Abstract
Transfusion of red blood cells (RBCs) is a life-saving intervention for anemic patients. Human induced pluripotent stem cells (iPSC) have the capability to expand and differentiate into RBCs (iPSC-RBCs). Here we developed a murine model to investigate the in vivo properties of human iPSC-RBCs. iPSC lines were produced from human peripheral blood mononuclear cells by transient expression of plasmids containing OCT4, SOX2, MYC, KLF4, and BCL-XL genes. Human iPSC-RBCs were generated in culture supplemented with human platelet lysate, and were CD34−CD235a+ CD233+ CD49dlow CD71low; about 13% of iPSC-RBCs were enucleated before transfusion. Systemic administration of clodronate liposomes (CL) and cobra venom factor (CVF) to NOD scid gamma (NSG) mice markedly promoted the circulatory survival of human iPSC-RBCs following transfusion. While iPSC-RBCs progressively decreased with time, 90% of circulating iPSC-RBCs were enucleated 1 day after transfusion (CD235a+ CD233+ CD49d− CD71−). Surprisingly, human iPSC-RBCs reappeared in the peripheral circulation at 3 weeks after transfusion at levels more than 8-fold higher than at 1 h after transfusion. Moreover, a substantial portion of the transfused nucleated iPSC-RBCs preferentially homed to the bone marrow, and were detectable at 24 days after transfusion. These results suggest that nucleated human iPSC-derived cells that homed to the bone marrow of NSG mice retained the capability to complete differentiation into enucleated erythrocytes and egress the bone marrow into peripheral blood. The results offer a new model using human peripheral blood-derived iPSC and CL/CVF-treated NSG mice to investigate the development and circulation of human erythroid cells in vivo.
1 |. INTRODUCTION
Transfusion of human red blood cells (RBCs) is the oldest form of cell therapy, and an irreplaceable life-saving medical treatment. Each year, more than 5 million patients in the US require over 13 million units of RBCs for transfusion due to major surgery, severe injury and bleeding, anemia, blood disorders, or cancer treatments.1,2
The supply of RBC units from volunteer blood donors is generally adequate and safe, but not always. For example, there are often shortages of specific blood units required in special situations3: group O Rh-negative RBCs are preferred for emergent transfusions of women of child-bearing potential; RBCs lacking erythroid allo-antigens (e.g., C-, E-, K-) are required for transfusion of many sickle cell patients.4 However, even when alloantigen-matching protocols are employed, transfusion-dependent patients can still generate alloantibodies that can make it difficult to find compatible RBC for future transfusion.4–8 Recent studies highlight this challenge and demonstrate that alloantibody formation against non-ABO(H) antigens can significantly increase transfusion complications,6,9 with a pronounced impact on mortality in patients with sickle cell disease in particular.10,11 While recent efforts are underway to reduce the deleterious sequalae of RBC alloantibodies in transfusion-dependent patients,12–15 alternative approaches that facilitate the availability of compatible RBCs for difficult to transfuse patients would be predicted to significantly improve outcomes.16
Additionally, while RBC transfusions are considered to be relatively safe procedures, adverse events continue to occur. Although rare, ABO-incompatible transfusions can be life-threatening.17 While the risk of ABO-incompatible transfusions can be abrogated by using group O units, these universal donor red cells are often in short supply. Other more common, and generally less serious, adverse reactions include febrile nonhemolytic, TRALI, and allergic transfusion reactions.18 These reactions can be largely eliminated by removing the causative non-RBC constituents from blood products, including cytokines, antibodies, and other plasma proteins.19 Furthermore, while current screening methods to mitigate risks of transfusion-transmitted viral infections have produced an extremely safe blood supply, RBC transfusions are not yet “zero-risk” with respect to infectious diseases.20 In addition, infectious disease outbreaks such as COVID-19 pandemic caused nationwide blood shortages. For these reasons, the production of cultured, group O RBCs of specified phenotypes promises to further improve the availability, efficacy, and safety of RBC transfusion therapies.
Human induced pluripotent stem cells (iPSC) can be produced by reprogramming adult peripheral blood mononuclear cells via transfection of OCT4, SOX2, KLF4, MYC, or LIN28 genes.21,22 The resulting iPSC, similar to embryonic stem cells, retain the capacity to self-renew, expand, and differentiate into all types of mature blood elements including RBCs.23–26 From a basic research perspective, iPSC provides a powerful model to dissect mechanisms of cell differentiation.
Clinically, the virtually unlimited growth potential of iPSC could facilitate a pipeline for large-scale production of iPSC-derived RBCs (iPSC-RBCs) in vitro. Furthermore, the appropriate selections of donors and/or application of genome-editing technologies make it feasible to create iPSC-RBCs of defined phenotypes (eg, group O, RhD-, C-, E-, K-negative),27 thus directly facilitating the transfusion management of difficult to transfuse patients. The use of defined culture media eliminates soluble factors implicated in the most common transfusion reactions. In the proper therapeutic niche, iPSC-RBCs would be high-value products due to their unique characteristics and the potential to alleviate shortages of specific RBC products.28
Here, we tested the effect of human platelet lysate (hPL) on the maturation of iPSC-RBCs, and investigated the in vivo behavior of the resulting iPSC-RBCs in the immunodeficient NOD scid gamma (NSG) mouse model. We also showed that iPSC homed to the bone marrow and continue development to mature RBCs, suggesting that this may represent a useful in vivo model to better understand the mechanisms directing erythroblasts to differentiate into mature RBCs.
2 |. METHODS
2.1 |. iPSC generation
Peripheral blood mononuclear cells were isolated from blood of healthy donors (n = 5). Erythroblasts were expanded in SFEM II medium (StemCell Technologies) containing human erythropoietin (EPO), holo-transferrin, stem cell factor (SCF) (R&D Systems), interleukin (IL)-3, and insulin-like growth factor-1 (PeproTech), as described.29 Expanded erythroblasts were reprogrammed to iPSC using three reprogramming plasmids (MOS, MMK and MBX containing OCT4, SOX2, MYC, KLF4, and BCL-XL genes) (Addgene), as described.29 iPSC were expanded in E8 medium (StemCell Technologies) containing ROCK inhibitor Y27632 (1:1000; Sigma), and immunophenotyped by flow cytometry, or stained by Giemsa banding for karyotype analyses.30 The use of human peripheral blood was approved by the Institutional Review Board of Emory University.
2.2 |. iPSC-RBCs production
iPSC was induced to hematopoietic progenitor cells (HPC) using the STEMdiff Hematopoietic kit (StemCell Technologies) following company’s instruction. HPC were harvested and cultured in SFEM II medium with StemSpan erythroid expansion supplement for 14 days to differentiate into erythroblasts. iPSC-derived erythroblasts were cultured for 8 days for maturation in SFEM II medium containing human EPO, holo-transferrin, heparin, and insulin as described,31 supplemented with either 10% human platelet lysate (hPL; produced as described32), human serum (Sigma), or plasma albumin (Grifols); this medium was also supplemented with human SCF (100 ng/mL) and IL-3 (10 ng/mL) for the first 4 days. Culture medium was refreshed every 2 days.
2.3 |. Flow cytometry
iPSC were profiled with phycoerythrin-conjugated (PE) anti-human SSEA4 (Millipore Corporation), TRA-1–60 and Nanog antibodies (1:100; BD Pharmingen) on a FACSCalibur Flow Cytometer (BD). iPSC-derived cells were analyzed with: fluorescein isothiocyanate-conjugated (FITC) anti-human CD235a (1:100; Caprico Biotechnologies); PE anti-human CD45 (1:100; BioLegend) and CD233 antibodies (1:100; Miltenyi Biotech); allophycocyanin-conjugated (APC) anti-human CD34, CD49d (Biolegend) and CD71 antibodies (1:100; Caprico Biotechnologies); and DNA-binding dye DRAQ5 (1:500; BioLegend). Peripheral blood, bone marrow cells, or cells isolated from murine spleen, liver, and lung were analyzed with anti-human CD235a (FITC), CD233 (PE), CD49d (APC) antibodies, and DRAQ5. Data were analyzed with FlowJo 10.7.1 software.
2.4 |. Mouse transfusion model
NSG mice (n = 5 per group, 8 weeks old, body weight >20 g) (The Jackson Laboratory) were pretreated with clodronate liposomes (CL) (100 μL/mouse; LIPOSOMA), cobra venom factor (CVF) (20 μg/mouse; Quidel Corporation), or combination of CL and CVF at both 1 day and 1 h before transfusion (50 μL/mouse of CL, 10 μg/mouse of CVF) by intravenous injection (IV).31 The mice were transfused by IV with 30 μL/mouse of normal human RBCs or 30 million iPSC-RBCs. After transfusion, the mice were continuously treated with CL (30 μL/mouse), CVF (10 μg/mouse), or the combination of CL and CVF every 3 days until sacrifice. Murine peripheral blood (before or after transfusion), femoral bone marrow, spleen, liver, and lung were collected for flow cytometry. The use of mice was approved by The Institutional Animal Care and Use Committee of Emory University.
2.5 |. Statistical analysis
Data were presented as mean ± SEM. p values were calculated using the one-way analysis of variance test. p value less than .05 was considered significant (*p < .05; **p < .01; ***p < .001).
3 |. RESULTS
3.1 |. Human platelet lysate promotes the maturation of iPSC-RBCs
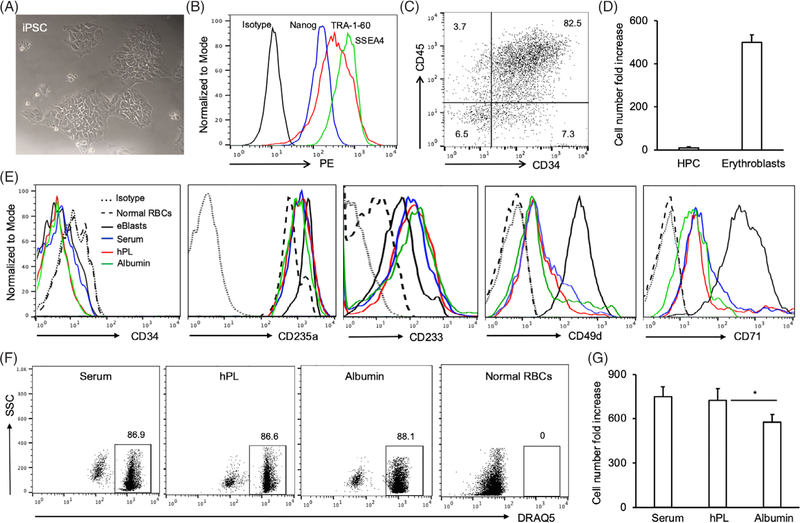
Human peripheral blood-derived iPSC (Figure 1A) were obtained by nucleofection of OCT4, SOX2, KLF4, MYC, and BCL-XL genes into primary human erythroblasts (Figure S1).29 The resulting iPSC lines expressed the pluripotency markers SSEA4, TRA-1–60, and Nanog (Figure 1B).33 The one line evaluated by cytogenetics showed a normal 46,XY male karyotype that was maintained for at least 20 passages (Figure S2). After the first stage of the differentiation protocol, iPSC-derived cells expressed CD34+ CD45+ consistent with an HPC-like phenotype (Figure 1C). We calculated that there was an average 10-fold increase in cell numbers during the first (HPC) differentiation phase, and an over 510-fold expansion by the end of the second phase (erythroblasts) (Figure 1D). At the start of the third culture phase, iPSC-derived erythroblasts were CD34− CD235a+ CD233+ CD49dhigh CD71high (Figure 1E). Following 8 days of maturation culture in the presence of either hPL, human serum, or albumin, iPSC-RBCs were CD34− CD235a+ CD233+ CD49dlow CD71low; in contrast, normal human RBCs were CD49d− CD71− (Figure 1E). Approximately, 13% of iPSC-RBCs were enucleated by the end of the maturation protocol, and the percentage was not affected by the presence of hPL, serum, or albumin (Figure 1F). However, the average fold increase in cell numbers was significantly higher in the presence of hPL or serum (730- and 750-fold, respectively) than when albumin was used (560-fold) (Figure 1G).
FIGURE 1.
Generation of human induced pluripotent stem cells (iPSC)-derived erythroblasts and iPSC-red blood cells (RBCs). Human iPSC clones were generated by introduction of OCT4, SOX2, KLF4, MYC, and BCL-XL genes into primary erythroblasts from health donors. (A) Human iPSC clones were photographed during culture in E8 medium (20X). (B) Human iPSC were collected after treatment with accutase, and subjected to flow cytometry analyses with anti-human TRA-1–60, SSEA4, and Nanog antibodies, or isotype. (C) Alternatively, Human iPSC were seeded and differentiated into hematopoietic progenitor cells (HPC) with STEMdiff Hematopoietic kit. After 12-day culture, the cells in suspension were harvested and subjected to flow cytometry analysis with anti-human CD45 and CD34 antibodies. The resulting dot plot is presented. (D) Human iPSC-derived HPC were continuously differentiated into erythroblasts with StemSpan erythroid expansion kit for 14 days. The numbers of human iPSC-derived HPC and erythroblasts were counted, and the cell number fold increase was calculated based on seeded iPSC number. (E) Human iPSC-RBCs were harvested after 8-day maturation culture in the presence of human serum (Serum), human platelet lysate (hPL), or human plasma albumin (Albumin). The cells were subjected to flow cytometry analyses with anti-human CD34, CD49d, CD71, CD233, and CD235a antibodies. Normal human RBCs and iPSC-derived erythroblasts before maturation served as control cells. Flow cytometry histograms are presented. (F) iPSC-RBCs were also stained with the DRAQ5 dye. Normal human RBCs served as DRAQ5− cells. (G) The cell number fold-increase of iPSC-RBCs after 8-day maturation in the presence of human serum, platelet lysate or albumin was calculated based on initial iPSC number. Data were from three independent experiments
3.2 |. Systemic administration of CL and CVF enhanced the survival of human RBCs and iPSC-RBCs in immunodeficient NSG mice
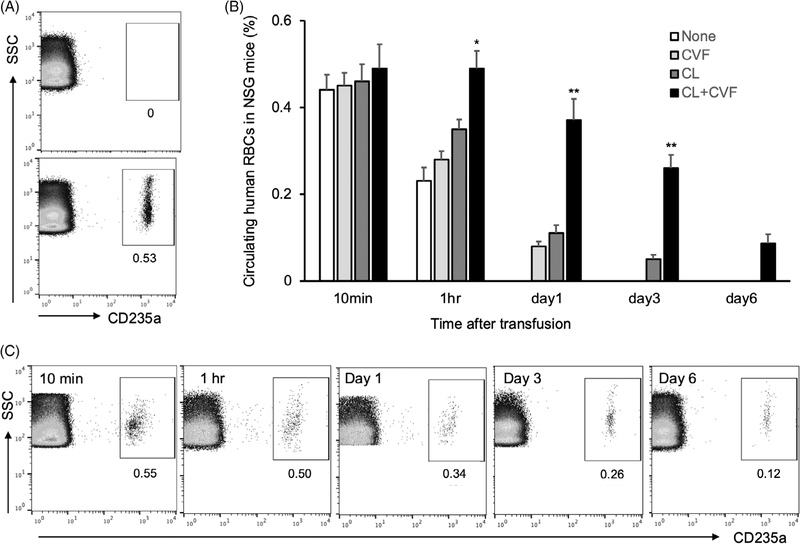
To study the circulation of iPSC-RBCs in vivo, we first needed to validate a mouse transfusion model. Similar to prior studies,31,34 we used NSG mice that were treated with CL and/or CVF. We first examined the circulation of normal human RBCs, which were identified using anti-human CD235a antibodies. Initial flow cytometry studies demonstrated that this antibody did not bind to murine RBCs collected from mice prior to transfusion (Figure 2A, top panel), but did recognize human RBCs at 10 min after transfusion (Figure 2A, bottom panel). Flow cytometric analyses further showed that combined administration of CL and CVF markedly enhanced the survival of human RBCs starting at 1 h after transfusion; the effects were significantly better than when using CL or CVF alone (Figure 2B). There was no significant difference in the percentage of circulating human RBCs among the four groups of NSG mice at 10 min after transfusion (Figure 2B). The percentage of circulating human RBCs in CL and CVF-treated mice decreased progressively over the first 6 days after transfusion. Nonetheless, human RBCs still persisted at greater than 0.1% of total RBCs at day 6 (Figure 2C), representing approximately 20% of the human RBCs circulating at 10 min after transfusion.
FIGURE 2.
Human red blood cells (RBCs) survival in NOD scid gamma (NSG) mice. (A) Peripheral blood of NSG mice was collected before (upper panel) or after (lower panel) transfusion of human blood and subjected to flow cytometry analyses with anti-human CD235a antibody. (B) Human blood was transfused into naïve NSG mice or mice treated with cobra venom factor (CVF) only, clodronate liposomes (CL) only, or combination of CL and CVF. Peripheral blood was collected at different time points after transfusion and used for flow cytometry analyses. The percentages of circulating human CD235a+ RBCs were calculated. Data were from three independent experiments. (C) Flow cytometric dot plots on the peripheral blood of NSG mice treated with CL and CVF were presented from one of the three independent experiments
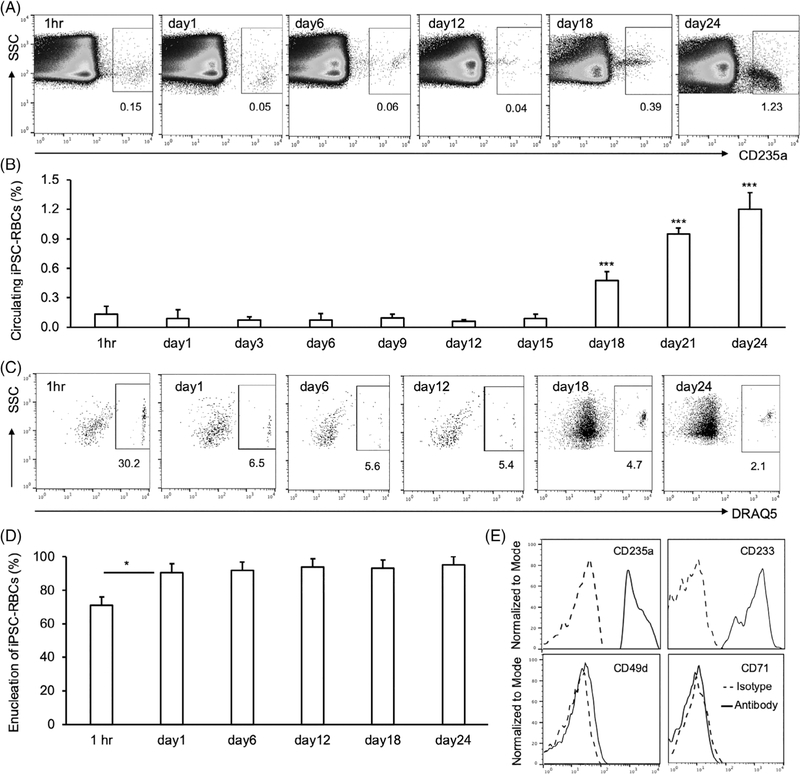
To follow the survival of human iPSC-RBCs in vivo, we transfused iPSC-RBCs into NSG mice pretreated with CL and CVF, as above. At 1 h after transfusion, 0.15% of circulating cells were human iPSC-RBCs (Figure 3A,B). Circulating human iPSC-RBCs decreased to 0.05% 24 h later and persisted at approximately this level through day 15 post transfusion (Figure 3B).
FIGURE 3.
Human induced pluripotent stem cells-red blood cells (iPSC-RBCs) survival in clodronate liposomes (CL)- and cobra venom factor (CVF)-treated NOD scid gamma (NSG) mice. (A) Human iPSC-RBCs were transfused into NSG mice treated with the combination of CL and CVF before and after transfusion. Murine peripheral blood was collected after transfusion at different time points for flow cytometry analyses with anti-human CD235a antibody. Gated CD235a+ cells represent transfused human iPSC-RBCs. (B) The percentages of circulating human iPSC-RBCs in murine peripheral blood at selected time points were calculated from three independent experiments. (C) Murine peripheral blood cells were further stained with dye DRAQ5. Nucleated human cells were gated on DRAQ5+ population, and enucleated human iPSC-RBCs were DRAQ5−. Flow cytometry dot plots at different time points were representative from three independent experiments. (D) Percentage of circulating enucleated human iPSC-RBCs at different time points after transfusion was calculated accordingly. (E) Alternatively, murine peripheral blood samples on day 24 after transfusion were also stained with anti-human CD235a, CD49d, CD71, CD233 antibodies, or isotype control. Flow cytometry histograms were represented from one of three independent experiments
3.3 |. Human iPSC-RBCs retain potent erythropoietic activity in vivo
Surprisingly, when monitoring the circulation of transfused iPSC-RBCs, we observed that levels of CD235a+ cells increased in mice after day 15; circulating iPSC-RBCs were 2-fold higher at day 18 than at 1 h after transfusion (Figure 3A,B). The percentage of human iPSC-RBCs in murine circulation continued to increase, and reached to 1.2% on day 24 after transfusion, 8-fold higher than the one at 1 h post transfusion (Figures 3A,B, S3). In contrast, normal human RBCs in the circulation of CL- and CVF-treated NSG mice continuously decreased, and became undetectable on day 24 after transfusion (Figure S4).
As shown above, only about 13% of human iPSC-RBCs were enucleated at the time of transfusion (Figure 1F). However, when the CD235a+ iPSC-RBCs identified in murine circulation after transfusion (gated as in Figure 3A) were stained with the DRAQ5 nuclear stain, the percentage of enucleated cells reached 70% at 1 h after transfusion (Figure 3C,D). On day 1 after transfusion, enucleated human iPSC-RBCs increased further to 95% of CD235a+ iPSC-RBCs, and persisted in murine circulation at approximately that level (Figure 3C,D). The iPSC-RBCs that reappeared in circulation after day 15 were 95%–98% enucleated. Additionally, flow cytometry on peripheral blood samples obtained at day 24 after transfusion showed that CD235a+ DRAQ5− enucleated circulating human iPSC-RBCs in NSG mice were CD233+CD49d−CD71− (Figure 3E), more closely matching the normal human RBC phenotype than iPSC-RBCs at the end of the culture period (Figure 1E).
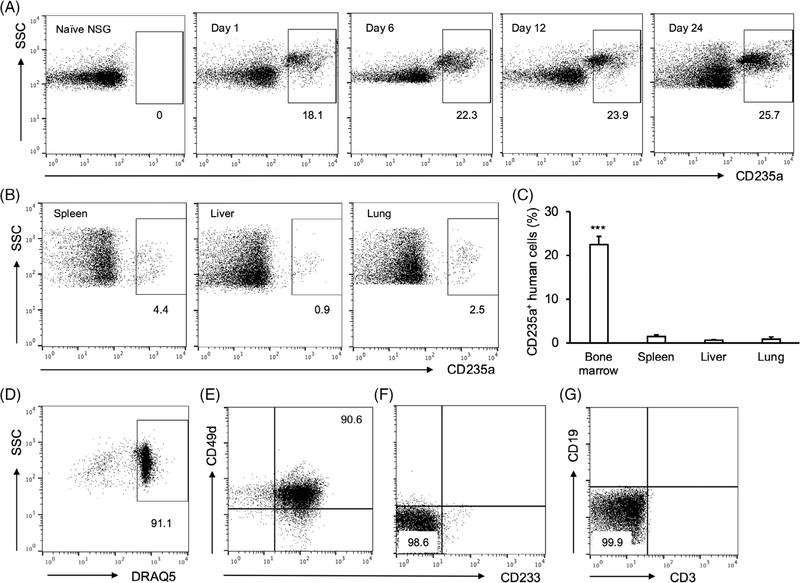
3.4 |. Human iPSC-RBCs preferentially migrated to murine bone marrow
We reasoned that the increase in iPSC-RBCs in the murine peripheral blood 2–3 weeks after transfusion may be due to homing of cells to the bone marrow where they differentiated to reseed the peripheral circulation with enucleated iPSC-RBCs. To check the distribution of iPSC-RBCs in vivo, we collected bone marrow, spleen, liver, and lung from NSG mice after transfusion. Flow cytometric analyses showed that a substantial amount of CD235a+ human cells migrated into bone marrow cells on day 1 after transfusion (Figures 4A, S5A). On day 24 after transfusion, more than 20% of bone marrow cells were CD235a+ human cells (Figures 4A,C, S5A). In contrast, only small population of iPSC-RBCs resided in spleen, liver, or lung at the time point (Figure 4B,C). Flow cytometry further revealed that more than 90% of human iPSC-RBCs in murine bone marrow were nucleated (DRAQ5+) (Figures 4D, S5B) and CD49d+ CD233+ (Figure 4E). The control murine bone marrow cells from naïve NSG mice were CD49d− CD233− (Figure 4F). There were no detectable human T cells or B cells in murine bone marrow of NSG mice transfused with human iPSC-RBCs (Figure 4G).
FIGURE 4.
Distribution of human induced pluripotent stem cells (iPSC)-red blood cells (RBCs) in NOD scid gamma (NSG) mice after transfusion. (A) Bone marrow cells were isolated from naïve NSG mice or clodronate liposomes (CL)- and cobra venom factor (CVF)-treated NSG mice transfused with human iPSC-RBCs on day 1, 6, 12, or 24 after transfusion. The cells were profiled by flow cytometry with anti-human CD235a antibody. Dot plots were represented from one of the three independent experiments. (B) Cells were also isolated from spleen, liver, or lung of CL- and CVF-treated NSG mice on day 24 after transfusion, and analyzed by flow cytometry with anti-human CD235a antibody. Dot plots were representative from one of the three independent experiments. (C) The percentages of human CD235a+ iPSC-RBCs in those tissues on day 24 were calculated. Data were from three independent experiments. (D) Bone marrow human CD235a+ cells (on day 24) were further stained with DRAQ5 dye, anti-human CD49d and CD233 antibodies. DRAQ5+ cells were gated and presented as a dot plot. (E) CD49d and CD233 double positive cells were gated from DRAQ5+ nucleated human iPSC-RBCs and presented as a dot plot. (F) Bone marrow cells from naïve NSG mice, which served as control cells, were stained with anti-human CD49d and CD233 antibodies and subjected to flow cytometry. (G) Alternatively, Bone marrow cells from NSG mice transfused with human iPSC-RBCs were stained with anti-human CD3 and CD19 antibodies before flow cytometry
4 |. DISCUSSION
Human iPSC-RBCs represent a potentially attractive alternative to donor-derived RBCs for transfusion, especially in niche situations where they could fill the need for products with difficult-to-source phenotypes. Additionally, they could represent a safer alternative to conventional RBC units, since they could be produced without antigens or soluble factors that cause some of the common and/or serious adverse reactions associated with RBC transfusion. However, much work remains to be done before iPSC-RBCs can be transfused to human recipients, including optimizing culture conditions for cGMP-compliant cell production, and confirming the in vivo survival of iPSC-RBCs.
In this study, we demonstrated that hPL promoted the maturation of human iPSC-RBCs in vitro. This product is a fibrinogen-depleted, cGMP-compliant cell culture supplement derived from plateletpheresis products of healthy donors.32 A freeze/thaw process used during manufacturing releases growth factors, cytokines, and other proteins from platelet granules.35 While hPL, like human serum, is a mixture of components including growth factors and cytokines, the concentrations of some proteins are higher in hPL than human serum.32 Furthermore, hPL has been utilized as a substitute of fetal bovine serum for the ex vivo growth and expansion under cGMP conditions of human cells such as mesenchymal stromal cells and stem cells.32,36 In this study, we examined the effect of cGMP-compliant hPL, human serum, or transfusion-grade albumin on the maturation of human iPSC-RBCs. There were no significant differences between hPL and human serum on iPSC-RBCs maturation, based on phenotype, or cell expansion. Human albumin has been used for erythroblast and RBC culture in vitro,37 but in this study it was less effective at promoting iPSC-RBCs expansion and maturation. This outcome is likely due to the lack of necessary growth factors in albumin preparations.38 These data suggest that hPL, a frequently utilized culture supplement for human cell therapy application, could also serve as a potent media supplement for human iPSC-RBCs cultures.
Erythroblasts undergo well-characterized changes as they differentiate into mature RBCs.39 For example, CD49d and CD71 decrease during latter stages of erythroid differentiation, and become undetectable in mature human RBCs.40 During the three-stage differentiation protocol used in these studies, we observed that cultured human iPSC-RBCs also lost these markers, becoming CD49dlow and CD71low, while maintaining high-level expression of CD233 and CD235a. Nonetheless, at the end of the culture period, the residual CD49d and CD71 expression contrasted with normal human RBCs that are negative for these markers. Interestingly, after transfusion, human iPSC-RBCs underwent further phenotypic change, becoming CD49d− and CD71−, while retaining CD233 and CD235a positivity. This result suggests that cultured human iPSC-RBCs can continue to mature in vivo beyond what is currently possible in culture.
Another characteristic of RBCs maturation is enucleation.41 Consistent with previous investigations,23 this study revealed detectable, but relatively low levels of enucleation for iPSC-RBCs at the end of the third culture stage. This inefficient enucleation may be due to the low-level expression of enucleation-related proteins on the surface of iPSC-derived erythroblasts, or other factors missing in culture.42 Surprisingly, however, cultured human iPSC-RBCs underwent rapid enucleation within 24 h after transfusion into CL- and CVF-treated immunodeficient NSG mice. More than 90% of circulating human iPSC-RBCs were enucleated, indicating that factors missing in culture could be provided after transfusion to complete the enucleation process. The enucleation of erythroblasts in bone marrow occurs in the niche of erythroblastic islands,41 involving the interaction between macrophages and erythroblasts, and the phagocytic removal of erythroid nucleus by central macrophages.43,44 It is likely that the enucleation occurs in bone marrow shortly post transfusion.
In this study, NSG mice were continuously treated with CL by intravenous injection before and after transfusion, with the intent of depleting murine phagocytic cells including macrophages. Nonetheless, even with repetitive CL dosing, enucleation occurred. This may indicate that either the elimination of macrophages was incomplete,45 or that other macrophage-independent pathways for the enucleation of human erythroid cells exist in vivo.
While NSG mice lack functional mature T cells, B cells, and NK cells,46 these immune system defects were insufficient to support significant human RBC circulation. We detected few circulating human RBCs in naïve NSG mice on day 1 after transfusion. However, consistent with previous publications, we found that combined injection of CL and CVF, a complement depleting agent,34 enhanced the percentage of circulating human RBC in NSG mice. These results confirmed that depletion of macrophages and complement synergically enhanced the survival of human iPSC-RBCs RBCs in murine peripheral circulation.
NSG mice treated with CL and CVF supported high levels of iPSC engraftment, preferentially in the bone marrow. The infused iPSCs that took up residence in the marrow persisted for at least 3 weeks after transfusion, where they constituted upward of 20% of marrow cells. Additionally, they appeared to retain the capability to complete differentiation into enucleated erythrocytes that egressed from the bone marrow into the peripheral blood. Bone marrow provides the required molecular and cellular niches for erythroid cell development, differentiation, and maturation.43 Phenotyping of marrow cells showed that the DRAQ5-positive human iPSCs were CD49 and CD233 double positive, bearing the typical surface markers of the late-stage erythroblasts in human bone marrow.47 Our findings suggest that bone marrow-residing human iPSC-RBCs in NSG mice retained the capability to complete differentiation into enucleated erythrocytes and egress from the bone marrow into the peripheral blood.
In summary, our data demonstrate that hPL serves as a potent media supplement for the GMP-compliant production of iPSC-RBCs. Additionally, iPSC-RBCs differentiated under these conditions can survive after transfusion into CL/CVF-treated NSG mice. While mature iPSC-RBCs from these cultures circulated comparably to normal human RBCs, nucleated cells took up residence in the bone marrow where at least a fraction of the cells were able to complete maturation. These findings offer a new model using human peripheral blood-derived iPSC and CL/CVF-treated NSG mice to investigate the development and circulation of human erythroid cells in vivo.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NIH (1RO1HL138656-01; T32 HL069769-11) (John D. Roback, Sean R. Stowell), Pediatric Research Alliance Award (John D. Roback), the Regenerative Engineering and Medicine Seed Grant (John D. Roback, Sean R. Stowell), and the Emory Department of Pathology SOUP Fund (Jiusheng Deng). The authors thank Shannon Bonds for collecting blood samples from healthy donors.
Funding information
Emory Department of Pathology SOUP Fund; Regenerative Engineering and Medicine Seed Grant; Pediatric Research Alliance Award; NIH, Grant/Award Number: 1RO1HL138656-01; NIH, Grant/Award Number: T32 HL069769-11
CONFLICT OF INTEREST
John D. Roback is a co-founder, consultant, and stock holder in Cambium Medical Technologies, which produces hPL for human use.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Shander A, Gross I, Hill S, Javidroozi M, Sledge S, College of American P, American Society of A, Society of Thoracic S, Society of Cardiovascular A, Society of Critical Care M. A new perspective on best transfusion practices. Blood Transfus. 2013;11(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connell NT. Transfusion medicine. Prim Care. 2016;43(4):651–659. [DOI] [PubMed] [Google Scholar]
- 3.Roberts N, James S, Delaney M, Fitzmaurice C. The global need and availability of blood products: a modelling study. Lancet Haematol. 2019;6(12):e606–e615. [DOI] [PubMed] [Google Scholar]
- 4.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120(3):528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4(2):327–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narbey D, Habibi A, Chadebech P, et al. Incidence and predictive score for delayed hemolytic transfusion reaction in adult patients with sickle cell disease. Am J Hematol. 2017;92(12):1340–1348. [DOI] [PubMed] [Google Scholar]
- 7.Vidler JB, Gardner K, Amenyah K, Mijovic A, Thein SL. Delayed haemolytic transfusion reaction in adults with sickle cell disease: a 5-year experience. Br J Haematol. 2015;169(5):746–753. [DOI] [PubMed] [Google Scholar]
- 8.Habibi A, Mekontso-Dessap A, Guillaud C, et al. Delayed hemolytic transfusion reaction in adult sickle-cell disease: presentations, outcomes, and treatments of 99 referral center episodes. Am J Hematol. 2016;91(10):989–994. [DOI] [PubMed] [Google Scholar]
- 9.Pirenne F, Yazdanbakhsh K. How I safely transfuse patients with sickle-cell disease and manage delayed hemolytic transfusion reactions. Blood. 2018;131(25):2773–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean CL, Maier CL, Chonat S, et al. Challenges in the treatment and prevention of delayed hemolytic transfusion reactions with hyperhemolysis in sickle cell disease patients. Transfusion. 2019;59(5):1698–1705. [DOI] [PubMed] [Google Scholar]
- 11.Dean CL, Maier CL, Roback JD, Stowell SR. Multiple hemolytic transfusion reactions misinterpreted as severe vaso-occlusive crisis in a patient with sickle cell disease. Transfusion. 2018;59:448–453. [DOI] [PubMed] [Google Scholar]
- 12.Arthur CM, Chonat S, Fasano R, et al. Examining the role of complement in predicting, preventing, and treating hemolytic transfusion reactions. Transfus Med Rev. 2019;33(4):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chonat S, Arthur CM, Zerra PE, et al. Challenges in preventing and treating hemolytic complications associated with red blood cell transfusion. Transfus Clin Biol. 2019;26(2):130–134. [DOI] [PubMed] [Google Scholar]
- 14.Chonat S, Quarmyne MO, Bennett CM, et al. Contribution of alternative complement pathway to delayed hemolytic transfusion reaction in sickle cell disease. Haematologica. 2018;103:e483–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chonat S, Graciaa S, Shin HS, et al. Eculizumab for complement mediated thrombotic microangiopathy in sickle cell disease. Haematologica. 2020;105(12):2887–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thein SL, Pirenne F, Fasano RM, et al. Hemolytic transfusion reactions in sickle cell disease: underappreciated and potentially fatal. Haematologica. 2020;105(3):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stowell CP, Stowell SR. Biologic roles of the ABH and Lewis histo-blood group antigens part I: infection and immunity. Vox Sang. 2019;114(5):426–442. [DOI] [PubMed] [Google Scholar]
- 18.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108(3):759–769. [DOI] [PubMed] [Google Scholar]
- 19.Frazier SK, Higgins J, Bugajski A, Jones AR, Brown MR. Adverse reactions to transfusion of blood products and best practices for prevention. Crit Care Nurs Clin North Am. 2017;29(3):271–290. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83(6):719–724. [PubMed] [Google Scholar]
- 21.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. [DOI] [PubMed] [Google Scholar]
- 23.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95(10):1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thom CS, Chou ST, French DL. Mechanistic and translational advances using iPSC-derived blood cells. J Exp Pathol. 2020;1(2):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz JP, Chen G, Haro Mora JJ, et al. Robust generation of erythroid and multilineage hematopoietic progenitors from human iPSCs using a scalable monolayer culture system. Stem Cell Res. 2019;41:101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Tian L, Dai X, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hockemeyer D, Jaenisch R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell. 2016;18(5):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Focosi D, Pistello M. Effect of induced pluripotent stem cell technology in blood banking. Stem Cells Transl Med. 2016;5(3):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowey SN, Huang X, Chou BK, Ye Z, Cheng L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat Protoc. 2012;7(11):2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arsham MS, Barch MJ, Lawce HJ. The AGT Cytogenetics Laboratory Manual. 4th ed. Wiley-Blackwell; 2017. [Google Scholar]
- 31.Liu S, Wu M, Lancelot M, et al. BMI1 enables extensive expansion of functional erythroblasts from human peripheral blood mononuclear cells. Mol Ther. 2021;29:1918–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Copland IB, Garcia MA, Waller EK, Roback JD, Galipeau J. The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials. 2013;34(32):7840–7850. [DOI] [PubMed] [Google Scholar]
- 33.Cevallos RR, Hossain ME, Zhang R, Hu K. Evaluating reprogramming efficiency and pluripotency of the established human iPSCS by Pluripotency markers. Methods Mol Biol. 2021;2239:235–249. [DOI] [PubMed] [Google Scholar]
- 34.Chen B, Fan W, Zou J, et al. Complement depletion improves human red blood cell reconstitution in Immunodeficient mice. Stem Cell Reports. 2017;9(4):1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roffi A, Filardo G, Assirelli E, et al. Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed Res Int. 2014;2014:692913 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieback K, Fernandez-Munoz B, Pati S, Schafer R. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Transfusion. 2019;59(11):3448–3460. [DOI] [PubMed] [Google Scholar]
- 37.Heshusius S, Heideveld E, Burger P, et al. Large-scale in vitro production of red blood cells from human peripheral blood mononuclear cells. Blood Adv. 2019;3(21):3337–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An X, Schulz VP, Mohandas N, Gallagher PG. Human and murine erythropoiesis. Curr Opin Hematol. 2015;22(3):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson-Luque R, Wang C, Ntumngia FB, et al. In-depth phenotypic characterization of reticulocyte maturation using mass cytometry. Blood Cells Mol Dis. 2018;72:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trakarnsanga K, Wilson MC, Griffiths RE, et al. Qualitative and quantitative comparison of the proteome of erythroid cells differentiated from human iPSCs and adult erythroid cells by multiplex TMT labelling and nanoLC-MS/MS. PLoS One. 2014;9(7):e100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3(4):a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow A, Huggins M, Ahmed J, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahmig S, Kronstein-Wiedemann R, Fohgrub J, et al. Improved human erythropoiesis and platelet formation in humanized NSGW41 mice. Stem Cell Rep. 2016;7(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Liu J, Xue F, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121(16):3246–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.