Abstract
Photodynamic therapy (PDT) is highly effective in treating tumors located near body surface, offering strong tumor suppression and low damage to normal tissue nearby. PDT is also effective for treating a number of other conditions. PDT not only provide a precise and selective method for the treatment of various diseases by itself, it can also be used in combination with other traditional therapies. Because PDT uses light as the unique targeting mechanism, it has simpler and more direct targeting capability than traditional therapies. The core material of a PDT system is the photosensitizer which converts light energy to therapeutic factors/substances. Different photosensitizers have their distinct characteristics, leading to different advantages and disadvantages. These could be enhanced or compensated by using proper PDT system. Therefore, the selected type of photosensitizer would heavily influence the overall design of a PDT system. In this article, we evaluated major types of inorganic and organic PDT photosensitizers, and discussed future research directions in the field.
Keywords: Photodynamic therapy, Photosensitizer, Tumour treatment, Combination therapy, Porphyrin
Graphical abstract
1. Introduction
Photodynamic therapy (PDT) is a relatively new therapeutic system that, when properly designed and applied, could be highly efficient in treating disease through targeted cell killing and has relatively low adverse effect [1], [2], [3]. The cell killing mechanism normally relies on the production of reactive oxygen species (ROS) [4] by PDT agent under the radiation of light of particular wavelength [5], ROS are a group of strong oxidative reagents capable of seriously affecting the physiological activity of cells and promoting apoptosis [6]. These reactions could be designed to occur only under stimulation of certain wavelengths of light. Hence, even when the PDT agents are absorbed by cells, PDT treatments are generally safe in the absence of light or under weak daylight. In this way, proper PDT therapies are generally regarded to be selective and safe. While most PDT researches focus on cancer treatment [7], PDT systems also have other clinical applications such as disinfection and skin disease treatment [8]. In general, PDTs are versatile and effective when used properly. Because it is relatively new in clinical application, there are still plenty of potential for further developments Table 1, 2 and 3.
Table 1.
Examples of application of inorganic photosensitizer-based systems.
| Material | Characteristic | Model | Radiation source | Ref. |
|---|---|---|---|---|
| Bi2S3@Bi Z-scheme | Regulation of electron energy level, and achieved high conversion efficiency | – | 808 nm laser irradiation | [20] |
| Se/N—CDs | Bind RNA selectively, RNA then acted as a carrier to transport photosensitizer to the nucleus of tumor cell | 4T1 tumor mice | LED irradiation (550 nm( | [22] |
| RuII-PtIV polypyridine Au(I) complexes |
Combined with chemotherapy and PTT to improve the effectiveness of cancer treatment | Drug resistant cancers | 480 and 595 nm light | [24] |
| – | 800 nm laser irradiation | [25] | ||
| MnFe2O4 Cr2Fe6O12 |
Metal compounds of Mn and Cr are made to NCs and the release of chemotherapeutic drug was pH-dependent | Michigan cancer foundation-7, breast cancer cells | UV light | [27] |
| g-C3N4 & PEG | This nanoplatform could produce oxygen via water splitting, and turn oxygen into ROS under light. It also improved biocompatibility due to the PEG modification | A-431 tumors | blue light | [29] |
| CuS | Does no produce ROS, but can be used as an excellent carrier of photosensitizer to achieve the combination of PTT and PDT | L929 normal fibroblast cells and HeLa cells | 808 nm laser irradiation | [30] |
| Iridium (III) complex | Self-assembled by electrostatic force with HA to form a spherical nanostructure called Irpy-HA, which is highly stable (keep its nanostructure at least 30 d). | 4T1.2 cells | 532 nm light | [31] |
| BPNS-GNBP | Act as carriers in PDT, and combined with PTT in the treatment of deep-seated tumor | HeLa cells | 808 nm laser irradiation | [34] |
Table 2.
Photosensitizer system of Porphyrin and porphyrin derivatives (including porphyrin precursors).
| Photosensitizer system | Treatment effect | Combo | Radiation source | Ref. |
|---|---|---|---|---|
| CuS@ICG | This system aims to both kill tumor cells and disrupt the tumor microenvironment for enhanced tumor suppression | PDT and PTT | 808 nm laser | [30] |
| Ppa | Ppa is a derivative of chlorophyll, which are the most abundant natural photosynthetic pigments | – | 660 nm-light irradiation | [41] |
| Responsive NPs including Ppa was designed, It could intelligently release ROS | – | [42] | ||
| The photosensitizer Ppa was functionalized with a 1O2-responsive vinyldithioether | – | [43] | ||
| Chlorin I & II | The structures of those two compounds are also stable under photo-bleaching test, and in vivo assessments displayed that they both have high tumor selectivity | – | Absorption peak around 650 nm | [44] |
| NP-sfb/ce6 | This system is a combination of immunotherapy and PDT, and it appears that the synergistic treatment of tumor is more effective than single cancer treatment | PDT and immunotherapy | 660 nm-light irradiation | [46] |
| 5-ALA | 5-ALA, a precursor of porphyrin, is reported to delay glioma progression and is expected to have great potential in PDT. | – | 410, 510, 545, 580 and 630 nm under 405 nm + 505 nm light source, 5-ALA as photodynamic drug produced the most ROS |
[49] |
| A nano delivery system where 5-ALA is loaded on PMO with PB coating (PB@PMO-5-ALA), It increased PpIX level in glioma cells by 75% comparing with unprotected 5-ALA in mice. | – | [52] | ||
| 5-ALA may also be used in the treatment of certain skin tumors such as SCCs | Activate immune response | [53] | ||
| PMO-PpIX | The PMO load PpIX by amide bond, and this structure showed better tumor inhibition in vitro than free PpIX | – | Illuminated with green laser light (532 nm) | [55] |
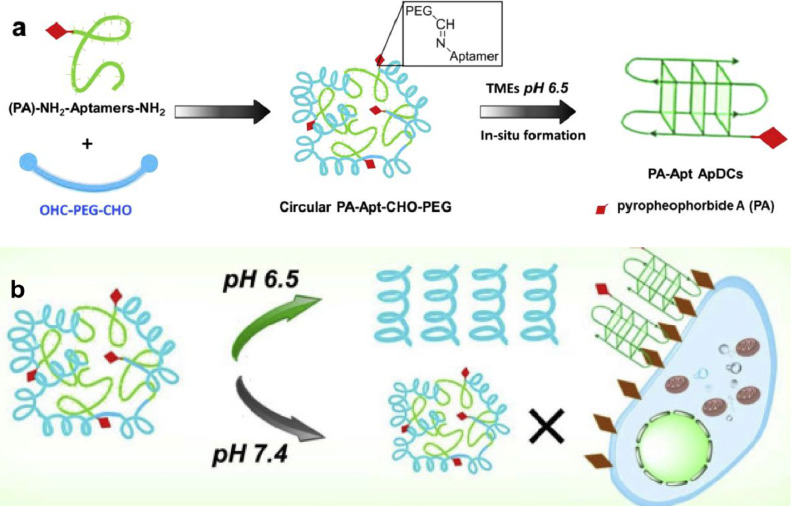
| PA-Apt–CHO–PEG | PA was first linked to aptamer, and the PA-aptamer molecules ware then combined with PEG to form circular PA-aptamer-PEG. The nanostructure is stable in normal physiological environment and bonds breaking in the acidic tumor microenvironment. | pH-dependent | 670 nm light | [57] |
| COF-366 | This NPs provide the combined therapy of PTT and PDT under a single wavelength light illumination with the monitoring of photoacoustic imaging, and makes the operation simpler and more convenient | PDT, PTT and photoacoustic imaging-guided | 635 nm laser | [60] |
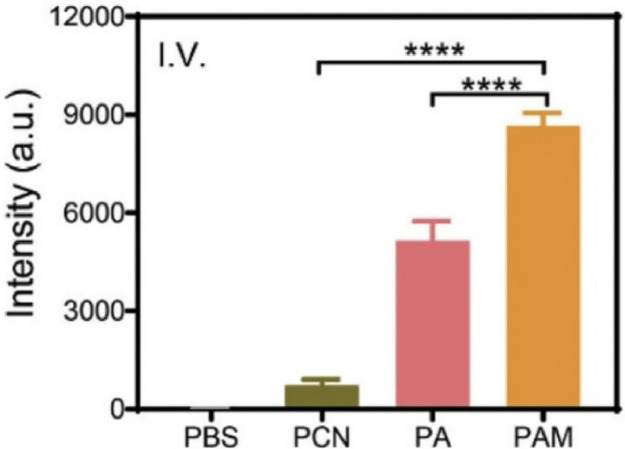
| Ag-MOFs@ TCPP | This structure was constructed by decorating AgNPs onto the porphyritic PCN and further camouflaging with the NM with inflammatory targeting ability (PAM) | PDT, PTT and metal ion therapy | 660 nm light irradiation | [64] |
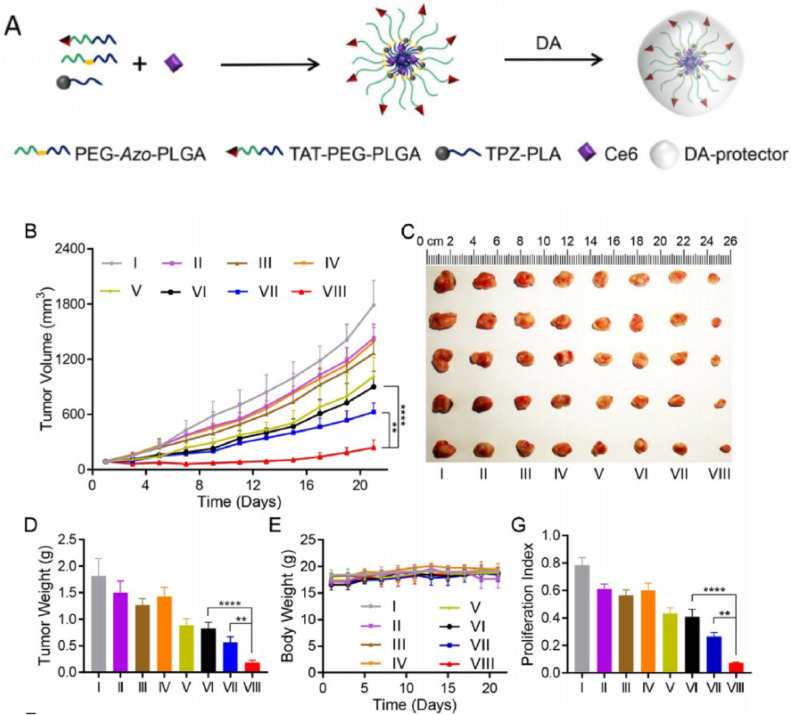
| TAT+AzoNPs@(Ce6+TPZ) | This system include TAT (targeting peptide), Ce6 (photosensitizer), TPZ (hypoxia activatable prodrug) and PEG-Azo-PLGA (amphiphilic polymers linked by azobenzene), which could induce hypoxia in tumor tissue under light stimulation by the releasing of TPZ. | PDT, chemotherapy and controlled drug delivery system | 660 nm light irradiation | [65] |
Table 3.
Examples of PDT for non-cancer diseases and conditions.
| Medicine | Research | Terapeutic purposes | Radiation source | Ref. |
|---|---|---|---|---|
| Tribenzoporphyrazines | This photosensitizer displayed high killing efficiency against S. aureus (and potentially other Gram-positive bacteria), and it showed improved bacteria cell wall permeability | Diseases caused by drug-resistant bacteria such ad S. aureus | 660 nm light irradiation | [67] |
| TiO2/Zn-HY | Inorganic photosensitizer complex which uses TiO2 NPs doped with Zn and hypericin. | Cutaneous Leishmaniasis caused by parasite L. amazonensis | Visible light | [68] |
| NE-CUR | A NE-CUR has devised which enhances performance of curcumin in PDT against vulvar intraepithelial neoplasia induced by HPV. | Intraepithelial neoplasia of vulva caused by HPV infection | Operating at 447±10 nm | [69,70] |
| 5-ALA | An effective 5-ALA based PDT system to treat MRSA infected wounds of diabetic mice, PDT kills bacteria with a mechanism that is different from antibiotics | Infection caused by drug-resistant bacteria such as MRSA-infected diabetic wounds | 635 nm irradiation | [71] |
| Methylene blue | A clinical report describes a case of palatal ulcer that is not sensitive to antibiotics but responds well to PDT and PBMT | Palatal ulcer | 660 nm-light irradiation | [72] |
| 5-ALA with MN | A clinical trial involving 41 AA patients, most of the patients treated with this PDT approach showed reduced severity of their symptoms | Immune-mediated disorder such as AA | 630 nm irradiation | [73] |
In PDT research, photosensitizers development is a major theme because of its importance. Photosensitizer is the substance that produces active intermediates when exposed to light [9,10], with some being able to show photo rejuvenation in vitro and in vivo [11]. Hence, the light-conversion efficiency of the photosensitizer largely determines the overall light-conversion efficiency of the whole PDT system [12]. The light-induced products generated by the photosensitizer also provide the starting point for the PDT to exhibit therapeutic effects. Therefore, photosensitizers are the core of a PDT system [13], and the choice of photosensitizer is highly-dependent on the purpose of the specific PDT. Apart from a well-selected photosensitizer, the other main factors affecting PDT efficiency are oxygen content (especially in tumors microenvironment [14]) and photosensitizer accumulation at target sites [15]. In most cancer PDT, ultimate photodynamic efficacy is supported by these 3 main factors. Hence, photosensitizers that are easily applicable to different delivery platforms are more favorable [16].
Although various types of photosensitizers are available, studies have shown that two major types of photosensitizers generally display excellent curative effects in PDT. These are: (1) inorganic nanoplatform, (2) porphyrin and porphyrin derivatives (including porphyrin precursors). These photosensitizers all have their unique characteristics, advantages and disadvantages. Delivery systems are often used to enhance their effect and improve their unfavored properties, which could result in decisive changes. Hence, it is important to design the photosensitizer, delivery system, and other related factors such as methods of administration, as integrated therapeutic strategy. In the following text, we will present more detailed descriptions of and discussions about some of the recent advances of PDT systems using these types of photosensitizers.
2. General properties of photosensitizers and correspondent PDT systems
Photosensitizer converts light into other forms of energy [17], such as heat energy and chemical energy [18]. During this process, other substances may be produced due to the interaction between substances in the environment and activated photosensitizer itself or released energy. The released energy and/or produced substance (normally ROS) thus could exhibit therapeutic effects. In this way, in areas where both light and photosensitizer could reach, certain medical purposes are achieved.
Based on this mechanism, some essential demands must be carefully counted in order to design clinically applicable PDT agents. First, because the PDT agent needs to locate to target tissue in order to be activated in situ, it is important for them to be biocompatible, and be able to be removed from the body or degraded later on. This is normally less a concern for porphyrin-based photosensitizers, but could be a problem for inorganic photosensitizers. Second, the agent should have adequate light conversion efficiency (and/or release more medically effective substances), otherwise impractical strength of light radiation will be required to exert demanded medical effects; in addition, the ability of photosensitizer to be active by specific wavelength of light is sometimes important as light with certain wavelengths could penetrate deeper into tissues. Here, inorganic photosensitizers often possess some advantages as they usually convert energy more efficiently. Third, for further improvement of overall therapeutic effect, sometimes it is important to modify the pharmacodynamics of the photosensitizers, increasing their concentration at target sites; and/or use PDT in combination with other drugs/therapies. For this purpose, various multifunctional PDT systems are often fabricated.
In summary, designing a proper PDT agent requires careful evaluation of a number of factors, and focusing on the clinical purpose of the PDT therapy. It is important to choose/generate the optimal photosensitizer and applying supportive strategies to compensate its disadvantages and exploit its advantages. In the next sections, some recent interesting PDT systems using different types of photosensitizers will be reviewed, demonstrating our advances in this field.
3. Inorganic photosensitizer-based systems
As introduced, inorganic photosensitizers often have high light conversion efficiency but suffers from poorer biocompatibility [19]. Here, the studies presented below employed various strategies (some of which are fairly interesting) to maintain the high efficiency and/or solve the biocompatibility problem of inorganic photosensitizers with some success.
3.1. Novel inorganic photosensitizers
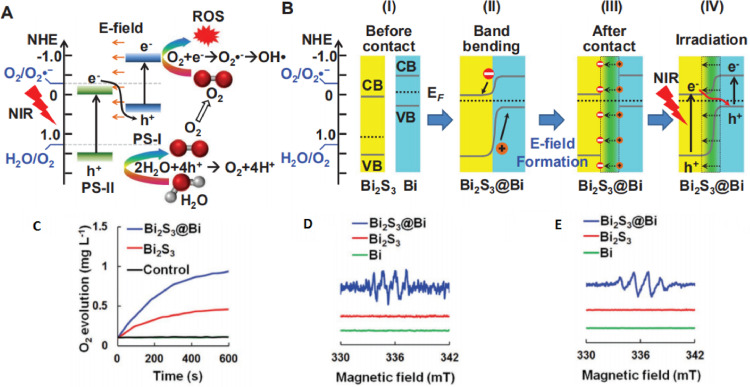
During light activation, the conversion of energy is accompanied by the transition of electron bands. Exploiting this transition, Zhang et al. reported novel Bi2S3@Bi Z-scheme heterostructured nanorods. Their work simultaneously implemented spatiotemporally synchronous O2 self-supply and production of ROS for hypoxic tumor therapy. The hole of the Bi2S3 valence band can react with water to supply O2 for the electron on the conduction band of Bi to produce ROS (Fig. 1A and 1B) [20]. The regulation of electron energy level makes it easier for electrons to be excited and then produce ROS [21]. By using this design of Bi2S3@Bi Z-scheme, they achieved high conversion efficiency, the production of oxygen and ROS was about twice than that of Bi2S3 (Fig. 1C-1E).
Fig. 1.
(A, B) Diagram of electron energy level change under NIR radiation. (C) O2 generation curve, (D) ESR spectra O2•−(DMSO), (E) OH•(water). Reproduced with permission from [20]. Copyrights 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
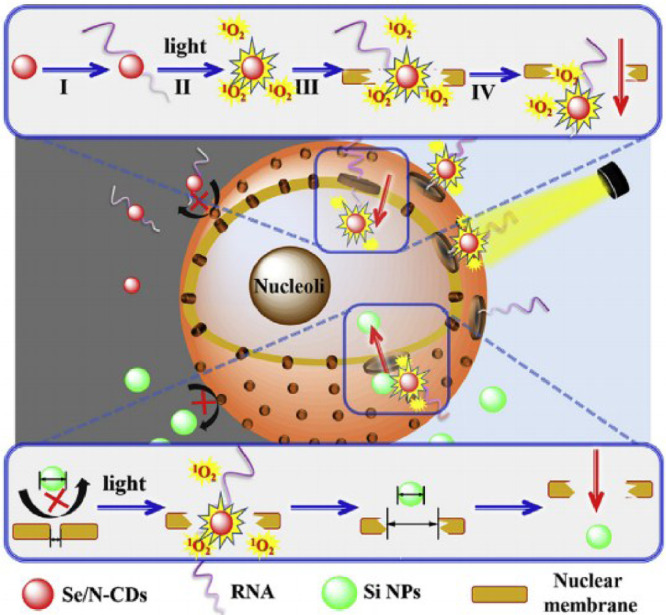
Du et al. have produced Se/N-doped carbon dots, which inhibit tumor growth by destroying DNA of tumor cell. Here, the photosensitizer Se/N-doped carbon dots were shown to bind RNA selectively. RNA then acted as a carrier to transport photosensitizer to the nucleus of tumor cell, and released ROS under light irradiation. Se/N—CDs near the nuclear membrane produced ROS that damaged the membrane under light irradiation, permitting the entry of more Se/N—CDs and thereby improving the conversion efficiency of photosensitization (Fig. 2) [22]. The system exhibited tumor inhibition effect with low organ damage with in model animals. Although the 1O2-generating efficiency of Se/N-doped carbon dots (10.6%) is lower than commercial photosensitizer, the nucleus-targeting mechanism is novel and sheds new lights on cancer treatment strategy.
Fig. 2.
Schematic diagram of light induced nanoparticles larger than nuclear pore into nucleus. Reproduced with permission from[22]. Copyrights 2019 Elsevier Ltd.
Another class of inorganic photosensitizers is the inorganic coordination compounds. Theoretical studies have shown that inorganic coordination compounds have certain photo-transmissibility. For example, a study by Rende et.al. have determined the main photophysical properties of some unsubstituted and iodine substituted phosphorus corrole complexes (Fig. 3), and the results showed that these compounds can indeed be employed as photosensitizers in PDT [23]. Here, density functional theory and its time-dependent formulation was applied to compute the photophysical properties (including photodynamic) of a series of unsubstituted and iodine substituted phosphorus corrole complexes. This study showed the benefit of using theoretical calculation as a guide to reduce screening experiments in the development of PDT drugs.
Fig. 3.
The main geometric parameters and chemical composition of the system studied. Reproduced with permission from[23]. Copyrights 2020 Wiley Periodicals, Inc.
Some metal ions complexes are widely used in chemotherapy for cancer treatment. Among them, polypyridine complexes have also gained much attention as photosensitizers for use in PDT. Hence, chemotherapy using metal ions could be combined with PDT to improve the effectiveness of cancer treatment. Gasser group have reported one such system using a RuII-PtIV polypyridine complex conjugate to treat drug resistant cancers. Because each component in this system has its individual targets, this system is multi-targeting and multi-action. It successfully produced strong cytotoxicity in drug resistant tumor cell upon 595 nm light irradiation [24].
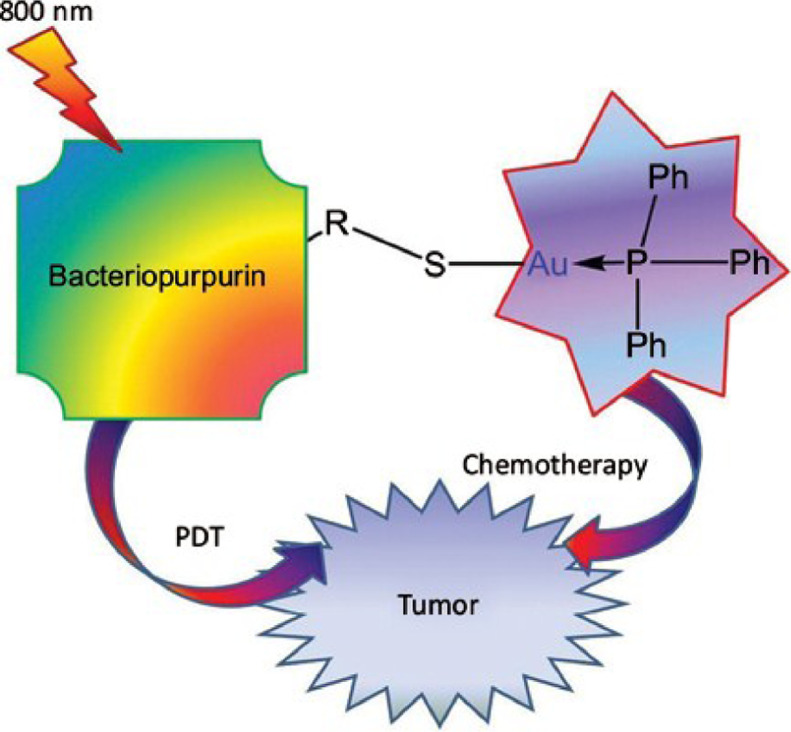
Au, another metal antitumor reagent, could also be combined with PDT to expand the therapeutic potential of photosensitizers to dual potency cytocidal agents. Elena et al. have introduced a photosensitizers system which was derivatives of bacteriopurpurin with thiolated Au(I) complexes, to realize a deep-seated tumor therapy program that can be activated by light (Fig. 4) [25]. Photosensitive systems that introduce metal ions can also be combined with chemotherapy and photothermal therapy (PTT) to enhance therapy [26].
Fig. 4.
Schematic diagram of PDT combined with chemotherapy with photoactivation. Reproduced with permission from [25]. Copyrights 2021 Bentham Science Publishers.
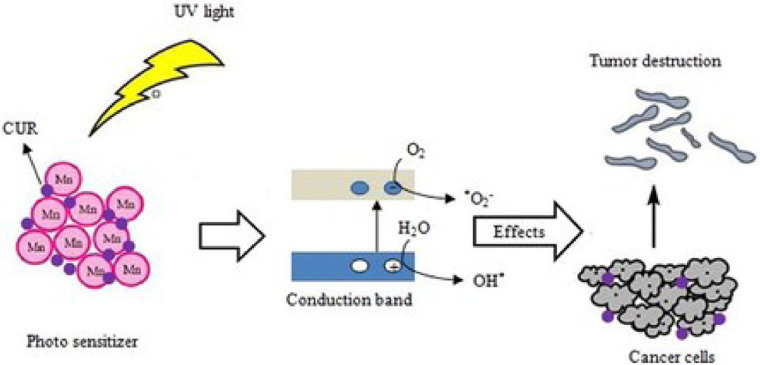
Some other studies directly used metal compounds as photosensitizers for PDT. MnFe2O4 and Cr2Fe6O12 nanocarriers (NCs) has been used as a photosensitizer in some reports to deliver chemotherapeutic drug such as curcumin and provide a new structure for PDT by Hossein et al. [27]. In this study, the metal compounds of Mn and Cr are made to NCs and the release of chemotherapeutic drug from NCs was pH-dependent (Fig. 5). According to the author, chemotherapeutic drug release from Mn compound can reach to over 90% at acidic media instead of 41% at neutral media. Therefore, the MnFe2O4 NC is proposed to be used as a pH-responsive carrier to deliver chemotherapy drugs in acidic tumor microenvironment, as well as a photosensitizer for PDT. This study provides new ideas for further research on multifunctional photosensitizer in PDT.
Fig. 5.
Schematic diagram of PDT photosensitizer system of Mn compound with photodynamic agent and pH response. Reproduced with permission from [27]. Copyrights 2021 Informa UK Limited.
3.2. Modifications of inorganic photosensitizers and photosensitizer delivery options
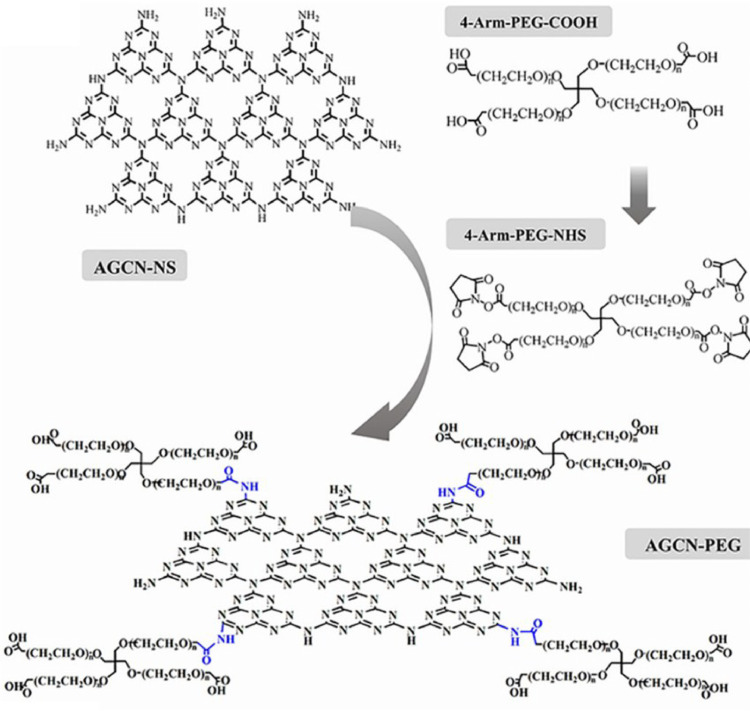
Moreover, inorganic nanomaterials generally have poor biocompatibility [28], so it generally requires certain modification such as the addition of the widely used poly ethylene glycol (PEG) chain. Cai et al. developed a photosensitizer called graphite phase carbon nitride (g-C3N4) and modified it with four-arm-PEG [29]. This nanoplatform was named AGCN-PEG (Fig. 6), which could produce oxygen via water splitting, and turn oxygen into ROS under light. It also improved biocompatibility due to the PEG modification. The researchers have compared the AGCN-PEG with PMPyP4, and while almost no ROS was produced in TMPyP4 group under hypoxic conditions, AGCN-PEG displayed a strong ability to produce ROS under the same conditions.
Fig. 6.
Characterization and preparation of four arm PEG-COOH. The structure diagram and principle of AGCN—NS modified by four arm PEG-COOH. AGCN—NS covalently binds to PEG via amide bond (blue). Reproduced with permission from [29]. Copyrights 2020 Elsevier Inc.
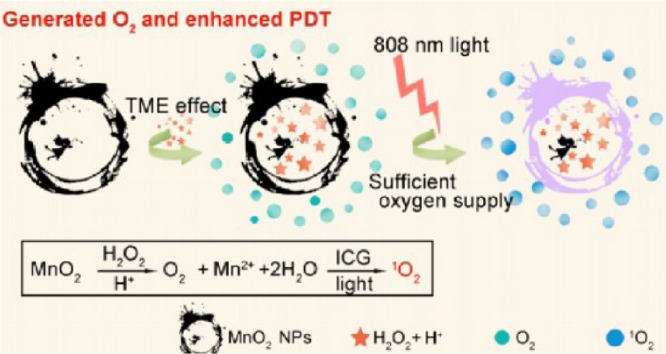
Beyond that, hyaluronic acid (HA) is an alternative modification choice. Gai et al. developed a nano platform called honeycomb MnO2, and used it to load the photosensitizer such as CuS nanoparticles (NPs) and indocyanine green (ICG). The particles were then wrapped in HA to grant the nanoplatform the capability to specifically bind CD44 on tumor cells [30]. In this experiment, the honeycomb MnO2 is not only the carrier of photosensitizer, but also the oxygen provider for PDT and heat generator of PTT. This system aims to both kill tumor cells and disrupt the tumor microenvironment for enhanced tumor suppression (Fig. 7). The highlight of this article is the high drug load rate, of which CuS NPs showed nearly 66.8% and ICG molecules showed up to 80.22% load rates. This system also showed good tumor inhibition in Kunming mice and no obvious damage was found in viscera.
Fig. 7.
Schematic diagram of the formation of O2 and further formation of 1O2 catalyzed by MnO2 in HA/ICG-CuS@hMnO2 under near-infrared light of 808 nm. Reproduced with permission from [30]. Copyrights 2020 Elsevier B.V.
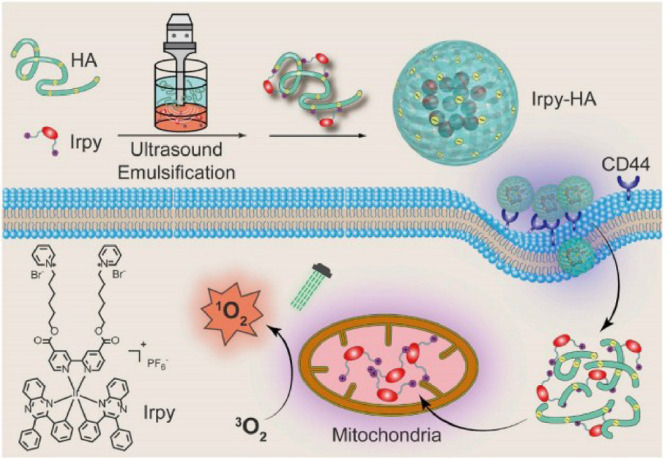
In another study by Li et al., an iridium (III) complex (Irpy) self-assembled by electrostatic force with HA to form a spherical nanostructure called Irpy-HA, which displayed excellent ability of singlet oxygen (1O2) generation [31]. Similarly, the HA target to the CD44 receptor in tumor cell membrane to enhance internalization and selective disassemble. After which, the iridium (III) gathered in mitochondria and produced the singlet oxygen under the green light irradiation (Fig. 8). The nanosphere of Irpy-HA is highly stable under condition, which can keep its nanostructure at least 30 d In vitro tumor cell experiment, over 90% tumor cells exhibited apoptosis and necrosis after PDT using Irpy-HA.
Fig. 8.
Schematic of Irpy-HA for PDT in this study. Reproduced with permission from[31]. Copyrights 2019 Elsevier Ltd.
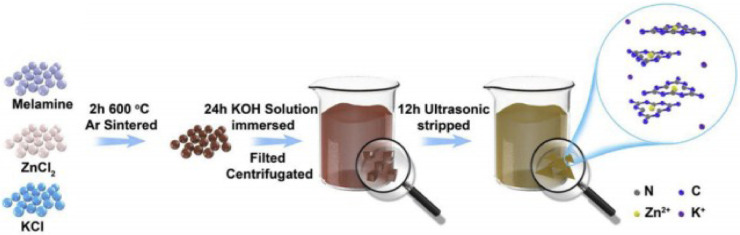
The limitation for medical application of many inorganic nanomaterial is their poor water solubility [32]. This could also be improved by proper modification. Cai et al. have reported a photosensitizer system of PDT that uses tunable water-soluble carbon nitride with alkali-metal cations modification. In this construct, g-C3N4 is only about atomic-thickness so that it can dissolve easily in water with alkali Zn2+ and K+ modification (Fig. 9). Then water-soluble g-C3N4 can tune the absorption edge from 460 nm to the entire visible-light region (663 nm), and reduce the band gap to 1.94 eV. It is worth mentioning that the synergistic effect of g-C3N4 and alkali metal ion have improved the production efficiency of ROS (45.16% vs ∼7.95% of pristine g-C3N4) [33]. This study accomplishes a considerable inactivation efficiency of virulent melanoma A375 by inhibiting the over expression of glutathione (GSH), and the authors claimed that they have overcome the limitation of g-C3N4 as a photosensitizer of PDT and revealed its multifunctional promise to cancer therapy.
Fig. 9.
Schematic of the preparation for Zn2+,K+-C3N4 nanosheets. Reproduced with permission from [33]. Copyrights 2020 Elsevier B.V.
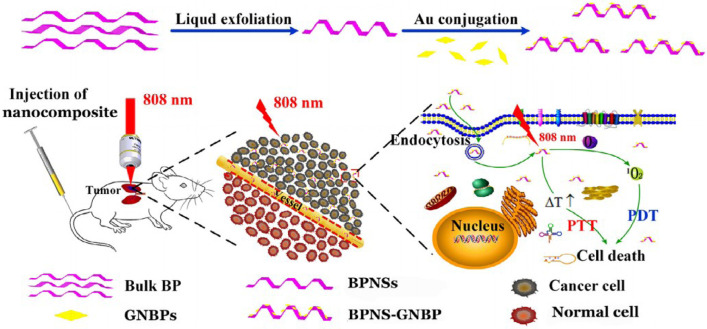
Except for being used as photosensitizers, some inorganic nanomaterials can also act as carriers in PDT. Lu team used black phosphorus nanosheets (BPNSs) to load gold nanobipyramid (GNBPs) and enhanced the curative effect of deep-seated orthotopic lung tumors [34]. The BPNS-GNBP system could simultaneously enhance 1O2 generation and hyperthermia by localized surface plasmon resonance (LSPR) in cancer therapy to overcome the problem of low 1O2 level at deep tumor tissue (Fig. 10). Results showed that BPNS-GNBP exhibited a higher photothermal conversion efficiency than only BPNS or GNBP, and the combined effect of PTT and PDT by this system also improved the overall tumor inhibition effect.
Fig. 10.
Preparation and application of LSPR and GNBP-BPNS nanocomposites reinforced PTT-PDT. Reproduced with permission from [34]. Copyrights 2020 Acta Materialia Inc, Published by Elsevier Ltd.
3.3. Summary
It is clear from above studies that there are still numerous possibilities and huge potential for inorganic photosensitizer development. The strategies that could combine light-conversion, tissue targeting, and/or drug delivery probably will become more attractive in future research, especially for tumor treatment. However, simpler designs with high efficiency will also have their clinical application scenarios (e.g. surface disinfection) as they could be more cost-effective and easier to use.
4. Porphyrin and porphyrin derivatives
Porphyrin is a family of light-absorption molecules with very ancient origin [35]. It is believed to be one of the first batch of biological molecules utilized by life on earth, and played vital part in the evolution history [36]. Chemically, porphyrins are organic molecules made up of 4 nitrogen-containing rings [37], which can form complexes with metal ions [38]. Because of its unique light-absorption capability, it forms essential component of omnipresent biomolecules like chlorophyll and heme, which are categorized as porphyrin derivatives. Thus, porphyrins are generally safe, though extremely high level of porphyrin inside of body could lead to porphyria. In fact, almost all organic photosensitizers are either porphyrin derivatives or molecules share very similar structures and mechanisms with porphyrins. However, porphyrins in their original forms generally have poor photodynamic activity, hence PDT systems mostly uses porphyrin derivatives, which could have higher efficiency, as the photosensitizer [39]. For example, chlorophyll and heme are widely used in PDT, since both are endogenous products of natural organisms with superior biocompatibility and high efficiency [40]. Below, we will review various strategies to further improve clinical performance of porphyrin-based PDTs such as using modified porphyrin derivatives or porphyrin precursors, designing specific delivery vehicle, and applying combined therapies.
4.1. Novel porphyrin derivatives and porphyrin precursors
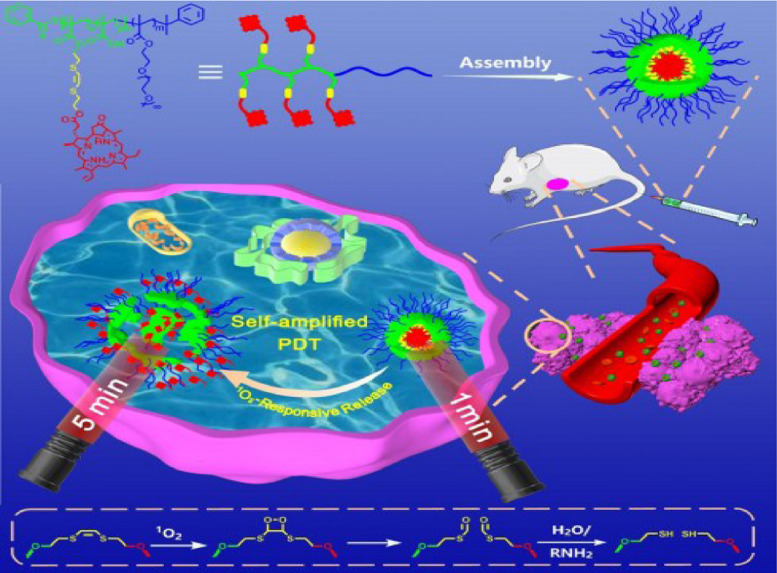
Zhang et al. have reported a photosensitizer that combines the block copolymer with pyro pheophorbide a (Ppa). Ppa is a derivative of chlorophyll, which are the most abundant natural photosynthetic pigments [41]. This photosensitizer is named POEGMA-b-P (MAA-co-VSPpaMA), which could intelligently release ROS when the host NPs accumulate in tumor tissue and is dissolved by cancer cells [42]. The photosensitizer Ppa was functionalized with a 1O2-responsive vinyldithioether [43] (Fig. 11). Besides, the linker of the Ppa and block copolymer is sensitive to ROS which would accelerate the break down process when ROS is generated. Therefore, they claimed their system as a self-amplified PDT.
Fig. 11.
Schematic of autoamplification PDT in NIR and mechanism of 1O2 stimulation of cleavage. Reproduced with permission from [43]. Copyrights 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
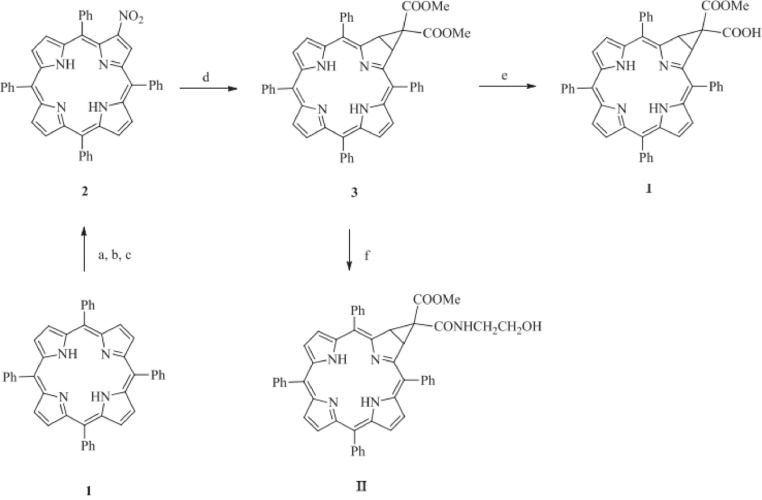
Chen et al. have developed a few photosensitizers of another type of porphyrin derivates called chlorin (i.e. 5,10,15,20-tetraphenylporphyrin and its substitution products). In their work, they generated novel photosensitizers such as 5,10,15,20-tetraphenyl-[2:3]- [(methoxycarbonyl,carboxy) methano] (named chlorin I) and 5,10,15,20-tetraphenyl-[2:3]- {[methoxycarbonyl, (2-hydroxyethyl) amide] methano} (named chlorin II) (Fig. 12). They characterized the structure of chlorin I & II with UV–vis, HNMR, CNMR and HRMS spectroscopies. The structures of those two compounds are also stable under photo-bleaching test [44]. Spectrometry analysis results showed that chlorin I and II both have an absorption peak around 650 nm. In vivo assessments displayed that both chlorin I and II have high tumor selectivity, and could induce tumor cell apoptosis under 650 nm laser irradiation. Both compounds displayed low tissue damage in histological examination, which was possibly benefited from their tumor selectivity. After comparing these two chlorin derivatives, the authors concluded that chlorin I suppresses tumor more effectively making it more appropriate to be developed as a potential anticancer photosensitizer agent.
Fig. 12.
The flowchart of synthetic of chlorin I and chlorinⅡ, a, b, c, d, e, f are available in references. Reproduced with permission from [44]. Copyrights 2020 Elsevier Masson SAS.
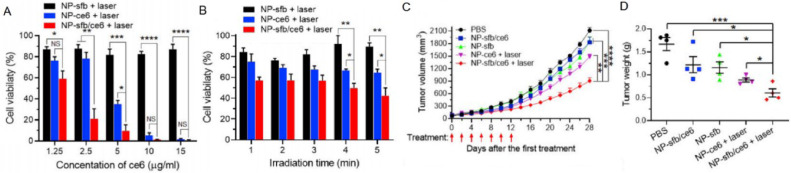
The chlorins can not only be used directly as a photosensitizer, but also as a carrier of other drugs [45]. Under such circumstances, the chlorin carrier system can play double roles in tumor therapy of PDT. Sun group have reported such a PDT system where sorafenib (Sfb) and chlorin e6 (ce6) were co-loaded in a NP which is responsive to ROS (NP-sfb/ce6) [46]. Sfb is an effective tumor suppressor, which could induce T cell-dependent local and systemic antitumor immune responses in organisms where CD8+ T cells quickly proliferate to kill the tumor cells. In this system, ROS produced by ce6 under 660-nm laser irradiation could destruct the NPs and release Sfb while the generated ROS can kill tumor cells at the same time. Therefore, this NP-sfb/ce6 system is a combination of immunotherapy and PDT and it appears that the synergistic treatment of tumor is more effective than single cancer treatment (Fig. 13). Hence, this combination strategy for tumor treatment shows good potential for improving antitumor effects of PDT.
Fig. 13.
(A, B) Evaluation of in vitro effects for NP-sfb/ce6 in PDT. (C) K1-Tumor growth curve after the first treatment. (D) The weights of K1-tumors after the treatment. Reproduced with permission from [46]. Copyrights 2020 Elsevier Ltd.
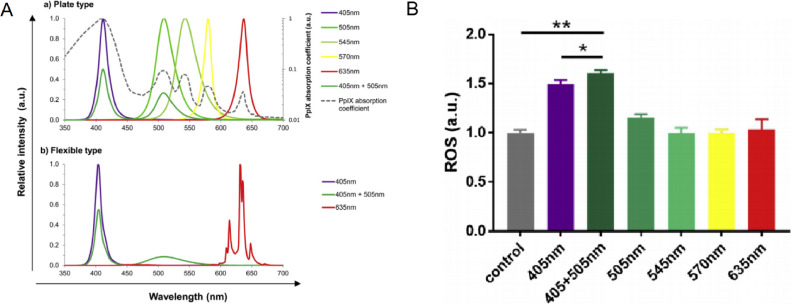
Glioma is a malignant brain tumor with extremely low 5-year survival rate and high recurrence [47]. Due to its sensitive location, glioma is often very difficult to treat [48]. Recently, 5-aminolevulinic acid (5-ALA), a precursor of porphyrin, is reported to delay glioma progression and is expected to have great potential in PDT [49]. It is shown that 5-ALA can be transformed into protoporphyrin IX (PpIX) in vivo, which is a highly effective photosensitizer like the heme and can produce free radicals and ROS with 635 nm laser light excitation. One problem of 5-ALA is that untransformed 5-ALA could induce edema in the tissue, especially under high doses of light about 635 nm (Fig. 14A). Because PpIX has 5 absorption wavelength peaks (410, 510, 545, 580 and 630 nm), hence using weaker multiple wavelength lights can improve the efficiency of 5-ALA based PDT and reduce toxicity. Based on this principle, Morita group developed a flexible light-emitting diode unit to improve 5-ALA PDT effects by emits light of multiple wavelengths. In this article, under 405 nm + 505 nm light source, 5-ALA as photodynamic drug produced the most ROS (Fig. 14B). The results showed that more ROS were produced at dual wavelengths so that the effect of PDT is better under the synergistic action [50].
Fig. 14.
(A) Elative spectral distribution of the plate-type LEDs (a) and flexible-type LEDs (b). (B) ROS produce by PpIX under different light sources. Reproduced with permission from [50]. Copyrights 2019 Japanese Society for Investigative Dermatology, Published by Elsevier B.V.
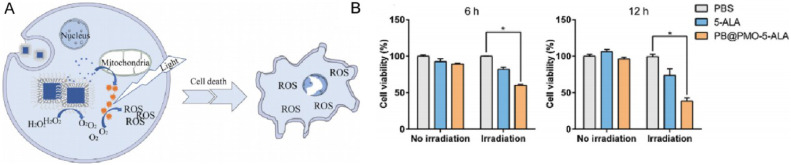
Tough 5-ALA has emerged as a promising modality for localized glioma ablation with limited damage to healthy brain tissues in experimental models, its inability to penetrate blood brain barrier (BBB) to reach glioma sites and hypoxic tumor microenvironment in glioma (low oxygen content in tumor site is an important factor restricting PDT [51]) hamper practical applications of 5-ALA based PDT. To solve these problems, Lu team designed a nano delivery system where 5-ALA is loaded on periodic mesoporous organosilica (PMO) with Prussian blue (PB) coating (PB@PMO-5-ALA) [52]. These NPs are ∼81 nm in diameter and displayed good biocompatibility. It increased PpIX level in glioma cells by 75% comparing with unprotected 5-ALA in mice, and effectively decompose hydrogen peroxide to oxygen in a temperature responsive manner (Fig. 15). The results from this study encourages further investigation of 5-ALA based glioma therapies.
Fig. 15.
(A) Decompose H2O2 to O2 in a temperature responsive manner, and increase the O2 in tumor site, then the tumors were killed by PDT. (B) Evaluation of in vitro effects for PB@PMO-5-ALA in PDT. Reproduced with permission from [52]. Copyrights 2020 Elsevier Inc.
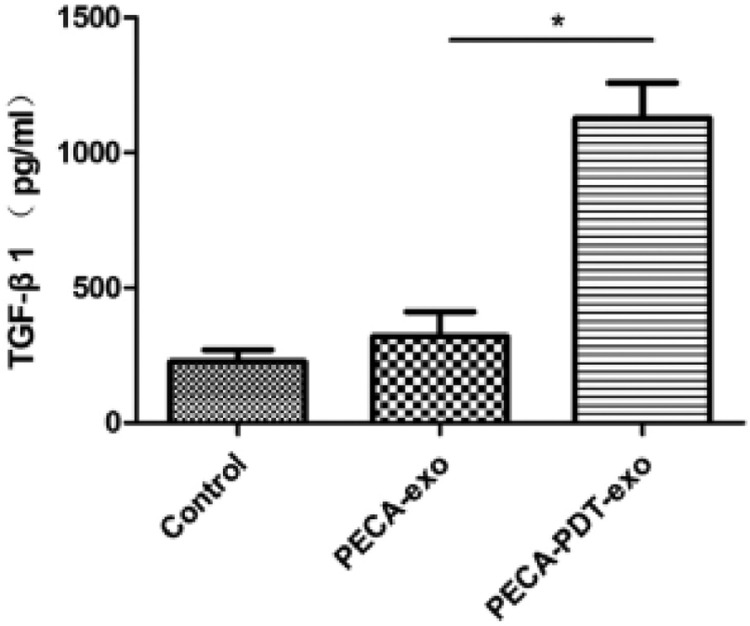
5-ALA may also be used in the treatment of certain skin tumors. Squamous cell carcinoma (SCCs) is a type of skin tumor with high incidence and a study from Wang group reported a PDT system using 5-ALA could inhibit this type of tumor efficiently. In this work, exosomes derived from ALA-PDT-treated SCCs were incubated with SCCs, fibroblasts and immature dendritic cells (DCs), separately discovered that exosomes derived from PDT-treated SCCs have ability to induce DCs maturation and IL-12 secretion, and promote the secretion of TGF-β1 from fibroblast, which improve anti-tumor immunity of the model animals (Fig. 16) [53]. This study revealed a novel PDT-enhanced immune reaction against cancer cells, and provides a promising PDT strategy for treating SCC.
Fig. 16.
TGF-β1 analyzed by ELISA after PDT in this study. Reproduced with permission from [53]. Copyrights 2020 Published by Elsevier B.V.
4.1. Delivery systems for porphyrin-based PDT
However, there is a practical problem for many porphyrin derivatives, as they generally have high protein affinity. Together with their high biocompatibility (due to their biological origin), they could spread in the whole body and sometimes stay in the body for too long by attaching to albumin and simultaneously avoiding macrophages clearance. This is an undesirable situation because the long-term accumulation of porphyrin derivatives in non-tumor tissues could lead to allergic symptoms under natural light. Therefore, drug delivery systems that could improve the biodistribution of porphyrin photosensitizers and increase their accumulation at tumor sites are important for clinical application.
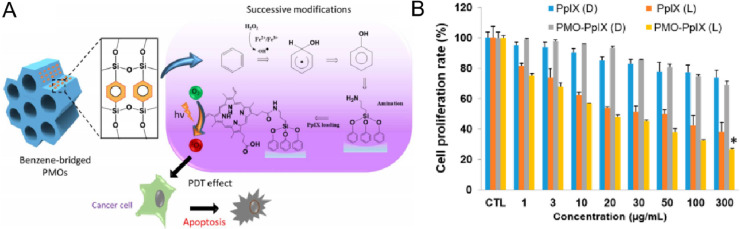
One way to generate such delivery system is developing highly functional materials. Lee group successfully developed an innovative PMO structure to encapsulate the PpIX for this purpose (Fig. 17A). PMO is a class of materials based on organic as well as inorganic hybrid nanocomposites, which has a considerable number of mesoporous and good biocompatibility. This structure was organo-bridged silsesquioxane ((R'O)3Si-R-Si(R'O)3)-integrated mesoporous silica frameworks, which have the provision of active sites for subsequent surface functionalizations [54]. In addition, PMO materials often have various available reactive groups that can be modified with other functional molecules to further improve their performance such as drug loading capacity and biocompatibility (like PMO—OH(II)-NH2 [55]). In this study, the PMO load PpIX by amide bond, and this structure (PMO-PpIX) showed better tumor inhibition in vitro than free PpIX (Fig. 17B).
Fig. 17.
(A) Schematic of the structure for PMO skeleton, the reaction pathway of benzene hydroxylation and protoporphyrin IX (PpIX) loading, and the mechanism of photoinduced biological effects. (B) Evaluation of in vitro effects for PMO-PpIX in MTT. Reproduced with permission from [55]. Copyrights 2020 by the authors. Licensee MDPI, Basel, Switzerland.
Good biocompatibility is necessary for successfully clinical application of PDT [56], and nano delivery system for PDT needs to meet this requirement as well. However, the majority types of nanomaterials are not suitable for direct in vivo applications due to their poor biocompatibility which would cause more damages than benefits during treatment. One of the most widely used method to improve biocompatibility of nanomaterials is to graft PEG onto their surface, as PEG is a well-recognized highly biocompatible molecule. In addition, in order to achieve efficient targeted tumor aggregation of photosensitizer by using NCs, they need to offer various pharmacological functions such as improving blood circulation and penetrating condense extracellular matrix in tumor microenvironment. One such example is reported by Chen group, where a circular aptamer-PEG system that could be activated by tumor microenvironment was generated. Here, photosensitizer pyrochlorophyll A (PA) was first linked to aptamer, and the PA-aptamer molecules ware then combined with PEG molecules to form circular PA-aptamer-PEG. The nanostructure of PA-aptamer-PEG is stable in normal physiological environment because the Schiff base bonds are stable at pH 7.4. In the acidic tumor microenvironment (pH≈6.5), these bonds break and smaller PA-aptamer fragments are released which are more likely to enter cells (Fig. 18) [57]. The PA-aptamer fragments in tumor cells works more efficiently under light stimulation, and aptamer could precisely label location of tumor cells for tumor imaging. Therefore, this system successfully functions as agent for both in vivo imaging and PDT.
Fig. 18.
Schematic for preparation of PEG-CHO-Apt-PA, and the acid response principle in TME (pH=6.5). Reproduced with permission from [57]. Copyrights 2020 Published by Elsevier Ltd.
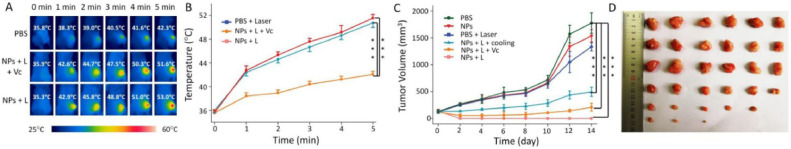
Another category of new materials with PDT potential is two-dimensional materials. Among these materials, covalent organic frameworks (COFs) have attracted much attention in recent years [58]. COFs have a monolayer carbon structure similar to graphene [59], which can also contain other elements such as nitrogen and oxygen. Interestingly, porphyrins can also be integrated into COFs by Schiff base reaction or other reactions. Chen group have reported a COF-based nano PDT system using porphyrin as photosensitizer, which could treat cancer by combined photoacoustic imaging-guided photodynamic and photothermal mechanisms [60]. In this study, the photosensitizer is called COF-366 NPs, which provide the combined therapy of PTT and PDT under a single wavelength light illumination with the monitoring of photoacoustic imaging, and makes the operation simpler and more convenient. The COF-366 was Synthesized by tetra (p-amino-phenyl) porphyrin (TAPP) and terephthaldehyde using published procedure [61]. According to the authors, this COF-366 NPs as a photosensitizer could achieved good phototherapy effect even in the face of large tumors. As COF-366 can simultaneously produce both heat and ROS, it achieves highly efficient tumor suppression in vivo (4T1 cells were planted in mice), reaching up to 70.4% tumor volume reduction in 6 h and the temperature can reached as high as 51 ℃ under the 635 nm laser irradiation for 5 min (Fig. 19). This work provides an illuminating example for applying COF material and a reference for other COF-based PDT design in the future.
Fig. 19.
Tumor inhibition in vivo. (A, B) Temperature change of tumor site after receiving light. (C, D) Tumor volume and tumor morphology after treatment in different group. Reproduced with permission from [60]. Copyrights 2019 Elsevier Ltd.
Similar to COFs, metal organic frames (MOFs) are also popular novel materials for drug delivery and other medical researches [62]. There are two main advantages for using MOFs: first, like COFs, MOFs themselves could be designed to possess pharmaceutical functions and can be further modified with various functional groups; second, MOFs can hold metal ions which are powerful tools in diseases diagnosis and treatment due to their unique physiochemical properties [63]. Because metal-based reagents are generally lack targeting ability, the target delivery potential of MOFs are critical in enhancing their performance. Zhang group has reported a MOF design which contains silver ions and could be activated by near infrared light (NIR) for tumor or infection treatments [64]. This structure was constructed by decorating silver nanoparticles (AgNPs) onto the porphyritic porous coordination network (PCN) and further camouflaging with the neutrophil membrane (NM) with inflammatory targeting ability (PAM). Using porphyrin as photosensitizer, NIR stimulation could produce singlet oxygen to partially degrade the MOF NPs and release cytotoxic Ag+ for metal ions therapy (MIT), which works together with the generated heat and ROS to kill target cells or pathogens. Results showed that PDT and MIT are mutually reinforcing in this system, as the incorporated AgNPs could also enhance the efficiency of PCN to produce 1O2 due to the localized electric field effect (Fig. 20).
Fig. 20.
Semiquantitative data of ROS production in vivo for different materials after intravenous illumination. Reproduced with permission from [64]. Copyrights 2020 Elsevier Ltd.
4.2. Combining PDT with other type of therapies
Besides, PDT can also be used with other therapies to improve the overall treatment efficiency and potentially reduce adverse effects. The basic principle of PDT is energy conversion from light irradiation, which may be employed for other chemical reactions. One of the applications is light triggered drug release system where release timing and location of chemotherapy drugs or other drugs could be precisely controlled. Jiang group reported a NC for effective synergistic bio-reductive chemotherapy using stepwise-activatable hypoxia triggered by light via photosensitizer [65]. This system include TAT (targeting peptide), chlorine e6 (photosensitizer), TPZ (hypoxia activatable prodrug tirapazamine) and PEG-Azo-PLGA (amphiphilic polymers linked by azobenzene) (Fig. 21A). Chlorine e6 (ce6) was used as the photosensitizer, which could induce hypoxia in tumor tissue under light stimulation by the releasing of TPZ. The Azo-benzene bonds in this system are stable under normal oxygen level and easy to break under hypoxic conditions. This would lead to degradation of the nanoplatform and release of loaded drugs. Hence, drug could be controlled and released at tissues experiencing light stimulation. In this way, tumor cells are under attack from both chemotherapy drug and PDT effects (e.g. hypoxia and heat), and damage to other normal tissues by chemotherapy could be reduced (Fig. 21B-21E and 21 G). In addition to this type of application, this drug-releasing NCs can also be used in combination with other response measures (such as pH-responses) to achieve higher targeted delivery efficiency. The PDT-based drug-controlled release system thus appears to be a promising research direction.
Fig. 21.
(A) the structure of the nano-drug delivery system. (B,C,D,E,G) In vivo efficacy of TAT+AzoNPs@ (Ce6+TPZ). (I) control group, (II) the system without light, (III) Free (Ce6+TPZ) with light, (IV) TAT+AzoNPs@ TPZ with light, (V) TAT+AzoNPs@ Ce6 with light, (VI) NNPs@(Ce6+TPZ) with light, (VII) AzoNPs@(Ce6+TPZ) with light, and (VIII) the system with light. Reproduced with permission from [65]. Copyrights 2020 Elsevier Ltd.
5. PDT for non-cancer diseases and conditions
Most of reports reviewed above were aimed to treat cancer, but application of PDT is not limited to cancer treatment. Because light stimulation is highly precise and easy to apply to shallow tissues, PDT could be used to treat a wide range of skin problems. As ROS generated by photosensitizer is often very effective against microbials [66], PDT is often used for bacterial and fungi related conditions [67]. In fact, currently, far more PDT treatments are conducted for non-cancer conditions (e.g. skin fungus infection) than cancer, partially attributed to the fact that many PDT treatment of skin problems does not need injection of PDT agent into tissues or blood vessels. Due to the high occurrence of skin-related conditions and other surface disinfection applications, it is important, and probably easier for clinical translation, to develop new PDT systems for non-cancer conditions. Therefore, it is worth mentioning some examples of PDT designed for these applications.
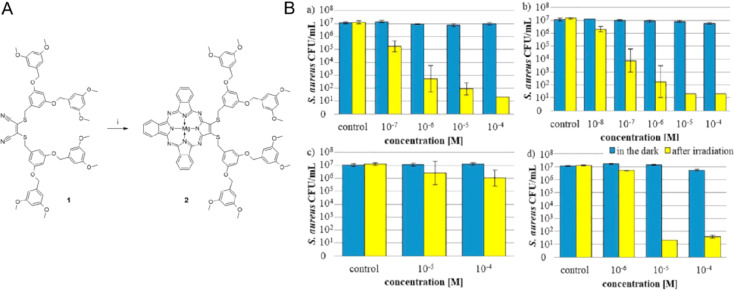
The team of Tomasz Goslinski reported a PDT system using tribenzoporphyrazines (a type of porphyrazine) with dendrimeric peripheral substituents as the photosensitizers for treating Staphylococcus aureus (S. aureus) infections (Fig. 22A). This photosensitizer displayed high killing efficiency against S. aureus (and potentially other Gram-positive bacteria), with log reductions of S. aureus number at 4–5 with very low concentration (∼10−6 M) and 660 nm light exposure for 20 min (Fig. 22B). Besides, it also showed improved bacteria cell wall permeability, hence this simple PDT system warrants further investigation.
Fig. 22.
(A) Synthesis route of new tribenzo porphyrin, the synthesis steps are shown in the original text. (B) The number of viable S.aureus after PDT using TBP. Reproduced with permission from [67]. Copyrights 2020 Elsevier B.V.
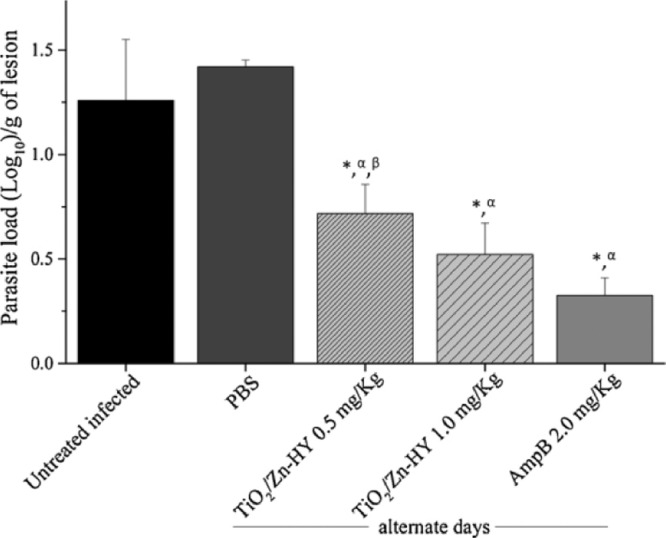
A study of Graminha group has designed an inorganic photosensitizer complex which uses TiO2 NPs doped with Zn and hypericin (TiO2/Zn-HY) to treat cutaneous leishmaniasis caused by parasite Leishmania amazonensis (L. amazonensis). TiO2/Zn-HY showed good anti leishmanial activity and low cytotoxicity against murine macrophage in visible light in vitro [68]. Under 22 mW/cm2 intensity and 52.8 J/cm2 fluency delivered blue and red light, the parasite level of L. amazonensis in infected BALB/c mice have decreased to 43%−58% of original level (Fig. 23). This study shows the potential of PDT in parasite treatment and provides a good inorganic photosensitizer candidate for further research.
Fig. 23.
Parasite load of mice infected with Amazon L. amazonensis treated with TiO2/Zn in PDT. Reproduced with permission from [68]. Copyrights 2020 Elsevier B.V.
Human papillomavirus (HPV) infection is a common cause of skin problems, including vulvar intraepithelial neoplasia. Curcumin is a nature bioactive compound with antineoplastic properties [69]. Rahal et al. has devised a nano emulsion of curcumin (NE-CUR) which enhances performance of curcumin in PDT against vulvar intraepithelial neoplasia induced by HPV. In this study, they have observed that about 90% of death cell were under the irradiated in the presence with NE-CUR [70]. This result suggested that this nano emulsion has attained good biocompatibility, and the large amounts of cell death occur only when exposed to light. As the authors state, this drug delivery system in PDT may become promising in treatment of HPV infection.
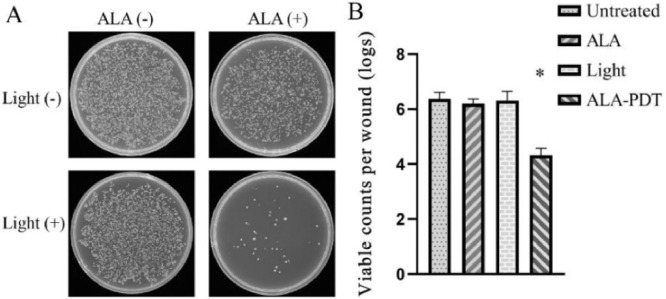
The porphyrin precursor 5-ALA has also been reported for treating conditions caused by drug-resistant bacteria infection such as methicillin-resistant S. aureus (MRSA). MRSA can cause serious infections in diabetic ulceration and common antibiotics are ineffective in treating such infections [71]. Wang et al. reported an effective 5-ALA based PDT system to treat MRSA infected wounds of diabetic mice. In the study, 5-ALA was made into a 10% solution and placed into the wounds which were infected with MRSA, and use 635 nm light was used as the irradiation source. Since PDT kills bacteria with a mechanism that is different from antibiotics, comparing with antibiotics methicillin, 5-ALA could effectively inhibit the growth of MRSA and treat related condition caused by MRSA infection (Fig. 24). The results indicate that ALA-PDT may become a potential treatment option for MRSA-infected diabetic wounds.
Fig. 24.
Analysis of viable bacteria count after PDT treatment. Reproduced with permission from [71]. Copyrights 2020 Elsevier B.V.
For the same reasons, PDT also has the potential to treat other infections by drug-resistant bacteria. A clinical report of Florence Institute of Superior Teaching describes a case of palatal ulcer that is not sensitive to antibiotics but responds well to PDT and photobioregulation therapy (PBMT) [72]. The results show that the combination of PDT and PBMT were able to enhance and accelerate the healing process and reduce the number of bacteria. This finding provides further evidence supporting the application of PDT for treating drug-resistant infections that are difficult to be managed by other traditional treatments.
In addition to bacterial infections, PDT can also be used to treat certain autoimmune diseases that are otherwise difficult to treat. Alopecia areata (AA) is a complex immune-mediated disorder, which has a high relapse rate with traditional treatment options. PDT combined with traditional therapy, like injection of drugs with microneedles (MN), may achieve better therapeutic effect. In a clinical trial involving 41 AA patients, 41 AA patients were divided into 3 groups, and groups of patients were treated with different treatments (only by MN, only by PDT, and PDT+MN). Most of the patients treated with this PDT approach showed reduced severity of their symptoms. Experiments showed that using MN administrated PDT with 5-ALA photosensitizer resulted in high competence against AA by effectively suppressing related immune reaction, which is likely due to precise local administration of therapeutic agents through skin by MN [73]. This study suggests that the combination of ALA-PDT with MN could be an alternative therapeutic option in the treatment of AA.
6. Discussion and conclusion
Due to its unique activation mechanism, PDT is highly effective at local stimulated areas, and could be non-active or only having limited activity in unstimulated regions. However, PDT agents normally have poor targeting capability (especially for penetrating deeper in tumors) and may cause damage if staying in certain tissues for too long. There are different ways to solve this problem. Apparently, developing new photosensitizers with significantly higher efficiency and better biocompatibility is the most direct solution. Unfortunately, completely new design which meets these demands is difficult to create. We have reviewed some recently reported novel photosensitizers, both inorganic and organic. These agents may not be ideal, but all have their advantages, hence could still provide useful reference for future photosensitizer design.
There are also other less direct ways to improve PDT effectiveness. One such way is supplementing targeting ability to PDT agents by using drug delivery system, which could enhance local PDT agent accumulation and reduce damage to normal tissues. On the other hand, plenty of combined therapies using PDT together with the likes of radiotherapy and chemotherapy are developed in order to improve the overall therapeutic effect. Yet another strategy is to use PDT agents as a switch for drug delivery systems, where PDT agents play dual roles. This is usually achieved by using active by-product of PDT (e.g. ROS and singlet oxygen) to induce designed chemical reactions, such as the breaking of certain bonds. All these different strategies are covered in this review. They offered examples for achieving different purposes and probably emphasizing on different aspects, with some provided higher overall anti-tumor effects, some displayed better biodistribution and pharmacodynamics, some designed simpler preparation processes, etc. It appears that, for inorganic photosensitizers, many researches focused on granting more function to the photosensitizers themselves; and for organic photosensitizers, main interest is to increase their local concentration, often relying on nano delivery vehicles. Combined therapies were explored for both types of photosensitizers, and provided some promising results.
In summary, PDT as an unique light-targeted therapy has high research and practical value, both owning to its therapeutic advantages and its mechanism. This review provides an brief introduction to PDT, focusing on photosensitizers which are the core of a PDT system. We covered most of the areas of current photodynamic research and clinical application. From the included research articles in this review, it is clear that PDT has the capability to be used in wider applications and there are still plenty of room for improved performance and lower toxicity for PDT. Many possibilities are still unexplored in this field and the directions investigated by the included studies are valuable clues for future research which could produce fruitful results. With the development and application of various effective and low biotoxicity photosensitizers and related delivery systems, the PDT could become an indispensable tool for the improvement of human health.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors acknowledge the financial support received from Sichuan Science and Technology Program (2019JDJQ0028). The authors are grateful for the funding.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2020.12.003.
Appendix. Supplementary materials
References
- 1.Lan M.H., Zhao S.J., Liu W.M., Lee C.S., Zhang W.J., Wang P.F. Photosensitizers for photodynamic therapy. Adv Healthc Mater. 2019;8 doi: 10.1002/adhm.201900132. [DOI] [PubMed] [Google Scholar]
- 2.McFarland S.A., Mandel A., Dumoulin-White R., Gasser G. Metal-based photosensitizers for photodynamic therapy: the future of multimodal oncology? Curr Opin Chem Biol. 2020;56:23–27. doi: 10.1016/j.cbpa.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JM, Fan TJ, Xie ZJ, Zeng QQ, Xue P, Zheng TT, et al. Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomater 2020;237:119827. [DOI] [PubMed]
- 4.Wu W.B., Mao D., Hu F., Xu S.D., Chen C., Zhang C.J., et al. A highly efficient and photostable photosensitizer with near - infrared aggregation - induced emission for image-guided photodynamic anticancer therapy. Adv Mater. 2017;29 doi: 10.1002/adma.201700548. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H., Zheng R.R., Fan G.L., Fan J.F., Zhao L.P., Jiang X.Y., et al. Mitochondria and plasma membrane dual-targeted chimeric peptide for single-agent synergistic photodynamic therapy. Biomater. 2019;188:1–11. doi: 10.1016/j.biomaterials.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Koszegi T., Sali N., Raknic M., Horvath-Szalai Z., Csepregi R., et al. A novel luminol-based enhanced chemiluminescence antioxidant capacity microplate assay for use in different biological matrices. J Pharmacol Toxicol Methods. 2017;88:153–159. doi: 10.1016/j.vascn.2017.09.256. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C., Chen W.X., Zhang T.X., Jiang X.Q., Hu Y. Hybrid nanoparticle composites applied to photodynamic therapy: strategies and applications. J Mater Chem A Mater. 2020;8:4726–4737. doi: 10.1039/d0tb00093k. [DOI] [PubMed] [Google Scholar]
- 8.Fonsecaa G.A.M.D., Dourado D.C., Barreto M.P., Cavalcanti M.F.X.B., Pavelski M.D., et al. Antimicrobial photodynamic therapy (aPDT) for decontamination of highspeed handpieces: a comparative study. Photodiagnosis Photodyn Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101686. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Ramírez D.R., Domínguez-Ríos R., Juárezb J., Valdés M., Hassan N., Quintero-Ramos A., et al. Biodegradable photoresponsive nanoparticles for chemo-, photothermaland photodynamic therapy of ovarian cancer. Mater Sci Eng C Mater Biol Appl. 2020;116 doi: 10.1016/j.msec.2020.111196. [DOI] [PubMed] [Google Scholar]
- 10.Lam Q., Kato M., Cheruzel L. Ru(II)-diimine functionalized metalloproteins: from electron transfer studies to light-driven biocatalysis. Biochim Biophys Acta Bioenerg. 2016;1857:589–597. doi: 10.1016/j.bbabio.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M., Yang X., Chang L., Chen J.Y., Luo J.F., Wu Y., et al. Low-level PDT treatment modulated photoaging mediated by UVA irradiation through regulating Bach2. Photodiagnosis Photodyn Ther. 2020;29 doi: 10.1016/j.pdpdt.2019.101606. [DOI] [PubMed] [Google Scholar]
- 12.Ayaza F., Ocakoglu K. Differential effects of aminochlorin derivatives on the phagocytic and inflammatory potentials of mammalian macrophages. Eur J Pharmacol. 2020;873 doi: 10.1016/j.ejphar.2020.172980. [DOI] [PubMed] [Google Scholar]
- 13.Anine C., Sathish S., Dhilip K., Heidi A. Effect of dose responses of hydrophilic aluminium (III) phthalocyanine chloride tetrasulphonate based photosensitizer on lung cancer cells. J Photochem Photobiol B. 2019;194:96–106. doi: 10.1016/j.jphotobiol.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Lane A.N., Higashi R.M., Fan T.W.M. Metabolic reprogramming in tumors: contributions of the tumor microenvironment. Genes Dis. 2020;7:185–198. doi: 10.1016/j.gendis.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eun H.J., Dae G.C., Min S.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm Sin B. 2016;6:297–307. doi: 10.1016/j.apsb.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kedzierska E., Knap-Czop K., et al. Photodynamic therapy- mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Zang F., Qin S., Ma C.B., Li W.J., Li J. Structure, function and applications of phycoerythrin: a unique light harvesting protein in algae. Chin Sci Bull Chin. 2020;65:565–576. [Google Scholar]
- 18.Niu F.J., Wang D.G., Li F., Liu Y.M., Shen S.H., Meyer T.J. Hybrid photoelectrochemical water splitting systems: from interface design to system assembly. Adv Energy Mater. 2020;10 [Google Scholar]
- 19.Cheng Q.L., Guo X.W., Hao X.J., Shi Z.S., Zhu S., Cui Z.C. Fabrication of robust antibacterial coatings based on an organicinorganic hybrid system. ACS Appl Mater Interfaces. 2019;11:42607–42615. doi: 10.1021/acsami.9b15031. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y., Kong X.P., Chang.Y Feng YL, Zheng R.X., Wu X.Q., et al. Spatiotemporally synchronous oxygen self-supply and reactive oxygen species production on Z-scheme heterostructures for hypoxic tumor therapy. Adv Mater. 2020;32 doi: 10.1002/adma.201908109. [DOI] [PubMed] [Google Scholar]
- 21.Lin H., Wang Q. Non-fullerene small molecule electron acceptors for high-performance organic solar cells. J Eng Chem. 2018;27:990–1016. [Google Scholar]
- 22.Xu N., Du J.J., Yao Q.C., Ge H.Y., Li H.S., Xu F., et al. Precise photodynamic therapy: penetrating the nuclear envelope with photosensitive carbon dots. Carbon N Y. 2020;159:74–82. [Google Scholar]
- 23.Alberto ME, Simone BCD, Liuzzi S, Marino T, Russo N, Toscano M. Iodine substituted phosphorus corrole complexes as possible photosensitizers in photodynamic therapy: insights from theory.Comput Chem 2020;41:1395–401. [DOI] [PubMed]
- 24.Karges J., Yempala T., Tharaud M., Gibson P.D., Gasser G. A multi-action and multi-target RuII-PtIV conjugate combining cancer-activated chemotherapy and photodynamic therapy to overcome drug resistant cancers. Angew Chem Int Ed. 2020;59:7069–7075. doi: 10.1002/anie.201916400. [DOI] [PubMed] [Google Scholar]
- 25.Grin M.A., Tikhonov S.I., Petrova A.S., Pogorilyy V.A., Noev A.N., Tatarskiy V.V., et al. New derivatives of bacteriopurpurin with thiolated Au (I) complexes: dual darkand light activated antitumor potency. Anticancer Agents Med Chem. 2020;20:49–58. doi: 10.2174/1871520619666190801102617. [DOI] [PubMed] [Google Scholar]
- 26.Wang M., chang M.Y., Chen Q., Wang D.M., Li C.X., Hou Z.Y., et al. Au2Pt-PEG-Ce6 nanoformulation with dual nanozyme activities for synergistic chemodynamic therapy/phototherapy. Biomater. 2020;252 doi: 10.1016/j.biomaterials.2020.120093. [DOI] [PubMed] [Google Scholar]
- 27.Aghajanzadeh M., Naderi E., Zamani M., Sharafi A., Naseri M., Danafar H. In vivo and in vitro biocompatibility study of MnFe2O4 and Cr2Fe6O12 as photosensitizer for photodynamic therapy and drug delivery of anti-cancer drugs. Drug Dev Ind Pharm. 2020;46:846–851. doi: 10.1080/03639045.2020.1757698. [DOI] [PubMed] [Google Scholar]
- 28.Li C., Wang J.C., Wang Y.G., Gao H.L., Wei G., Huang Y.Z., et al. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Q., Chen Y., Hao L.Y., Zhou R.H., Li Y.J., Li Q.R., et al. Pegylated carbon nitride nanosheets for enhanced reactive oxygen species generation and photodynamic therapy under hypoxic conditions. Nanomedicine. 2020;25 doi: 10.1016/j.nano.2020.102167. [DOI] [PubMed] [Google Scholar]
- 30.Sun Q.Q., Wang Z., Liu B., Jia T., Wang C., Yang D., et al. Self-generation of oxygen and simultaneously enhancing photodynamic therapy and MRI effect: an intelligent nanoplatform to conquer tumor hypoxia for enhanced phototherapy. Chem Eng J. 2020;390 [Google Scholar]
- 31.Deng Y., Pan S.J., Zheng J.J., Hong Y.L., Liu J., Chang H.Z., et al. Electrostatic self-assembled Iridium (III) nano-photosensitizer for selectively disintegrated and mitochondria targeted photodynamic therapy. Dyes Pigm. 2020;175 [Google Scholar]
- 32.Xu T., Zhao S.J., Lin C.W., Zheng X.L., Lan M.H. Recent advances in nanomaterials for sonodynamic therapy. Nano Res. 2020;13:2898–2908. [Google Scholar]
- 33.Wu Y.Z., Yang D.X., Xu W., Song R.J., Li M.M., Wang Y., et al. Tunable water-soluble carbon nitride by alkali-metal cations modification: enhanced ROS-evolving and adsorption band for photodynamic therapy. Appl Catal B. 2020;269 [Google Scholar]
- 34.Wang J., Zhang H., Xiao X., Liang D., Liang X.Y., Mi L., et al. Gold nanobipyramid-loaded black phosphorus nanosheets for plasmon-enhanced photodynamic and photothermal therapy of deep-seated orthotopic lung tumors. Acta Biomater. 2020;107:260–271. doi: 10.1016/j.actbio.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Tian J., Huang B.X., Nawaz M.H., Zhang W.A. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord Chem Rev. 2020;420 [Google Scholar]
- 36.Majumdur M., Goswami T., Misra A. Multifunctional magnetic materials of organic origin for biomedical applications: a theoretical study. ChemistrySelect. 2018;3:933–939. [Google Scholar]
- 37.Steinberg M.L., Cosloy S.D. The facts on file dictionary of biotechnology and genetic engineering. Libr J. 1994;119:62. [Google Scholar]
- 38.Alka A., Shetti V.S., Ravikanth M. Coordination chemistry of expanded porphyrins. Coord Chem Rev. 2019;401 [Google Scholar]
- 39.Rajora M.A., Lou J.W.H., Zheng G. Advancing porphyrin's biomedical utility via supramolecular chemistry. Chem Soc Rev. 2017;46:6433–6469. doi: 10.1039/c7cs00525c. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y.T., Xu J.M., Hu X.X., Xia X., Dong Q., Liu Z.K. Zinc-substituted hemoglobin with specific drug binding sites and fatty acid resistance ability for enhanced photodynamic therapy. Nano Res. 2019;12:1880–1887. [Google Scholar]
- 41.Zhang C., Zhao W.J., Sasaki S.I., Tamiaki H., Wang X.F. A chlorophyll derivative-based bio-solar energy conversion and storage device. Electrochim Acta. 2020;347 [Google Scholar]
- 42.Liu Z.Y., Cao T.Y., Xue Y.D., Li M.T., Wu M.S., Engle J.W., et al. Self-amplified photodynamic therapy through the 1O2-mediated internalization of photosensitizers from a Ppa-bearing block copolymer. Angew Chem Int Ed. 2020;59:3711–3717. doi: 10.1002/anie.201914434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erbas-Cakmak S., Akkaya E.U. Cascading of molecular logic gates for advanced functions: a self- reporting, activatable photosensitizer. Angew Chem Int Ed. 2013;52:11364–11368. doi: 10.1002/anie.201306177. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y.H., Li M.Y., Sajjad F.Z., Wang J.H., Meharban F.Z., Gadoora M.A., et al. Synthesis and pharmacological evaluation of chlorin derivatives for photodynamic therapy of cholangiocarcinoma. Eur J Med Chem. 2020;189 doi: 10.1016/j.ejmech.2020.112049. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal A., Samaroo D., Jovanovic I.R., Singh S., Tuz M.P., Mackiewicz M.R. Porphyrinoid-based photosensitizers for diagnostic and therapeutic applications: an update. J Porphyr Phthalocyanines. 2019;23:729–765. [Google Scholar]
- 46.Sun X., Gao Z.Y., Mao K.R., Wu C.X., Chen H.M., Wang J.L., et al. Photodynamic therapy produces enhanced efficacy of antitumor immunotherapy by simultaneously inducing intratumoral release of sorafenib. Biomater. 2020;240 doi: 10.1016/j.biomaterials.2020.119845. [DOI] [PubMed] [Google Scholar]
- 47.Gao H.L. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu K., Whitham E., Kichenadasse G. Potential role of cannabidiol for seizure control in a patient with recurrent glioma. J Clin Neurosci. 2020;71:275–276. doi: 10.1016/j.jocn.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Vermandel M., Quidet M., Vignion-Dewalle A.S., Leroy H.A., Leroux B., Mordon S., et al. Comparison of different treatment schemes in 5-ALA interstitial photodynamic therapy for high-grade glioma in a preclinical model: an MRI study. Photodiagnosis Photodyn Ther. 2019;25:166–176. doi: 10.1016/j.pdpdt.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Masuda H., Kimura M., Nishioka A., Kato H., Morita A. Dual wavelength 5-aminolevulinic acid photodynamic therapy using a novel flexible light-emitting diode unit. J Dermatol Sci. 2019;93:109–115. doi: 10.1016/j.jdermsci.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Wang G., Gao J.Y., Fu Y.K., Ren Z.H., Huang J., Li X., et al. Implantable composite fibres with Self-supplied H2O2 for localized chemodynamic therapy. Chem Eng J. 2020;388 [Google Scholar]
- 52.Wang X.F., Tian Y., Liao X., Tang Y.X., Ni Q.Q., Sun J., et al. Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J Colloid Interface Sci. 2020;565:483–493. doi: 10.1016/j.jcis.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Z.J., Zhang H.Y., Zeng Q.Y., Wang P.R., Zhang G.L., Ji J., et al. Exosomes from 5-aminolevulinic acid photodynamic therapy-treated squamous carcinoma cells promote dendritic cell maturation. Photodiagnosis Photodyn Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101746. [DOI] [PubMed] [Google Scholar]
- 54.Attia M.F., Swasy M.I., Ateia M., Alexis F., Whitehead D.C. Periodic mesoporous organosilica nanomaterials for rapid capture of VOCs. Chem Commun. 2020;56:607–610. doi: 10.1039/c9cc09024j. [DOI] [PubMed] [Google Scholar]
- 55.Lin C.H., Kumar K.R., Busa P., Lee C.H. Hydrophobicity-tuned periodic mesoporous organo-silica nanoparticles for photodynamic therapy. Int J Mol Sci. 2020;21:2586. doi: 10.3390/ijms21072586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu T.T., Mei X., Wang Y.J., Weng X.S., Liang R.Z., Wei M. Two-dimensional nanomaterials: fascinating materials in biomedical field. Sci Bull. 2019;64:1707–1727. doi: 10.1016/j.scib.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y., Zhu W.J., Cheng L., Cai R., Yi X., He J.X., et al. Tumor microenvironment (TME)-activatable circular aptamer-PEG as an effective hierarchical-targeting molecular medicine for photodynamic therapy. Biomater. 2020;246 doi: 10.1016/j.biomaterials.2020.119971. [DOI] [PubMed] [Google Scholar]
- 58.Lv S.W., Liu J.M., Wang Z.H., Ma H., Li C.Y., Zhao N., et al. Recent advances on porous organic frameworks for the adsorptive removal of hazardous materials. J Environ Sci. 2019;80:169–185. doi: 10.1016/j.jes.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Yang Q., Luo M.L., Liu K.W., Cao H.M., Yang H.J. Covalent organic frameworks for photocatalytic applications. Appl Catal B. 2020;276 [Google Scholar]
- 60.Wang D.W., Zhang Z., Lin L., Liu F., Wang Y.B., Guo Z.P., et al. Porphyrin-based covalent organic framework nanoparticles for photoacoustic imaging-guided photodynamic and photothermal combination cancer therapy. Biomater. 2019;223 doi: 10.1016/j.biomaterials.2019.119459. [DOI] [PubMed] [Google Scholar]
- 61.Yuasa M., Oyaizu K., Yamaguchi A. Micellar cobaltporphyrin nanorods in alcohols. J Am Chem Soc. 2004;126:11128–11129. doi: 10.1021/ja0486216. [DOI] [PubMed] [Google Scholar]
- 62.Yang G., Bing Y., Yu C., Long M. A new Dy(III)-based metal-organic framework with polar pores for pH-controlled anticancer drug delivery and inhibiting human osteosarcoma cells. J Coord Chem. 2019;72:262–271. [Google Scholar]
- 63.Cheng L., Yuan C., Shen S., Yi X., Gong H., Yang K., et al. Bottom-up synthesis of metal-ion-doped WS2 nanoflakes for cancer theranostics. ACS Nano. 2015;9:11090–11101. doi: 10.1021/acsnano.5b04606. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Cheng Q., Li C.H., Zeng X., Zhang X.Z. Near infrared light-triggered metal ion and photodynamic therapy based on AgNPs/porphyrinic MOFs for tumors and pathogens elimination. Biomater. 2020;248 doi: 10.1016/j.biomaterials.2020.120029. [DOI] [PubMed] [Google Scholar]
- 65.Ihsanullah K.M., Kumar B.N., Zhao Y.Y., Muhammad H., Liu Y., Wang L., et al. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomater. 2020;245 doi: 10.1016/j.biomaterials.2020.119982. [DOI] [PubMed] [Google Scholar]
- 66.Wang T., Hu J.H., Luo H., Li H.Y., Zhou J.H., Zhou L., et al. Photosensitizer and autophagy promoter coloaded ROS-responsive dendrimer-assembled carrier for synergistic enhancement of tumor growth suppression. Small. 2018;14 doi: 10.1002/smll.201802337. [DOI] [PubMed] [Google Scholar]
- 67.Mlynarczyk D.T., Dlugaszewska J., Falkowski M., Popenda L., Kryjewski M., Szczolko W., et al. Tribenzoporphyrazines with dendrimeric peripheral substituents and their promising photocytotoxic activity against staphylococcus aureus. J Photochem Photobiol B. 2020;204 doi: 10.1016/j.jphotobiol.2020.111803. [DOI] [PubMed] [Google Scholar]
- 68.Sepúlveda A.A.L., Velásquez A.M.A., Linares I.A.P., Almeida L.D., Fontana C.R., Garcia C., et al. Efficacy of photodynamic therapy using TiO2 nanoparticles doped with Zn and hypericin in the treatment of cutaneous Leishmaniasis caused by Leishmania amazonensis. Photodiagnosis Photodyn Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101676. [DOI] [PubMed] [Google Scholar]
- 69.Abolfazl S., Yunes P., Thomas J., Amirhossein S. Biological properties of metal complexes of curcumin. Biofactors. 2019;45:304–317. doi: 10.1002/biof.1504. [DOI] [PubMed] [Google Scholar]
- 70.Bonfim C.M.D., Monteleoni L.F., Calmon M.D.F., Candido N.M., Provazzi P.J.S., Lino V.D.S., et al. Antiviral activity of curcumin-nanoemulsion associated with photodynamic therapy in vulvar cell lines transducing different variants of HPV-16. Artif Cells Nanomed Biotechnol. 2020;48:515–524. doi: 10.1080/21691401.2020.1725023. [DOI] [PubMed] [Google Scholar]
- 71.Huang J.H., Guo M.Q., Wu M.F., Shen S.Z., Shi L., Cao Z., et al. Effectiveness of a single treatment of photodynamic therapy using topical administration of 5-aminolevulinic acid on methicillin-resistant staphylococcus aureus-infected wounds of diabetic mice. Photodiagnosis Photodyn Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101748. [DOI] [PubMed] [Google Scholar]
- 72.Rafael M., Costa L.L.L., Pinto M.J.E., Goncalves M.L.M., Calil B.S., Benini P.M.A. The combination of antimicrobial photodynamic therapy and photobiomodulation therapy for the treatment of palatal ulcers: a case report. J Lasers Med Sci. 2020;11:228–233. doi: 10.34172/jlms.2020.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giorgio C.M., Babino G., Caccavale S., Russo T., Rosa A.B.S., Alfano R., et al. Combination of photodynamic therapy with 5-aminolaevulinic acid and microneedling in the treatment of Alopecia Areata resistant to conventional therapies: our experience with 41 patients. Clin Exp Dermatol. 2020;45:323–326. doi: 10.1111/ced.14084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.