Abstract
Background:
The estimation of influenza-associated excess mortality in developing countries can help improve the estimate of global mortality burden attributable to influenza virus infections. The present study estimates the influenza-associated excess respiratory mortality in mainland China for the 2010/11 through 2014/15 seasons.
Methods:
We collected the provincial weekly influenza surveillance data and population mortality data from the Chinese Center for Disease Control and Prevention for the analysis. The influenza-associated excess respiratory mortality rates (EMR) for 22 out of 31 provinces in the country were estimated using the linear regression models. Extrapolation of EMRs was performed using random-effect meta-regression models for provinces without valid data for a direct estimation of EMR.
Findings:
It was estimated that an annual average of 88,100 (95% confidence interval (CI): 84,200, 92,000) influenza-associated excess respiratory deaths occurred in China in the five years studied, corresponding to 8·2% (95% CI: 7·9%, 8·6%) of respiratory deaths. The mean EMRs per season for influenza A(H1N1)pdm09, A(H3N2), and B viruses were 1·6 (95% CI: 1·5, 1·7), 2·6 (95% CI: 2·4, 2·8), and 2·3 (95% CI: 2·1, 2·5) per 100,000 person-seasons, respectively. The estimated EMR was 1·5 (95% CI: 1.1, 1·9) for people <60 years of age, which was substantially smaller than the rate of 38·5 (95% CI: 36·8, 40·2) per 100,000 person-seasons for those ≥60 years of age. Approximately 80% of influenza-associated excess respiratory deaths occurred in people aged ≥60 years of age.
Interpretation:
Influenza was associated with substantial excess respiratory mortality in China the estimates of which varied across provinces whereas comparable to what has been previously reported. Continuous and high-quality surveillance data across the whole country are needed to improve the estimation of the disease burden attributable to influenza in China.
INTRODUCTION
Influenza virus infections lead to considerable morbidity and mortality each year.1, 2 At the global level, influenza has been estimated to cause approximately 290,000 – 650,000 respiratory deaths annually.3 Many influenza virus infections are never laboratory confirmed due to delays in healthcare seeking and lack of laboratory testing in many locations. Therefore, statistical analyses correlating temporal patterns in influenza circulation and mortality in populations are most often used to quantify the mortality burden associated with influenza.1, 3–5
The mortality burden associated with influenza virus infections varies across populations and geographical locations.1, 3 Most of the available estimates of disease burden attributable to influenza are from higher income locations, while the burden in lower and middle income locations are less well understood.6 In China, the expanded national immunization program does not include influenza vaccination, and over the past 15 years the vaccine coverage has gradually increased to approximately 2%.7 Influenza virus activity varies substantially across provinces in China, with winter epidemics in northern provinces but spring and summer epidemics in southern provinces.8 Two studies estimated the national or regional excess mortality associated with seasonal influenza viruses and influenza A(H1N1)pdm09 in or before 2009/10 in mainland China by major cause of death.9, 10 Seven studies provided estimates of the influenza-associated mortality burden from several particular cities in the country including Beijing,11 Shanghai,12 Yancheng,13 Guangzhou,14–16 and Hefei.17 There were no national estimates of influenza-associated mortality since the 2009 pandemic. Here, we estimate the influenza-associated excess respiratory mortality for mainland China for the period 2010/11 through 2014/15.
METHODS
Sources of data
There are 31 provinces or autonomous regions or municipalities in mainland China (here we use “provinces” to indicate the major administrative divisions in mainland China including provinces, autonomous regions, and municipalities for simplicity). We obtained the weekly mortality and population data in 161 Disease Surveillance Points (DSPs) in China for the years 2005–2015 from the Chinese Center for Disease Control and Prevention (China CDC).9 Given the possibility in re-configuration of the administrative areas in study sites, we collected local data to complement the analysis on the data from the China CDC (e.g. Shanghai, Supplementary Materials 1). For the quality of the study, we excluded the DSPs with an annual average mortality rate less than 0.4% between 2005 and 2015 and those with the annual mortality rate less than 0.3% in any given years.18 Data of 121 DSPs in 30 provinces remained. The data of Hainan province were excluded because the mortality rates of the DSPs in this province did not met the inclusion criteria mentioned above. Next, we cleaned out the outliers in the data and imputed the deleted data points using statistical techniques. Details of the definition of outlier and the statistical techniques used for the imputation were provided in the Supplementary Materials 2. We extracted respiratory deaths based on codes J00-J99 under the 10th edition of the International Classification of Diseases. The data on respiratory mortality and population were stratified by age group (those aged <60 years and ≥60 years) and the data were aggregated by province. The overall annual population data of each province and national annual respiratory mortality data were compiled from the China Statistical Yearbook (Figure S1).
The influenza surveillance data on weekly proportion of samples testing positive for influenza virus by type/subtype (lab%) for 31 provinces were extracted from the National Sentinel Hospital-based Influenza Surveillance Network.9 Data of Tibet were excluded because the number of hospitals in the Influenza Surveillance Network was small and the data were not sufficient enough for the analysis. Details on the National Sentinel Hospital-based Influenza Surveillance Network were provided in the Supplementary Materials 3. Meteorological data on ambient temperature and relative humidity were obtained from the China Meteorological Data Sharing Service System. We used meteorological data of all monitoring stations within the same province to estimate the data of temperature and relative humidity for the province as a whole. Absolute humidity was derived from the temperature and relative humidity.5
Data analysis
We used the lab% (by type/subtype) as the influenza virus activity proxy, intended to reflect patterns in incidence of infections with influenza A(H1N1)pdm09, A(H3N2), and B. An influenza season was defined in this study as the time period between October and September in the following year. The number of hospitals included in the Influenza Surveillance Network expanded after the 2009 pandemic. We observed that the surveillance data on the proportions of influenza virus detection were relatively less stable prior to 2009, probably because of the smaller number of surveillance sites. To avoid the potential impact from the changes in the influenza surveillance on the estimation of the influenza-associated excess mortality in this study, we restricted our analyses to the post-pandemic period, and studied the five years 2010/11 through 2014/15. In addition, we calculated the ratios of the standard deviations of the detrended time-series of the respiratory death rates and lab% to the means of time-series. A larger ratio, particularly the one exceeding the threshold, indicated instability in the measurements. We excluded the data of seven provinces (i.e. Hebei, Ningxia, Shanxi, Qinghai, Xinjiang, Yunnan, and Inner Mongolia) from the analysis because the ratios were larger than thresholds (further details provided in the Supplementary Materials 4, Figure S2, and Table S1). Further, we deleted an abnormally increased data point in lab% in Jiangxi in late 2011 from the analysis as indicated in Figure S3. The influenza-associated excess respiratory mortality rate (EMR) was first estimated at the provincial level, and then at the national level (Figure S4).
Stage 1: Single-province mortality estimates
A linear model was applied, assuming an additive association between influenza incidence and mortality which could be more biologically plausible than a multiplicative association.1, 4 Meanwhile, the respiratory mortality rates in all ages and in persons ≥60 years of age approximately followed a normal distribution. Therefore, linear regression models were selected to investigate the association between the mortality rates and the influenza virus activity in this study in order to estimate the EMR between the 2010/11 and 2014/15 seasons for each province.4, 5 Respiratory mortality rates for all ages combined, and in those aged ≥60 years as a specific subgroup, were used as the dependent variables in the models, and regressed against the proxies for influenza A(H1N1)pdm09, A(H3N2), and B. A natural cubic spline of calendar week was used to allow for linear and non-linear changes in the mortality rate over time. Natural cubic spline functions were also used to control for the effects of temperature and absolute humidity. We assumed that there was a lag of one week between the exposure variables, including influenza virus activity and climatic variables, and the model outcome respiratory mortality rate.5
The influenza-associated excess respiratory deaths for all ages and people aged ≥60 years were estimated by subtracting the predicted number of respiratory deaths assuming that the influenza virus activity proxy was 0 (E(Y|X=0)) from the predicted number of respiratory deaths using the observed influenza virus activity proxy (E(Y|X=x_obs)).5 Y indicates the predicted respiratory death rate; X represents influenza virus activity proxy; x_obs refers to the observed influenza virus activity proxy. We were not able to directly estimate excess respiratory mortality in children or younger adults because of the smaller numbers of deaths reported. Thus, the influenza-associated excess respiratory deaths were estimated for population aged <60 years by subtracting the estimated respiratory deaths for those aged ≥60 years from the estimated excess respiratory deaths for all ages. The excess respiratory mortality rate was estimated as the number of influenza-associated excess respiratory deaths per 100,000 population. Technical details on how to estimate single-province mortality were presented in the Supplementary Materials 5.
Stage 2: National burden estimates
Random-effect meta-regression models were fitted for the extrapolation of (mean) EMRs per season for the provinces where data were not available or directly analyzed. We considered one group of economy-related variables: gross domestic product per capita (GDP), population density, and regional classification (i.e. west, center, and east), one groups of variables which indicated healthcare infrastructure, access to healthcare services as well as healthcare needs in the population: number of sentinel hospitals per 100,000 persons, number of hospitals per capita, number of hospital beds per 1,000 persons, number of outpatients per capita, and a climate-related variable: latitude. We selected the model from those with statistically significant independent variables and based on the Akaike Information Criterion (AIC) (Supplementary Materials 6).
The national excess respiratory mortality rates by age group, influenza virus types/subtype, combination of age groups and influenza virus types/subtypes, and combination of influenza seasons and influenza virus types/subtypes were estimated. The national excess respiratory mortality rates each season were estimated as the population-weighted EMRs of 31 provinces. The proportion of respiratory deaths due to influenza was also estimated (Supplementary Materials 7). In addition we performed a sensitivity analysis to 1) extrapolate the influenza-associated excess respiratory mortality for the nine excluded provinces simply based on the model with the smallest value of AIC to explore potential impact of selection of the meta-regression model on the national overall estimate of influenza-associated excess respiratory mortality; 2) estimate the national influenza-associated excess respiratory mortality assuming a lag of two weeks between influenza, climatic factors and respiratory mortality; 3) estimate the national influenza-associated excess respiratory mortality using the data of more DSPs (Supplementary Materials 8, Figure S5, and Table S2). All analyses were conducted using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
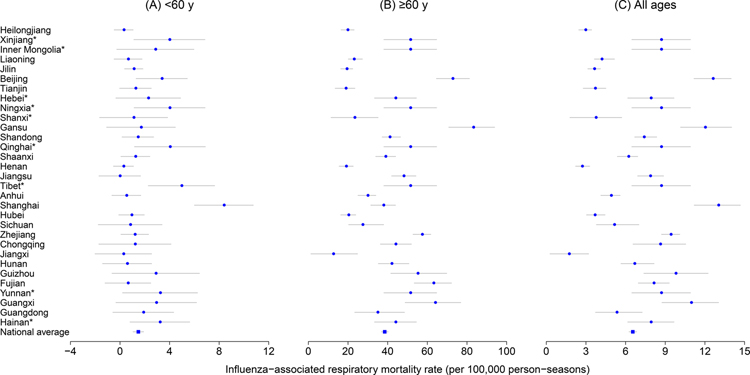
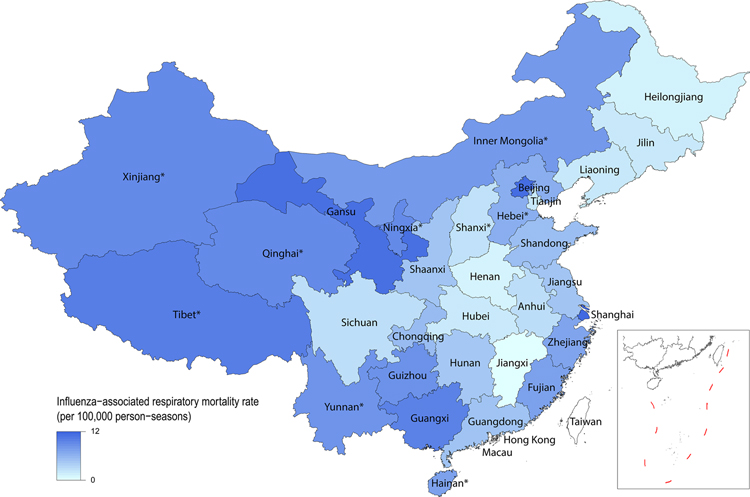
Our study used the data collected from 22 out of 31 provinces in mainland China, representing 83·0% of the total population, to estimate the influenza-associated excess respiratory mortality rates. The age-specific estimates of mean excess respiratory mortality rates per season varied across the 22 provinces, with a median of 1·1 (range: 0·0, 8·4) and 38·4 (range: 12·5, 83·2) per 100,000 person-seasons for people aged <60 years and those aged ≥60 years, respectively (Figure 1 and Table S3). The age-standardized EMRs per season in the 22 provinces ranged between 1·7 (95% CI: −0·7, 4·2) per 100,000 person-season in Jiangxi and 11·9 (95% CI: 9·7, 14·1) per 100,000 person-season in Shanghai (Figure 2 and Table S3).
Figure 1.
Mean age-specific influenza-associated excess respiratory mortality rates (EMRs) per 100,000 person-seasons for all influenza in mainland China between the 2010/11 through 2014/15 seasons. * the excess respiratory mortality rate for the province was estimated using a random-effect meta-regression model based on estimates from the 22 provinces. Dots indicate the point estimates of excess respiratory mortality rates. Grey lines indicate the 95% confidence intervals of the estimated EMRs.
Figure 2.
Mean age-standardized influenza-associated excess respiratory mortality rates (EMRs) per 100,000 person-seasons for all influenza in mainland China between the 2010/11 through 2014/15 seasons. * means the excess respiratory mortality rates for the province was estimated using a random-effect meta-regression model based on estimates from the 22 provinces.
The contributions of infections of different influenza virus types/subtypes to respiratory mortality varied across the 22 provinces, with a median of the mean excess respiratory mortality rates per season to be 1·6 (range: 0·3, 5·2), 2·9 (range: −0·1, 9·7), and 1·9 (range: −0·8, 6·4) per 100,000 person-seasons for influenza A(H1N1)pdm09, A (H3N2), and B viruses, respectively (Table S4).
Heterogeneity was observed in the estimated all-age average excess respiratory mortality rates per season of the 22 provinces (Q = 784; P < 0·0001; I2 = 98%). A random-effect meta-regression model with regional classification was used for the extrapolation of the average excess respiratory mortality rates for the nine provinces which had no reliable data available (Table S5). The results of meta-regression analysis indicated that the average excess mortality was higher in the west (Z = 3·274; P = 0·0011) and the east of China (Z = 3·109; P = 0·0019) than that in the center of China (Table S5).
Overall we estimated that an annual average of 88,100 (95% CI: 84,200, 92,000) influenza-associated excess respiratory deaths occurred in mainland China between 2010/11 and 2014/15, corresponding to 8·2% (95% CI: 7·9%, 8·6%) of all respiratory deaths. Accordingly, the average all-age and age-standardized excess respiratory mortality rates per season were estimated to be 6·5 (95% CI: 6·3, 6·8) and 5·9 (95% CI: 5·5, 6·3) per 100,000 persons, respectively.
The estimate of the overall rate of influenza-associated excess respiratory deaths for older adults was 38·5 (95% CI: 36·8, 40·2) per 100,000 person-seasons, which was substantially higher than that for those <60 years of age (1·5 (95% CI: 1.1, 1·9) per 100,000 person-seasons). We estimated that 17,200 (95% CI: 12,200, 22,000) and 71,000 (95% CI: 67,800, 74,100) influenza-associated excess respiratory deaths occurred in those aged <60 years and ≥60 years during an influenza season, which corresponded to 20% and 80% of all influenza-associated excess respiratory deaths, respectively.
The national average annual influenza-associated excess respiratory death rates were estimated to be 1·6 (95% CI: 1·5, 1·7), 2·6 (95% CI: 2·4, 2·8), and 2·3 (95% CI: 2·1, 2·5) per 100,000 person-seasons for influenza A(H1N1)pdm09, A(H3N2), and B viruses, respectively. Similar to the results for all ages, the average annual mortality burden of influenza A(H3N2) and B virus infections for people aged ≥60 years was higher than those for influenza A(H1N1)pdm09 virus infection (Table).
Table.
Average annual influenza-associated excess respiratory excess mortality rates per 100,000 person-seasons by age group and by influenza virus type/subtype in mainland China between the 2010/11 through 2014/15 seasons.
| Age group | Average annual influenza-associated excess respiratory mortality rate (per 100,000 person-seasons) |
|||||||
|---|---|---|---|---|---|---|---|---|
| A(H1N1)pdm09 | (95% CI) | A(H3N2) | (95% CI) | B | (95% CI) | All influenza | (95% CI) | |
|
| ||||||||
| <60 y | 0·4 | (0·2, 0·5) | 0·7 | (0·5, 1·0) | 0·4 | (0·1, 0·6) | 1.5 | (1.1, 1.9) |
| ≥60 y | 9·7 | (9·0, 10·4) | 14·4 | (13·3, 15·4) | 14·4 | (13·4, 15·5) | 38·5 | (36·8, 40·2) |
| All ages | 1·6 | (1·5, 1·7) | 2·6 | (2·4, 2·8) | 2·3 | (2·1, 2·5) | 6·5 | (6·3, 6·8) |
Abbreviation: 95% CI, 95% confidence interval.
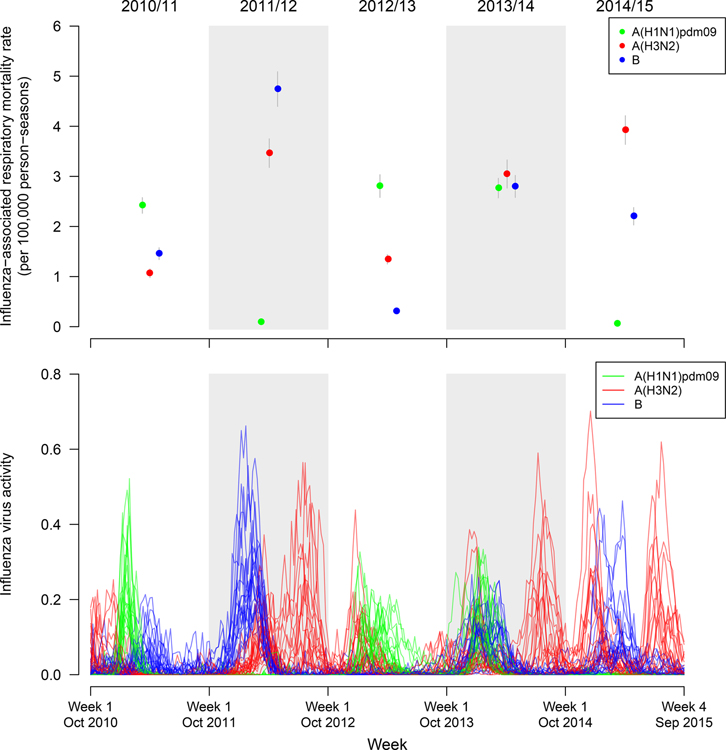
Variations were observed in the influenza virus activity and the annual estimated national EMRs across five influenza seasons (Figure 3 and Table S6). Specifically, the estimated annual national excess respiratory mortality rates ranged between 0·1 (95 CI: 0·1, 0·1) and 2·8 (95% CI: 2·6, 3·0) per 100,000 persons, between 1·1 (95% CI: 1·0, 1·2) and 3·9 (95% CI: 3·6, 4·2) per 100,000 persons, and between 0·3 (95% CI: 0·3, 0·3) and 4·7 (95% CI: 4·4, 5·1) per 100,000 persons for influenza A(H1N1)pdm09, A(H3N2), and B viruses, respectively (Figure 3 and Table S6). It was estimated that the national excess respiratory mortality rates ranged between 4·5 (95% CI: 4·2, 4·7) and 8·6 (95% CI: 8·2, 9·0) per 100,000 persons in 2012/13 and 2013/14, respectively (Table S6).
Figure 3.
Annual national estimates of the all-age influenza-associated excess respiratory excess mortality rates (EMRs) per 100,000 person-seasons by influenza virus type/subtype in mainland China from the 2010/11 through 2014/15 seasons (upper panel) and the weekly detections of influenza viruses from 22 provinces included in the original modeling analysis activity (lower panel, each line refers to the virus activity observed in a province). Dots indicate the point estimates of excess respiratory mortality rates. Grey lines indicate the 95% confidence intervals of the estimated EMRs.
Results from the sensitivity analysis indicated that no substantial variations were observed in the estimates of the influenza-associated excess respiratory mortality from different models or from the analysis on a varied set of DSP sites. The average annual estimates of influenza-associated excess respiratory mortality rates were robust to the selection of meta-regression models with different independent variables for extrapolation of the excess respiratory mortality for the nine provinces excluded from the original modeling analysis, the models with a different time lag between influenza virus activity, climatic factors and respiratory mortality, and more DSPs included for the estimation (Table S7).
DISCUSSION
We estimated that 88,100 (95% CI: 84,200, 92,000) influenza-associated excess respiratory deaths occurred in mainland China during an influenza season, corresponding to 8·2% (95% CI: 7·9%, 8·6%) of annual respiratory deaths. Accordingly, the mean all-age excess respiratory mortality rate in mainland China was 6·5 (95% CI: 6·3, 6·8) per 100,000 person-seasons, which fell within the estimate for the Western Pacific Region reported in a global study (95% CrI: 3·6, 7·5 per 100,000 individuals),3 despite differences in modelling approaches. Our estimate was also comparable to those previously published in other countries or regions or cities. Specifically, our point estimate of the overall influenza-associated excess respiratory mortality rate was similar to that reported in Hong Kong SAR (6·3 per 100,000 person-years),19 but was higher than those reported in Yancheng, a subtropical city in eastern China (4·6 per 100,000 person-years),13 the United States (3·6 per 100,000 person-seasons),4 and Hefei, a subtropical city in China (2·7 per 100,000 person-years),17 but was smaller than that reported in Western Kenya (10 per 100,000 person-years).20 Influenza virus infections may not only cause respiratory deaths, but also lead to deaths by triggering or exacerbating underlying chronic conditions such as cardiovascular diseases.21 Therefore, the estimated excess respiratory deaths in this study only reflected a fraction of all influenza–associated deaths.1
Spatial variations were observed in the estimates of the influenza-associated excess respiratory mortality among the provinces studied. We found that the estimates in provinces located in the west and east of China were higher than those in provinces in the center of China. The higher mortality burden of influenza in the west of China could be due to a lack of access to healthcare services, especially in rural areas,22, 23 while the relatively higher mortality burden of influenza in the east of China could be due to higher population density, which might increase the risk of influenza transmission. Higher influenza-associated excess respiratory mortality in Shanghai and Beijing might be attributable to a high population density resulting from population movement largely from other provinces driven by patients seeking advanced healthcare, migrant workers or tourists,24, 25 which was likely to increase the risk of influenza transmission in the population and therefore the observed mortality burden in the cities.
We found that the majority (80%) of the influenza-associated excess respiratory deaths occurred in people aged ≥60 years, similar to other previous studies.10, 13 People in older age would be more likely to have underlying health conditions and therefore at a higher risk of severe disease after influenza virus infections.26 The observed variations in the proportions of the influenza-associated excess respiratory mortality in older adults from various studies could be due to the difference in the proportion of older adults in the population as well as other factors such as the baseline prevalence of respiratory diseases, circulating influenza virus types/subtypes in the study period, duration of influenza epidemics, and clinical practice in coding causes of death, etc. Seasonal influenza vaccination is the most effective intervention in protection against influenza virus infection in outpatients and inpatients.27 Many high-income countries conducted influenza vaccination program in older adults with the goal of reducing the influenza-associated mortality in this high-risk group. China in general has low vaccination coverage in the population, although vaccine uptake in some specific age groups is higher in cities such as Beijing where the local government fully subsidized the vaccination in older adults.7 Further efforts, such as improving healthcare services/access for the less developed region, more targeted interventions for older adults, including vaccination, early treatment with antivirals and hand hygiene may also be needed to mitigate the mortality burden of influenza in mainland China.
The virus-specific estimates of excess respiratory mortality rates in our study indicated that influenza A(H3N2) and B viruses were on average associated with a relatively higher respiratory mortality burden than influenza A(H1N1)pdm09 virus. A higher excess mortality was also found to be associated with influenza A(H3N2) virus infection compared with other influenza virus types/subtypes in the United States4 and Hong Kong SAR.5 Possible explanation included 1) more severe clinical symptoms caused by seasonal influenza A(H3N2) virus infections are more likely to increase mortality burden;2, 28 2) higher level of virulence was observed in this subtype;29 3) faster antigenic drift of the virus lead to a higher incidence of infections among the population, which may also contribute to the higher mortality burden.30 The varied mortality burden of influenza virus types/subtypes across different study locations could be attributable to the distinct circulating influenza virus types/subtypes during the study period, transmission dynamic of and population immunity against different influenza virus types/subtypes.9
Some limitations of this study should be mentioned. First, we estimated the influenza-associated excess respiratory mortality in China based on data from five influenza seasons. The long-term trends of influenza-associated mortality burden should be assessed in the future with data of a longer time span. Second, the current analyses were based on the data from 22 out of 31 provinces/municipalities in mainland China which might not fully represent the influenza-associated excess respiratory mortality burden over the country as a whole. Third, the quality of mortality data reported by DSPs varying across provinces might influence the estimated EMRs while our sensitivity analysis did not imply a substantial impact on the final estimates of this study. Fourth, our analysis only provided combined estimates of the influenza-associated excess respiratory deaths in children and adults as people <60 years of age instead of the two groups separately because of the small number of deaths reported in each of the age group. In addition, we did not include pathogens such as respiratory syncytial virus (RSV) into our model when estimating the EMRs. RSV was suggested in previous studies to be associated with an increase in mortality of older population while the impact might not be as substantial as that attributable to influenza. The lack of national RSV surveillance data in China did not allow us to investigate the mortality impact of RSV in this study. Lastly, the potential cluster effect of the estimates from geographically close provinces was not considered in the current analyses, which might lead to narrower interval estimates.
CONCLUSION
Human seasonal influenza viruses were associated with substantial mortality burden in population in China, and the burden varied across age groups and provinces. Our estimates of the influenza-associated excess respiratory deaths provided critical information for optimization of vaccination strategies in the country.
Supplementary Material
Research in context.
Evidence before this study
Previous studies have shown that influenza virus causes substantial mortality burden globally in humans. Estimation of influenza-associated disease burden is the basis for implementation of interventions in control of influenza. We searched PubMed on 13 April 2019 using the key words “influenza OR flu”, “Chinese OR China”, “mortality OR death” and “burden OR impact” without language restrictions. Among the 111 articles identified, there are eight relevant published studies on estimation of influenza-associated mortality burden in China. Two studies estimated the national or regional excess mortality associated with seasonal influenza viruses and influenza A(H1N1)pdm09 in or before 2009/10 in mainland China by major cause of death. Feng et al. estimated annual influenza-associated excess all-cause mortality of 18·0 and 11·3 per 100,000 in the Northern and Southern cities, respectively, in 2003–08. Yu et al. studied the 2009/10 pandemic and estimated 11·4–12·1 influenza-associated circulatory and respiratory deaths per 100,000 persons per year, with some variability by region. The other six studies provided estimates of the influenza-associated mortality burden from several particular cities in the country including Beijing, Shanghai, Yancheng, Guangzhou, and Hefei. Wu et al. reported an average of 19·1 influenza-associated all-cause deaths per 100,000 persons per year in Beijing between 2007 and 2013. Zhang et al. reported an average of 4·6 influenza-associated excess respiratory deaths per 100,000 persons per year in the city of Yancheng in eastern China, between 2011 and 2015. Yu et al. estimated that there were an average of 0·23 influenza-associated pneumonia and influenza deaths per 100,000 persons per year in Shanghai between 2010 and 2015. Wang et al. estimated an average of 15 influenza-associated all-cause deaths per 100,000 persons per year in Guangzhou between 2010 and 2012, while Yang et al. estimated an average of 10·6 influenza-associated all-cause deaths per 100,000 persons per year in Guangzhou between 2004 and 2006. Liu et al. estimated an average of 9·9 influenza-associated all-cause deaths per 100,000 persons per year in the city of Hefei in Southern China between 2010 and 2015. In summary, there were no national estimates of influenza-associated mortality since the 2009 pandemic, while pre-pandemic estimates at the national level and post-pandemic estimates in selected cities were generally consistent with the recent global estimate on influenza impact of 4·0–8·8 influenza-associated excess respiratory deaths per 100,000 population per year, and the highest influenza-associated mortality rates occurring in older adults.
Added value of this study
We provide the first estimates of influenza-associated mortality for China at the provincial level and overall. We estimated that on average 88,100 (95% confidence interval (CI): 84,200, 92,000) influenza-associated excess respiratory deaths occurred in China every year during 2010/11–2014/15 seasons, accounting for 8·2% (95% CI: 7·9%, 8·6%) of all reported respiratory deaths. Approximately 80% of the influenza-associated excess respiratory deaths occurred in population ≥60 years of age, leading to 38·5 (95% CI: 36·8, 40·2) excess respiratory deaths per 100,000 person-seasons which was substantially higher than the estimate in individuals <60 years of age (1·5, 95% CI: 1·1, 1·9 per 100,000 person-seasons). Influenza A(H1N1)pdm09, A(H3N2), and B viruses were attributable to 1·6 (95% CI: 1·5, 1·7), 2·6 (95% CI: 2·4, 2·8), and 2·3 (95% CI: 2·1, 2·5) respiratory deaths per 100,000 person-seasons, respectively.
Implications of available evidence
Influenza causes substantial mortality impact in China, associated with 8·2% (95% CI: 7·9%, 8·6%) of all respiratory deaths. Influenza is a vaccine-preventable disease, and our findings imply that increased use of influenza vaccines in China could have a clear public health impact.
ACKNOWLEDGMENTS
We thank the staff members at the influenza sentinel hospital network and the Disease Surveillance Points in each province in China for the administrative work and data collection.
FUNDING
This work was supported by the National Science Fund for Distinguished Young Scholars (81525023), National Science and Technology Major Project of China (2017ZX10103009-005; 2018ZX10713001-005), the National Institute of Health Research using Official Development Assistance (ODA) funding (16/137/109), the Research Grants Council of the Hong Kong Special Administrative Region, China (project numbers: 17106617 and T11-705/14N), the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant number U54 GM088558), and the China-US Collaborative Program on Emerging and Re-emerging Infectious Disease (6 NU2GGH000961-05-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of NHS, the National Institute for Health Research or the Department of Health, and the National Institute of General Medical Sciences or the National Institutes of Health. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
YHJ has received investigator-initiated research funding from Sanofi Pasteur, GlaxoSmithKline, and Yichang HEC Changjiang Pharmaceutical Company. BJC has received honoraria from Sanofi Pasteur and Roche.
REFERENCES
- 1.Li L, Wong JY, Wu P, et al. Heterogeneity in estimates of the impact of influenza on population mortality: a systematic review. Am J Epidemiol 2018; 187: 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292: 1333–40. [DOI] [PubMed] [Google Scholar]
- 3.Iuliano A, Roguski K, Chang H, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391: 1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein E, Viboud C, Charu V, Lipsitch M. Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology 2012; 23: 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis 2012; 206: 1862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Influenza (Seasonal). 2019. http://www.who.int/mediacentre/factsheets/fs211/en/ (accessed June 21, 2019).
- 7.Yang J, Atkins KE, Feng L, et al. Seasonal influenza vaccination in China: landscape of diverse regional reimbursement policy, and budget impact analysis. Vaccine 2016; 34: 5724–35. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Alonso WJ, Feng L, et al. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: spatio-temporal modeling of surveillance data. PLoS Med 2013; 10: e1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Feng L, Viboud CG, et al. Regional variation in mortality impact of the 2009 A(H1N1) influenza pandemic in China. Influenza Other Respir Viruses 2013; 7: 1350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng L, Shay DK, Jiang Y, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bull World Health Organ 2012; 90: 279–88b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Wei Z, Greene CM, et al. Mortality burden from seasonal influenza and 2009 H1N1 pandemic influenza in Beijing, China, 2007–2013. Influenza Other Respir Viruses 2018; 12: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Wang C, Chen T, et al. Excess pneumonia and influenza mortality attributable to seasonal influenza in subtropical Shanghai, China. BMC Infect Dis 2017; 17: 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Xiong Q, Wu P, Chen Y, Leung NHL, Cowling BJ. Influenza-associated mortality in Yancheng, China, 2011–15. Influenza Other Respir Viruses 2018; 12: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Fu C, Li K, et al. Influenza associated mortality in Southern China, 2010–2012. Vaccine 2014; 32: 973–8. [DOI] [PubMed] [Google Scholar]
- 15.Wong CM, Peiris JS, Yang L, et al. Effect of influenza on cardiorespiratory and all-cause mortality in Hong Kong, Singapore and Guangzhou. Hong Kong Med J 2012; 18 Suppl 2: 8–11. [PubMed] [Google Scholar]
- 16.Yang L, Ma S, Chen PY, et al. Influenza associated mortality in the subtropics and tropics: results from three Asian cities. Vaccine 2011; 29: 8909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XX, Qin G, Li X, et al. Excess mortality associated with influenza after the 2009 H1N1 pandemic in a subtropical city in China, 2010–2015. Int J Infect Dis 2017; 57: 54–60. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. National Disease Surveillance Point System Mortality Surveillance Dataset 2012. Beijing: Popular Science Press. 2013 Dec, Page 4. [Google Scholar]
- 19.Wu P, Presanis AM, Bond HS, Lau EHY, Fang VJ, Cowling BJ. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998–2013. Sci Rep 2017; 7: 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emukule GO, Spreeuwenberg P, Chaves SS, et al. Estimating influenza and respiratory syncytial virus-associated mortality in Western Kenya using health and demographic surveillance system data, 2007–2013. PLoS One 2017; 12: e0180890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siriwardena AN. Increasing evidence that influenza is a trigger for cardiovascular disease. J Infect Dis 2012; 206: 1636–8. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Shi L, Liang H, Ding G, Xu L. Urban-rural disparities in health care utilization among Chinese adults from 1993 to 2011. BMC Health Serv Res 2018; 18: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Xu Y, Ren J, Sun L, Liu C. Inequality in the distribution of health resources and health services in China: hospitals versus primary care institutions. Int J Equity Health 2017; 16: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J, Lau EH, Li K, et al. Effect of Live Poultry Market Closure on Avian Influenza A(H7N9) Virus Activity in Guangzhou, China, 2014. Emerg Infect Dis 2015; 21: 1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z Research on strengthening the management of migrant population in Shanghai. 2013. http://www.gzsk.org.cn/URBAN-INSIGHT/Magazine/2013/201306/201306-145_split_1.pdf (accessed July 21, 2019).
- 26.Gordon A, Reingold A. The burden of influenza: a complex problem. Curr Epidemiol Rep 2018; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castilla J, Godoy P, Dominguez A, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57: 167–75. [DOI] [PubMed] [Google Scholar]
- 28.Frank AL, Taber LH, Wells JM. Comparison of infection rats and severity of illness for influenza A subtypes H1N1 and H3N2. J Infect Dis 1985; 151: 73–80. [DOI] [PubMed] [Google Scholar]
- 29.Wright PF, Thompson J, Karzon DT. Differing virulence of H1N1 and H3N2 influenza strains. Am J Epidemiol 1980; 112: 814–9. [DOI] [PubMed] [Google Scholar]
- 30.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008; 453: 615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.