Abstract
Ovine scrapie is a member of the transmissible spongiform encephalopathies (TSEs), a heterogeneous family of fatal neurologic disorders characterized by deposition of an abnormal isoform (prion protein [PrP] PrP-Sc) of a cellular sialoglycoprotein in neural tissue. PrP-Sc is detectable in some lymphoid tissues of infected sheep months or years before development of clinical disease. Detection of PrP-Sc in these tissues is the basis for live-animal testing. In this study, we characterize the performance of a preclinical diagnostic test for ovine scrapie based on a monoclonal antibody (MAb)-based immunohistochemistry assay of nictitating membrane (“third eyelid”)-associated lymphoid tissue. The results of third eyelid immunohistochemistry assay agreed with the scrapie status of the sheep for 41 of 42 clinical suspects with confirmed scrapie and 174 of 175 sheep without scrapie. Third eyelid sampling agreed with the scrapie status for 36 of 41 clinically normal sheep positive for PrP-Sc immunostaining of brain tissue, including 27 sheep with positive biopsy specimens that progressed to clinical disease with confirmed scrapie 3 to 20 months after biopsy. The assay used MAb F89/160.1.5, which binds to residues 142 to 145 of ovine PrP. This antibody can be used in combination with MAb F99/97.6.1, which binds to residues 220 to 225. One or both MAbs in this cocktail recognize PrP sequences conserved in most mammalian species in which natural TSEs have been reported. Immunohistochemistry assay of routinely formalin-fixed lymphoid tissues with a cocktail of pan-specific MAbs is a practical, readily standardized live-animal and preclinical test for ovine scrapie.
Scrapie is a fatal neurodegenerative disorder of sheep and goats characterized by accumulation of prions, novel transmissible agents largely composed of prion protein (PrP) PrP-Sc, an abnormally folded isoform of the normal cellular PrP, PrP-C (20). Ovine scrapie is the prototype for a heterogeneous group of PrP-Sc-associated disorders, notably, bovine spongiform encephalopathy (BSE) (28) and the related human disorder, variant Creutzfeldt-Jakob disease (30). BSE has a broad experimental host range and has possibly been transmitted to domestic cats (1) and to a variety of nondomestic felids, ruminants (27), and nonhuman primates (2) in zoological parks. PrP-Sc accumulation is also noted in chronic wasting disease of captive and free-ranging mule deer, white-tailed deer, and elk in limited areas of the United States (4, 31).
Ovine scrapie is endemic in many parts of the world, and control measures have been hampered by the long incubation time and the lack of a preclinical or live-animal diagnostic test. Scrapie is diagnosed by detection of neuronal vacuolation and degeneration in formalin-fixed tissues of the central nervous system and by immunohistochemical detection of PrP-Sc in the brains of sheep (14). Immunohistochemistry assay is particularly useful for the diagnosis of scrapie in sheep early in the clinical stage of the disease, when histologic changes are mild, or for examination of autolyzed tissues. Immunoassay for PrP-Sc is also useful for identification of sheep in the preclinical stage of the disease because PrP-Sc (10, 23) accumulates in peripheral lymph nodes well before involvement of the central nervous system (CNS). A transmissible agent was detected in the tonsil, retropharyngeal lymph node, and mesenteric portal lymph node of naturally infected Suffolk sheep in the United States at age 10 to 14 months (5), with clinical signs typically appearing at 35 to 45 months of age (32). In clinically normal Swifter × Texel crossbred lambs that are homozygous for PrP scrapie susceptibility genotype 136VV171QQ and that typically develop clinical signs at 25 months of age, PrP-Sc was detected in the tonsil, occasionally as early as 4 months of age (23).
On the basis of these observations, antemortem and preclinical postmortem diagnostic tests based on detection of PrP-Sc in peripheral lymphoid tissue have been proposed (10, 21, 23). PrP-Sc colocalizes with PrP-C in lymphoid tissue germinal centers (13). Assay conditions which differentiate between the isoforms include formalin fixation, which reduces PrP-C reactivity, followed by formic acid and heat pretreatments, which enhance PrP-Sc reactivity (7, 12). Under these conditions, immunohistochemistry assay of peripheral lymphoid tissue with standardized reagents would allow screening of flocks of live sheep and surveys of clinically normal mature sheep culled through slaughter channels. Slaughter and necropsy samples typically include the brain at the level of the obex as well as the tonsil and retropharyngeal lymph node, the extraneural tissues with the earliest and most abundant accumulation of PrP-Sc and the highest titer of a transmissible agent (5, 25). Nictitating membrane (“third eyelid”) lymphoid tissue can be collected under local anesthesia and has been proposed as a readily accessible source of lymphoid tissue from live animals (16). In this study, a monoclonal antibody (MAb)-based immunohistochemistry assay for PrP-Sc in third eyelid-associated lymphoid tissue of sheep was evaluated to begin validation of this assay as a preclinical antemortem diagnostic test for scrapie.
MATERIALS AND METHODS
MAb F89/160.1.5 and MAb F99/97.6.1 production.
A previously characterized MAb, MAb F89/160.1.5 (16), was produced in an artificial capillary cell culture system (CellMax; CellCo Inc.). Supernatants were clarified by low-speed centrifugation and were stored at −80°C. This antibody binds to an epitope that includes ovine residues 142 to 145 (amino acid sequence, IHFG).
MAb F99/97.6.1 was generated by immunization with a synthetic peptide, NH2-CITQYQRESQAYYQR-COOH, representing residues 217 to 231 of the ovine PrP (9), coupled to maleimide-activated keyhole limpet hemocyanin (Pierce Chemical Company). Five 6-week-old BALB/c mice were each inoculated subcutaneously in two sites with a total of 10 μg of conjugated peptide emulsified in 200 μl of Freund's complete adjuvant. Two booster inoculations of 10 μg of conjugated peptide in 200 μl of Freund's incomplete adjuvant were administered at 14-day intervals. Three days before cell fusion, mice were immunized intravenously with 10 μg of conjugated peptide in phosphate-buffered saline without adjuvant. Cell fusion and cloning by limiting dilution were performed by standard protocols (33). Supernatants from primary and cloned hybridomas were screened by recombinant ovine PrP-C enzyme-linked immunosorbent assay (17). Clone 6.1 from cell line F99/97 was selected and transferred to an artificial capillary cell culture system (CellMax; CellCo Inc.) for in vitro production of the MAb supernatant. The heavy-chain isotype was identified by enzyme-linked immunosorbent assay and MAb concentration by immunodiffusion. Epitope analysis was performed with overlapping hexamers bound to nitrocellulose (PepScan) detected by enhanced chemiluminescence. Specificity was determined by immunohistochemistry assay with brain (obex) and lymphoid tissue (tonsil) sections from infected and uninfected sheep.
Samples from infected and uninfected control sheep.
Positive controls included third eyelids from sheep in the United States and the United Kingdom with clinical signs compatible with scrapie. Sheep were submitted for necropsy following clinical diagnosis on the basis of behavioral changes, ataxia, wool loss, pruritis, or weight loss. Scrapie was diagnosed in sheep from the United Kingdom by conventional histologic examination of the brain stem at the level of the obex. Scrapie was diagnosed in U.S. sheep by histology and immunohistochemistry assays of brain tissue (15). Breeds, when reported, included Suffolk, Hampshire, and Southdown (U.S. sheep) and Welsh, Cheviot, Finn Dorset, Suffolk, Romney, Vendeen, Clun, Bleu de Main, Swaledale, Cambs, and Charollais and crosses of these breeds (for samples from sheep from the United Kingdom). Eyelid biopsy specimens collected from sheep in three U.S. flocks with no reported exposure to scrapie were used as negative controls.
Samples from clinically normal sheep in scrapie-exposed flocks.
To determine the characteristics of this assay with sheep early in the course of the disease, the third eyelid test was evaluated with clinically normal sheep in 14 U.S. flocks with a confirmed history of scrapie. Breeds, when reported, included Suffolk, Southdown, and Suffolk crossbred sheep. Only sheep 14 months of age or older were included in this study. Sheep were sampled at necropsy following euthanasia in scrapie control programs or by antemortem biopsy with subsequent transfer to quarantine facilities for observation until death from any cause. Brains, third eyelid lymphoid tissues, tonsils, and lymph nodes (minimally retropharyngeal and submandibular) were collected at necropsy from all sheep. Sheep were defined as having scrapie on the basis of detection of PrP-Sc in the medulla oblongata at the level of the obex. Sheep were defined as not having scrapie if PrP-Sc was not detected by immunohistochemistry assay of brain and tonsil tissue and retropharyngeal and submandibular lymph nodes. Sheep over 14 months of age that met this definition are likely to be uninfected but may be in the first 14 months of infection. The lymph nodes but not the brains of small number of sheep that died from other causes or euthanatized in control programs were positive by immunostaining. These probably represent animals with early cases of scrapie, but they were excluded from this study because their scrapie status could not be determined in the absence of validated lymphoid tissue-based diagnostic tests.
Postmortem third eyelid lymphoid tissue sampling.
Lymphoid tissue was prepared by one of two techniques from third eyelids collected at necropsy.
(i) Method I.
Third eyelids were removed at necropsy and placed bulbar surface upward on a card before fixing the tissue in buffered formalin for periods between 1 and 22 days. The third eyelids were then cut into strips 2 to 3 mm wide, processed, and embedded edge on in paraffin wax, with several pieces to a block. Eleven of 31 samples contained less than four germinal centers and were not included in this study.
(ii) Method II.
The entire third eyelid was collected from necropsy specimens. Cartilage from the center and the medial edge of the third eyelid was removed with curved iris scissors, and the remaining tissue was positioned intact in the cassette for routine processing. The tissue was positioned in the paraffin block with the bulbar surface placed toward the eventual cutting surface of the paraffin block. This technique yielded at least four germinal centers per section for approximately 85% of the postmortem samples collected during the past 2 years.
Antemortem biopsy specimen collection and handling.
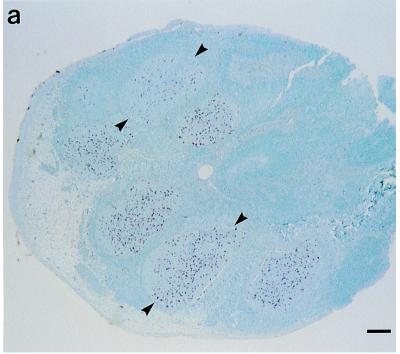
Third eyelid lymphoid tissue biopsy specimens were collected as described previously (16). Briefly, one eye was pretreated with topical proparacaine approximately 10 min before sampling and again at the time of sampling. The sheep were restrained in a semireclining position, preferably in a chute or a portable restraint apparatus such as that used for hoof trims (Sheep Deck Chair; Premier 1, Washington, Iowa). The third eyelid was gently retracted with disposable forceps. The lymphoid tissue was visualized near the medial edge of the bulbar surface of the third eyelid as bilateral patches of raised, slightly pink tissue. One lymphoid patch was gently grasped with a second set of disposable forceps, and a partial-thickness biopsy specimen was collected with disposable curved Metzenbaum scissors. Antemortem biopsy specimens were positioned on nylon sponges in histopathology cassettes without further trimming. The percentage of biopsy specimens with at least four germinal centers varied among flocks and was usually dependent on the level of training provided to the veterinarians and the availability of a restraint apparatus for handling the sheep. Trained personnel testing 30 to 60 sheep in a day using a restraint apparatus have collected biopsy specimens in which 80 to 90% contain at least four germinal centers (Fig. 1a), with many samples containing 12 to 15 germinal centers.
FIG. 1.
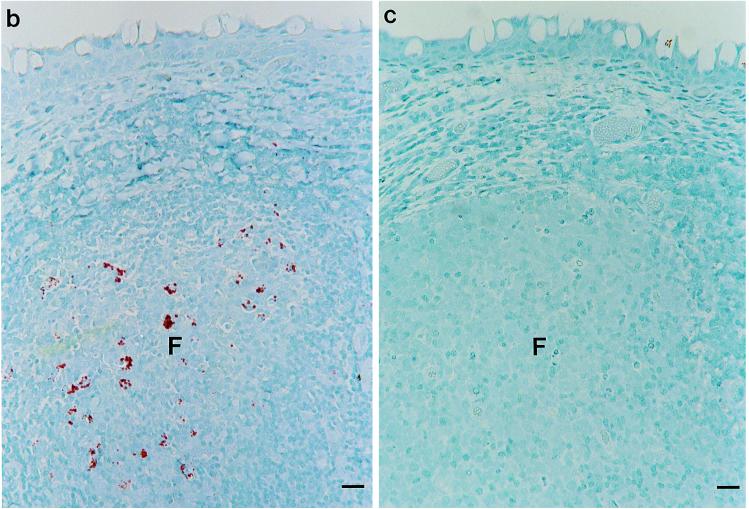
Immunohistochemical detection of PrP-Sc in third eyelid lymphoid tissue with a cocktail of MAbs F89/160.1.5 and F99/97.6.1. (a) Third eyelid biopsy specimen from a sheep with preclinical scrapie. Multiple lymphoid follicles (arrowheads) have punctate PrP-Sc immunoreactivity (red). Bar, 250 μm. (b) Third eyelid of a sheep with preclinical scrapie. The lymphoid follicle (F) within the germinal center (red) has multifocal, coarse, granular PrP-Sc immunoreactivity. The top is the conjunctival surface. Bar, 30 μm. (c) Third eyelid of an uninfected sheep. No PrP-Sc immunoreactivity was observed in the lymphoid follicle (F). The top is the conjunctival surface. Bar, 30 μm.
Immunohistochemistry assay.
Slides with samples collected in the United Kingdom were stained by a standard protocol developed for PrP-Sc detection in CNS tissue (7). Briefly, slides were dewaxed, rehydrated, and treated in 98% formic acid for 20 min prior to hydrated autoclaving for 30 min at 122°C. After blocking with normal goat serum (dilution, 1:66), sections were then immunostained with rabbit antiserum 971F diluted 1:8,000 or with MAb F89/160.1.5 diluted 1:2,000 (1.8 μg/ml) and 1:4,000 (0.9 μg/ml) at 4°C for 20 h. The sections were rinsed and treated with biotinylated goat anti-rabbit or rabbit anti-mouse (Vector Laboratories) immunoglobulin G (IgG) diluted 1:200, followed by treatment with Vector Elite ABC, and the color was developed with diaminobenzidine.
Sections from samples collected in the United States were immunostained by a capillary gap immunoassay method as described previously (15) or by automated immunostaining (Ventana Medical Systems). For the latter procedure, rehydrated sections were exposed to 98% formic acid for 5 min, washed in 0.01 M Tris-HCl (pH 7.6), and autoclaved at 121°C for 25 min in 0.1 M Tris-HCl (pH 7.6). Primary MAb F89/160.1.5 was applied at a concentration of 3 to 5 μg/ml for 32 min at 37°C, followed by application of goat anti-mouse IgG for 8 min at 37°C and streptavidin-horseradish peroxidase for 8 min at 37°C and detection with 3-amino-9-ethylcarbazole–H2O2. Tissues from sheep with and without scrapie were used as controls with each run. Negative antibody controls included an unrelated mouse IgG1 MAb and MAb F89/160.1.5 absorbed with a 10- to 40-fold molar excess of the peptide immunogen. Slides were coded, and the results were read by at least two observers who had no knowledge of the sheep's clinical status or the flock of origin.
Replicate sets of 146 lymphoid tissues (third eyelid lymphoid, retropharyngeal lymph node, or tonsil tissue), composed of 88 samples from sheep without scrapie and 58 samples from sheep with scrapie, were immunostained by the Ventana method with MAb F89/160.1.5 alone (5 μg/ml) or with a cocktail of MAb F89/160.1.5 and MAb F99/97.6.1 at 5 μg of each antibody per ml.
Positive staining of third eyelid lymphoid tissue was identified as strong particulate and cytoplasmic staining in germinal centers of lymphoid follicles as described previously (16) (Fig. 1b). Eyelid samples were considered positive if one or more follicles showed such staining. Samples were considered negative if no immunostaining was observed in samples that contained four or more germinal centers (Fig. 1c).
Data analysis.
Estimates of specificity and sensitivity were calculated separately for the clinically suspect sheep (see Table 1), for the clinically normal infected sheep (see Table 2), and for the uninfected sheep exposed to scrapie (see Tables 1 and 2). Exact confidence intervals were calculated at the 95% level.
TABLE 1.
Immunohistochemistry assay results for third eyelid lymphoid tissue from sheep with clinical signs suggestive of scrapiea and from sheep with no reported exposure to scrapie
| Scrapie agent status | No. of sheep | No. of sheep with the following result by IHC of third eyelid lymphoid tissueb
|
|
|---|---|---|---|
| Positive | Negative | ||
| Infectedc | 42 | 41 | 1 |
| Uninfectedd | 7 | 1 | 6 |
| No exposure to scrapiee | 48 | 0 | 48 |
Clinical signs included wool rubbing, weight loss, and/or ataxia.
Results of immunohistochemistry (IHC) assay performed with MAb F89/160.1.5.
PrP-Sc immunostaining of the medulla oblongata at the level of the obex (U.S. samples, n = 28) or routine histopathology of brain (samples from the United Kingdom; n = 14) was found for sheep with scrapie.
No lesions characteristic of scrapie (samples from the United Kingdom, n = 5 with a negative eyelid immunostaining result and n = 1 with a positive eyelid immunostaining result) or the lack of detection of PrP-Sc in brain, tonsil, retropharyngeal lymph node, or submandibular lymph node (U.S. samples; n = 1) was found for sheep without scrapie.
Sheep from three flocks with no reported exposure to scrapie for the last 5 years.
TABLE 2.
Immunohistochemistry results for third eyelid lymphoid tissue from clinically normal sheep in flocks with scrapie
| Scrapie agent status | Outcome | No. of sheep | No. of sheep with the following IHC results with eyelid tissuea
|
|
|---|---|---|---|---|
| Positive | Negative | |||
| Uninfectedb | Euthanatized while healthy | 120 | 0 | 120 |
| Infectedc | Euthanatized while healthy | 13 | 9 | 4 |
| Progressed to clinical diseased | 28 | 27 | 1 | |
Results of immunohistochemistry assay performed with MAb F89/160.1.5.
Sheep without scrapie showed no PrP-Sc in brain, tonsil, retropharyngeal lymph node, or submandibular lymph node.
Sheep with scrapie were positive by PrP-Sc immunostaining of the medulla oblongata at the level of the obex.
Sheep were clinically normal when sampled and progressed to clinical disease and were confirmed to have scrapie 3 to 20 months after a positive result with an eyelid biopsy specimen.
Codon 171 genotyping.
Whole blood in EDTA or frozen brain tissue was available from 119 sheep in this study. Following PCR amplification, codon 171 genotyping was performed by a DNA mismatch binding assay (GeneCheck Inc.) (3) or by restriction endonuclease digestion of PCR products amplified with primers modified to incorporate a restriction site at alleles encoding glutamine at position 171. The entire open reading frame of the PrP gene in 48 of these samples was determined by automated dideoxynucleotide sequence analysis.
RESULTS
Immunohistochemical analysis of third eyelid lymphoid tissues from sheep with and without scrapie.
In the positive control group (Table 1), 14 of 14 sheep with scrapie in the sample of sheep from the United Kingdom were considered positive by third eyelid immunohistochemistry assay. In two animals that were negative with antiserum 971F, positive staining was obtained with MAb F89/160.1.5. Six sheep were found to not have scrapie by routine histopathology of the brain. Five eyelid samples from these six sheep were PrP-Sc negative. In one animal, with no histologic evidence of scrapie, PrP-Sc was detected in the third eyelid. Without submission of additional lymphoid tissues, it cannot be determined whether the result for this animal represents a false-positive eyelid test or a true-positive test for an animal with an early case of scrapie, in which accumulation of PrP-Sc in lymphoid tissue preceded CNS involvement. Among the animals in the U.S. sample of sheep, 27 of 28 scrapie-infected sheep were considered positive by examination of third eyelid tissues. One sheep in the U.S. sample was found to have scrapie by histopathology assay of brain tissue and immunohistochemistry assay of brain tissue and lymph nodes, but the eyelid tissue was considered PrP-Sc negative. Eyelid biopsy specimens from all 48 sheep with no reported exposure to scrapie were considered PrP-Sc negative.
Immunohistochemical analysis of third eyelid lymphoid tissues from clinically normal sheep.
The results of immunohistochemical analysis of third eyelids and scrapie status of the 161 clinically normal sheep in infected flocks are shown in Table 2. One hundred twenty sheep were considered to not have scrapie on the basis of immunohistochemistry assay of the brain, tonsil, retropharyngeal lymph node, and submandibular lymph node. All 120 sheep were considered negative on the basis of third eyelid immunohistochemistry assay. Forty-one of 161 clinically normal sheep from infected flocks were diagnosed with scrapie by immunohistochemistry assay of brain tissue. Twenty-eight of the 41 sheep were sampled by eyelid biopsy and 13 sheep were examined at necropsy following depopulation of high-risk sheep in scrapie control programs. PrP-Sc was detectable in both the brains and the third eyelid sections of 27 of the 28 sheep biopsied and 9 of the 13 sheep sampled at necropsy. One sheep sampled by biopsy and four sheep from the depopulated flocks were positive by immunostaining of the brain but negative by immunohistochemistry assay of the third eyelid. Three of these five false-negative samples were from sheep with positive immunostaining of tonsil and retropharyngeal lymph nodes but not the third eyelid. Two samples with false-negative results were from sheep with positive immunostaining of the brain but not the tonsil, retropharyngeal lymph node, or submandibular lymph node.
The prognostic value of the test was evaluated with sheep held in quarantine facilities for observation following antemortem eyelid testing. In the study described in our previous report (17), six clinically normal sheep with positive eyelid biopsy specimens progressed to clinical disease and were confirmed to have scrapie within 7 months of testing. In this study, we identified and quarantined an additional 45 eyelid biopsy-positive sheep and 10 eyelid biopsy-negative sheep from infected flocks. Twenty-seven eyelid biopsy-positive and one eyelid biopsy-negative sheep progressed to confirmed scrapie within 3 to 20 months following third eyelid biopsy. Seventeen sheep with positive biopsy specimens collected after July 1998 remain in quarantine; all sheep with positive biopsy specimens collected prior to July 1998 have died and were confirmed to have scrapie or were clinically affected as of May 2000.
Data from the sheep in this study provide a preliminary estimate of the diagnostic sensitivity and specificity needed to determine the theoretical number of samples required for complete validation (11). The sensitivity of the assay with clinically suspect, infected sheep (Table 1) was 97.6% (95% confidence interval, 87.4 to 99.9%), and the sensitivity of the assay with clinically normal infected sheep (Table 2) was 87.8% (95% confidence interval, 73.85 to 95.9%). The specificity of the test, estimated with data for the sample of 127 uninfected sheep exposed to scrapie in the United States and the United Kingdom (Tables 1 and 2), was 99.2% (95% confidence interval, 95.7 to >99.9%). Allowing a 2% error and a 95% confidence interval that preliminary estimates of 87% sensitivity and 94% specificity are correct, the minimum number of infected reference sheep for validation of the assay is 1,085 and the minimum number of uninfected reference sheep is 542 (11). Large-scale trials of U.S. sheep, including both necropsy sampling and long-term quarantine of test-positive and test-negative sheep, are under way. The final sample size, particularly of uninfected reference sheep, will be considerably larger than the minimum to include variables such as breed, PrP genotype, age at sampling, and age at the time of presumed exposure to scrapie.
Comparison of third eyelid and tonsillar immunostaining.
Tonsils were available from 9 sheep from the United Kingdom (3 uninfected and 6 infected sheep) and 47 U.S. sheep (31 uninfected and 16 infected sheep) and were immunostained by the same method used for the third eyelid samples. For all nine sheep from the United Kingdom there was agreement between the results for the third eyelids and the tonsils. For three U.S. sheep, the brains and tonsils were positive by immunostaining, but third eyelid tissues were negative. Results of third eyelid and tonsil immunostaining for the remaining 44 U.S. sheep were identical.
Immunohistochemistry of lymphoid tissues with a cocktail of MAbs F89/160.1.5 and F99/97.6.1.
Paired slides of 146 lymphoid tissue sections (88 samples from sheep with scrapie and 58 samples from sheep without scrapie) were coded and immunostained by the Ventana method with MAb F89/160.1.5 alone (5 μg/ml) or with a cocktail of MAb F89/160.1.5 and MAb F99/97.6.1 at 5 μg of each antibody per ml. The level of agreement between the two staining methods was 100%, and the distribution of immunostaining of lymph nodes with the combination of MAb F89/160.1.5 and MAb F99/97.6.1 (Fig. 1b) did not differ from that observed with MAb F89/160.1.5 alone (17).
PrP genotypes of clinically affected and preclinically affected infected sheep.
All samples analyzed by DNA sequence analysis were homozygous for sequences encoding the epitopes for MAb F89/160.1.5 (amino acid sequence, IHFG; codons 142 to 145) and MAb F99/97.6.1 (amino acid sequence, QYQRES; codons 220 to 225). All sheep positive for PrP-Sc by immunostaining of brain or lymph nodes, whether from clinical suspects or clinically normal animals, were homozygous for glutamine at PrP codon 171 (171QQ), the previously reported genotype of naturally and experimentally infected U.S. Suffolk sheep (18, 19, 29). However, there was considerable variation in alleles 136 and 154, and the sample size was insufficient to draw statistical conclusions of the significance of the peripheral PrP-Sc distribution in sheep of these genotypes. The association of particular genotypes with the accumulation of PrP-Sc in third eyelids, other lymphoid tissues, and brains of a large sample of U.S. sheep is under investigation.
DISCUSSION
Detection of PrP-Sc in the brain is a useful adjunct to histopathology for the diagnosis of ovine scrapie, and PrP-Sc detection in lymphoid tissue is particularly useful for the identification of scrapie-affected sheep during the early, preclinical stage of scrapie. The tonsil, third eyelid lymphoid tissue, and retropharyngeal lymph node accumulate PrP-Sc detectable by immunohistochemistry assay (17, 22) early in the course of infection and are suitable tissues for live-animal and postmortem examination of clinically normal sheep. Tonsillar tissue contains a larger number of germinal centers than third eyelid tissue and is therefore a preferred tissue for samples from slaughterhouses and necropsy evaluation of sheep without clinical signs of scrapie when paired with samples collected from the medulla oblongata at the level of the obex. The retropharyngeal lymph node may be collected more easily than the small ovine tonsil in some slaughter settings. Eyelid biopsy specimens from live sheep, readily collected with disposable instruments after the animals have received a topical anesthetic, are suitable for antemortem screening of flocks.
MAb-based assays are preferable to those based on rabbit or goat antisera, which cannot be standardized for widespread use due to limitations in quantity and specificity. However, use of an antibody with specificity restricted to a single epitope might lead to false-negative findings for sheep of some genotypes. MAb F89/160.1.5 binds to an epitope conserved in 15 of the 16 ovine PrP gene sequences deposited with GenBank (GenBank accession no. AF180389) but polymorphic at site 143 (H to R) in an allele identified in Dorset crossbred sheep. The addition of MAb F99/97.6.1 provides a reagent cocktail predicted to recognize all the reported ovine alleles, as well as the reported PrP alleles of cattle, humans, deer, elk, mink, domestic cats, kudu, bison, and a number of nonhuman primates, the species with naturally occurring transmissible spongiform encephalopathies (TSEs) or PrP-Sc accumulation. The single exception is the nyala (Tragelaphus angasi). Furthermore, at least one of these epitopes is found in most species of domestic and nondomestic ruminants, carnivores, and nonhuman primates for which PrP sequence data are available (see Appendix). The conservation of the epitopes recognized by MAbs F89/160.1.5 and F99/97.6.1 should make this reagent cocktail suitable for immunoassays of tissues from most species with naturally occurring TSEs and for surveillance of other species exposed to TSEs under field conditions or in zoological gardens.
Lymphoid tissue-based preclinical diagnostic testing is feasible for the subset of TSEs in which PrP-Sc accumulates in lymph nodes early in the incubation period, particularly ovine scrapie, chronic wasting disease of mule deer (24), and variant Creutzfeldt-Jakob disease (8). In contrast, the infectivity of the distal ileum of cattle experimentally infected with the BSE agent, probably within Peyer's patches (26), has been confirmed, but detection of PrP-Sc by immunoassay of peripheral lymphoid tissues has not been reported. Furthermore, a subset of sheep with scrapie in this and previous studies (6, 25) showed accumulation of PrP-Sc or a transmissible agent in CNS tissue but not lymphoid tissue. The value of diagnostic tests for ovine scrapie based on detection of PrP-Sc in lymphoid tissue will depend in part on an emerging understanding of the pathogenesis of scrapie, including the relative effects of differences in breed and host genetics, the dose and route of infection, and the strain of the agent on the timing and degree of involvement of the third eyelid lymphoid tissue, tonsil, Peyer's patches, and CNS tissues.
The third eyelid test for PrP-Sc in live sheep is a practical, readily standardized assay that will be useful for the diagnosis of scrapie, in surveillance programs, and in research studies to determine the transmission, epidemiology, and pathogenesis of a naturally occurring TSE. Examination of a sufficiently large sample for precise determination of the diagnostic sensitivity and specificity of the assay as a diagnostic test for scrapie in U.S. sheep is in progress.
ACKNOWLEDGMENTS
This work was supported by the Agricultural Research Service, U.S. Department of Agriculture (grant CWU 5348-32000-011-00D).
We are grateful to P. Steiner, W. Harwood, S. Munro, A. Anderson, and J. Bulgin for excellent assistance; to J. Wilesmith for critical review of the manuscript; and to private, state, and federal veterinarians and to sheep producers for samples and helpful discussion.
Appendix
The following are species in which the PrP gene product contains the epitopes IHFG and/or QYQRES, corresponding to ovine residues 142 to 145 and 220 to 225 (sequences were derived from GenBank). Species with naturally occurring TSEs or PrP-Sc accumulation include Ovis aries, Capra hircus, Ros taurus, Homo sapiens, Felis catus, Hippotragus niger, Odocoileus hemionus, Odocoileus virginianus, Cervus elephus, Macaca mulatta, Mustela visa, Tragelaphus strepsiceros, and Bison bonasus. Species with no reported naturally occurring TSEs include the nonhuman primate species Aotus trivirgatus, Ateles geoffroyi, Callicebus moloch, Cebus apella, Cercocebus spp., Cercopithecus spp., Chlorocebus aethiops, Colobus guereza, Gorilla gorilla, Hylobates lar, Macaca spp., Mandrillus sphinx, Pan troglodytes, Papio hamadryus, Pongo pygmaus, Saimiri sciureus, and Theropithecus gelada; the domestic and nondomestic hoofed stock Addax nasomaculatus, Antilocapra americana, Bos javanicus, Camelus dromedarius, Giraffa camelopardus, Sus scrofa, and Equus caballus; and the carnivores and rodents Canis familiaris, Mustela putorius, and Mus musculus.
REFERENCES
- 1.Aldhous P. Spongiform encephalopathy found in cat. Nature. 1990;345:194–194. doi: 10.1038/345194c0. [DOI] [PubMed] [Google Scholar]
- 2.Bons N, Mestre-Frances N, Belli P, Cathala F, Gajdusek D C, Brown P. Natural and experimental oral infection of nonhuman primates by bovine spongiform encephalopathy agents. Proc Natl Acad Sci USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debbie P, Young K, Pooler L, Lamp C, Marietta P, Wagner R. Allele identification using immobilized mismatch binding protein: detection and identification of antibiotic-resistant bacteria and determination of sheep susceptibility to scrapie. Nucleic Acids Res. 1997;25:4825–4829. doi: 10.1093/nar/25.23.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guiroy D C, Williams E S, Yanagihara R, Gajdusek D C. Immunolocalization of scrapie amyloid (PrP27-30) in chronic wasting disease of Rocky Mountain elk and hybrids of captive mule deer and white-tailed deer. Neurosci Lett. 1991;126:195–198. doi: 10.1016/0304-3940(91)90552-5. [DOI] [PubMed] [Google Scholar]
- 5.Hadlow W J, Kennedy R C, Race R E. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 6.Hadlow W J, Rice R E, Kennedy R C, Ecklund C M. Natural infection of sheep with scrapie virus. In: Prusiner S B, Hadlow W J, editors. Slow transmissible diseases of the nervous system. New York, N.Y: Academic Press, Inc.; 1979. pp. 3–12. [Google Scholar]
- 7.Haritani M, Spencer Y I, Wells G A H. Hydrated autoclave pretreatment enhancement of prion protein immunoreactivity in formalin-fixed-bovine spongiform encephalopathy-affected brain. Acta Neuropathol (Berlin) 1992;87:86–90. doi: 10.1007/BF00386258. [DOI] [PubMed] [Google Scholar]
- 8.Hill A F, Zeidler M, Ironside J, Collinge J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet. 1997;349:99. doi: 10.1016/S0140-6736(97)24002-X. [DOI] [PubMed] [Google Scholar]
- 9.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of the prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol. 1995;76:2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami Y, Ito M, Isomura H, Momotani E, Sasaki K, Muramatsu Y, Ishiguro N, Shinagawa M. Pre-clinical and clinical diagnosis of scrapie by detection of PrP protein in tissues of sheep. Vet Rec. 1991;128:271–275. doi: 10.1136/vr.128.12.271. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson R. Principles of validation of diagnostic assays for infectious diseases. In: Reichard R, editor. OIE manual of standards for diagnostic tests and vaccines. Paris, France: Office International des Epizooties; 1996. pp. 8–15. [Google Scholar]
- 12.Kitamoto T, Ogomori K, Tateishi J, Prusiner S B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Investig. 1987;57:230–236. [PubMed] [Google Scholar]
- 13.McBride P A, Eickelenboom P, Kraal G, Fraser H, Bruce M E. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J Pathol. 1992;168:413–418. doi: 10.1002/path.1711680412. [DOI] [PubMed] [Google Scholar]
- 14.Miller J M, Jenny A L, Taylor W D, Marsh R F, Rubenstein R, Race R E. Immunohistochemical detection of prion protein in sheep with scrapie. J Vet Diagn Investig. 1993;5:309–316. doi: 10.1177/104063879300500301. [DOI] [PubMed] [Google Scholar]
- 15.Miller J M, Jenny A L, Taylor W D, Race R E, Ernst D R, Katz J B, Rubenstein R. Detection of prion protein in formalin-fixed brain by hydrated autoclaving immunohistochemistry for the diagnosis of scrapie in sheep. J Vet Diagn Investig. 1994;6:366–368. doi: 10.1177/104063879400600315. [DOI] [PubMed] [Google Scholar]
- 16.O'Rourke K I, Baszler T V, Miller J M, Spraker T R, Sadler-Riggleman I, Knowles D P. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol. 1998;36:1750–1755. doi: 10.1128/jcm.36.6.1750-1755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Rourke K I, Baszler T V, Parish S M, Knowles D P. Preclinical detection of PrP-Sc in nictitating membrane lymphoid tissue of sheep. Vet Rec. 1998;142:489–491. doi: 10.1136/vr.142.18.489. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke K I, Holyoak G R, Clark W W, Mickelson J R, Wang S, Melco R P, Besser T E, Foote W C. PrP genotypes and experimental scrapie in orally inoculated Suffolk sheep in the United States. J Gen Virol. 1997;78:975–978. doi: 10.1099/0022-1317-78-4-975. [DOI] [PubMed] [Google Scholar]
- 19.O'Rourke K I, Melco R P, Mickelson J R. Allelic frequencies of an ovine scrapie susceptibility gene. Anim Biotech. 1996;7:155–162. [Google Scholar]
- 20.Prusiner S B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 21.Race R, Ernst D, Jenny A, Taylor W, Sutton D, Caughey B. Diagnostic implications of detection of proteinase K-resistant protein in spleen, lymph nodes, and brain of sheep. Am J Vet Res. 1992;53:883–889. [PubMed] [Google Scholar]
- 22.Schreuder B E C, van Keulen L J M, Vromans M E W, Langeveld J P M, Smits M A. Preclinical test for prion disease. Nature. 1996;381:563. doi: 10.1038/381563a0. [DOI] [PubMed] [Google Scholar]
- 23.Schreuder B E C, van Keulen L J M, Vromans M E W, Langeveld J P M, Smits M A. Tonsillar biopsy and PrPSc detection in the preclinical diagnosis of scrapie. Vet Rec. 1998;142:564–568. doi: 10.1136/vr.142.21.564. [DOI] [PubMed] [Google Scholar]
- 24.Spraker T R, Miller M W, Williams E S, Getzy D M, Adrian W J, Schoonveld G G, Spowart R A, O'Rourke K I, Miller J M, Merz P A. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis. 1997;33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 25.van Keulen L J M, Schreuder B E C, Meloen R H, Mooij-Harkes G, Vromans M E W, Langeveld J P M. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J Clin Microbiol. 1996;34:1228–1231. doi: 10.1128/jcm.34.5.1228-1231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G A, Hawkins S A, Green R B, Austin A R, Dexter I, Spencer Y I, Chaplin M J, Stack M J, Dawson M. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet Rec. 1998;142:103–106. doi: 10.1136/vr.142.5.103. [DOI] [PubMed] [Google Scholar]
- 27.Wells G A H, McGill I S. Recently described scrapie-like encephalopathies of animals: case definitions. Res Vet Sci. 1992;53:1–10. doi: 10.1016/0034-5288(92)90076-e. [DOI] [PubMed] [Google Scholar]
- 28.Wells G A H, Scott A C, Johnson C T, Gunning R D, Hancock R D, Jeffrey M, Dawson M, Bradley R. A novel progressive spongiform encephalopathy in cattle. Vet Rec. 1987;121:419–420. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- 29.Westaway D, Zuliani V, Cooper C M, Da Costa M, Neuman S, Jenny A L, Detwiler L, Prusiner S B. Homozygosity for prion protein alleles encoding glutamine-171 renders sheep susceptible to natural scrapie. Genes Dev. 1994;8:959–969. doi: 10.1101/gad.8.8.959. [DOI] [PubMed] [Google Scholar]
- 30.Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P G. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 31.Williams E S, Young S. Spongiform encephalopathies in cervidae. Rev Sci Tech OIE. 1982;11:551–567. doi: 10.20506/rst.11.2.611. [DOI] [PubMed] [Google Scholar]
- 32.Wineland N E, Detwiler L, Salmon D. Epidemiologic analysis of reported scrapie in sheep in the United States: 1,117 cases (1947–1992) J Am Vet Med Assoc. 1998;212:713–718. [PubMed] [Google Scholar]
- 33.Yokoyama W M. Production of monoclonal antibodies. In: Coligan J E, editor. Current protocols in immunology. New York, N.Y: Wiley Interscience; 1994. pp. 2.2.1–2.5.17. [Google Scholar]