isolates), New Zealand and Australia (5.3%), and the United States (3.1%).
Where more than one restriction enzyme is given, respective data for each restriction enzyme are given with regard to number of isolates and number of isolates of RFLP type.
The aims of this study were to determine whether there is evidence of segregation of M. avium subsp. paratuberculosis strains among sheep, cattle, and other species in Australia and whether there are likely to be geographic or farm enterprise differences in the strains of M. avium subsp. paratuberculosis present in animals naturally affected with paratuberculosis.
MATERIALS AND METHODS
Tissue samples for bacterial purification from intestine.
Merino and Merino-crossbred sheep with paratuberculosis were selected from a range of farms and geographic regions in New South Wales and Victoria, Australia. Wherever possible, multiple sheep were selected from each farm. Sheep with suspected paratuberculosis were collected from farms and transported to veterinary laboratories for necropsy and confirmation of infection by histopathology. As large-scale liquid culture of ovine strains of M. avium subsp. paratuberculosis is currently not technically possible, the organism was purified from the intestinal lining of affected sheep (6). Briefly, the terminal 8 m of small intestine was removed from each sheep immediately after euthanasia, cut into 2-m lengths, rinsed through with water to remove all intestinal contents, placed in a plastic bag, and frozen at −20°C. Intestinal samples from New South Wales and Victoria were transported frozen to the Elizabeth Macarthur Agricultural Institute (EMAI). Intestinal samples were also fixed in 10% (vol/vol) buffered neutral formalin, embedded in paraffin, sectioned to a thickness of 5 μm, stained with haematoxylin and eosin and by a Ziehl-Neelsen method, and examined microscopically. Intestine that lacked lesions consistent with ovine paratuberculosis and that did not contain acid-fast bacilli in the lamina propria was discarded. M. avium subsp. paratuberculosis was purified from mucosal homogenates from the intestinal tissues using differential and gradient centrifugation.
Culture of isolates.
Isolates of M. avium subsp. paratuberculosis were collected from routine diagnostic and research accessions of laboratories in New South Wales (EMAI) and Victoria (Victorian Institute of Animal Science). Isolates of M. avium subsp. paratuberculosis from animals were obtained from feces or tissues using double incubation-centrifugation methods and culture in modified BACTEC 12B medium (Becton Dickinson, Sparks, Md.) or on Herrold's egg yolk medium with mycobactin J (HEYM+MJ) slopes (1, 36). Two lyophilized human isolates were obtained originally from a female with Crohn's disease (20). At EMAI, lyophilized cultures were reconstituted in 250 μl of sterile distilled water, and the entire volume was inoculated on HEYM+MJ slopes. These were incubated for 4 to 6 months. Isolated colonies were subcultured into 120 ml of modified Watson-Reid broth (pH 5.5) (per liter: l-asparagine, 5 g; d-glucose, 10 g; glycerol, 63 ml; ammonium hydrogen citrate, 2 g; ferric ammonium citrate, 75 mg; KH2PO4, 2 g; NaCl, 2 g; MgSO4 · 7H2O, 1 g; ZnSO4 · 7H2O, 10 mg; CaCl2 · 2H2O, 20 mg; CoCl2 · 6H2O, 2 mg; mycobactin J, 2 mg; pyruvate, 4.1 g) and incubated for 4 to 8 months. Isolated colonies were also subcultured from archived HEYM+MJ slopes stored at room temperature. In this case colonies were collected onto a sterile cotton swab moistened with modified Watson-Reid broth. The swab was placed in 120 ml of modified Watson-Reid broth for 1 min and then withdrawn, and the broth was incubated for 4 to 8 months. If growth was not apparent the procedure was repeated by subculture to a 10-ml volume of modified Watson-Reid broth, and later the entire volume was added to 120 ml of this medium. At the Victorian Institute of Animal Science lyophilized cultures were reconstituted in 0.4 ml of donor horse serum (CSL Pty Ltd., Parkville, Australia) and 0.1 ml of PANTA Plus (Becton Dickinson) and incubated overnight. An aliquot of 0.3 ml was inoculated into modified BACTEC 12B medium and incubated. When the growth index was greater than 500, 2 ml from the BACTEC 12B medium was inoculated into 60 ml of modified Watson-Reid broth and incubated. Isolates were also recovered from stored HEYM+MJ slopes: colonies were suspended in 1.2 ml of phosphate-buffered saline and 0.1 ml of PANTA Plus, incubated overnight, and then transferred directly to flasks containing 60 ml of modified Watson-Reid broth. Modified Watson-Reid broth cultures were incubated for 5 to 10 months. All cultures were incubated in air at 37°C.
Cultures were harvested by centrifugation at 15,000 × g for 30 min at room temperature. Pellets were washed three times with sterile phosphate-buffered saline pH 7.2, and once with sterile distilled water. Bacterial pellets were dispensed in 0.5-g aliquots and stored at −20°C.
Extraction and purification of DNA.
Cells were lysed using lysozyme and mutanolysin and digested using proteinase K. DNA was extracted using cetyltrimethylammonium bromide and purified using chloroform-isoamyl alcohol extraction and alcoholic precipitation (6). The yield and quality of genomic DNA was assessed by electrophoresis in agarose gel with ethidium bromide staining.
RFLP analysis.
DNA was digested with BstEII, separated in 1% (wt/vol) agarose gel at 35 V for 20 h, transferred under vacuum to a positively charged nylon membrane, and probed with an IS900 fragment labeled with digoxigenin. The probe was the 229-bp PCR product produced from M. avium subsp. paratuberculosis strain 316V using primers P25 and P26 (24) located between bp 53 and 281 on IS900, and labeling was undertaken using a random hexamer labeling kit (Boehringer GmbH, Mannheim, Germany). The membrane was developed using a chemiluminescent kit (Boehringer). The banding pattern was identified by matching with published figures in two sources (10, 30). Unfortunately, there are discrepancies in nomenclature between these two sources. For reason of scientific precedent, names were first allocated to isolates according to the patterns of Collins et al. (10). Remaining isolates were named according to Pavlik et al. (30), but for previously unrecognized patterns, S was used to indicate an isolate similar to existing S isolates, C was used for isolates similar to existing C isolates, U was used to signify a new or unknown type, and lastly, consecutive numbers were allocated (e.g., SU1, SU2, CU1). Type CU3 was allocated to an isolate in a separate study in our laboratory.
IS900 and IS1311 PCR-REA.
PCR to detect IS900, an element specific for M. avium subsp. paratuberculosis (16), using primers P90 and P91 (24) as described (46) and REA of the product with AlwI to distinguish IS900 from related elements (11) were used to confirm the identity of isolates as M. avium subsp. paratuberculosis for cases in which a previously unrecognized IS900 RFLP pattern was obtained or no RFLP pattern was obtained. IS1311 PCR and REA with HinfI and MseI were undertaken to determine whether isolates were of the C or S type (21, 44).
RESULTS
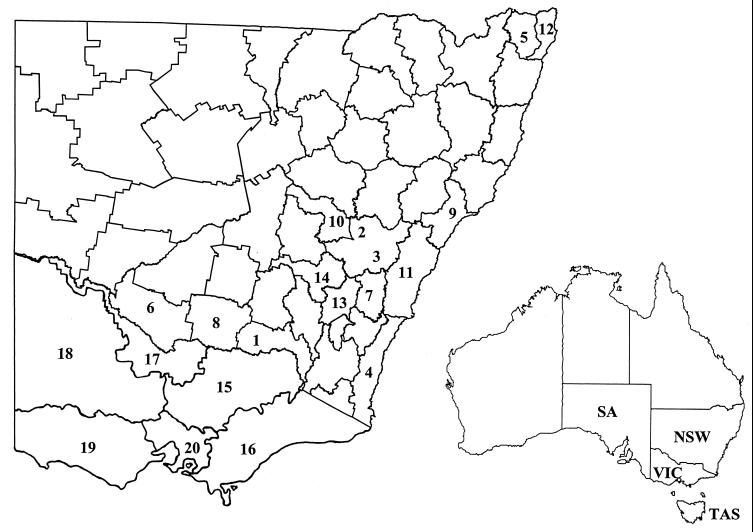
DNA analysis was undertaken on 328 isolates comprising 160 derived from cultures from several species and 168 derived from scrapings of the intestinal mucosae of sheep. The geographical origins of the host animals included New South Wales, Victoria, Tasmania, and South Australia, Australia (Table 2; Fig. 1). The majority of isolates came from New South Wales and Victoria, and overall more than 96 farms were represented. The isolates were obtained between 1981 and 1998, with the majority being from the period 1995 to 1998.
TABLE 2.
Host and geographical origin of the isolates of M. avium subsp. paratuberculosis used in this study
| Sample and host | Yr(s) of collection | No. of isolates obtained ina:
|
Total no. of isolates | |||
|---|---|---|---|---|---|---|
| NSW | VIC | TAS | SA | |||
| Intestinal scrapings (sheep) | 1995–1998 | 147 | 21 | 168 | ||
| Cultured isolates | ||||||
| Sheep | 1986 | 1 | 1 | |||
| Cattle | 1985–1997 | 44 | 100 | 7 | 151 | |
| Goat | 1981–1982 | 2 | 2 | |||
| Alpaca | 1996–1997 | 2 | 1 | 3 | ||
| Rhinoceros | 1995 | 1 | 1 | |||
| Human | 1984 | 2 | 2 | |||
| Total no. of isolates | 195 | 125 | 7 | 1 | 328 | |
| Total no. of farms | 57 | 38 | NRb | 1 | 96 | |
Abbreviations: NSW, New South Wales; VIC, Victoria; TAS, Tasmania; SA, South Australia.
NR, not recorded.
FIG. 1.
Locations for collection of samples for isolation and strain typing of M. avium subsp. paratuberculosis. NSW, New South Wales; VIC, Victoria; SA, South Australia; TAS, Tasmania. Regions 1 to 14 are within NSW: 1, Albury; 2, Bathurst; 3, Carcoar; 4, Bega; 5, Casino; 6, Deniliquin; 7, Goulburn; 8, Jerilderie; 9, Maitland; 10, Molong; 11, Moss Vale; 12, Tweed Lismore; 13, Yass; 14, Young. Regions 15 to 20 are within VIC: 15, North East; 16, Gippsland; 17, North Irrigation; 18, North West; 19, South West; 20, Port Phillip.
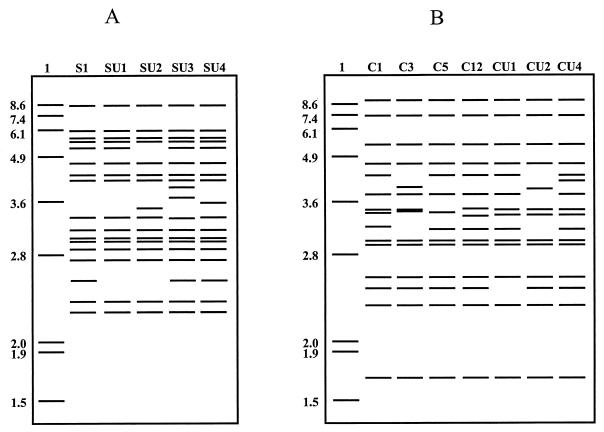
Of the 328 isolates, an IS900 RFLP type was determined for 300 and an IS1311 PCR-REA type was determined for 324. Twelve RFLP types were found: C1, C3, C5, C12, CU1, CU2, CU4, S1, SU1, SU2, SU3, and SU4 (Fig. 2). There was insufficient DNA for RFLP typing of some isolates from cattle from New South Wales which grew poorly after subculture in liquid medium (n = 19), but these were typed using IS1311 PCR-REA (see below). There were also IS900 PCR-positive isolates (n = 9) that did not produce bands in Southern blots due to degradation of DNA, but eight of these were typed successfully using IS1311 PCR-REA (see below).
FIG. 2.
Schematic diagram of RFLP types. (A) Sheep strains; (B) cattle strains. Lane 1, molecular size markers in kb; other lanes, RFLP types as designated.
Most of the isolates examined were from sheep and cattle; however, several isolates from other species were also typed. Two of three isolates from alpacas were cattle RFLP types (C1 and C3), while the third, which could not be typed by RFLP analysis due to degraded DNA, was determined to be a C type by IS1311 PCR-REA. A rhinoceros isolate was RFLP type C5 and two isolates from dairy goats were C3, while two isolates from a human with Crohn's disease were C1 and C5.
Where a sufficient number of isolates was available (for sheep and cattle), RFLP type was analyzed by host species, farm enterprise, geographic location, and also by farm and individual animal when multiple isolates were obtained.
Five RFLP types were identified in sheep, all of which were farmed for wool and/or meat (Table 3; Fig. 1). In New South Wales, S1 was most prevalent, comprising 137 of 142 isolates for which an RFLP type could be determined. Consequently the other RFLP types, namely, SU1, SU2, SU3, and SU4, were rare. Most of the isolates were obtained from farms in regions that are considered to have the highest prevalence of ovine paratuberculosis (Bathurst, Carcoar, and Goulburn [Fig. 1]) (136 isolates from 26 farms), but other regions were also represented (Molong, Yass, and Young [Fig. 1]) (12 isolates from 3 farms). S1 was present in each region; the other RFLP types were found only in the high-prevalence regions where relatively large numbers of isolates had been examined.
TABLE 3.
IS900 BstEII RFLP type for isolates of M. avium subsp. paratuberculosis from sheep
| RFLP type | No. of isolates obtained ina:
|
Total no. of isolates | |
|---|---|---|---|
| NSW | VIC | ||
| S1 | 137 | 21 | 158 |
| SU1 | 1 | 1 | |
| SU2 | 1 | 1 | |
| SU3 | 2 | 2 | |
| SU4 | 1 | 1 | |
| IS1311 PCR-REA type Sb | 5c | 6 | |
| Total | 148 | 21 | 169 |
Abbreviations: NSW, New South Wales; VIC, Victoria.
RFLP bands were not obtained due to degraded DNA.
One isolate not tested.
S1 was the only RFLP type found among the 21 isolates from sheep from four farms in the Gippsland and Port Phillip regions in Victoria (Table 3; Fig. 1).
Seven RFLP types were identified in cattle, namely, C3, C1, C5, CU1, C12, CU2, and CU4 (in decreasing order of prevalence) (Table 4). C3 and C1 collectively accounted for about 85% and C5 accounted for about 8% of the isolates for which an RFLP type was determined, while the other types were uncommon. There were striking geographical and farm enterprise differences in distribution of RFLP type. C1 comprised 47% of the 100 isolates from cattle from Victoria but was not present among the 25 isolates from cattle from New South Wales for which an RFLP type was obtained. C3 was not found in beef cattle in Victoria, even though it was the most common type in dairy cattle in that state and the most common type in beef and dairy cattle in New South Wales.
TABLE 4.
IS900 BstEII RFLP type for isolates of M. avium subsp. paratuberculosis from cattle
| RFLP | No. of cattle in region with RFLP typea
|
Total no. of isolates | ||||
|---|---|---|---|---|---|---|
| NSW
|
VIC
|
TASb | ||||
| Beef | Dairy | Beef | Dairy | |||
| C3 | 3 | 21 | 34 | 6 | 64 | |
| C1 | 26 | 21 | 47 | |||
| C5 | 5 | 5 | 10 | |||
| CU1 | 1 | 3 | 4 | |||
| C12 | 2 | 1 | 3 | |||
| CU2 | 1 | 1 | ||||
| CU4 | 1 | 1 | ||||
| IS1311 PCR-REA type Cc | 4 | 15 | 2 | 21 | ||
| Total | 7 | 37 | 34 | 66 | 7 | 151 |
Abbreviations: NSW, New South Wales; VIC, Victoria; TAS, Tasmania.
Farm enterprise not recorded.
Insufficient DNA for RFLP typing (n = 19) or RFLP bands were not obtained due to degraded DNA.
The isolates from cattle in New South Wales came from 26 farms (7 beef, 19 dairy enterprise) from nine geographic regions (Albury, Bega, Casino, Deniliquin, Jerilderie, Maitland, Molong, Moss Vale, and Tweed Lismore [Fig. 1]). Apart from a single example of CU1, C3 was the only RFLP type found.
The 100 isolates from cattle in Victoria came from 31 farms (4 beef, 27 dairy enterprise) in five geographic regions (Fig. 1). RFLP type C1 was found in each region; C3 was found in three regions; and C5, CU1, and C12 were each found in two regions. Multiple RFLP types were found in each region and enterprise where more than one isolate had been examined. In Gippsland C1, C3, C5, and C12 were found in dairy cattle (among 30 isolates from 15 farms), while C1, C5, and CU4 were found in beef cattle (5 isolates from 1 farm). In the North Irrigation region C1, C3, and CU1 were found in dairy cattle (9 isolates from 4 farms). C1, C5, and C12 were present in beef cattle in the Northeast region (28 isolates from 2 farms). In the Southwest region C1, C3, and CU1 were found in dairy cattle (26 isolates from 7 farms), while C1 was found in beef cattle (1 isolate). A single isolate from a dairy cow in the Northwest region was C1. It appears that a diverse range of RFLP types is present in both beef and dairy cattle in most regions of Victoria, because multiple RFLP types were found even where only relatively few isolates were examined.
Of the 96 individually identified farms represented in this study, 42 contributed more than one isolate; 23 had 2 to 5 isolates, 13 had 6 to 10 isolates, 4 had 11 to 15 isolates, 1 had 16 to 20 isolates, and 1 had 26 isolates. Only one RFLP type was detected on 34 of the 42 farms. Two RFLP types were detected on each of six farms (three sheep and three cattle farms), while three types were detected on each of two cattle farms (Table 5).
TABLE 5.
IS900 BstEII RFLP types for isolates of M. avium subsp. paratuberculosis on farms where more than one type occurred
| Farm no. | Host | Regiona | RFLP type
|
IS1311 PCR-REA typeb | Total no. of animals | Total no. of isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | SU1 | SU2 | SU4 | C1 | C3 | C5 | C12 | CU1 | CU4 | ||||||
| 1 | Sheep | Bathurst, NSW | 11 | 1 | 1 S | 13 | 13 | ||||||||
| 2 | Sheep | Carcoar, NSW | 3 | 1 | 1c | 5 | 5 | ||||||||
| 3 | Sheep | Bathurst, NSW | 6 | 1 | 7 | 7 | |||||||||
| 4 | Cattle dairy | Gippsland, VIC | 8 | 5 | 1 | 1 C | 11 | 15 | |||||||
| 5 | Cattle dairy | North Irrigation, VIC | 1 | 4 | 5 | 5 | |||||||||
| 6 | Cattle dairy | Southwest, VIC | 2 | 1 | 3 | 3 | |||||||||
| 7 | Cattle beef | Gippsland, VIC | 2 | 2 | 1 | 4 | 5 | ||||||||
| 8 | Cattle beef | Northeast, VIC | 23 | 3 | 16 | 26 | |||||||||
Abbreviations: NSW, New South Wales; VIC, Victoria.
RFLP bands were not obtained due to insufficient or degraded DNA.
Not tested.
Among Victorian cattle there were 13 individuals (from six farms) from which isolates of M. avium subsp. paratuberculosis had been obtained from different samples (feces, intestine, or mesenteric lymph node) and/or on different occasions (Table 6). Only one animal yielded isolates that did not have the same RFLP pattern, indicating that RFLP pattern is stable and that within-animal mixed infection is probably uncommon.
TABLE 6.
IS900 BstEII RFLP typing of multiple isolates of M. avium subsp. paratuberculosis from individual cattle
| Sample and time of collection | Total no. of cattle | No. of cattle with same RFLP type | No. of cattle with a different RFLP type |
|---|---|---|---|
| Feces (2 to 4 samples), collected within 1 month | 3 | 2 | 1a |
| Intestine and lymph node samples collected at necropsy | 4 | 4 | 0 |
| Feces and 2 to 3 samples of intestine or lymph node, collected 2 to 15 months apart | 6 | 6 | 0 |
The RFLP types were C5 and CU4.
Of the 300 isolates for which an IS900 RFLP type was assigned in this study, 297 were examined also by IS1311 PCR-REA, and there was complete agreement between the two tests with respect to genotype (Table 7). In addition, isolates that could not be typed by IS900 RFLP analysis due to lack of DNA (n = 19) or degraded DNA (n = 8) were typed successfully by IS1311 PCR-REA. In these cases the typing results matched the species of origin (21 isolates of C type derived from cattle, five isolates of S type derived from sheep) or expected outcome (one isolate of C type derived from an alpaca). Thus overall, all isolates from sheep were S type by IS1311 PCR-REA, whereas all isolates from cattle, goats, alpacas, a rhinoceros, and a human were C type by this test.
TABLE 7.
Comparison of results of typing methods for isolates of M. avium subsp. paratuberculosis
| IS1311 PCR-REA type | IS900 BstEII RFLP type
|
Total no. of isolates | |||
|---|---|---|---|---|---|
| S | C | NTa | NBb | ||
| S | 160 | 5 | 165 | ||
| C | 137 | 19 | 3 | 159 | |
| NT | 3 | 1 | 4 | ||
| Total | 163 | 137 | 19 | 9 | 328 |
NT, not tested due to insufficient DNA.
NB, RFLP bands were not obtained due to degraded DNA.
DISCUSSION
Greater understanding of the epidemiology of mycobacterial infections has followed the application of molecular techniques for identifying and typing mycobacterial isolates. The techniques of REA, pulsed-field gel electrophoresis, PCR, and RFLP are powerful means of typing mycobacterial species such as M. tuberculosis and M. avium to track the transmission of diseases (5). However, there is a high degree of genetic conservation among some mycobacterial species, for example M. leprae, which limits the application of molecular epidemiology (5). Indeed, little useful heterogeneity was revealed in initial attempts to type M. avium subsp. paratuberculosis using REA with more than 30 isolates from widely separate geographic regions failing to segregate into clearly distinct groups (8, 43). However, the simplified patterns obtained in RFLP analysis using IS900 probes were more rewarding (10), there now being many studies reporting genetic diversity (Table 1). RFLP analysis is a robust and reproducible method, and the results for individual isolates are stable over time, enabling direct comparison of data obtained in different laboratories.
In the present study a range of strains of M. avium subsp. paratuberculosis were identified, including RFLP patterns not previously recognized, and clear differences were found in the segregation of strains between host species, geographic regions, and farm enterprises in Australia. Consequently the findings extend those resulting from a smaller Australian survey in which several other RFLP types were found (C22, CU3, C15, and S4) (12). There was a lack of diversity of RFLP type among isolates of M. avium subsp. paratuberculosis from sheep in Australia, with almost all being S1. There were occasional examples of other types (SU1, SU2, and SU4), but these were mostly from farms where S1 was dominant. The exception was SU3, which was responsible for infection in two of two sheep examined from a farm in the Goulburn region of New South Wales. The nature of the differences between these S strains is uncertain, but they may represent minor variants of S1 arising by mutation and persisting within farms.
There was lack of diversity among isolates of M. avium subsp. paratuberculosis from cattle from New South Wales, with type C3 being predominant. There was only one other type in New South Wales, although the relatively small sample size for New South Wales may have precluded detection of uncommon C strains such as CU3 and C15 which have also been found (12). The earlier report of type C1 from cattle in New South Wales (12) requires qualification, as the isolate was obtained from a bull after importation from Canada (44). In contrast to the pattern in New South Wales, six RFLP types were found in Victorian cattle, with C1, C3, and C5 being most common and CU1, CU4, and C12 being quite uncommon. C22 was found in one animal in another survey (12). Although C3 was the most prevalent type overall, in Victoria it was confined to dairy cattle. It is notable that there are strong differences in farming practices between Victoria and New South Wales. For example, cattle dairy enterprises in Victoria are based on seasonal calving, whereas all-year-round calving is practiced in New South Wales. As a consequence there are differences in pasture management and livestock trading between these regions. If confirmed by further sampling, the differences observed between RFLP types in cattle in New South Wales and Victoria and between dairy and beef cattle enterprises in Victoria have potential application in epidemiological studies. The reasons for the absence of type C1 in New South Wales cattle and C3 in Victorian beef cattle require further investigation but probably relate to trading practices in the cattle industries, there being less trade between than within these industries.
Multiple RFLP types were found within geographical regions and even among cattle from the same farm in Victoria. However, in most cases the same type was recovered from individual cattle over periods as long as 15 months, indicating stability in RFLP type and apparent rarity of multistrain infection (Table 6). The occurrence of multiple strains of M. avium subsp. paratuberculosis on some farms and within one animal mirrors findings in other countries. Bauerfeind et al. (3) found different RFLP types in sheep and cattle on the same farm in Morocco and up to 3 types were found on farms with cattle in Argentina (25), while Pavlik et al. (28) reported several RFLP types in one cow and one sheep.
The data in this study highlight differences between continents in the prevalence and diversity of IS900 RFLP types. The most common strain in cattle in Argentina was C17 (25), a strain rare in Europe and not seen at all in Australia. A greater diversity of strains of M. avium subsp. paratuberculosis was found in the northern hemisphere (30) compared to Australia. This may reflect the relative isolation of the livestock industries as well as tight quarantine controls on animal importation in Australia, both of which limit international trade in livestock and therefore the potential to acquire different strains of M. avium subsp. paratuberculosis.
The results of this study are broadly consistent with those of earlier studies in which C strains have been found in cattle and other species while S strains have predominated in sheep, at least in some countries (Table 1). There are a number of possible explanations for observations that cattle and sheep are often affected by distinct strains of M. avium subsp. paratuberculosis. Firstly, the different strains may have some degree of host specificity, preference, or adaptation. They may have different means of infection or environmental survival under different conditions which tend to favor infection of one or other host species. Secondly, the pathogenesis of the disease may differ between host species, for example in route of infection, age of susceptibility, or infectious dose. Thirdly, the grazing behaviors of sheep and cattle are different, as are the forms of their dung, and this may influence exposure to infective material on pasture and result in isolation of infection within one or another species. Finally, farm management practices may tend to reduce the chance of spread of infection between the two species, by influencing the degree of contact between the species at times in their life history when they are susceptible.
Segregation of M. avium subsp. paratuberculosis strains between host species currently occurs in Australia. Furthermore, there was an indication that segregation of certain C strains may occur within the cattle industries and between geographic areas. The data are suggestive of artificial barriers to the spread of strains associated simply with the opportunity for transmission afforded by trade or other movements of livestock. It is this feature of the data which makes it difficult to conclude that cross-species transmission does not pose a risk in disease control programs in Australia. The apparent segregation of strains within sheep and cattle populations may simply reflect there having been a lack of opportunity to exchange infection rather than a real biological barrier to infection. We were unable to assess whether there had been overlap of paratuberculous cattle with healthy sheep and vice versa in this study. The occurrence of C strains of M. avium subsp. paratuberculosis in sheep in other countries (Table 1) may merely reflect the existence of greater opportunities for transmission between cattle and sheep under particular management conditions and over a longer period of time than in Australia, where ruminant industries were established much later than in Europe and elsewhere.
There were several biases inherent in this study. Firstly, only culturable isolates were examined from cattle, making it less likely that ovine strains would be detected in this species. Most of the isolates from cattle were obtained in the period prior to the availability of methodologies suitable for recovery of ovine strains from fecal or tissue samples (45, 46). Nevertheless there are very few examples in Australia of negative culture results from cattle that had histological lesions of paratuberculosis, suggesting that ovine strains of M. avium subsp. paratuberculosis are likely to be uncommon in cattle. Secondly, the samples examined from sheep in this study tended to be from cases of multibacillary paratuberculosis. The question of whether multibacillary and paucibacillary paratuberculosis might be caused by different RFLP types could not be answered due to the design of this study. However, this possibility seems unlikely because both forms of paratuberculosis occur within the same flocks, there may be a progression of lesions from paucibacillary to multibacillary paratuberculosis during the pathogenesis of the disease, and the two forms of lesion in advanced cases of paratuberculosis may simply represent different types of immunopathology (7).
The discovery of differences in the IS1311 gene sequence between isolates of M. avium subsp. paratuberculosis from sheep and cattle enabled the development of a rapid typing test that required little DNA compared to IS900 RFLP analysis (21, 44). It is clear from the present study that the technique is applicable also to degraded DNA samples that are unsuitable for RFLP analysis. The test may be used in any diagnostic laboratory with PCR capability. It is now being used routinely in Australia for strain typing directly from fecal samples, fresh tissue samples (unpublished), formalin-fixed paraffin-embedded tissue samples, and cultures in liquid and solid media (21, 47).
The data in this study provide useful insight into the RFLP types of M. avium subsp. paratuberculosis present in sheep and cattle populations in Australia. There was no evidence for current or widespread exchange of M. avium subsp. paratuberculosis infection between sheep and cattle and as in many countries, paratuberculosis in other species was due to C strains. While cross-species transmission cannot be ignored, it is probably unlikely to prevent successful control of paratuberculosis in Australia. This conclusion must be moderated by the question of the frequency of opportunity for cross-species transmission in the past. Although mixed grazing of sheep and cattle is common in Australia, there is considerable variation in management practices between farms and regions. If the opportunity for transmission between these species had been limited, cross-species transmission would not be reflected in the present data. According to current recommendations in Australia, cattle may be used to graze pasture recently inhabited by paratuberculous sheep. It may be that this practise provides an opportunity for indirect transmission that has not existed commonly in the past. Ongoing monitoring of the types of M. avium subsp. paratuberculosis responsible for paratuberculosis in sheep, cattle, and other species is therefore being undertaken as part of a national approach to control this disease.
ACKNOWLEDGMENTS
This project was funded by NSW Agriculture, Agriculture Victoria, Meat and Livestock Australia, and the Woolmark Company.
Thanks are due to Stephen Ottaway (Orange Agricultural Institute), Graeme Eamens, Elissa Choy, Mark Turner, Vanessa Saunders, Aparna Vadali (EMAI), Pat Kluver, Wendy McDonald (Agriculture Victoria), and the many livestock producers who cooperated with us during this research project.
REFERENCES
- 1.Anonymous. Laboratory methods in veterinary mycobacteriology for the isolation and identification of mycobacteria. Ames, Iowa: National Animal Disease Laboratory; 1972. [Google Scholar]
- 2.Armstrong M C. Johne's disease of sheep in the South Island of New Zealand. N Z Vet J. 1956;4:56–59. [Google Scholar]
- 3.Bauerfeind R, Benazzi S, Weiss R, Schliesser T, Willems H, Baljer G. Molecular characterization of Mycobacterium paratuberculosis isolates from sheep, goats, and cattle by hybridization with a DNA probe to insertion element IS900. J Clin Microbiol. 1996;34:1617–1621. doi: 10.1128/jcm.34.7.1617-1621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard P M, Henderson D, Daniels M J, Pirie A, Buxton D, Greig A, Hutchings M R, McHendrick I, Rhind S, Stevenson K, Sharp J M. Evidence of paratuberculosis in fox (Vulpes vulpes) and stoat (Mustela erminea) Vet Rec. 1999;145:612–613. [Google Scholar]
- 5.Butcher P D, Hutchinson N A, Doran T J, Dale J W. The application of molecular techniques to the diagnosis and epidemiology of mycobacterial diseases. J Appl Bacteriol. 1996;81:53S–71S. [PubMed] [Google Scholar]
- 6.Choy E, Whittington R J, Marsh I, Marshall J, Campbell M T. A method for purification and characterisation of Mycobacterium avium subsp. paratuberculosis from the intestinal mucosa of sheep with Johne's disease. Vet Microbiol. 1998;64:51–60. doi: 10.1016/s0378-1135(98)00252-1. [DOI] [PubMed] [Google Scholar]
- 7.Clarke C J, Little D. The pathology of ovine paratuberculosis: gross and histological changes in the intestine and other tissues. J Comp Pathol. 1996;114:419–437. doi: 10.1016/s0021-9975(96)80017-x. [DOI] [PubMed] [Google Scholar]
- 8.Collins D M, de Lisle G W. Restriction endonuclease analysis of various strains of Mycobacterium paratuberculosis isolated from cattle. Am J Vet Res. 1986;47:2226–2229. [PubMed] [Google Scholar]
- 9.Collins D M, Gabric D M, de Lisle G W. Identification of a repetitive DNA sequence specific to Mycobacterium paratuberculosis. FEMS Microbiol Let. 1989;60:175–178. doi: 10.1016/0378-1097(89)90503-x. [DOI] [PubMed] [Google Scholar]
- 10.Collins D M, Gabric D M, de Lisle G W. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol. 1990;28:1591–1596. doi: 10.1128/jcm.28.7.1591-1596.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cousins D V, Whittington R J, Marsh I, Masters A, Evans R J, Kluver P. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS900-like sequences detectable by IS900 polymerase chain reaction: implications for diagnosis. Mol Cell Probes. 1999;14:431–442. doi: 10.1006/mcpr.1999.0275. [DOI] [PubMed] [Google Scholar]
- 12.Cousins D V, Williams S N, Hope A, Eamens G J. DNA fingerprinting of Australian isolates of Mycobacterium avium subsp. paratuberculosis using IS-900 RFLP. Aust Vet J. 1999;78:184–190. doi: 10.1111/j.1751-0813.2000.tb10590.x. [DOI] [PubMed] [Google Scholar]
- 13.de Lisle G W, Collins D M. Paratuberculosis in farmed deer: case reports and DNA characterisation of isolates of Mycobacterium paratuberculosis. J Vet Diagn Investig. 1993;5:567–571. doi: 10.1177/104063879300500411. [DOI] [PubMed] [Google Scholar]
- 14.de Lisle G W, Collins D M, Huchzermeyer H F A K. Characterization of ovine strains of Mycobacterium paratuberculosis by restriction endonuclease analysis and DNA hybridization. Onders J Vet Res. 1992;59:163–165. [PubMed] [Google Scholar]
- 15.Fridriksdottir V, Gunnarsson E, Sigurdarson S, Gudmundsdottir K B. Paratuberculosis in Iceland: epidemiology and control measures, past and present. In: Manning E J B, Collins M T, editors. Proceedings of the Sixth International Colloquium on Paratuberculosis. Madison, Wis: Melbourne, International Association for Paratuberculosis; 1999. pp. 105–108. [Google Scholar]
- 16.Green E P, Tizard M L V, Moss M T, Thompson J, Winterbourne D J, McFadden J J, Hermon-Taylor J. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 1989;17:9063–9073. doi: 10.1093/nar/17.22.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greig A, Stevenson K, Perez V, Pirie A A, Grant J M, Sharp J M. Paratuberculosis in wild rabbits (Oryctolagus cuniculus) Vet Rec. 1997;140:141–143. doi: 10.1136/vr.140.6.141. [DOI] [PubMed] [Google Scholar]
- 18.Greig A, Stevenson K, Henderson D, Perez V, Hughes V, Pavlik I, Hines II M E, McKendrick I, Sharp J M. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J Clin Microbiol. 1999;37:1746–1751. doi: 10.1128/jcm.37.6.1746-1751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunnarsson E. Isolation of Mycobacterium paratuberculosis from sheep and cattle in Iceland. Acta Vet Scand. 1979;20:191–199. doi: 10.1186/BF03546611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightfoot D, Dwyer B, Sievers I, Jackson K. Isolation of mycobactin dependent mycobacteria from clinical specimens. In: Milner A R, Wood P R, editors. Johne's disease. Melbourne, Australia: CSIRO; 1989. pp. 142–145. [Google Scholar]
- 21.Marsh I, Whittington R, Cousins D. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium based on polymorphisms in IS1311. Mol Cell Probes. 1999;13:115–126. doi: 10.1006/mcpr.1999.0227. [DOI] [PubMed] [Google Scholar]
- 22.McCausland I P. Apparent Johne's disease in a sheep. Aust Vet J. 1980;56:564. doi: 10.1111/j.1751-0813.1980.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 23.McEwen A D. Investigations on Johne's disease of sheep. J Comp Pathol. 1952;52:69–87. [Google Scholar]
- 24.Millar D S, Withey S J, Tizard M L V, Ford J G, Hermontaylor J. Solid-phase hybridization capture of low-abundance target DNA sequences: application to the polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp silvaticum. Anal Biochem. 1995;226:325–330. doi: 10.1006/abio.1995.1232. [DOI] [PubMed] [Google Scholar]
- 25.Moreira A R, Paolicchi F, Morsella C, Zumarraga M, Cataldi A, Fabiana B, Alicia A, Piet O, van Soolingen D, Isabel R M. Distribution of IS900 restriction fragment length polymorphism types among animal Mycobacterium avium subsp. paratuberculosis isolates from Argentina and Europe. Vet Microbiol. 1999;70:251–259. doi: 10.1016/s0378-1135(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 26.Murray A, Moriaty K M, Scott D B. A cloned DNA probe for the detection of Mycobacterium paratuberculosis. N Z Vet J. 1989;37:47–50. doi: 10.1080/00480169.1989.35556. [DOI] [PubMed] [Google Scholar]
- 27.Pálsson P A. Paratuberculosis in Icelandic sheep and its control by vaccination. Bull Off Int Epizooties. 1962;58:65–79. [Google Scholar]
- 28.Pavlik I, Bejckova L, Pavlas M, Rozsypalova Z, Koskova S. Characterization by restriction endonuclease analysis and DNA hybridization using IS900 of bovine, ovine, caprine and human dependent strains of Mycobacterium paratuberculosis isolated in various localities. Vet Microbiol. 1995;45:311–318. doi: 10.1016/0378-1135(94)00130-o. [DOI] [PubMed] [Google Scholar]
- 29.Pavlik I, Horvathova A, Bartl J, Rychlik I. Restriction fragment length polymorphism with the probe IS900 in Mycobacterium paratuberculosis. Epidemiol. Sante Anim. 1997. pp. 31–32. [Google Scholar]
- 30.Pavlik I, Horvathova A, Dvorska L, Bartl J, Svastova P, du Maine R, Rychlik I. Standardisation of restriction fragment length polymorphism analysis for Mycobacterium avium subspecies paratuberculosis. J Microbiol Methods. 1999;38:155–167. doi: 10.1016/s0167-7012(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 31.Riemann H, Zaman M R, Ruppanner R, Aalund O, Jorgensen J B, Worsaae H, Behymer D. Paratuberculosis in cattle and free-living exotic deer. J Am Vet Med Assoc. 1979;174:841–843. [PubMed] [Google Scholar]
- 32.Ris D R, Hamel K L, Ayling J M. Can sheep become infected by grazing pasture contaminated by cattle with Johne's disease? N Z Vet J. 1987;35:137. doi: 10.1080/00480169.1987.35414. [DOI] [PubMed] [Google Scholar]
- 33.Ris D R, Hamel K L, Weaver A M. Natural transmission of Johne's disease to feral goats. N Z Vet J. 1988;36:98–99. doi: 10.1080/00480169.1988.35496. [DOI] [PubMed] [Google Scholar]
- 34.Seaman J T, Thompson D R. Johne's disease in sheep. Aust Vet J. 1984;61:227–229. doi: 10.1111/j.1751-0813.1984.tb05995.x. [DOI] [PubMed] [Google Scholar]
- 35.Stamp J T, Watt J A. Johne's disease in sheep. J Comp Pathol. 1954;64:26–40. doi: 10.1016/s0368-1742(54)80005-1. [DOI] [PubMed] [Google Scholar]
- 36.Stephens L R. Johne's disease (paratuberculosis) In: Corner L A, Baghust T J, editors. Australian standard diagnostic techniques for animal diseases. East Melbourne, Australia: Standing Committee on Agriculture and Resource Management, Commonwealth Scientific and Industrial Research Organisation Publications; 1987. [Google Scholar]
- 37.Taylor A W. Ovine paratuberculosis (Johne's disease of sheep) J Comp Pathol. 1945;55:41–44. [Google Scholar]
- 38.Taylor A W. Varieties of Mycobacterium johnei from sheep. J Pathol Bacteriol. 1951;63:333–336. doi: 10.1002/path.1700630217. [DOI] [PubMed] [Google Scholar]
- 39.Taylor A W. The experimental infection of cattle with varieties of Mycobacterium johnei isolated from sheep. J Comp Pathol. 1953;63:368–373. doi: 10.1016/s0368-1742(53)80038-x. [DOI] [PubMed] [Google Scholar]
- 40.Thoresen O F, Olsaker I. Distribution and hybridisation patterns of the insertion element IS900 in clinical isolates of Mycobacterium paratuberculosis. Vet Microbiol. 1994;40:293–303. doi: 10.1016/0378-1135(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 41.Watt J A A. Johne's disease in a bovine associated with the pigmented strain of Mycobacterium johnei. Vet Rec. 1954;27:387. [Google Scholar]
- 42.Whipple D, Kapke P, Vary C. Identification of restriction fragment length polymorphisms in DNA from Mycobacterium paratuberculosis. J Clin Microbiol. 1990;28:2561–2564. doi: 10.1128/jcm.28.11.2561-2564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whipple D L, Kapke P A, Andrews R E. Analysis of restriction endonuclease fragment patterns of DNA from Mycobacterium paratuberculosis. Vet Microbiol. 1989;19:189–194. doi: 10.1016/0378-1135(89)90084-9. [DOI] [PubMed] [Google Scholar]
- 44.Whittington R, Marsh I, Choy E, Cousins D. Polymorphisms in IS1311, an insertion sequence common to Mycobacterium avium and M. avium subsp. paratuberculosis, can be used to distinguish between and within these species. Mol Cell Probes. 1998;12:349–358. doi: 10.1006/mcpr.1998.0194. [DOI] [PubMed] [Google Scholar]
- 45.Whittington R J, Marsh I, McAllister S, Turner M J, Marshall D J, Fraser C A. Evaluation of modified BACTEC 12B radiometric medium and solid media for the culture of Mycobacterium avium subsp. paratuberculosis from sheep. J Clin Microbiol. 1999;37:1077–1083. doi: 10.1128/jcm.37.4.1077-1083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittington R J, Marsh I, Turner M J, McAllister S, Choy E, Eamens G J, Marshall D J, Ottaway S. Rapid detection of Mycobacterium paratuberculosis in clinical samples from ruminants and in spiked environmental samples by modified BACTEC 12B radiometric culture and direct confirmation by IS900 PCR. J Clin Microbiol. 1998;36:701–707. doi: 10.1128/jcm.36.3.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittington R J, Reddacliff L, Marsh I, Saunders V. Detection of Mycobacterium avium subsp. paratuberculosis in formalin-fixed paraffin-embedded intestinal tissue by IS900 polymerase chain reaction. Aust Vet J. 1999;77:392–397. doi: 10.1111/j.1751-0813.1999.tb10315.x. [DOI] [PubMed] [Google Scholar]