Abstract
Because the Neisseria meningitidis serogroup B (NMSB) capsule is poorly immunogenic in humans, immunization strategies have focused on noncapsular antigens. Both PorA and to a lesser extent PorB are noncapsular protein antigens capable of inducing protective bactericidal antibodies, and vaccines based on the outer membrane protein (OMP) components of serogroup B meningococci have been shown to be effective in clinical trials. Multiple PorA antigens seem to be needed to prevent endemic meningococcal disease around the world, and a hexavalent PorA-based meningococcal vaccine has recently been developed in The Netherlands. To evaluate the distribution of NMSB PorA and PorB antigens in the United States, serosubtyping and serotyping were done on 444 NMSB strains isolated in the active surveillance areas of the United States (total population, 32 million) during the period 1992 to 1998. A total of 244 strains were isolated from sporadic cases of meningococcal disease, and 200 strains were isolated from an epidemic in Oregon. A panel of 16 mouse monoclonal antibodies reactive with PorA and 15 monoclonal antibodies reactive with PorB were used. Among the NMSB isolates obtained from sporadic cases, the most prevalent serosubtypes were P1.7,16 (14.3%), P1.19,15 (9.8%), P1.7,1 (8.6%), P1.5,2 (7.8%), P1.22a, 14 (7.8%), and P1.14 (5.3%) and the most prevalent serotypes were 4,7 (27.5%), 15 (16%), 14 (8.6%), 10 (6.1%), 1 (4.9%), and 2a (3.7%). A multivalent PorA-based OMP vaccine aimed at the six most prevalent serosubtypes could have targeted about half of the sporadic cases of NMSB disease that occurred between 1992 and 1998 in the surveillance areas. Twenty serosubtypes would have had to be included in a multivalent vaccine to achieve 80% coverage of strains causing sporadic disease. The relatively large number of isolates that did not react with murine monoclonal antibodies indicates that DNA sequence-based variable region typing of NMSB will be necessary to provide precise information on the distribution and diversity of PorA antigens and correlation with nonserosubtypeable isolates. The high degree of variability observed in the PorA and PorB proteins of NMSB in the United States suggests that vaccine strategies not based on OMPs should be further investigated.
Neisseria meningitidis is a common cause of meningitis and sepsis in children and adults. Effective vaccines for most of the major disease-causing serogroups (C, Y, W135, and A) have been developed using their antigenically unique capsular polysaccharides, leaving Neisseria meningitidis serogroup B (NMSB) as the only major disease-associated serogroup for which there is no licensed vaccine in the United States. NMSB causes about one-third of all invasive disease in the United States, has epidemic potential, and compared to other serogroups causes a disproportionately large number of cases among infants.
Unlike other major meningococcal serogroups, serogroup B capsular polysaccharide is poorly immunogenic in humans, and consequently most research has focused on the use of noncapsular antigens as vaccine candidates. The PorA and to a lesser extent PorB outer membrane proteins (OMPs) have been shown to be major immunogens. They have also been used to serologically classify N. meningitidis into serosubtypes and serotypes, respectively. PorA (class 1 OMP) is expressed by most meningococcal isolates and is encoded by the porA gene. PorB is expressed by all meningococcal isolates, and class 2 and 3 OMPs are encoded by alleles of the single-copy porB gene (2, 31). PorA and PorB function as porins with eight surface-exposed loop regions, designated loops I to VIII (34). The murine serotype-specific monoclonal antibodies (MAbs) recognize the epitopes located in the variable regions of loops I, V, VI, and VII of PorB, designated VR1 through VR4. The serosubtype-specific MAbs recognize the epitopes located in loops I (VR1) and IV (VR2) of PorA (2, 10, 14, 16, 27, 28, 32).
OMP vaccines based on unique epidemic serogroup B strains have been shown to be efficacious among older children and adults (3, 4, 8, 30). Subsequent immunogenicity studies of vaccines have suggested that protection may be strain specific and that bactericidal activity is principally directed toward epitopes on the PorA antigens (18, 19, 22, 25, 33, 38). Following the recognition that more than a single PorA protein would be needed to prevent and control endemic meningococcal disease, researchers in The Netherlands developed a hexavalent PorA-based meningococcal vaccine (21, 35). This vaccine contains six different PorA OMPs (serosubtypes P1.7,16; P1.5,2; P1.19,15; P1.7h,4; P1.5c,10; and P1.12,13). Available data suggest that, if clinically effective, this combination of PorA would provide substantial coverage against NMSB strains currently in The Netherlands and some other European countries (5, 21, 36). Since invasive N. meningitidis strains are not routinely serosubtyped or serotyped in the United States, we used a set of NMSB strains collected through the Active Bacterial Core surveillance project, a part of the activities of the Centers for Disease Control and Prevention (CDC) Emerging Infections Program, to determine the distribution of serosubtypes and serotypes in the United States. This study will aid in deciding if an OMP vaccine is feasible, defining how many and which PorA OMPs would need to be included in a multivalent OMP vaccine for control of endemic NMSB disease in the United States.
MATERIALS AND METHODS
Active surveillance.
All isolates analyzed in this study were collected through active, laboratory-based surveillance for invasive disease caused by N. meningitidis that is part of an ongoing multistate Active Bacterial Core surveillance project now coordinated by the CDC's Emerging Infections Program (26). Between 1992 and 1998, staff of the CDC collaborated with investigators in state and local health departments and universities in 4 to 10 geographically dispersed areas of the United States; participating areas included sites in California, Connecticut, Georgia, Maryland, Minnesota, Missouri, New York, Oklahoma, Oregon, and Tennessee. Because the active surveillance was not conducted continuously in all surveillance areas, the aggregate population under surveillance varied from 12.8 million in 1995 to 32 million in 1998.
A case of meningococcal disease was defined as the isolation of N. meningitidis from a normally sterile site, such as blood or cerebrospinal fluid, of a resident of the surveillance area. Between 1 January 1992 and 31 December 1998, 1,696 cases of meningococcal disease were detected in the surveillance areas, for an average annual incidence of 1.1 cases per 100,000 members of the population.
N. meningitidis strains.
From a total of 470 serogroup B meningococcal disease cases identified in the United States in the period from 1992 to 1998, 444 NMSB strains were available for analysis. All strains were isolated from blood or cerebrospinal fluid. Two hundred forty-four isolates were collected from patients with sporadic meningococcal disease: 70 in California, 37 in Georgia, 34 in Maryland, 31 in Minnesota, 26 in Tennessee, 24 in Connecticut, 11 in Oklahoma, 9 in Missouri, and 2 in New York. The additional 200 isolates were collected during the meningococcal disease epidemic in the state of Oregon, ongoing since 1994 (6, 9).
The reference strains B16B6, 126E, 2996, 2396, M981, M982, M136, M990, M992, M1027, M1080, BB393, 870227, 882066, S3032, S3436, S3446, H355, H44/76, 6557, 51/90, and 503/93 were kindly provided by C. E. Frasch, Center for Biologics Evaluation and Research, Bethesda, Md.; W. D. Zollinger, Walter Reed Army Medical Center, Washington, D.C.; J. T. Poolman, SmithKline Beecham Biologicals, Rixensart, Belgium; C. T. Sacchi, Institute Adolfo Lutz, Sao Paulo, Brazil; and P. Kriz, National Institute of Public Health, Prague, Czech Republic.
Dot blot analysis.
Strains were tested with a set of 16 murine MAbs produced against the variable regions of PorA and 15 murine MAbs produced against the variable regions of PorB. The MAbs and their respective sources are shown in Table 1. These MAbs have been evaluated in 11 laboratories, and results confirmed a high level of specificity (23). The dot blotting analysis was performed as previously described (37). Briefly, the whole-cell suspensions were dotted onto 0.45-μm-pore-size nitrocellulose membrane strips (Schleicher & Schuell, Inc., Keene, N.H.). After drying for 10 min at room temperature, the strips were transferred to Accutran eight-well incubation trays (Schleicher & Schuell) and blocked for 30 min with 1 ml of 3% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) in phosphate-buffered saline (PBS; Gibco BRL, Grand Island, N.Y.). MAbs were pipetted directly into the blocking buffer at dilutions ranging from 1 in 8,000 to 1 in 64,000. After overnight incubation at room temperature on a rotator, the strips were washed three times with PBS and incubated for 2 h with goat anti-mouse immunoglobulin G conjugated to peroxidase (dilution, 1 in 4,000) (Sigma). Strips were washed three times with PBS and developed with the substrate 3-amino-9 ethyl-carbazole (Sigma) and hydrogen peroxidase.
TABLE 1.
MAbs used for serosubtyping and serotyping of NMSB isolates by dot blot analysis
| PorA serosubtype (MAb) | Reference strain | PorB serotype (MAb) | Reference strain |
|---|---|---|---|
| P1.1 (MN14C2.3)a | M1080 | 1 (MN3C6B)a | M1080 |
| P1.2 (OD6-4)b | B16B6 | 2a (5D4-5)b | B16B6 |
| P1.3 (5G8-B2-F9)b | BB393 | 2b (2H10-2)b | 2996 |
| P1.4 (MN20B9.34)a | 882066 | 2c (6-D9-5.6-F3)b | 2396 |
| P1.5 (MN22A9.19)a | B16B6 | 4 (5DC4-C8-G8)b | M981 |
| P1.6 (MN19D6.13)a | M990 | 5 (7BG5-H2)b | M992 |
| P1.7 (MN14C11.6)a | M1080 | 7 (F22-8B5/1D10)c | 870227 |
| P1.9 (MN5A10.7)a | M982 | 9 (F24-11F5/3B4)c | M982 |
| P1.10 (MN20F4.17)a | 870227 | 10 (F11-6D12/1C5)c | 126E |
| P1.12 (MN20A7.10)a | S3032 | 11 (9-1-P11)b | M136 |
| P1.13 (MN25H10.75)a | 51/90 | 14 (MN5C8C)a | S3446 |
| P1.14 (MN21G3.17)a | S3446 | 15 (8-B5-5-B9)b | H355 |
| P1.15 (95-718)a | H355 | 17 (F4-3C1/1A6)c | 6557 |
| P1.16 (OF11-4)a | H44/76 | 21 (6B11-C2-F1)b | M1027 |
| P1.19 (7A2-11)b | H355 | 22 (IA5D90)d | 503/93 |
| P1.22a (F4-1F1/1G11)c | 6557 |
I. M. Feavers, National Institute for Biological Standards and Control, Potters Bar, United Kingdom.
W. D. Zollinger, Walter Reed Army Medical Center, Washington, D.C.
C. T. Sacchi, Institute Adolfo Lutz, Sao Paulo, Brazil.
P. Kriz, National Institute of Public Health, Prague, Czech Republic.
Statistical analysis.
The χ2 test was used for analysis of potential association of particular serosubtypes and serotypes with a variety of demographic and other denominators (temporal and geographic distribution, patients' age groups, etc).
RESULTS
The distribution of NMSB serosubtypes and serotypes causing meningococcal disease in the United States in the period from 1992 to 1998 are shown in Tables 2 and 3, respectively. Among all strains isolated from sporadic cases of NMSB disease (n = 244), 37 (15.2%) were nonserosubtypeable (Table 2) and 63 (25.8%) were nonserotypeable (Table 3). Among the serosubtypeable strains, the six most prevalent serosubtypes were P1.7,16 (14.3%), P1.19,15 (9.8%), P1.7,1 (8.6%), P1.5,2 (7.8%), P1.22a,14 (7.8%), and P1.14 (5.3%). Among the serotypeable strains, the six most prevalent serotypes were 4,7 (27.5%), 15 (16.0%), 14 (8.6%), 10 (6.1%), 1 (4.9%), and 2a (3.7%). Serosubtype P1.7,16 accounted for 78.5% and serotype 15 accounted for 69.0% of the total number of strains isolated in Oregon from 1992 to 1998 (Tables 2 and 3), reflecting the epidemic caused by the phenotype B:15:P1.7,16 (6, 9).
TABLE 2.
Serosubtypes (PorA) of NMSB strains isolated during laboratory-based active surveillance of sporadic cases of meningococcal disease in geographically diverse regionsa
| Serosubtype (PorA) | United States
|
Oregon
|
Europe
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. of strains | % of strains | Cumulative % | No. of strains | % of strains | Cumulative % | No. of strains | % of strains | |
| P1.7,16 | 35 | 14.3 | 14.3 | 157 | 78.5 | 78.5 | 222 | 13.5 |
| P1.19,15 | 24 | 9.8 | 24.1 | 6 | 3.0 | 81.5 | ||
| P1.7,1 | 21 | 8.6 | 32.7 | 2 | 1.0 | 82.5 | 29 | 1.8 |
| P1.5,2 | 19 | 7.8 | 40.5 | 71 | 4.3 | |||
| P1.22a,14 | 19 | 7.8 | 48.3 | 4 | 2.0 | 84.5 | ||
| P1.14 | 13 | 5.3 | 53.6 | 7 | 3.5 | 88.0 | 54 | 3.3 |
| P1.7 | 11 | 4.5 | 58.1 | 3 | 1.5 | 89.5 | 57 | 3.5 |
| P1.5 | 8 | 3.3 | 61.4 | 1 | 0.5 | 90.0 | 46 | 2.8 |
| P1.7,13 | 8 | 3.3 | 64.7 | 1 | 0.5 | 90.5 | ||
| P1.1 | 7 | 2.9 | 67.6 | 1 | 0.5 | 91.0 | 19 | 1.5 |
| P1.4 | 5 | 2.0 | 69.6 | 3 | 1.5 | 92.5 | 494 | 30.0 |
| P1.6 | 4 | 1.6 | 71.2 | 72 | 4.4 | |||
| P1.9 | 4 | 1.6 | 72.8 | 1 | 0.5 | 93.0 | 39 | 2.4 |
| P1.7,4 | 3 | 1.2 | 74.0 | 1 | 0.5 | 93.5 | ||
| P1.13 | 3 | 1.2 | 75.2 | 41 | 2.5 | |||
| P1.15 | 3 | 1.2 | 76.4 | 194 | 11.8 | |||
| P1.16 | 3 | 1.2 | 77.6 | 7 | 3.5 | 97.0 | 91 | 5.5 |
| P1.3,6 | 2 | 0.8 | 78.4 | |||||
| P1.5,10 | 2 | 0.8 | 79.2 | |||||
| P1.10 | 2 | 0.8 | 80.0 | 66 | 4.0 | |||
| P1.3 | 1 | 0.4 | 80.4 | |||||
| P1.5,15 | 1 | 0.4 | 80.8 | |||||
| P1.7,3,6 | 1 | 0.4 | 81.2 | |||||
| P1.12,13 | 1 | 0.4 | 81.6 | 13 | 0.8 | |||
| P1.12,16 | 1 | 0.4 | 82.0 | |||||
| P1.12 | 1 | 0.4 | 82.4 | 2 | 1.0 | 98.0 | 23 | 1.4 |
| P1.19 | 1 | 0.4 | 82.8 | |||||
| P1.19,14 | 1 | 0.4 | 83.2 | |||||
| P1.22a,4 | 1 | 0.4 | 83.6 | |||||
| P1.22a,13 | 1 | 0.4 | 84.0 | |||||
| P1.22a | 1 | 0.4 | 84.4 | |||||
| P1.2 | 27 | 1.7 | ||||||
| Other | 86 | 5.2 | ||||||
| NSSTb | 37 | 15.2 | 100 | 4 | 2.0 | 100 | NAd | |
| Total | 244 | 100c | 200 | 100 | 1,647 | 100c | ||
Regions included states of the United States (California, Connecticut, Georgia, Maryland, Minnesota, Missouri, New York, Oklahoma, and Tennessee) where isolates were collected from 1992 to 1998, Oregon, where there is an ongoing outbreak, and Europe. Data for Europe are from reference 7.
NSST, nonserosubtypeable isolates.
The sum of the percentages does not equal 100 due to rounding of figures.
NA, data not available.
TABLE 3.
Serotypes (PorB) of NMSB strains isolated during laboratory-based active surveillance of sporadic cases of meningococcal disease in geographically diverse regionsa
| Serotype (PorB) | United States
|
Oregon
|
||
|---|---|---|---|---|
| No. of strains | % of strains | No. of strains | % of strains | |
| 4,7 | 67 | 27.5 | 36 | 18.0 |
| 15 | 39 | 16.0 | 138 | 69.0 |
| 14 | 21 | 8.6 | 5 | 2.5 |
| 10 | 15 | 6.1 | 2 | 1.0 |
| 1 | 12 | 4.9 | 2 | 1.0 |
| 2a | 9 | 3.7 | ||
| 7 | 6 | 2.5 | 3 | 1.5 |
| 17 | 3 | 1.2 | 1 | 0.5 |
| 21 | 2 | 0.8 | ||
| 2b | 2 | 0.8 | ||
| 2c | 2 | 0.8 | ||
| 4,14 | 1 | 0.4 | ||
| 4,21 | 1 | 0.4 | ||
| 17,7 | 1 | 0.4 | ||
| NSb | 63 | 25.8 | 13 | 6.5 |
| Total | 244 | 99.9 | 200 | 100 |
Regions included states of the United States (California, Connecticut, Georgia, Maryland, Minnesota, Missouri, Oklahoma, and Tennessee) where isolates were collected between 1992 and 1998 and Oregon, where there is an ongoing outbreak.
NS, nonserotypeable.
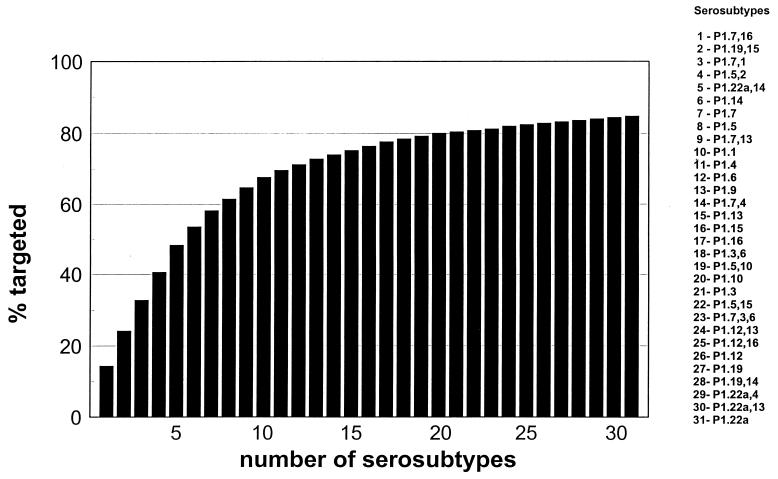
The results show that a vaccine aimed at the six most prevalent serosubtypes could have targeted 53.6% of the sporadic cases of NMSB disease that occurred in the United States between 1992 and 1998 (Table 2). No statistically significant differences were found in the distribution of individual serosubtypes and/or serotypes by patients' age groups, and no particular temporal clustering was observed. The numbers of strains with particular serosubtypes were too small to allow for analysis of their geographic distribution (data not shown). Contrary to observations for endemic disease, the same hexavalent vaccine could have targeted 88% of the epidemic cases of serogroup B meningococcal disease that occurred in Oregon during the same time period (Table 2). To achieve comparable (80%) coverage of sporadic disease, a total of 20 serosubtypes would have to be included in a multivalent PorA-based vaccine (Fig. 1). Excluding the 37 nonserosubtypeable isolates, the results suggest that 8, 11, or 16 serosubtypes would have to be added to prevent 70, 80, and 90% of sporadic meningococcal disease caused by NMSB, respectively.
FIG. 1.
Number of serosubtypes that would have to be added in a multivalent OMP-based vaccine versus percentages of isolates of sporadic NMSB disease targeted, including 37 (15%) nonserosubtypeable isolates, collected in the United States from 1992 to 1998.
DISCUSSION
NMSB is responsible for approximately one-third of the endemic meningococcal disease in the United States and a higher proportion of disease among young children (26). Because protection has been shown to be largely serosubtype specific (18, 22, 33), the identification of prevalent serosubtypes is important in evaluating the possibility of using multivalent OMP-based vaccines to control endemic NMSB disease. The results of this study provide information on the prevalence and distribution of serosubtypes and serotypes of NMSB in the United States from 1992 to 1998. In addition, the study indicates that a multivalent OMP-based vaccine aimed at the six most prevalent serosubtypes (P1.7,16; P1.19,15; P1.7,1; P1.5,2; P1.22a,14; and P1.14) would target only half of the sporadic cases of NMSB disease that occurred between 1992 and 1998 in the United States.
Information about the diversity of serosubtypes in other parts of the world is presently available only to limited geographic areas. Among the 1,647 NMSB strains from 23 European countries that were serosubtyped in 1996, serosubtype P1.4 was identified in 30% of them (Table 2) (7). In contrast, this serosubtype, associated with an increase of endemic meningococcal cases in The Netherlands since 1983 (1, 29) and the recent epidemic in New Zealand (15), was seen in only 2% of isolates in our study. Isolates of the serosubtype P1.7,16, the most common in the United States in the observed period, ranked second in Europe (Table 2) and has been responsible for an outbreak of meningococcal disease in Norway since the mid-1970s (3, 25, 38). In the period of July 1977 to June 1998, 5,523 laboratory-confirmed cases of meningococcal disease were reported in 20 European countries, with serogroup B being predominant (61%) (20). Serosubtyping was available for 1,236 of the NMSB isolates, and as in 1996, serosubtypes P1.4 and P1.7,16 predominated. Consequently, a vaccine developed in response to an epidemic in Europe, New Zealand, or other parts of the world will not necessarily be the optimal vaccine to prevent NMSB disease in the United States. For instance, the hexavalent PorA vaccine (21) contains three serosubtypes (P1.7h,4; P1.5c,10; and P1.12,13) that are not among the most prevalent in the United States. On the other hand, data from the NMSB OMP-based vaccine trial in Chile (33) suggested that some serum bactericidal activity against strains other than the vaccine strain may have been elicited, although the specific surface components responsible for the cross-protection have not been identified.
The data from this study show that a large number of serosubtypes (20 or more) might have to be included in a multivalent OMP-based vaccine to target 80% of sporadic disease caused by NMSB. This estimate is based solely on reactivity with mouse MAbs, as there are few data about human responses to PorA immunogens. Consequently, there are reasons to suggest that this estimate may be either too high or too low. First, we can hypothesize that vaccination with a vaccine containing P1.5,2 would protect not only against serogroup B strains of this PorA, but also against the strains of other serogroups that express P1.5,2. This PorA type has been identified in one-third of serogroup C and Y isolates in the United States, collected in the same time period as the NMSB isolates (data not shown).
Second, a relatively large number of strains in our study did not react with the currently available MAbs. There are several mechanisms that explain the lack of reactivity with murine MAbs. Probably the most important mechanism is the horizontal genetic exchange among N. meningitidis strains causing generation and spread of antigenic variants of OMPs. By this mechanism, entire genes may have been exchanged among strains, thus explaining why some strains with divergent molecular fingerprints share identical porA genes and, conversely, why some strains with similar chromosome structures exhibit porA genes with different nucleotide sequences and serological properties (11).
Finally, if a significant proportion of nonserosubtypeable strains are variants of a prototype included in a vaccine, then some protection might be expected after immunization because nonreactivity with the murine MAb does not necessarily equate to lack of coverage of a particular variant. As shown in Table 2, the number of serosubtypes needed for 80% coverage of sporadic disease would be smaller (n = 11) if the nonserosubtypeable isolates were included in the coverage or excluded from the analysis. Contrary to that, the estimates of the coverage levels may be too high, if we consider that different PorA variants that are still reactive with the murine MAb may not exhibit cross-reactivity with the human antibodies. The OMP's genetic variation in PorA not only restricts the usefulness of serosubtyping because many isolates become nonreactive to MAbs but, more importantly, it may also have significant implications for vaccine design (1, 12, 14, 17, 24, 28, 32). Therefore, sequencing of the porA variable regions of the nonserosubtypeable strains and additional knowledge about the extent of cross-protection for variants of individual PorA types are needed to provide precise information on the diversity and distribution of PorA antigens and correlation with nonserosubtypeable isolates.
Serogroup B meningococcus is the most common cause of meningococcal disease in many countries, and epidemics continue to occur around the world. Previously evaluated monovalent OMP-based vaccines have not been shown to be efficacious in young children (4, 8). Several strategies of vaccine development have been proposed for prevention of serogroup B meningococcal disease such as production of strain-specific OMP-based vaccines at the onset of an outbreak and use of multivalent vaccines based on highly endemic and epidemic strain-specific PorA antigens (39). Due to the substantial variations detected in genes coding for PorA, even multivalent OMP-based vaccines may not be effective in preventing the majority of endemic disease. Also, technical and logistical parameters may limit how many different PorA types may be included in such a vaccine and how rapidly a vaccine could be manufactured and licensed to allow for its timely implementation. Therefore, research is under way to address alternative strategies for development of serogroup B vaccines (13; B. Manberg, E. A. Hoiby, and E. Wedege, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 213, 1997). Continuous surveillance of meningococcal disease as well as coordination and commitment of public health personnel, government personnel, and the vaccine industry will be required for successful development of vaccines against all NMSB isolates.
ACKNOWLEDGMENTS
We thank Corie A. Noble for technical support. We also thank Carl E. Frasch, Wendell D. Zollinger, Ian M. Feavers, Jan T. Poolman, Claudio T. Sacchi, and Paula Kriz for providing the monoclonal antibodies and prototype strains.
Appendix
The members of The Active Bacterial Core Surveillance Team are as follows: from the California Emerging Infections Program, Berkeley, G. Rothrock, N. Mukerjee, P. Daily, L. Gelling, and D. Vugia; from Vanderbilt University Medical Center, Nashville, Tenn., B. Barnes and C. Gilmore; from Veterans Affairs Medical Services and the Emory University School of Medicine, Atlanta, Ga., M. Farley, W. Baughman, S. Whitfield, and M. Bardsley; from John Hopkins University, Baltimore, Md., L. Billmann; from the Maryland Department of Health and Mental Hygiene, Baltimore, D. Dwyer; from the Connecticut Emerging Infections Program, Hartford, J. Hadler, P. Mshar, N. Barrett, C. Morin, and Q. Phan; from the Minnesota Emerging Infections Program, Minneapolis, M. Osterholm, R. Danila, J. Rainbow, C. Lexau, L. Triden, K. White, and J. Besser; from the Oregon Emerging Infections Program, Portland, K. Stefonek, J. Donegon, and S. Ladd-Wilson; and from the CDC, G. Ajello, M. Berkowitz, B. Plikaytis, M. Reeves, K. Robinson, and S. Schmink.
REFERENCES
- 1.Bart A, Dankert J, van der Ende A. Antigenic variation of the class I outer membrane protein in hyperendemic Neisseria meningitidis strains in The Netherlands. Infect Immun. 1999;67:3842–3846. doi: 10.1128/iai.67.8.3842-3846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bash M C, Lesiak K B, Banks S D, Frash C E. Analysis of Neisseria meningitidis class 3 outer membrane protein gene variable regions and type identification using genetic techniques. Infect Immun. 1995;63:1484–1490. doi: 10.1128/iai.63.4.1484-1490.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjune G, Høiby E A, Grønnesby J K, Arnesen O, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 4.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, Moran E, Hankins W, Gilly J, Mays J The Chilean National Committee for Meningococcal Disease. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine. 1995;9:821–829. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright K, Morris R, Rumke H, Fox A, Borrow R, Begg N, Richmond P, Poolman J. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (Por A) outer membrane proteins. Vaccine. 1999;17:2612–2619. doi: 10.1016/s0264-410x(99)00044-4. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Serogroup B meningococcal disease—Oregon, 1994. Morb Mortal Wkly Rep. 1995;44:121–124. [PubMed] [Google Scholar]
- 7.Connolly M, Noah N. Surveillance of bacterial meningitis in Europe 1996. London, United Kingdom: King's European Meningitis Surveillance Centre; 1997. [Google Scholar]
- 8.De Moraes J C, Perkins B A, Camargo M C C, Hidalgo N T R, Barosa H A, Sacchi C T, Land Gral I M, Gattas V L, Vasconcelos H D G, Plikaytis B D, Wenger J D, Broome C V. Protective efficacy of a serogroup B meningococcal vaccine in São Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 9.Diermayer M, Hedberg K, Hoesly F, Fischer M, Perkins B, Reeves M, Fleming D. Epidemic serogroup B meningococcal disease in Oregon. The evolving epidemiology of the ET-5 strain. JAMA. 1999;281:1493–1497. doi: 10.1001/jama.281.16.1493. [DOI] [PubMed] [Google Scholar]
- 10.Feavers I M, Sucker J, McKenna A J, Heath A B, Maiden M C J. Molecular analysis of the serotyping antigens of Neisseria meningitidis. Infect Immun. 1992;60:3620–3629. doi: 10.1128/iai.60.9.3620-3629.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feavers I M, Heath A B, Bygraves J A, Maiden M C J. Role of the horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol Microbiol. 1992;6:489–495. doi: 10.1111/j.1365-2958.1992.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 12.Feavers I M, Fox A J, Gray S, Jones D M, Maiden M C J. Antigenic diversity of meningococcal outer membrane protein PorA has implications for epidemiological analysis and vaccine design. Clin Diagn Lab Immunol. 1996;3:444–450. doi: 10.1128/cdli.3.4.444-450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frash C E. Vaccines for the prevention of meningococcal disease. Clin Microbiol Rev. 1989;2:S134–S138. doi: 10.1128/cmr.2.suppl.s134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiden M C J, Sucker J, McKenna A J, Bygraves J A, Feavers I M. Comparison of the class 1 outer membrane proteins of eight serological reference strains of Neisseria meningitidis. Mol Microbiol. 1991;5:727–736. doi: 10.1111/j.1365-2958.1991.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin D R, Walter S J, Baker M G, Lennon D R. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J Infect Dis. 1998;177:497–500. doi: 10.1086/517385. [DOI] [PubMed] [Google Scholar]
- 16.McGuinness B T, Barlow A K, Clarke I N, Farley J E, Anilionis A, Poolman J T, Heckels J E. Deduced amino acid sequences of class 1 protein (PorA) from three strains of Neisseria meningitidis. Synthetic peptides define the epitopes responsible for serosubtype specificity. J Exp Med. 1990;171:1871–1882. doi: 10.1084/jem.171.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuinness B T, Lambden P R, Heckels J E. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol Microbiol. 1993;7:505–514. doi: 10.1111/j.1365-2958.1993.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 18.Milagres L G, Gorla M C, Sacchi C T, Rodrigues M M. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect Immun. 1998;66:4755–4761. doi: 10.1128/iai.66.10.4755-4761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Næss L M, Oftung F, Aase A, Wetzler L M, Sandin R, Michaelsen T E. Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine. Infect Immun. 1998;66:959–965. doi: 10.1128/iai.66.3.959-965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noah N, Henderson B. Surveillance of bacterial meningitis in Europe 1997/98. London, United Kingdom: Communicable Disease Surveillance Centre; 1998. pp. 1–36. [Google Scholar]
- 21.Peeters C C A M, Rümke H C, Sundermann L C, Rouppe van der Voort E M, Meulenbelt J, Schuller M, Kuipers A J, van der Ley P, Poolman J T. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine. 1996;14:1009–1015. doi: 10.1016/0264-410x(96)00001-1. [DOI] [PubMed] [Google Scholar]
- 22.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Høiby E A, Rosenqvist E, Holst J, Nøkleby H, Sotolongo F, Sierra G, Huergo C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 23.Poolman J T, Kriz-Kuzemenska P, Ashton F, Bibb W, Dankert J, Demina A, Frøholm L O, Hassan-King M, Jones D M, Lind I, Prakash K, Xujing H. Serotypes and subtypes of Neisseria meningitidis: results of an international study comparing sensitivities and specificities of monoclonal antibodies. Clin Diagn Lab Immunol. 1995;2:69–72. doi: 10.1128/cdli.2.1.69-72.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenqvist E, Høiby E A, Wedege E, Caugant D A, Frøholm L O, McGuinness B T, Brooks J, Lambden P R, Heckels J E. A new variant of serosubtype P1.16 in Neisseria meningitidis from Norway, associated with increased resistance to bacterial antibodies induced by a serogroup B outer membrane protein vaccine. Microb Pathog. 1993;15:197–205. doi: 10.1006/mpat.1993.1070. [DOI] [PubMed] [Google Scholar]
- 25.Rosenqvist E, Høiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Rønnild E, Bjune G, Nøkleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenstein N E, Perkins B A, Stephens D, Lefkowitz L, Cartter M L, Danila R, Cieslak P, Shutt K A, Popovic T, Schuchat A, Harrison L H, Reingold A L The Active Bacterial Core Surveillance Team. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180:1894–1901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 27.Sacchi C T, Lemos A P S, Whitney A M, Solari C A, Brandt M E, Melles C E A, Frasch C E, Mayer L W. Correlation between serological and sequencing analyses of the PorB outer membrane protein in the Neisseria meningitidis serotyping system. Clin Diagn Lab Immunol. 1998;5:348–354. doi: 10.1128/cdli.5.3.348-354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacchi C T, Lemos A P S, Brandt M E, Whitney A M, Melles C E A, Solari C A, Frasch C E, Mayer L W. Proposed standardization of Neisseria meningitidis PorA variable-region typing nomenclature. Clin Diagn Lab Immunol. 1998;5:845–855. doi: 10.1128/cdli.5.6.845-855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholten R J P M, Bijlmer H A, Poolman J T, Kuipers B, Caugant D A, Van Alphen L, Dankert J, Valkenburg H A. Meningococcal disease in The Netherlands, 1958–1990: a steady increase in the incidence since 1982 partially by new serotypes and subtypes of Neisseria meningitidis. Clin Infect Dis. 1993;16:237–246. doi: 10.1093/clind/16.2.237. [DOI] [PubMed] [Google Scholar]
- 30.Sierra G V G, Campa H C, Varacel N M, Garcia I L, Izquierdo P L, Sotolongo P F, Casanueva G V, Rico C O, Rodriguez C R, Terry M H. Vaccines against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–210. [PubMed] [Google Scholar]
- 31.Smith N H, Smith J M, Spratt B G. Sequence evolution of the porB gene of Neisseria gonorrhoeae and Neisseria meningitidis: evidence of positive Darwinian selection. Mol Biol Evol. 1995;12:363–370. doi: 10.1093/oxfordjournals.molbev.a040212. [DOI] [PubMed] [Google Scholar]
- 32.Suker J, Feavers I M, Maiden M C J. Monoclonal antibody recognition of members of the meningococcal P1.10 variable region family: implications for serological typing and vaccine design. Microbiology. 1996;142:63–69. doi: 10.1099/13500872-142-1-63. [DOI] [PubMed] [Google Scholar]
- 33.Tappero J W, Lagos R, Ballesteros A M, Plikaytis B, Williams D, Dykes J, Gheesling L L, Carlone G M, Høiby E A, Holst J H, Nøkleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman J T, Perkins B A. Immunogenicity of 2 serogroup B outer membrane protein meningococcal vaccines. A randomized controlled trial in Chile. JAMA. 1999;281:1520–1527. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 34.van der Ley P, Heckels J E, Virji M, Hoogerhout P, Poolman J T. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun. 1991;59:2963–2971. doi: 10.1128/iai.59.9.2963-2971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Ley P, Poolman J T. Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect Immun. 1992;60:3156–3161. doi: 10.1128/iai.60.8.3156-3161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Voort E R, van der Ley P, van der Biezen J, George S, Tunnela O, van Dijken H, Kuipers B, Poolman J. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. 1996. Infect Immun. 1996;64:2745–2751. doi: 10.1128/iai.64.7.2745-2751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedege E, Høiby E A, Rosenqvist E, Frøholm L O. Serotyping and subtyping of Neisseria meningitidis isolates by co-agglutination, dot-blotting and ELISA. J Med Microbiol. 1990;31:195–201. doi: 10.1099/00222615-31-3-195. [DOI] [PubMed] [Google Scholar]
- 38.Wedege E, Høiby E A, Rosenqvist E, Bjune G. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccines and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect Immun. 1998;66:3223–3231. doi: 10.1128/iai.66.7.3223-3231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenger J D. Serogroup B meningococcal disease. New outbreaks, new strategies. JAMA. 1999;281:1541–1543. doi: 10.1001/jama.281.16.1541. [DOI] [PubMed] [Google Scholar]