Abstract
Background and aims
Hepatitis C virus (HCV) treatment is essential for eliminating HCV in people who inject drugs (PWID), but has limited coverage in resource-limited settings. We measured the cost-effectiveness of a pilot HCV screening and treatment intervention using directly observed therapy among PWID attending harm reduction services in Nairobi, Kenya.
Design
We utilized an existing model of HIV and HCV transmission among current and former PWID in Nairobi to estimate the cost-effectiveness of screening and treatment for HCV, including prevention benefits versus no screening and treatment. The cure rate of treatment and costs for screening and treatment were estimated from intervention data, while other model parameters were derived from literature. Cost-effectiveness was evaluated over a life-time horizon from the health-care provider’s perspective. One-way and probabilistic sensitivity analyses were performed.
Setting
Nairobi, Kenya.
Population
PWID.
Measurements
Treatment costs, incremental cost-effectiveness ratio (cost per disability-adjusted life year averted).
Findings
The cost per disability-adjusted life-year averted for the intervention was $975, with 92.1% of the probabilistic sensitivity analyses simulations falling below the per capita gross domestic product for Kenya ($1509; commonly used as a suitable threshold for determining whether an intervention is cost-effective). However, the intervention was not cost-effective at the opportunity cost-based cost-effectiveness threshold of $647 per disability-adjusted life-year averted. Sensitivity analyses showed that the intervention could provide more value for money by including modelled estimates for HCV disease care costs, assuming lower drug prices ($75 instead of $728 per course) and excluding directly-observed therapy costs.
Conclusions
The current strategy of screening and treatment for hepatitis C virus (HCV) among people who inject drugs in Nairobi is likely to be highly cost-effective with currently available cheaper drug prices, if directly-observed therapy is not used and HCV disease care costs are accounted for.
Keywords: Chronic hepatitis C, cost-effectiveness, direct-acting antiviral treatment, Kenya, low-income setting, people who inject drugs
INTRODUCTION
Globally, 71 million people were chronically infected with hepatitis C virus (HCV) in 2015 [1]. most of whom live in lower- and middle-income countries (LMIC) where there is limited testing and treatment [1,2].
People who inject drugs (PWID) have a high prevalence of HCV infection (52% antibody-positive) [3] globally and contribute an estimated 43% of incident HCV infections [4]. In Kenya, the estimated seroprevalence of HCV is 3% in the general population [5], but 11–36% among PWID [6-10]. To ensure that Kenya can achieve the World Health Organization HCV elimination targets [11], interventions to scale-up HCV case-finding and directly acting antiviral (DAA) treatment must target PWID. Despite international guidelines recommending these interventions for PWID [12,13], coverage is limited in Kenya and LMICs [1,14].
Testing, referral and treatment of PWID can be challenging due to patient-level and system-wide factors [15], particularly in LMIC with poor availability of services for PWID. However, the increasing acceptability and availability of harm reduction services in settings such as Kenya [16], and recent advances in the simplification of HCV testing and treatment, presents opportunities for expanding HCV treatment among PWID in LMICs [17]. This could improve access and reduce the costs of expanding HCV treatment to PWID.
Recent systematic reviews highlight the cost-effectiveness of HCV treatment for PWID in high-income countries, but evidence from LMICs is limited [18,19]. Model-based analyses evaluated the cost-effectiveness of HCV screening and DAA-based treatment among PWID in LMICs [20], including Tanzania [21], but relied mainly on data from literature and expert opinion. The lack of empirical data makes the realism of these analyses uncertain, and their generalizability to other LMICs unclear. Cost-effectiveness analyses of ‘real-world’ HCV testing and treatment interventions for PWID in LMICs are needed for guiding policy on the expansion of these interventions. In this study, we evaluated the impact and cost-effectiveness of a pilot HCV screening and DAA-based treatment intervention among people who use drugs (PWUD) in Nairobi, Kenya.
METHODS
Study design
The cost-effectiveness of the HCV screening and DAA-based intervention was assessed in comparison to usual care. Although the intervention was for PWUD, all HCV infections diagnosed in this setting were assumed to be through injecting drug use. Before the pilot programme there was negligible screening and treatment for HCV among PWID, as confirmed by the Kenyan Ministry of Health (Helgar Musyoki, January 2021) and the Kenyan testing and linkage to care for injecting drug users (TLC-IDU) study survey from 2015 that found no PWID reported previously being treated for HCV [10]. We therefore used ‘no screening and treatment’ as the comparator. A health-care provider’s perspective was assumed as it estimates the costs and effects incurred from the health service, and so provides guidance to decision-makers on whether to invest in HCV screening and treatment in Kenya.
Ethical approval for this study was obtained from the Kenya Medical Research Institute Scientific and Ethics Review Unit (reference: KEMRI/RES/7/3/1).
Setting and intervention
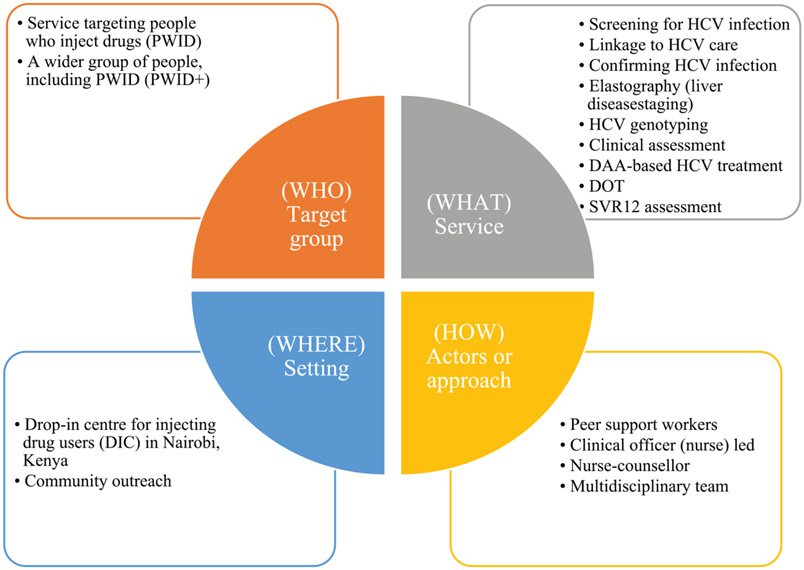
Patient characteristics and resource utilization in the base-case analysis were collected from a pilot intervention aimed at demonstrating the ‘real-world’ effectiveness of DAA-based HCV treatment among PWID in Nairobi. The pilot treatment programme was established in 2016 by Médecins Sans Frontières in collaboration with Médecins du Monde. Médecins du Monde offered point-of-care screening for HCV antibodies to PWUD as part of harm reduction services provided through its drop-in centre and outreach activities in Nairobi (Fig. 1 shows the model of care). Blood samples for all HCV-seropositive clients were sent to an external laboratory for HCV confirmatory testing, genotyping and other pre-treatment tests. Patients received a transient elastography (fibroscan) at a nearby private hospital. Treatment eligibility was based on international guidelines [22-24]. Eligible clients were treated with daclatasvir and sofosbuvir (86.4%) or ledipasvir and sofosbuvir (13.6%), delivered within the drop-in centre using directly observed therapy (DOT). All clients on treatment attended the clinic every day, where a clinical officer dispensed and observed them taking drugs. The clinical officer provided counselling sessions or medical reviews at each visit, including family planning, pre-treatment, treatment initiation and life-style and re-infection advice. Transport costs were reimbursed and included in the analysis. Peer support and defaulter tracing was facilitated through peer educators. After treatment completion, patients were followed-up for ~12 weeks, whereupon the sustained virological response (SVR12) was assessed to determine treatment success.
Figure 1.
Summary representation of the model of care for HCV screening and treatment with direct-acting antivirals in Nairobi, Kenya. HCV= hepatitis C virus; DAA = direct-acting antiviral; SVR12 = sustained virological response; DOT = directly observed therapy
We also used data from the TLC-IDU study (NCT01557998) [8,10] to estimate costs of an alternative HCV screening and treatment intervention in Kenya. The study cohort and intervention cost analysis are described in the Supporting information. These costs were used in the sensitivity analysis.
Mathematical model structure
We utilized an existing dynamic compartmental model of HIV and HCV transmission among current and former PWID in Nairobi [25] to evaluate health outcomes and costs of the HCV treatment intervention in comparison to no treatment. The model allowed us to capture both the individual (preventing or slowing down HCV disease progression) and population benefits (preventing new infections) of treatment.
The model incorporates the transmission of HIV and HCV due to injecting drug use as well as HIV transmission due to sexual risk behaviour (Supporting information, Fig. S1). The population is stratified by injecting status (PWID and former PWID), sex, HIV infection state (susceptible, acute HIV infection, chronic HIV infection, pre-AIDS, AIDS), HIV treatment status [on/off antiretroviral therapy (ART)], HCV infection state (susceptible, previously exposed, chronic HCV infection and chronic HCV undergoing treatment), HCV disease progression states (METAVIR fibrosis stages F0–F4, decompensated cirrhosis or hepatocellular carcinoma) and harm reduction state [on/off medically assisted therapy (MAT) and/or needle and syringe exchange programme (NSP)]. The model was calibrated using approximate Bayesian computation to detailed data for Nairobi from the Kenya AIDS indicator surveys [26], national polling booth surveys among PWID from 2015 and 2016 [27,28], national MAT and NSP programme data and a series of cross-sectional bio-behavioural surveys conducted during 2012–15 by the TLC-IDU study [29]. Data on HIV and HCV disease progression rates and efficacy of NSP, MAT and ART came from the literature (Table 1). The calibrated model included uncertainty in all model parameters, which was propagated into all model projections. We used data on MAT status, current injecting status, HIV co-infection and fibrosis stages of all patients treated in the intervention to parameterize treatment numbers within each compartment of the model (Table 1).
Table 1.
Key model parameters and calibration data.
| Parameter | Prior parameter distribution/calibration range | Source |
|---|---|---|
| Cohort characteristics | ||
| PWID population size* | 9750–17 150 | [30] |
| Proportion of PWID who are female* | 14.7% (95% CI = 13.1–16.4) | TLC-IDU [10] |
| Average duration of injecting drug use (years) | Uniform: 1.75–7.0 | TLC-IDU [10] |
| HIV prevalence among male PWID in 2015* | 9.6% (95% CI = 8.2–11.0) | TLC-IDU [10] |
| HIV prevalence among female PWID in 2015* | 29.1% (95% CI = 19.8–38.4) | TLC-IDU [10] |
| ART coverage among HIV-positive PWID in 2015* | 65.7% (95% CI = 60.3–71.0) | TLC-IDU[10] |
| Proportion of PWID on ART that are virally suppressed | Normal: 34.3% (95% CI = 28.3–40.2) | TLC-IDU [10] |
| HCV antibody prevalence among PWID in 2015* | 10.9% (95% CI = 8.4–13.3) | TLC-IDU [10] |
| Proportion of HCV infections that spontaneously clear among HIV-negatives among HIV-positives | Uniform: 0.22–0.29 | [31] |
| Uniform: 0.115–0.193 | [32] | |
| Efficacy of interventions | ||
| Relative reduction in HCV transmission risk if on OST | Log-normal: 0.50 (95% CI = 0.40–0.63) | [33] |
| Relative reduction in HCV transmission risk if on NSP | Log-normal: 0.44 (95% CI = 24–0.80) | [33] |
| Relative reduction in HIV transmission risk if on OST | Log-normal: 0.46 (95% CI = 0.32–0.67) | [34] |
| Relative reduction in HIV transmission risk if on NSP | Log-normal: 0.42 (95% CI = 0.22–0.81) | [35] |
| HCV disease progression rates | ||
| F0–F1 (per year) | Normal (0.128, 0.0245) | [36] |
| F1–F2 (per year) | Normal (0.059, 0.012) | [36] |
| F2–F3 (per year) | Normal (0.078, 0.0112) | [36] |
| F3–F4 (per year) | Normal (0.116, 0.0232) | [36] |
| Relative increase in HCV disease progression from F0 to F4 if HIV infected | ||
| Without ART | Log-normal: 2.489 (95% CI = 1.811–3.420) | [37] |
| With ART | Log-normal: 1.723 (95% CI = 1.059–2.804) | [37] |
| Annual probability of HCV progression from F4 to decompensated cirrhosis | Beta (14.6168, 360.1732) | [38] |
| Annual probability of HCV progression from F4 to hepatocellular carcinoma | Beta (1.9326, 136.1732) | [38] |
| Annual probability of HCV progression from decompensated cirrhosis to hepatocellular carcinoma | Beta (1.9326, 136.1732) | [38] |
| Annual probability of mortality from decompensated cirrhosis | Beta (147.03, 983.97) | [38] |
| Factor increase in mortality rate from decompensated cirrhosis if HIV co-infected | Log-normal: 2.26% (95% CI = 1.51–3.38) | [39,40] |
| Annual probability of mortality from hepatocellular carcinoma | Beta (117.1033, 155.23) | [38] |
| Relative risk of progression from F4 to decompensated cirrhosis following SVR | Log-normal: 0.07% (95% CI = 0.03–0.2) | [41] |
| Relative risk of progression from F4 to hepatocellular carcinoma following SVR | Log-normal: 0.23% (95% CI = 0.16–0.35) | [42] |
| Disability weights | ||
| HIV disease states | ||
| Acute infection | Equal to ART value | No GBD estimate so assumed equal to ART |
| Chronic infection | Equal to ART value | No GBD estimate so assumed equal to ART |
| HIV: symptomatic, pre-AIDS | Uniform (0.184, 0.377) | [43] |
| AIDs: not on ART | Uniform (0.406, 0.743) | [43] |
| HIV/AIDs: receiving ART | Uniform (0.052, 0.111) | [43] |
| HCV disease states | ||
| METAVIR FO | Not sampled | |
| METAVIR F1-F3 | [67] Assumed linear disability increase from F0 to F4 | |
| METAVIR F4 | Uniform (0.078, 0.159) | [67] No GBD estimate so used value for moderate abdominopelvic problem |
| Decompensated cirrhosis | Uniform (0.123, 0.250) | [67] Decompensated Cirrhosis of the liver |
| Hepatocellular carcinoma | Uniform (0·307, 0·600) | [67] Cancer: metastatic |
| HIV/HCV co-infection | Not sampled | Disability weights were compounded multiplicatively |
| HCV disease state costs ($ | ||
| METAVIR FO | 38 | [44] Sensitivity analysis |
| METAVIR F1–F3 | 76 | [44] Sensitivity analysis |
| METAVIR F4 | 89 | [44] Sensitivity analysis |
| Decompensated cirrhosis | 994 | [44] Sensitivity analysis |
| Hepatocellular carcinoma | 1827 | [44] Sensitivity analysis |
AIDS = acquired immune deficiency syndrome; ART = antiretroviral therapy; GBD = Global Burden of Disease; HCV = hepatitis C virus; HIV = human immunodeficiency virus; KAIS = Kenya AIDS Indicator Survey; NSP = needle and syringe exchange programme; OST = opioid substitution therapy; PWID = people who inject drugs; SVR = sustained virologic response; TLC-IDU = test and linkage to care for injecting drug users.
Calibration data.
Intervention costs
HCV screening and treatment costs were estimated from intervention data using a retrospective, cohort-based, micro-costing approach from the health-care provider’s perspective in 2018 dollars. A detailed review of the treatment protocol and interviews with staff identified activities undertaken in the screening and treatment intervention. Resources accounted for each activity included staff time (doctors, nurses, counsellors), diagnostic and clinical tests, medicines, overheads (management, buildings, support staff, utilities and consumables) and reimbursed transport costs for patients. Staff time for clinical staff was estimated for each activity using staff time-sheets, supplemented through interviews. Patient-level data on resource use including clinic visits, tests and medicines were obtained from the Research Electronic Data Capture clinical database [45].
Costs for staff and consumables, including test kits, were obtained from study financial records and supplemented through interviews with key personnel (finance, logistics and programme managers). Costs for the DAA medicines represent the prices paid by Médecins Sans Frontières in Kenya at the time. Unit costs for outsourced laboratory tests were obtained from relevant laboratories.
Up-to-date unit costs were applied for each resource. Historical costs were adjusted for inflation to 2018 prices [46]. Local currency prices were converted to dollars using the average market-based exchange rate for 2016–17 [47] (1 $ = 103 Kenya Shillings). The cost of each activity is the sum of costs for all resources used for that activity, i.e. labour, consumables and overheads. The activity costs were multiplied by the frequency that a patient received each activity and summed to give the estimated total cost per patient.
The costs of HCV screening included the rapid test for HCV antibodies, and when positive, the HCV confirmatory test. The average cost per diagnosis was calculated based on the number of antibody and confirmatory tests performed per individual diagnosed with chronic infection.
Costs of HCV-related disease
Information on cost of health care for HCV-related disease was not available for Kenya, so were not included in the base-case analysis.
HCV treatment outcome
We estimated the proportion of patients who achieved an SVR at 12 weeks among all those that initiated therapy using patient-level data from the intervention.
HCV disability weights
In the absence of Kenya-specific health utility values, we applied the Global Burden of Disease estimates of disability weights to HCV disease states in the model to estimate disability-adjusted life-years (DALYs) as health outcomes (Table 1) [43]. We assumed that patients with METAVIR score F0 were not associated with disability. A linear increase in disability was modelled for F1–F3 based on the estimate for F4 (cirrhosis), which was assumed to be equivalent to the value for a moderate abdominopelvic problem. The estimate for decompensated cirrhosis was used. A direct estimate for hepatocellular carcinoma was not available, so a value for metastatic cancer was used.
Cost-effectiveness
We estimated the incremental cost-effectiveness ratio (ICER) in terms of cost per DALY averted. We used a 3% discount rate for both costs and DALYs, following current guidance for LMICs [48,49]. We used a 50-year time horizon to capture the long-term effects of chronic HCV infection and population prevention benefits associated with disease transmission. The estimated ICER was compared to the 2018 gross domestic product (GDP) per capita for Kenya ($ 1509) [50], which is commonly used as a threshold for determining whether an intervention is cost-effective [51]. We also compared the ICER to an empirical opportunity cost-based cost-effectiveness threshold for Kenya of $647 per DALY averted [52]. This analysis was not pre-registered; however, we followed standard guidelines for economic evaluations [48,49] and methods we have used in previous analyses [25].
Sensitivity analyses
To quantify the effect of parameter uncertainty on model results, a probabilistic sensitivity analysis was conducted using the uncertainties of individual parameters and performing random independent parameter draws from their probability distributions to generate 3000 simulations of costs and DALYs (Table 1 and Supporting information, Tables S2-S5). These simulation results were used to estimate the probability that the intervention was cost-effective over different cost-effectiveness thresholds.
Sensitivity analyses were performed to evaluate the impact of varying our assumptions for key parameters on cost-effectiveness. We performed one-way sensitivity analyses on the following model parameters: time horizon (25 or 100 years), discount rates (0 or 6%, as recommended by the World Health Organization [48]), SVR12 (70/ 95%), higher HCV seroprevalence among PWID in Kenya (13%) [10], a lower cost of HCV rapid diagnostic test ($1.16) and HCV confirmatory test ($50) using estimates from the TLC-IDU study. We also evaluated the effect of varying HCV seroprevalence from 2.8% (observed in the general population) [5] to 70% (highest observed in PWID) [53] to reflect the probable variation across Kenya. We also evaluated the effect of including health-care costs for HCV-related disease using modelled estimates from Tanzania [44], adjusted for Kenya using purchasing power parity conversion factors [54].
The intervention employed DOT to improve adherence to HCV treatment; however, evidence shows that PWID can adhere to ART [55] and HCV treatment without DOT [56-59]. In addition, the costs for DOT could have been lower if it had been integrated with the provision of MAT, which 69.1% of treated patients were taking. Therefore, in two scenarios we explored the effects of either assuming a shorter time for each DOT (5 versus 20 minutes used in the base-case) or excluding the costs of DOT altogether. The shorter time was based on interviews with pharmacists in a local MAT clinic, where a similar programme (TLC-IDU) was piloted. We also evaluated the impact of assuming costs incurred by this other pilot intervention, assuming similar treatment outcomes (see Supporting information).
The average price of DAAs used by the intervention was $728 per treatment. We assessed the effect of reducing DAA prices to levels currently being paid by Médecins Sans Frontières ($75 per 12-week treatment), but not in Kenya. We also evaluated the simultaneous effect of the cheaper DAA price, accounting for health-care costs and the exclusion of DOT costs on cost-effectiveness of the intervention.
RESULTS
Patient characteristics
The HCV cascade of care in the intervention is shown in Fig. 2. A total of 1673 people [33.8% PWID, 58.8% PWUD (non-injecting), 6.9% other key populations and 0.5% general population] were screened for HCV between January 2016 and April 2018, with 124 (7.7%) HCV-seropositive and 96 (77.4%) HCV RNA-positive. Eighty-one individuals (84.4%) initiated DAA treatment; their fibrosis distribution and treatment outcomes are shown in Table 2. The mean age for the diagnosed patients was 37.0 years and 88.9% were male. Nearly half (43.2%) the patients who initiated treatment were co-infected with HIV, all of whom were receiving ART, and most had early stages of fibrosis (Table 2). Most patients (72%) had a history of past drug/substance use, 24.7% reported current use and data were missing for 2.5%. Because 90.6% of patients with past drug/substance use were on MAT we assumed that they had ongoing drug use, while the remainder were assumed to be ex-PWID; 66.7% of current users were also on MAT. Of the 15 diagnosed clients not started on treatment, nine were lost to follow-up before treatment initiation, two were excluded because of high HIV viral load, three for comorbidities and information was missing for one patient (data not shown in Table 2). A total of 79 clients completed treatment, 77 were assessed for SVR12 and 73 achieved SVR12 (90.1% of all patients who initiated treatment and 92.4% of those assessed for SVR12). SVR12 was 89.1% in HCV mono-infected versus 91.4% in HIV-HCV co-infected patients.
Figure 2.
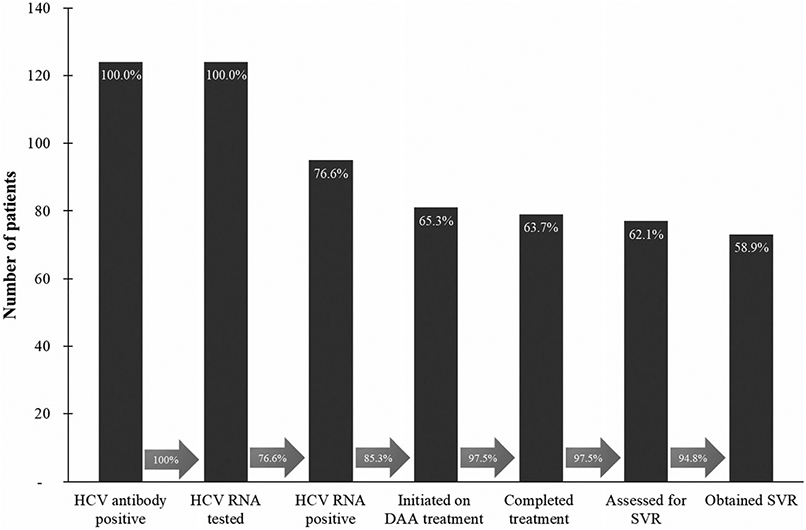
HCV cascade of care in the Meédecins du Monde/Médecins Sans Frontières intervention in Nairobi, Kenya. DAA = direct-acting antiviral; SVR = sustained virological response; HCV = hepatitis C virus; RNA = ribonucleic acid. Arrows between bars represents the proportion of patients going from one step of the cascade to the next, e.g. 85.3% of those confirmed with chronic HCV-initiated HCV treatment
Table 2.
Distribution of cohort of diagnosed patients who initiated treatment and achieved SVR by fibrosis stages (n = 81); F0-F4 are METAVIR scores estimated using APRI scores.
| Disease stage | n (% of total) | HIV-positive (% of group) |
Finished treatment (% of group) |
Assessed for SVR (% of group) |
Achieved SVR (% of group) |
|---|---|---|---|---|---|
| F0 | 56 (69.1%) | 19 (33.9%) | 55a (98.2%) | 53b (96.4%) | 50c (94.3%) |
| F1 | 11 (13.6%) | 4 (36.4%) | 10d (90.9%) | 10 (100%) | 10 (100%) |
| F2 | 3 (3.7%) | 1 (33.3%) | 3 (100%) | 3 (100%) | 3 (100%) |
| Unknown | 11 (13.6%) | 11 (100%) | 11 (100%) | 11 (100%) | 10e (90.9%) |
| Total | 81 (100%) | 35 (43.2%) | 79 (97.5%) | 77 (97.5%) | 73 (94.8%) |
One patient died during treatment.
Two not assessed for SVR.
One patient died, two failed treatment.
One patient lost to follow-up during treatment.
One patient failed treatment. HIV = human immunodeficiency virus; SVR = sustained virological response; METAVIR = meta-analysis of histological data in viral hepatitis; APRI = aminotransferase/platelet ratio.
Treatment costs
The average cost per diagnosis was estimated to be $574 (accounting for testing HCV seronegative patients and HCV confirmatory tests in seropositive patients), while the cost of treatment was $5164 [standard deviation (SD) = $785] per patient. The total cost of finding and treating HCV was $5739 per patient treated. The distribution of costs is shown in Table 3. Visit costs include costs incurred during all visits made in preparation for, during and after treatment. These included baseline assessments, treatment initiation, on-treatment follow-up (excluding DOT), end of treatment, post-treatment follow-up and SVR assessment. DOT costs include the costs associated with daily visits made by patients to take medications under supervision. The major cost driver was DOT, contributing 57.2%% of the total intervention cost. Other contributing costs were DAAs (12.8%), clinic visits (10.9%), screening and diagnosis (10.0%), laboratory investigations (7.2%) and elastography (3.4%). Treatment costs were $429 higher for HCV/HIV coinfected compared to HCV mono-infected patients largely due to differences in DAA drugs used, laboratory and clinic visit costs (Fig. 3).
Table 3.
Average cost of HCV screening and treatment using DAA-based regimens.
| Average cost (SD) | ||||||
|---|---|---|---|---|---|---|
| Fibrosis stage (n) |
Visits | Laboratory | Fibroscan | DAA | DOT | Total costb |
| Full cohort (64) | 626.47 (73.15) | 412.06 (104.51) | 114.21 (13.01) | 727.66 (301.08) | 3284.54 (566.37) | 5164.92 (785.34) |
| F0 (47) | 622.12 (42.78) | 380.25 (74.44) | 115.67 (0) | 636.06 (154.95) | 3269.38 (492.63) | 5023.49 (560.17) |
| F1 (10) | 586.42 (42.01) | 417.36 (100.34) | 115.67 (0) | 600.46 (99.53) | 3122.74 (376.19) | 4842.66 (538.14) |
| F2 (3) | 599.09 (0) | 412.04 (45.81) | 115.67 (0) | 615.41 (113.79) | 3236.17 (0) | 4978.39 (142.91) |
| aUnknown(11) | 695.31 (151.56) | 562.88 (121.75) | 105.15 (34.88) | 1335.12 (307.41) | 3533.91 (987.5) | 6232.37 (1169.54) |
One patient was retreated on sofosbuvir/daclatasvir and the rest of the patients received sofosbuvir/ledipasvir, which was more expensive.
Excludes diagnosis cost ($574 per patient treated) which varies according to HCV seroprevalence and HCV RNA prevalence—currently based on antibody prevalence of 7.7% and chronic prevalence of 77.4%. Costs are mean [standard deviation (SD)] in 2018 $. DAA = directly acting antivirals; DOT = directly observed therapy; HCV = hepatitis C virus.
Figure 3.
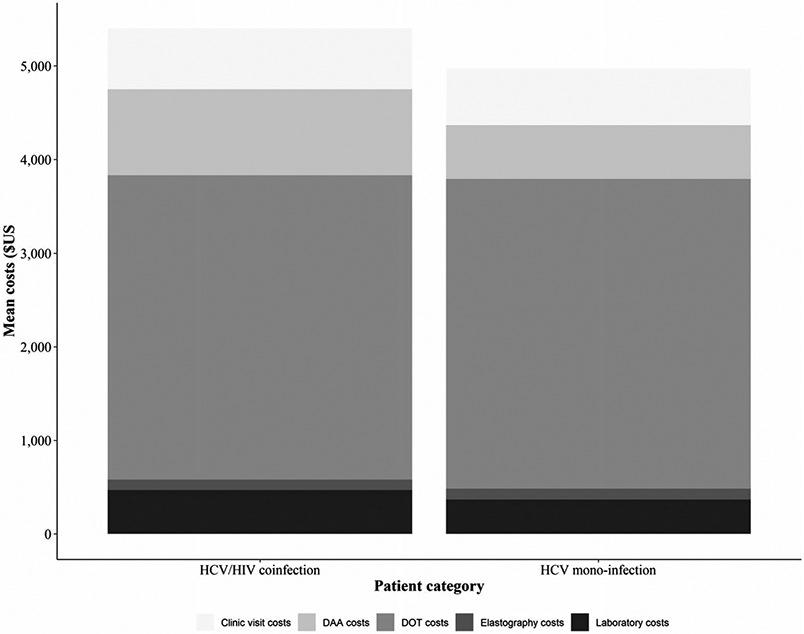
Average cost of HCV screening and treatment using DAA-based regimens by HIV status. Costs are presented in 2018 $. DAA = direct-acting antiviral; DOT = direct-observed therapy; HCV = hepatitis C virus; HIV = human immunodeficiency
Base-case cost-effectiveness
The calibrated model fitted the data well, suggesting slowly decreasing HIV and slowly increasing HCV epidemics among PWID in Nairobi, with an estimated chronic HCV prevalence of 6.7% [95% credible interval (CrI) = 5.9–8.2] in 2016. The intervention is estimated to avert 5.9% (95% CrI = 4.2–8.1%) of all new HCV infections during 2016–30.
We estimated that the HCV screening and treatment intervention undertaken during 2016–18 incurred a total cost of $463 629 and would avert 475 DALYs over 50 years, discounted at 3.0% per annum, resulting in an ICER of $975 per DALY averted (Table 4). The ICER is less than one times the 2018 GDP per capita for Kenya ($1509), demonstrating that this intervention is potentially cost-effective at this cost-effectiveness threshold. However, the intervention was not cost-effective at the opportunity cost-based threshold of $647 per DALY averted.
Table 4.
Base-case costs, effects and incremental cost-effectiveness ratios for HCV screening and DAA-based treatment compared to no treatment per person.
| Treatment strategy | Costs, $; mean(95% CrI) |
$ effects; mean (95% CrI) |
$ ICER; mean (95% CrI) |
||
|---|---|---|---|---|---|
| Total costs | Incremental costs | Total DALYs | DALYs averted |
$/DALY | |
| Base-case | |||||
| No treatment | 0 | – | 901509 | – | – |
| DAA-based treatment | 463 629 (393 669–539 366) | 463 629 (393 669–539 366) | 901 034 (722 592–1032 582) | 475 (296–673) | 975 (661–1601) |
CrI = credible interval; DALY = disability-adjusted life-year; DAA = direct-acting antiviral; ICER = incremental cost-effectiveness ratio; HCV = hepatitis C virus.
Sensitivity analysis
In one-way sensitivity analyses, the base-case ICER was most sensitive to the time horizon (Fig. 4). Reducing the time horizon to 25 years increased the ICER to $3708/DALY averted, rendering the intervention not cost-effective, while increasing the time horizon to 100 years reduced the ICER to $355/DALY averted. Assuming discount rates of 0% or 6% improved (ICER = $342/DALY) or reduced (ICER = $2514/DALY) cost-effectiveness, respectively. Assuming a 13% HCV seroprevalence among PWID in Kenya reduced the cost of case-finding from $574 to $434, slightly improving cost-effectiveness of the intervention (ICER = $951/DALY). The intervention could remain cost-effective at the GDP per capita cost-effectiveness threshold over all the HCV seroprevalences evaluated, including the lowest (2.8%; ICER = $1114/DALY). Reducing the costs of HCV point of care and confirmatory tests reduced the cost per case diagnosed to $349 and $469, respectively, resulting in ICERs of $937 and $958 per DALY averted, respectively. Accounting for costs of HCV disease care reduced the ICER to $670 per DALY averted.
Figure 4.
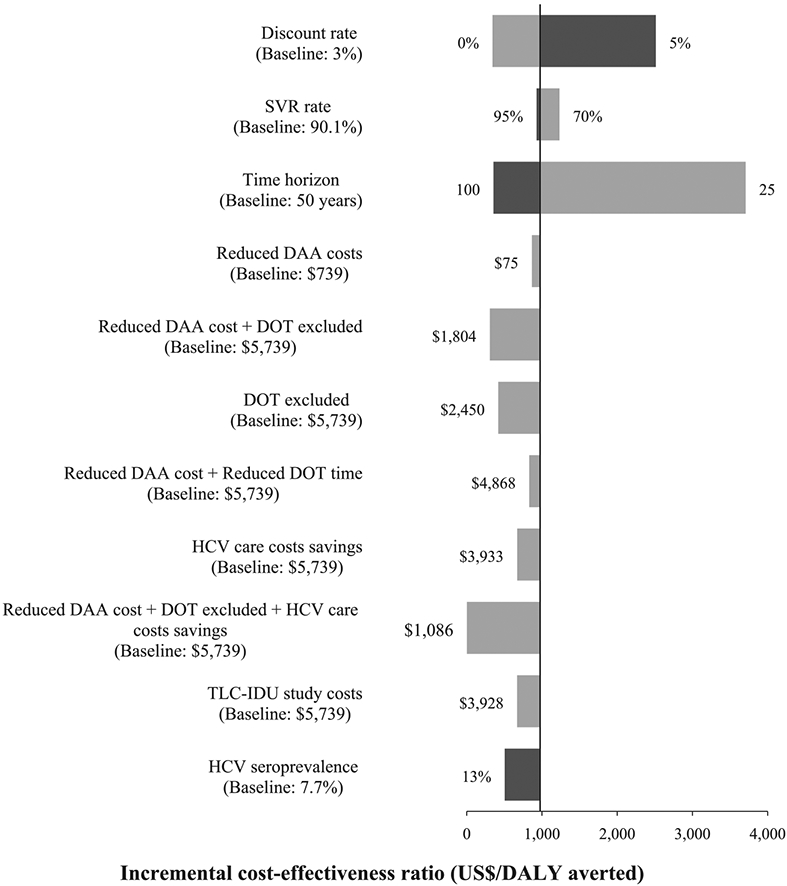
Univariate sensitivity analysis showing the effect of various changes in parameter values or model assumptions (listed on left-hand side) on the incremental cost-effectiveness ratio (ICER, cost per DALY averted). The vertical line shows the base-case ICER per DALY averted. Numbers at the end of each bar are the new values used for each parameter, with grey bars giving the new ICER for decreases in parameters and black for increases in a parameter. The baseline cost of $5739 is the estimated total cost per individual treated in the intervention. DAA = direct-acting antivirals; DALY = disability-adjusted life-year; DOT = directly observed therapy; SVR = sustained virological response; TLC-IDU = test and linkage to care for injecting drug users
The ICER for the base-case scenario is reduced by a shorter time for DOT ($939/DALY), a reduced price for DAAs ($866/DALY) or a combination of both ($830/DALY). The ICER is further reduced ($418/DALY) if DOT is not used, assuming no adverse effect on HCV treatment outcomes. A reduction in DAA prices and the exclusion of DOT costs resulted in an ICER of $307/DALY averted. Lastly, accounting for HCV disease care costs, a reduction in DAA prices and the exclusion of DOT costs resulted in improved value for money ($2/DALY averted).
The probabilistic sensitivity analysis suggests that 92.1% of the simulated ICERs for the base-case scenario fall below the GDP per capita cost-effectiveness threshold (Supporting information, Figs S3 and S4), but only 1.8% fall below the opportunity cost-based threshold. This increases to 36.7% when we account for HCV health-care costs, 99.0% if we assume the cheaper DAA cost and no DOT and 100% if we account for all three.
DISCUSSION
Main findings
This study provides important evidence that the implementation of testing and DAA-based HCV treatment interventions among PWID can be cost-effective in a LMIC setting. Our results suggest that the intervention undertaken in Nairobi involving DOT cost $975/DALY averted, less than the one-times GDP per capita (US$1509) cost-effectiveness threshold for Kenya. The intervention could provide improved value for money with simplification of the care pathway, integration with other services such as MAT and lower prices for DAAs. The intervention would become nearly cost-saving (ICER = $2/DALY averted) if, in addition to reduced DAA prices and accounting for HCV health-care costs, DOT is eliminated altogether (assuming no drop in SVR).
Strengths and limitations
This study draws major strength from using ‘real-world’ data on PWID screened, diagnosed and treated as part of a pilot intervention in Nairobi. This enabled collection of patient-level data on resource utilization and estimation of the full costs of screening and DAA-based HCV treatment. We used testing, linkage-to-care and effectiveness data (SVR12 rates) derived directly from the intervention. These strengths make our results probably transferable to PWID populations in other parts of Kenya and the SSA region. Secondly, a dynamic compartmental model allowed us to capture both the individual (prevention of HCV disease progression) and population benefits (reducing HCV transmission) of HCV treatment.
However, the interpretation of our findings requires consideration of potential limitations. First, the generalizability of our results may be limited because they are based on a closely managed, intensive model of care for testing, linkage-to-care treatment, DOT and follow-up using dedicated staff in a harm reduction service, all of which may have contributed to the observed successful treatment outcomes. However, they could be generalizable to other SSA settings with similar HCV prevalence in PWID, where PWID are provided harm reduction services through similar drop-in centres and MAT services. Secondly, we did not evaluate the effect of including health-care costs associated with HCV-related disease in the base-case analysis, which is likely to make our projections conservative. Accounting for these cost savings decreased the ICER by a third, reflecting improved value for money. Thirdly, the use of costs and outcome data from a single population of PWID may limit the generalizability of our results. Fourthly, in the absence of Kenya specific utility weights, we applied the Global Burden of Disease study disability weights to estimate DALYs. Some of these disability weights were not specific to HCV disease states, possibly limiting their accuracy; however, they are widely used in the literature [60,61], allowing comparison of our results with other studies, while previous analyses suggest they may not impact upon decisions [62].
Comparison with other studies
To our knowledge, this is the first study to evaluate the cost-effectiveness of a ‘real-world’ implementation of HCV testing and DAA-based treatment among PWID in an LMIC setting. This represents a significant addition to existing evidence, which currently focuses upon high-income countries [18]. Model-based analyses have evaluated the cost-effectiveness of HCV screening and DAA-based treatment among PWID in LMICs [20] and recently in Tanzania [21], and found them to be cost-effective or cost-saving if DAA costs are low enough. However, unlike our study, their costs or outcomes were not derived from an actual intervention.
CONCLUSIONS AND IMPLICATIONS
Our analysis suggests that screening and treatment of HCV with DAA-based regimens among PWID in Nairobi, Kenya is associated with significant costs largely due to DOT and expensive DAAs. Despite this, the intervention is cost-effective in its current format. Large improvements in cost-effectiveness can be easily achieved through accessing cheaper DAAs and streamlining or removing DOT. In this study, therapy was dispensed by a clinical officer, which could be simplified through using trained peer educators who already support harm reduction services. Although eliminating DOT could improve cost-effectiveness, it is important to assess whether it would be similarly effective for treating PWID. Fortunately, prior studies suggest this should be the case, with 94.0% retention and 90.0% achieving SVR12 in a randomized control trial setting in New York [57,63], 94.9% retention in the TLC-IDU study in Kenya (NCT01557998) and 93–98% retention and 85–87% achieving SVR12 in other real-world studies not using DOT [58,59,64]. The development of innovative approaches to enhance and monitor adherence to DAAs in PWID that may be more cost-effective than DOT presents opportunities to further optimize models of care in this setting [65].
Our findings support the development of similar and optimized HCV screening and treatment strategies for PWID in Kenya and other LMIC. Numerous centres provide harm reduction services and MAT in Kenya. Such centres provide opportunities to establish similar treatment interventions for HCV that could enable Kenya and other LMIC with such services to substantially reduce their HCV burden.
Supplementary Material
Table S1 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Table S2 Model parameters and calibration data not included in the manuscript.
Table S3 Parameters for mortality rates amongst ex-PWID.
Table S4 Baseline characteristics of patients in the MSF/MdM HCV cohort.
Table S5 Characteristics of patients who completed treatment in the MSF/MdM HCV screening and treatment intervention.
Table S6 Staff types and staff times for the different activities in the MSF/MdM HCV screening and treatment intervention.
Table S7 Activities, resources and estimated unit costs in the MSF/MdM HCV screening and treatment intervention.
Table S8 Unit costs for visits, laboratory tests, test kits and medicines in the MSF/MdM HCV screening and treatment intervention.
Table S9 Total unit costs for screening tests by result ($) in the MSF/MdM HCV screening and treatment intervention.
Table S10 Baseline characteristics of patients in the TLC study cohort analyzed.
Table S11 Characteristics of patients who completed 12-week post-treatment follow-up in the TLC study cohort.
Table S12 Staff types and staff times for the different activities in the TLC HCV screening and treatment intervention.
Table S13 Activities, resources and estimated unit costs in the TLC HCV screening and treatment intervention.
Table S14 Unit costs for visits, laboratory tests, test kits and medicines in the TLC HCV screening and treatment intervention.
Table S15 Total unit costs for screening tests by result ($) in the TLC HCV screening and treatment intervention.
Figure S1. Model schematic of (a) harm reduction interventions; (b) HIV transmission and treatment; (c) HCV transmission and treatment.
Figure S2. Model projections for HCV and HIV prevalence in Nairobi. HCV = hepatitis C infection, HIV = human immunodeficiency virus.
Figure S3. Cost-effectiveness plane showing the incremental costs and disability-adjusted life years averted based on 3000 simulations. DALYs – disability adjusted life years, GDP – gross domestic product.
Figure S4. Cost-effectiveness acceptability curve for direct-acting antiviral-based HCV treatment in comparison to no treatment. DALY – disability adjusted life year.
Figure S5. Cost-effectiveness acceptability curves showing the effect of parameter assumptions on the probability of cost-effectiveness for direct-acting antiviral-based HCV treatment in comparison to no treatment. DALY – disability adjusted life year.
Figure S6. Incremental cost-effectiveness ratio (ICER) for HCV screening and treatment among PWID compared to no screening over a range of HCV seroprevalences. ICER: incremental cost-effectiveness ratio; DALY: disability-adjusted life years; HCV: hepatitis C virus; USD, United States dollar.
Acknowledgements
Funding for this study was provided by Unitaid (grant SPHQ14-LOA-217) and Médecins Sans Frontières. P.V., H.F. and J.S. are supported by the National Institute for Health Research Health Protection Research Units (NIHR HPRUs) in Evaluation of Interventions and Behavioural Science at the University of Bristol in partnership with Public Health England (PHE). M.H., P.V. and H.F. also acknowledges support from the NIHR-funded EPIToPe project. P.V., H.F. and J. S. also acknowledge support from the US National Institute for Drug Abuse (NIDA grant number R01 AI147490, R01 DA033679, R01 DA037773, R21 DA046809 and R01 DA047952). P.V., J.S., B.M. and H.F. acknowledge support from Global Fund to Fight AIDS, Tuberculosis and Malaria, grant/award number: QPB-H-KANCO grant number 861. M.A., P.C., A.K. acknowledge support from grants (numbers R01DA032080 and R01DA032080-05S1, awarded to Principal Investigators A.K. and P.C.) from the National Institute on Drug Abuse.
Footnotes
Declaration of interests
H.F. has received an honorarium from MSD. P.V. and J.W. have received investigator-initiated untied grants from Gilead and P.V. has received honorarium from Gilead and Merck.
Supporting Information
Additional supporting information may be found online in the Supporting information section at the end of the article.
References
- 1.World Health Organization (WHO). Global Hepatitis Report 2017. Geneva: WHO; 2017. Available at: https://www.who.int/publications/i/item/global-hepatitis-report-2017 [Google Scholar]
- 2.World Health Organization (WHO). Progress Report on Access to Hepatitis C Treatment: Focus on Overcoming Barriers in Low- and Middle-Income Countries. Geneva: WHO; 2018. https://apps.who.int/iris/handle/10665/260445 [Google Scholar]
- 3.Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5: e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trickey A, Fraser H, Lim AG, Peacock A, Colledge S, Walker JG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol 2019; 4: 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonderup MW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis C in sub-Saharan Africa: the current status and recommendations for achieving elimination by 2030. Lancet Gastroenterol Hepatol 2017; 2: 910–9. [DOI] [PubMed] [Google Scholar]
- 6.Muasya T, Lore W, Yano K, Yatsuhashi H, Owiti FR, Fukuda M, et al. Prevalence of hepatitis C virus and its genotypes among a cohort of drug users in Kenya. East Africa Med J 2008; 85: 318–25. [DOI] [PubMed] [Google Scholar]
- 7.Mwatelah RS,Lwembe RM, Osman S, Ogutu BR, Aman R, Kitawi RC, et al. Co-infection burden of hepatitis C virus and human immunodeficiency virus among injecting heroin users at the Kenyan coast. PLOS ONE 2015; 10: e0132287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ClinicalTrials.gov. National Library of Medicine (U.S.). Testing and Linkage to Care for Injecting Drug Users in Kenya. Identifier: NCT01557998. 2018. Available at: https://ClinicalTrials.gov/show/NCT01557998.
- 9.Kenya Aids NGOs Consortium (KANCO). KANCO Annual Report 2016. Nairobi: KANCO; 2016. [Google Scholar]
- 10.Akiyama MJ, Cleland CM, Lizcano JA, Cherutich P, Kurth AE Prevalence, estimated incidence, risk behaviours, and genotypic distribution of hepatitis C virus among people who inject drugs accessing harm-reduction services in Kenya: a retrospective cohort study. Lancet Infect Dis 2019; 19: 1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. Geneva: WHO; 2016. https://apps.who.int/iris/handle/10665/246177 [Google Scholar]
- 12.World Health Organization (WHO) Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 13.Day E, Hellard M, Treloar C, Bruneau J, Martin NK, Øvrehus A, et al. Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int 2019; 39: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill AM, Nath S, Simmons B The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad 2017; 3: 117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol 2013; 19: 7846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone K, Shirley-Beavan S The Global State of Harm Reduction 2018. London, UK: Harm Reduction International; 2018. https://www.hri.global/global-state-of-harm-reduction-reports [DOI] [PubMed] [Google Scholar]
- 17.Ford N, Wiktor S, Kaplan K, Andrieux-Meyer I, Hill A, Radhakrishnan P, et al. Ten priorities for expanding access to HCV treatment for people who inject drugs in low- and middle-income countries. Int J Drug Policy. 2015; 26(11): 1088–93. 10.1016/j.drugpo.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Cipriano LE, Goldhaber-Fiebert JD Population Health and Cost-Effectiveness Implications of a “Treat All” Recommendation for HCV: A Review of the Model-Based Evidence. MDM Policy Pract. 2018; 3(1): 238146831877663. 10.1177/2381468318776634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan JR, Servidone M, Easterbrook P, Linas BP Economic evaluation of HCV testing approaches in low and middle income countries. BMC Infect Dis 2017; 17: 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mabileau G, Scutelniciuc O, Tsereteli M, Konorazov I, Yelizaryeva A, Popovici S, et al. Intervention packages to reduce the impact of HIV and HCV infections among people who inject drugs in Eastern Europe and Central Asia: a modeling and cost-effectiveness study. Open Forum Infect Dis 2018; 5: ofy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott N, Mohamed Z, Rwegasha J, Mbwambo J, Lemoine M, Hellard M Upscaling prevention, testing and treatment to control hepatitis C as a public health threat in Dar Es Salaam, Tanzania: a cost-effectiveness model. Int J Drug Policy 2021; 88: 102634. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection. Updated version, April 2016: Guidelines. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver (EASL) EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 2014; 60: 392–420. [DOI] [PubMed] [Google Scholar]
- 24.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62: 932–54. [DOI] [PubMed] [Google Scholar]
- 25.Stone J, Fraser H, Walker JG, Mafirakureva N, Mundia B, Cleland C, et al. Modelling the impact of prevention and treatment interventions on HIV and Hepatitis C Virus Transmission Among People who Inject Drugs in Kenya. medRxiv 2020; 10.1101/2021.02.02.21251008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenyan Ministry of Health and National AIDS Control Council. Kenya Aids Response Progress Report 2016. Nairobi: Kenyan Ministry of Health and National AIDS Control Council; 2016. https://nacc.or.ke/wp-content/uploads/2016/11/Kenya-AIDS-Progress-Report_web.pdf [Google Scholar]
- 27.National STI/AIDS Control Programme,Nairobi, Ministry of Health Kenya National Behavioral Assessment of Key Populations in Kenya Polling Booth Survey Report. Nairobi: National STI/AIDS Control Programme Ministry of Health Kenya; 2015. [Google Scholar]
- 28.Musyoki H, Bhattacharjee P, Blanchard AK, Kioko J, Kaosa S, Anthony J, et al. Changes in HIV prevention programme outcomes among key populations in Kenya: data from periodic surveys. PLOS ONE 2018; 13: e0203784–e0203784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurth AE, Cleland CM, Des Jarlais DC, Musyoki H, Lizcano JA, et al. HIV prevalence, estimated incidence, and risk behaviors among people who inject drugs in Kenya. J Acquir Immune Defic Syndr 2015; 70: 420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris P. A, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42(2): 377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenya National Bureau of Statistics. Key statistics: Consumer Price Indices (CPI) and Inflation Rates. Available at: https://www.knbs.or.ke/download/inflation-trends-1961-present/ (accessed 30 October 2018).
- 32.Central Bank of Kenya. Foreign Exchange Rates. Available at: https://www.centralbank.go.ke/rates/forex-exchange-rates/ (accessed 30 October 2018).
- 33.Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015; 3: e712–e723. [DOI] [PubMed] [Google Scholar]
- 34.Baltussen RMPM, Adam T, Tan-Torres Edejer T, Hutubessy RCW, Acharya A, Murray CJL Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis, Vol. 1. Geneva: World Health Organization; 2003. https://apps.who.int/iris/handle/10665/42699 [Google Scholar]
- 35.Wilkinson T, Sculpher MJ, Claxton K, Revill P, Briggs A, Cairns JA, et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health 2016; 19: 921–8. [DOI] [PubMed] [Google Scholar]
- 36.World Bank. World Bank national accounts data, and OECD National Accounts data files. GDP per capita (current US$). Available at: https://data.worldbank.org/indicator/NY.GDP.PCAPCD?locations=KE (accessed 30 October 2018). [Google Scholar]
- 37.Bertram MY, Lauer JA, de Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Org 2016; 94: 925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochalek J, Lomas J, Claxton K Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data. BMJ Glob Health 2018; 3: e000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ndetei D, Ongecha-Owuor FA, Malow RM, Onyancha J, Mutiso V, Kokonya DD, et al. Next priorities for intervention in Kenya: results from a cohort study of drug use, HIV and HCV patterns in five urban areas. Int Psychol Rep 2006; 10: 16–9. [Google Scholar]
- 40.Chhatwal J, Chen Q, Bethea ED, Ladd MA, Mueller PP, Hutin Y, et al. Hep C calculator: an online tool for cost-effectiveness analysis of DAAs. Lancet Gastroenterol Hepatol 2018; 3: 819. [DOI] [PubMed] [Google Scholar]
- 41.World Bank. World Development Indicators 2020. Washington, DC: World Bank; 2020. https://databank.worldbank.org/source/world-development-indicators [Google Scholar]
- 42.Feelemyer J, Des Jarlais D, Arasteh K, Uuskula A Adherence to antiretroviral medications among persons who inject drugs in transitional, low and middle income countries: an international systematic review. AIDS Behav 2015; 19: 575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham EB, Hajarizadeh B, Amin J, Litwin AH, Gane E, Cooper C, et al. Adherence to once-daily and twice-daily direct-acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy Clin Infect Dis 2020; 71: e115–e124. [DOI] [PubMed] [Google Scholar]
- 44.Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouscaillou J, Kikvidze T, Butsashvili M, Labartkava K, Inaridze I, Etienne A, et al. Direct acting antiviral-based treatment of hepatitis C virus infection among people who inject drugs in Georgia: a prospective cohort study Int J Drug Policy 2018; 62: 104–11. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M, Janjua NZ, Shafiq TKI, Chowdhury EI, Sarker S, Sharful SI, et al. Piloting Hepatitis C Virus Treatment in People Who Inject Drugs (PWID) in Bangladesh. Int J Drug Policy 2019; 74: 69–75. [DOI] [PubMed] [Google Scholar]
- 47.Neumann P J, Anderson JE, Panzer AD, Pope EF, D’Cruz BN, Kim DD, et al. Comparing the cost-per-QALYs gained and cost-per-DALYs averted literatures. Gates Open Res 2018; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panzer AD, Emerson JG, D’Cruz B, Patel A, Dabak S, Isaranuwatchai W, et al. Growth and capacity for cost-effectiveness analysis in Africa. Health Econ 2020; 29: 945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng X, Kim DD, Cohen JT, Neumann PJ, Ollendorf DA Using QALYs versus DALYs to measure cost-effectiveness: how much does it matter? Int J Technol Assess Health Care 2020; 36: 96–103. [DOI] [PubMed] [Google Scholar]
- 50.Norton BL, Akiyama MJ, Agyemang L, Heo M, Pericot-Valverde I, Litwin AH. Low Adherence Achieves High HCV Cure Rates Among People Who Inject Drugs Treated With Direct-Acting Antiviral Agents. Open Forum Infect Dis. 2020; 7(10). 10.1093/ofid/ofaa377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kikvidze T, Luhmann N, Avril E, Butsashvili M, Labartkava K, Etienne A, et al. Harm reduction-based and peer-supported hepatitis C treatment for people who inject drugs in Georgia. Int J Drug Policy 2018; 52: 16–9. [DOI] [PubMed] [Google Scholar]
- 52.Akiyama MJ, Agyemang L, Arnsten JH, Heo M, Norton BL, Schackman BR, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy BMC Infect Dis 2018; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ministry of Health. Kenya Most at risk Populations Size Estimate Consensus report. Seattle, WA: Institute for Health Metrics and Evaluation;I 2013. [Google Scholar]
- 54.Micallef JM, Kaldor JM, Dore GJ Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepatol 2006; 13: 34–41. [DOI] [PubMed] [Google Scholar]
- 55.Smith DJ, Jordan AE, Frank M, Hagan H Spontaneous viral clearance of hepatitis C virus (HCV) infection among people who inject drugs (PWID) and HIV-positive men who have sex with men (HIV+ MSM): a systematic review and meta-analysis. BMC Infect Dis 2016; 16: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017; 9: CD12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345: e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, van Velzen E, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol 2014; 43: 235–48. [DOI] [PubMed] [Google Scholar]
- 59.Smith DJ, Combellick J, Jordan AE, Hagan H Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta-analysis. Int J Drug Policy 2015; 26: 911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thein H-H, Yi Q, Dore GJ, Krahn MD Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008; 22: 1979–91. [DOI] [PubMed] [Google Scholar]
- 61.Shepherd J, Shepherd J, Jones J, Hartwell D, Davidson P, Price A, et al. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation, in NIHR health technology assessment programme: executive summaries. NIHR Journals Library 2007; 11: 1–205, iii. [DOI] [PubMed] [Google Scholar]
- 62.Merchante N, Girón-González JA, González-Serrano M, Torre-Cisneros J, García-García JA, Arizcorreta A, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS 2006; 20: 49–57. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Dieguez M, Montes ML, Pascual-Pareja JE, Quereda C, von Wichmann M, Berenguer J, et al. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS 2011; 25: 899–904. [DOI] [PubMed] [Google Scholar]
- 64.van der Meer AJ, Veldt BJ, Eeld JJ, Wedemeyer H, Dufour JE, Lammert E, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308: 2584–93. [DOI] [PubMed] [Google Scholar]
- 65.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158: 329–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Table S2 Model parameters and calibration data not included in the manuscript.
Table S3 Parameters for mortality rates amongst ex-PWID.
Table S4 Baseline characteristics of patients in the MSF/MdM HCV cohort.
Table S5 Characteristics of patients who completed treatment in the MSF/MdM HCV screening and treatment intervention.
Table S6 Staff types and staff times for the different activities in the MSF/MdM HCV screening and treatment intervention.
Table S7 Activities, resources and estimated unit costs in the MSF/MdM HCV screening and treatment intervention.
Table S8 Unit costs for visits, laboratory tests, test kits and medicines in the MSF/MdM HCV screening and treatment intervention.
Table S9 Total unit costs for screening tests by result ($) in the MSF/MdM HCV screening and treatment intervention.
Table S10 Baseline characteristics of patients in the TLC study cohort analyzed.
Table S11 Characteristics of patients who completed 12-week post-treatment follow-up in the TLC study cohort.
Table S12 Staff types and staff times for the different activities in the TLC HCV screening and treatment intervention.
Table S13 Activities, resources and estimated unit costs in the TLC HCV screening and treatment intervention.
Table S14 Unit costs for visits, laboratory tests, test kits and medicines in the TLC HCV screening and treatment intervention.
Table S15 Total unit costs for screening tests by result ($) in the TLC HCV screening and treatment intervention.
Figure S1. Model schematic of (a) harm reduction interventions; (b) HIV transmission and treatment; (c) HCV transmission and treatment.
Figure S2. Model projections for HCV and HIV prevalence in Nairobi. HCV = hepatitis C infection, HIV = human immunodeficiency virus.
Figure S3. Cost-effectiveness plane showing the incremental costs and disability-adjusted life years averted based on 3000 simulations. DALYs – disability adjusted life years, GDP – gross domestic product.
Figure S4. Cost-effectiveness acceptability curve for direct-acting antiviral-based HCV treatment in comparison to no treatment. DALY – disability adjusted life year.
Figure S5. Cost-effectiveness acceptability curves showing the effect of parameter assumptions on the probability of cost-effectiveness for direct-acting antiviral-based HCV treatment in comparison to no treatment. DALY – disability adjusted life year.
Figure S6. Incremental cost-effectiveness ratio (ICER) for HCV screening and treatment among PWID compared to no screening over a range of HCV seroprevalences. ICER: incremental cost-effectiveness ratio; DALY: disability-adjusted life years; HCV: hepatitis C virus; USD, United States dollar.