Abstract
Purpose:
There is increasing evidence that coffee consumption is related to reduced risks for some cancers, but the evidence for renal cancer is inconclusive. Therefore, we conducted a meta-analysis to summarize the cohort evidence of this relationship.
Methods:
A literature search was performed in PubMed and Embase through February 2021. Meta-analyses using a random effects model were conducted for reported relative risk estimates (RRs) relating coffee intake and renal cancer incidence or mortality. We also performed a two-stage random-effects exposure-response meta-analysis. Between-study heterogeneity was assessed.
Results:
In a meta-analysis of the ten identified cohort studies, we found a summary RR of 0.88 [95% confidence interval (CI) 0.78–0.99] relating the highest vs. the lowest category of coffee intake and renal cancer, with no significant between-study heterogeneity observed (I2=35%, p=0.13). This inverse association remained among studies of incident cancers (RR 0.85, 95% CI 0.76–0.96) and studies adjusting for smoking and body mass index (RR 0.87, 95% CI 0.77–0.99).
Conclusions:
Our findings from this meta-analysis of the published cohort evidence are suggestive of an inverse association between coffee consumption and renal cancer risk.
Keywords: coffee, kidney cancer, cohort study, meta-analysis
Background
Renal cancer is estimated to have accounted for 2.4% of all estimated worldwide cancer cases in 2018 (1) and was the sixth and tenth most commonly diagnosed cancer among US men and women respectively (2). The US incidence rate of cancers of the kidney and renal pelvis in 2017 (15.6 per 100,000) has increased 24% since 2000 (12.5 per 100,000) and 120% since 1975 (7.8 per 100,000) (3), partly because of incidental detection of asymptomatic tumors using medical imaging (4). Modifiable risk factors of renal cancer established to date include excess body weight (5, 6), hypertension (7) and tobacco smoking (8). There is also accumulating evidence supporting an inverse association with alcohol consumption (9).
Coffee, one of most popular beverages worldwide, contains antioxidants and anticarcinogenic compounds (10, 11) and has been associated with reduced risks of some malignancies such as cancers of the liver, colon/rectum, and endometrium (12, 13). The epidemiologic evidence relating coffee intake to renal cancer risk, however, has been inconclusive. A previous meta-analysis (14) including 16 case-control and six cohort studies found no significant association between coffee intake and renal cancer risk. However, a weak inverse relationship was observed in an analysis restricted to cohort studies, which are less susceptible to bias than case-control studies (15). Since the publication of that meta-analysis (14) several large cohort analyses investigating coffee consumption and renal cancer risk have been published (16–19). To better understand the cohort evidence of this relationship, we conducted a new meta-analysis focusing on evidence from this study design.
Methods
Search strategy
We performed a literature search in PubMed, Embase, and The Cochrane Library for studies published through February 2021, using the Medical Subject Headings (MeSH) terms or key words “cancer”, “tumor”, “carcinoma”, “malignant neoplasm”, “renal cancer”, “kidney cancer”, “renal cancer” “coffee”, “caffeine” and “beverages” (Supplementary File 1). Additionally, the reference lists of all retrieved articles, including previous relevant meta-analysis and review articles, were checked to identify additional studies. We followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines throughout the design, conduct, analysis, and reporting of this meta-analysis (20).
Study selection, data extraction, and quality assessment
We included cohort studies which reported relative risks (RRs) with corresponding 95% confidence intervals (CIs) estimating the association between coffee intake (any kind) and renal cancer. The studies reporting RRs and 95% CIs for more than three categories of coffee intake as well as category-specific number of participants and number of renal cancers were further eligible for exposure-response meta-analysis. We excluded review articles, abstracts, editorial letters, non-English articles, and relevant studies of coffee and cancer risk that did not include renal cancer specifically (Fig. 1).
Fig. 1.
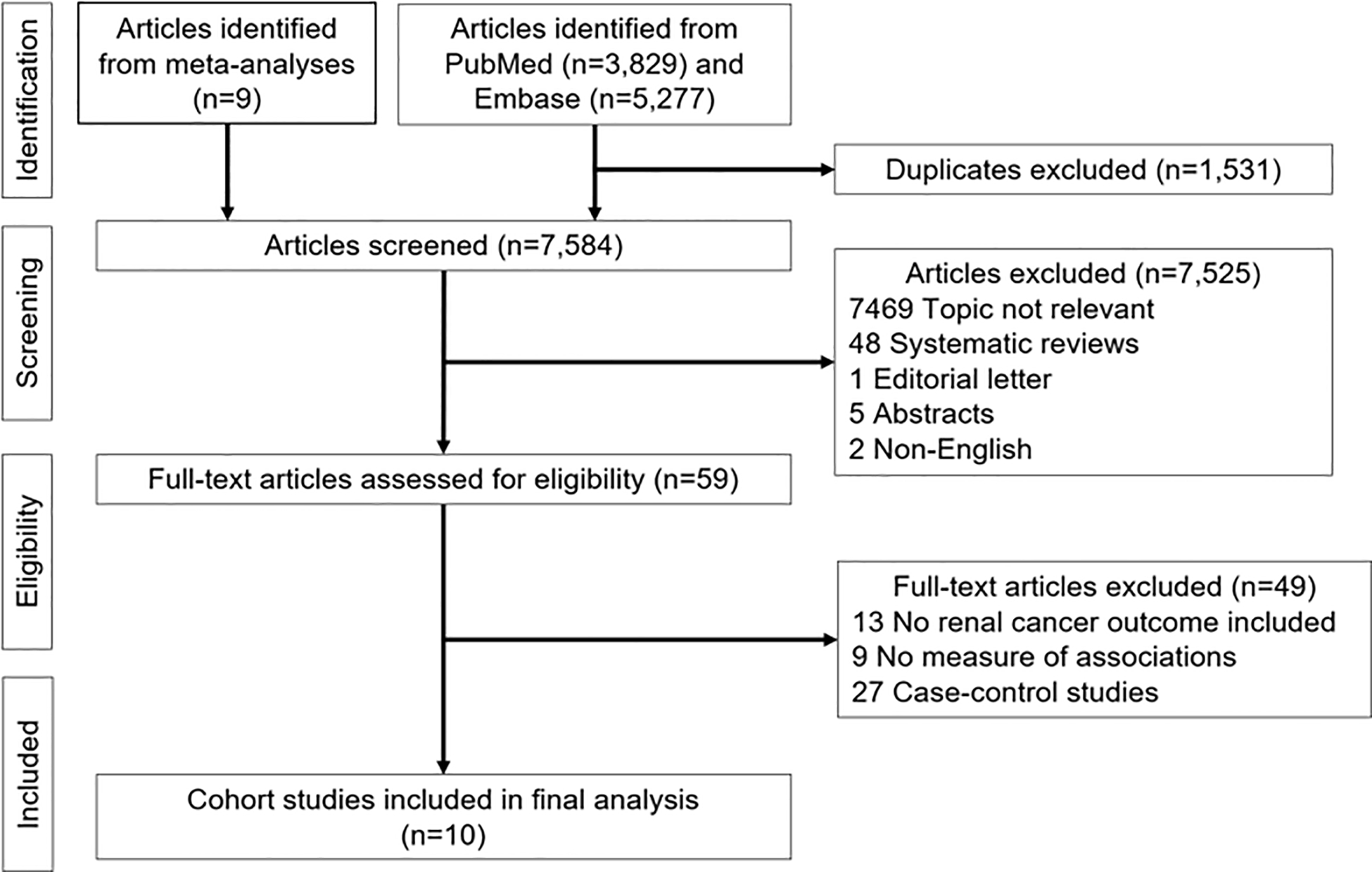
Literature search results for publications related to coffee consumption and risk of renal cancer
From each selected study we extracted the following information: authors, publication year, study geographic region, number of years of follow-up, type of endpoint (incident cases, deaths), renal cancer definition, number of participants, number of cases, scales of coffee intake (categorical and/or continuous number of drinks per day), most fully adjusted RRs and corresponding 95% CIs, and adjusted variables in the multivariable models. For the exposure-response meta-analysis, we extracted category-specific ranges of coffee intake, its most fully adjusted RRs and corresponding 95% CIs. All extracted data were cross-checked by the authors.
We performed quality assessments using the Newcastle-Ottawa scale (21) for cohort studies which addresses subject selection, study comparability, and the assessment of outcome.
Statistical analysis
Meta-analyses for RRs contrasting the highest vs. lowest level of coffee intake per day were performed using a random effects model. We additionally performed a two-stage random-effects exposure-response meta-analysis to examine linear and a potential non-linear relationships between coffee intake and risk of renal cancer using the Greenland and Longnecker method (22, 23) for covariance approximation and the multivariate DerSimonian and Laird method (24, 25) to estimate summary RRs. For the analysis of a linear relationship, we calculated the median value of coffee intakes in each exposure category and matched it to the category-specific RR. For categories with undefined upper boundary (e.g. ≥4 cups/day), we calculated the median value assuming the range of the category to be the same as other defined intervals. For non-linear exposure-response meta-analysis, we used a restricted cubic spline model with 3 knots at fixed percentiles of the distribution (25%, median, 75%) (26). We excluded studies that did not report the number of participants or renal cancer cases for each category of coffee intake (18, 27, 28) from our analysis of linear/non-linear relationships to avoid biases in the estimates for the variances of the log RRs (29). We used a Wald-type test to calculate a P value for non-linearity testing that null hypothesis that the coefficient of the second spline was equal to zero (29).
As a sensitivity analysis, we restricted meta-analyses to study findings having non-drinkers as a reference group (18, 19, 27) instead of the least coffee drinkers (e.g. <1 cup/day or ≤2 cups/day) (16, 17, 28, 30–33). We also examined the summary risk of renal cancer excluding a study for which we manually calculated the effect estimates (30), a study having a different scale for coffee intake other than cup/day (e.g. occasions per day (31)), and a study which did not adjust for additional confounders other than age and sex (27). Similarly, we conducted influence analyses, repeating our meta-analysis after excluding each study one at a time.
Potential publication bias was evaluated by the asymmetrical shape of a funnel plot and by the p-value from Egger’s test (34). Between-study heterogeneity was assessed using Cochran Q-statistic and quantified by Higgins I2 statistic and associated 95% CIs (35). Sources of heterogeneity were explored by performing meta-regression and subgroup analyses by sex (women, men), study region (US/Canada, other regions), outcome definition (kidney cancer, renal cell carcinoma), type of outcome (incidence, mortality), smoking status (never smokers, ever smokers), BMI (study-specific BMI cutpoints splitting participants into categories of healthy weight and overweight/obesity; see Table 1 footnote) and adjustment for established risk factors for renal cancer (smoking (8), BMI (5, 6), alcohol consumption (9)).
Table 1.
Subgroup meta-analyses of coffee intake and risk of renal cancer comparing the highest vs. lowest levels
| Subgroups | No. of studies | No. of renal cancer cases/No. of participants | Summary RR (95% CI) | P for Heterogeneity | I2 (95% CI) | P for interaction |
|---|---|---|---|---|---|---|
| All studies | 10 | 8,399/3,577,921 | 0.88 (0.78, 0.99) | |||
| Study region | 0.45 | |||||
| North America (US, Canada) | 4 | 5,752/1,608,068 | 0.84 (0.76, 0.93) | 0.47 | 0.0% (0.0%, 81.8%) | |
| Others | 6 | 2,647/1,969,853 | 0.93 (0.72, 1.21) | 0.07 | 51.1% (0.0%, 80.5%) | |
| Sex | 0.90 | |||||
| Men | 4 | 3,166/36,4925 | 0.89 (0.69, 1.15) | 0.08 | 56.1% (0.0%, 85.5%) | |
| Women | 4 | 1,874/416,352 | 0.85 (0.72, 1.02) | 0.38 | 3.3% (0.0%, 85.2%) | |
| Outcome definition | 0.40 | |||||
| Kidney cancer | 7 | 5,081/2,313,831 | 0.91 (0.81, 1.01) | 0.55 | 0.0% (0.0%, 70.8%) | |
| Renal cell carcinoma | 3 | 3,318/1,264,090 | 0.78 (0.54, 1.14) | 0.03 | 72.2% (6.0%, 91.8%) | |
| Type of outcome | 0.35 | |||||
| Incidence | 8 | 6,444/2,540,508 | 0.85 (0.76, 0.96) | 0.26 | 21.7% (0.0%, 63.7%) | |
| Mortality | 2 | 1,955/1,037,413 | 1.37 (0.50, 3.72) | 0.06 | 71.1% (0.0%, 93.5%) | |
| Smoking status | 0.15 | |||||
| Never smokers | 4 | 1,043/570,922 | 0.79 (0.59, 1.08) | 0.02 | 75.5% (19.3%, 92.6%) | |
| Ever smokers | 4 | 1,991/529,504 | 0.96 (0.87, 1.06) | 0.29 | 18.5% (0.0%, 91.5%) | |
| BMI | 0.96 | |||||
| Healthy weight* | 4 | 1,909/518,784 | 0.98 (0.91, 1.05) | 0.20 | 35.8% (0.0%, 77.7%) | |
| Overweight or obesity* | 4 | 3,115/613,343 | 0.96 (0.88, 1.05) | 0.01 | 71.5% (18.8%, 90.0%) | |
| Adjustments for renal cancer risk factors (smoking and BMI) | 0.70 | |||||
| Yes | 7 | 8,018/3,323,097 | 0.87 (0.77, 0.99) | 0.14 | 38.2% (0.0%, 74.0%) | |
| No | 3 | 381/254,824 | 1.12 (0.59, 2.14) | 0.13 | 50.9% (0.0%, 85.8%) |
RR: relative risk, BMI: body mass index
Gapstur et al. (16) categorized individuals’ smoking status as never, former, or current smokers but only presented stratified results for never and former smokers. We included the RR of former smokers in the analysis for ever smokers. Number of participants for never and former smokers were manually calculated using % presented in Table 1. Number of renal cancer cases were only presented in the study for never and former smokers combined (n=189) so this table does not include renal cancer cases stratified by smoking from Gapstur et al. (16)
Lukic et al. (17) did not present number of participants stratified by smoking status.
Park et al. (18) did not present number of renal cancer cases stratified by sex and Lee et al. (28) did not present number of participants by sex so this table does not include these numbers.
Lee et al. (28) presented stratified RR by smoking status (never, past, and current smokers) for an increment of one cup of coffee so we calculated 4 cups/day vs. nondrinkers for never and current smokers.
Four studies (18, 19, 28, 32) provided results of analyses stratified by BMI using different cut-points. 30kg/m2, Park et al. (18); 25kg/m2, Rhee et al. (19), Lee et al. (28); 25.4kg/m2, Allen et al.(32). Lee et al. (28) and Park et al.(18) did not present number of participants by BMI categories so they are not included in this table.
All statistical analyses were conducted with the metaphor (36) and dosresmeta (37) packages in R (R Foundation for Statistical Computing, Vienna, Austria) (38). P values less than 0.05 were considered statistically significant.
Results
The results of our systematic review of the published literature are summarized in Fig. 1. From our collection of screened articles (n=7,584) we excluded irrelevant topic articles (n=7,469), systematic reviews (n=48), editorial letter (n=1), abstracts (n=5), and non-English articles (n=2). Among 59 full-text articles assessed for eligibility, we further excluded relevant articles not reporting measures of association (n=9), relevant articles including only non-renal cancer outcomes (n=13), and case-control studies (n=27). Following these exclusions, ten cohort studies remained. These remaining studies had estimated Newcastle-Ottawa total quality scores of 9 (n=9) or 8 (n=1) (Supplementary Table 1).
In a meta-analysis of the ten identified studies (16–19, 27, 28, 30–33), which collectively included 8,399 renal cancer cases, we found a summary RR of 0.88 (95% CI 0.78–0.99) relating the highest study category of coffee intake (vs. the lowest category or non-drinkers) and renal cancer risk (Fig. 2), with no significant between-study heterogeneity observed (I2=35%, 95% CI 0–69.1%, p=0.13). In the influence analyses, we observed the summary RRs ranged from 0.86 (95% CI 0.76–0.97, excluding Allen et al. (32), lowest RR; Fig. 3) to 0.91 (95% CI 0.80–1.03, excluding Rhee et al. (19), highest RR). Visual inspection of the funnel plot suggested that the studies were nearly symmetrically distributed around the log of the summary estimate (Fig. 4), and Egger’s tests presented that there was no significant evidence of publication bias (p=0.78).
Fig. 2.
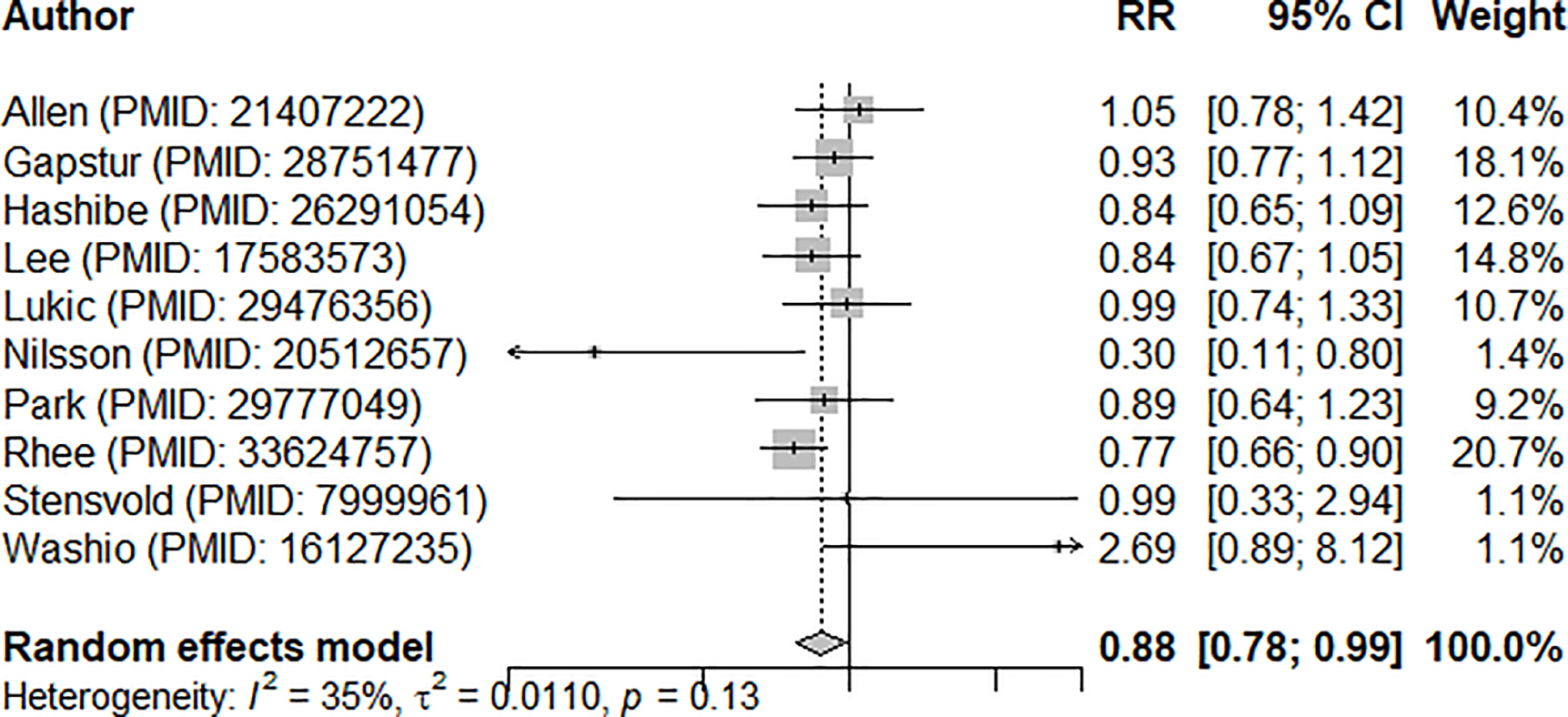
Forest plot of meta-analysis summarizing cohort evidence relating coffee intake and risk of renal cancer (comparing highest vs. lowest levels)
RR: relative risk, CI: confidence interval
Fig. 3.
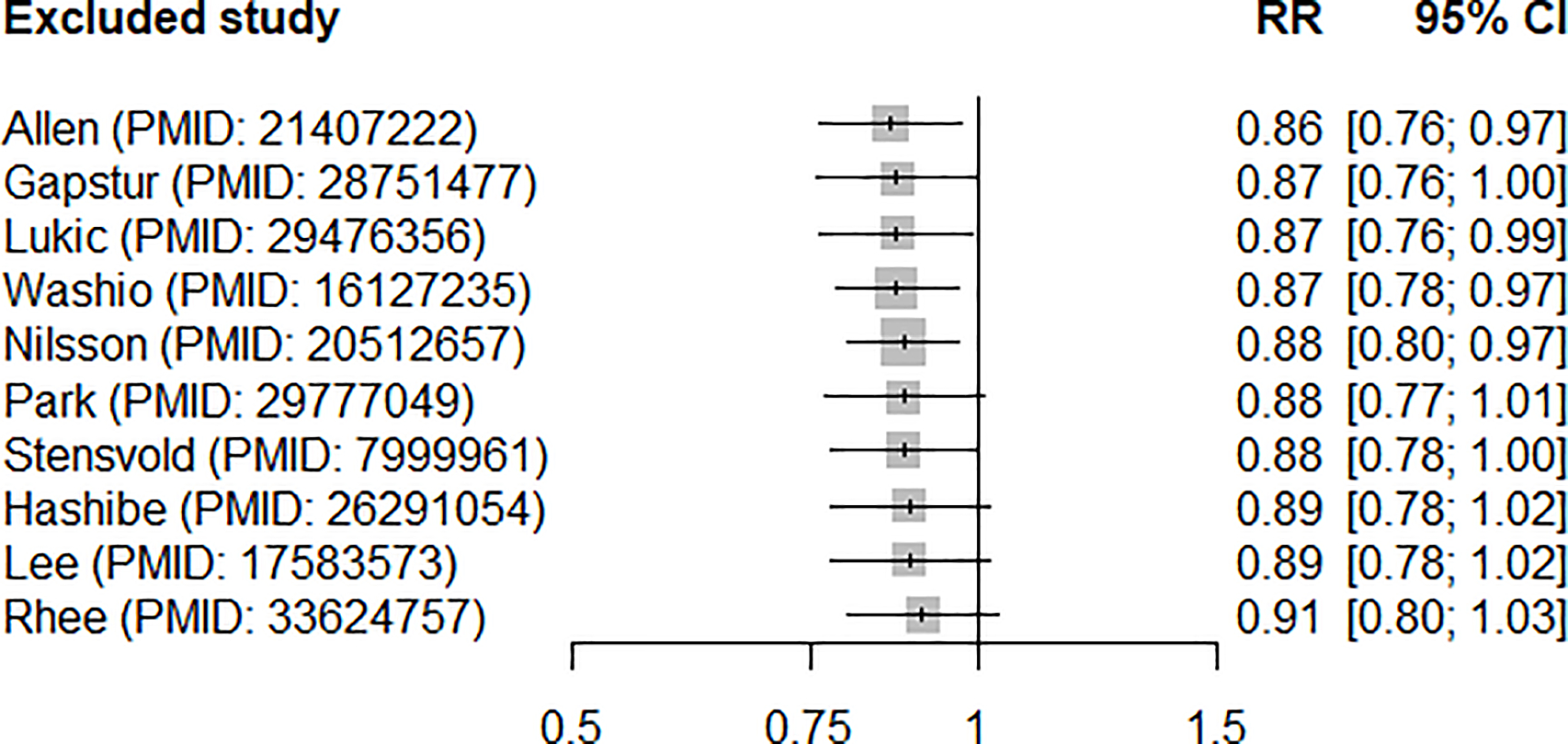
Results of meta-analyses of coffee intake (highest vs. lowest) and risk of renal cancer after excluding each study one at a time
RR: relative risk, CI: confidence interval
Fig. 4.
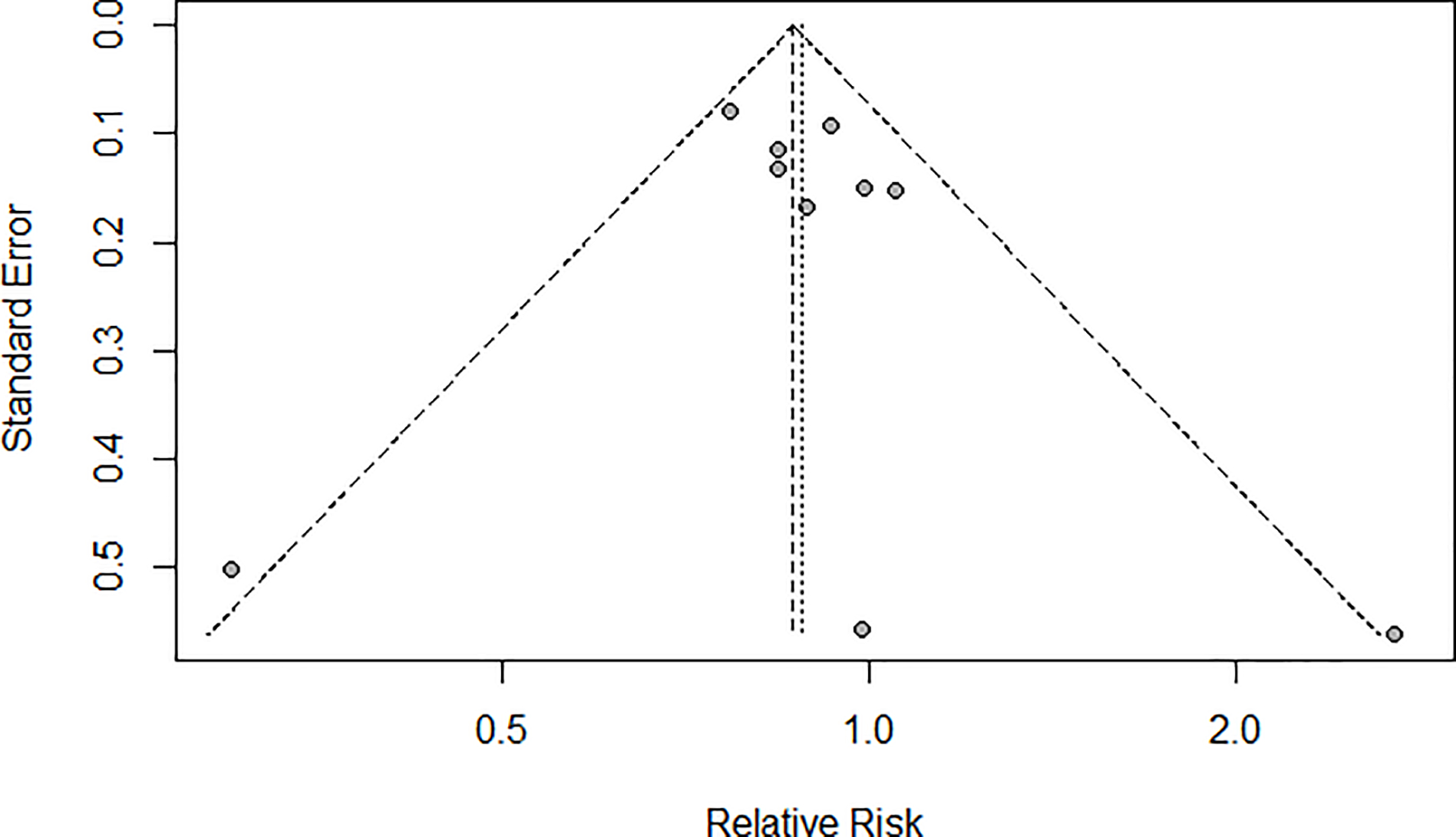
Funnel plot of meta-analysis on coffee intake (highest vs. lowest) and risk of renal cancer
In the exposure-response meta-analysis, which included seven studies (16, 17, 19, 30–33), the test for non-linear effects was not statistically significant (p for non-linearity=0.13, Supplementary Figure 1). In computing a linear exposure-response relationship, we estimated that one additional cup of coffee per day was associated with a 3.0% decrease in the risk of renal cancer (summary RR 0.97, 95% CI 0.94–1.00).
The meta-regression tests showed that none of the evaluated study characteristics (sex, study region, outcome definition, type of outcome, smoking status, BMI, adjustment of renal cancer risk factors) significantly modified the summary RR of coffee intake on the risk of renal cancer (Table 1). The summary RR of comparing the highest vs. lowest coffee intake and risk of renal cancer was comparable for men and women (0.89, 95% CI 0.69–1.15 and 0.85, 95% CI 0.72–1.02, respectively; p for interaction=0.90). There was no difference in the association by outcome definition (p=0.40) as more than 90% of kidney cancers are renal cell carcinoma (39). In meta-analyses of results stratified by smoking status, a stronger inverse association was observed among never smokers (RR 0.79, 95% CI 0.59–1.08) than ever smokers (RR 0.96, 95% CI 0.87–1.06), although a test of interaction was not statistically significant (p=0.15). The inverse association remained when restricted to studies of incident renal cancer (17, 19, 28, 30–33) (RR 0.85, 95% CI 0.76–0.96) and those which adjusted for both smoking and BMI (16–19, 28, 31, 32) (RR 0.87, 95% CI 0.77–0.99) as well as studies with additional adjustment for alcohol consumption (16–19, 28) (RR 0.85, 95% CI 0.78–0.94).
In sensitivity analyses we found that the summary RRs of the highest vs. lowest meta-analysis were similar after excluding a study with manually calculated RR (30) (summary RR 0.88, 95% CI 0.78–1.00), a study with a different scale for coffee intake (31) (RR 0.88, 95% CI 0.80–0.97), and a study only adjusting for age and sex (27) (RR 0.87, 95% CI 0.78–0.96). We observed a similar association when we restricted studies to those having non-drinkers as a reference group (18, 19, 27) (summary RR of highest vs. lowest 0.90, 95% CI 0.64–1.27).
Discussion
In this meta-analysis of ten cohort studies, we found a 22% lower risk of renal cancer in the highest coffee intake group compared to the lowest category with no significant between-study heterogeneity. The inverse associations remained when we restricted to studies with incident renal cancer as the outcome and those adjusting for smoking, BMI and alcohol intake. The linear exposure-response analysis of seven studies estimated a 3% lower risk of renal cancer for one additional cup of coffee per day. There was no evidence of publication bias in our study.
Our findings suggest that the cohort evidence for an inverse association between coffee intake and renal cancer is stronger than that observed in the previous meta-analysis (14) (summary RR 0.88, 95% CI 0.70–1.10). We additionally conducted a linear exposure-response analysis and found a 3% lower RR of renal cancer for one additional cup of coffee a day. It is notable that two studies (18, 28) which could not be included in the exposure-response analysis due to missing information on case frequencies across exposure categories reported similar RRs per cup per day (0.97, 95% CI 0.93–1.01 (28); 0.95, 95% CI 0.90–1.01 (18), respectively). Interestingly, an identical magnitude of association was observed in previous meta-analyses of coffee in relation to total cancer (40) and endometrial cancer (13) (RR 0.97 per one cup of coffee/day, 95% CI 0.96–0.98 in each meta-analysis).
We observed a non-significantly stronger inverse association among never smokers (RR 0.79, 95% CI 0.59–1.08) compared to coffee RRs among smokers (RR 0.96, 95% CI 0.87–1.06) for the four studies (16, 17, 19, 28) that presented RRs stratified by smoking status. We further observed an inverse association among seven studies that presented smoking-adjusted RRs (summary RR 0.87, 95% CI 0.77–0.99) (16–19, 28, 31, 32), but no association among three studies that provided RRs without adjustment of smoking (RR 1.12, 95% CI 0.59, 2.14) (27, 30, 33). The potential for residual confounding towards the null from smoking may at least partially account for the null association among ever smokers, as coffee consumption is more common among smokers than non-smokers (41). We found a low level of between-study heterogeneity overall, although we observed a slightly stronger inverse association after excluding Allen et al. (32) (which reported substantially higher coffee intakes than other studies; Supplementary Table 1) and a slightly weaker, non-significant association after excluding Rhee et al. (19). None of the study characteristics appeared to materially affect the summary effect estimates.
Our findings are compatible with the hypothesis that coffee intake may be associated with a lower renal cancer risk. There are several biologic mechanisms that potentially play a role in mediating such an effect from coffee. Cafestol and kahweol, natural diterpenes extracted from coffee beans, have been shown to have a role in anticarcinogenic activity (11). Particularly, it has been found that cafestol modulates multiple proteins in apoptotic response of Caki-1 human renal cancer cell lines in an in vivo study (42). Coffee intake has been associated with lower risks of chronic kidney disease (CKD) (43–45) and lower estimated glomerular filtration rate, which are themselves risk factors for renal cancer (46). Additionally, coffee intake has been related to higher insulin sensitivity and lower risk of type 2 diabetes (47), another renal cancer risk factor. Finally, coffee intake may dilute the concentration of carcinogens in renal epithelial cells by increasing urine volume (28).
There are several strengths to this meta-analysis. We substantially updated the cohort evidence in the recent meta-analysis (14) by including four additional studies involving over 5,600 incident renal cancer cases (16–19, 30). Our restriction to cohort-based evidence helped avoid summarizing potentially biased risk estimates due to recall or selection bias in case-control studies. Unlike with the previous meta-analysis, we performed exposure-response meta-analyses and assessed the robustness of our findings through several sensitivity and subgroup analyses. We observed consistent findings in the influence test and found no evidence of publication bias.
Our study has some limitations to be noted. First, variation in the definition of cup size could lead to misclassification of coffee consumption. Poole et al. (48) found that when standardized by volume (227 mL), 84% of study participants had correctly classified reported intakes, 8% underestimated, and 8% overestimated, compared to reported cups. Second, we were not able to evaluate potential differences in renal cancer risk by coffee type (caffeinated vs. decaffeinated) or brewing method (boiled vs. filtered) due to the limited number of studies providing such results. Among studies providing evidence, it is unclear whether coffee type or brewing method modifies the association. Nilsson et al (31) reported a stronger inverse association for filtered coffee intake (hazard ratio (HR) 0.46, 95% CI 0.17–1.24 for coffee intake ≥4 occasions/day vs. <1 occasion/day) than boiled coffee (0.90, 95% CI 0.28–2.97). However, Lukic et al (17) found similar associations for both brewing methods. Both Gapstur et al (16) and Rhee et al (19) did not find significant differences in renal cancer risk for caffeinated and decaffeinated coffee. Third, selected studies mainly included studies conducted in North America and Europe; it is unclear whether the observed findings are generalizable to Asian or African populations.
In conclusion, in this meta-analysis of ten cohort studies we observed an inverse association between higher coffee consumption and lower renal cancer risk. Additional large prospective investigations of coffee consumption and renal cancer risk in diverse populations are needed to clarify this relationship, as well as experimental and molecular studies to elucidate possible anti-neoplastic mechanisms related to coffee consumption.
Supplementary Material
Funding:
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology & Genetics.
Abbreviations:
- MeSH
Medical Subject Headings
- MOOSE
Meta-analysis Of Observational Studies in Epidemiology
- RR
relative risk
- CI
confidence intervals
- CKD
chronic kidney disease
- BMI
body mass index
Footnotes
Conflict of interests/Competing interests: The authors declare no conflict of interest.
Code availability: Researchers who are interested in R codes for the analyses may contact the authors.
Ethics approval and consent to participate: the systematic review and meta-analysis do not require ethical approval or consent to participate.
Consent for publish: NA
Contributor Information
Jongeun Rhee, Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Rachel K. Lim, Brown University, Providence, RI, USA
Mark P. Purdue, Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA
Data availability:
All data reported in this manuscript are found in the literature as cited in the text.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. (2018) Cancer statistics, 2018. CA Cancer J Clin. 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. (April 2020) SEER Cancer Statistics Review, 1975–2017. National Cancer Institute. Bethesda, MD. [Google Scholar]
- 4.Chow W-H, Devesa SS, Warren JL, Fraumeni JF Jr. (1999) Rising incidence of renal cell cancer in the United States. JAMA. 281: 1628–31. [DOI] [PubMed] [Google Scholar]
- 5.Bergström A, Hsieh C, Lindblad P, Lu C, Cook N, Wolk A. (2001) Obesity and renal cell cancer–a quantitative review. Br J Cancer. 85: 984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjørge T, Tretli S, Engeland A. (2004) Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 160: 1168–76. [DOI] [PubMed] [Google Scholar]
- 7.Hidayat K, Du X, Zou S-Y, Shi B-M. (2017) Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 35: 1333–44. [DOI] [PubMed] [Google Scholar]
- 8.Cumberbatch MG, Rota M, Catto JW, La Vecchia C. (2016) The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 70: 458–66. [DOI] [PubMed] [Google Scholar]
- 9.Song DY, Song S, Song Y, Lee JE. (2012) Alcohol intake and renal cell cancer risk: a meta-analysis. Br J Cancer. 106: 1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Ruiz JÁ, Leake DS, Ames JM. (2007) In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem. 55: 6962–9. [DOI] [PubMed] [Google Scholar]
- 11.Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber W, Schilter B. (2002) Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol. 40: 1155–63. [DOI] [PubMed] [Google Scholar]
- 12.Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. (2017) Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukic M, Guha N, Licaj I, et al. (2018) Coffee drinking and the risk of endometrial cancer: an updated meta-analysis of observational studies. Nutr Cancer. 70: 513–28. [DOI] [PubMed] [Google Scholar]
- 14.Wijarnpreecha K, Thongprayoon C, Thamcharoen N, Panjawatanan P, Cheungpasitporn W. (2017) Association between coffee consumption and risk of renal cell carcinoma: a meta-analysis. Intern Med J. 47: 1422–32. [DOI] [PubMed] [Google Scholar]
- 15.Gibson TM, Ferrucci LM, Tangrea JA, Schatzkin A. (2010) Epidemiological and clinical studies of nutrition. Semin Oncol. 37: 282–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gapstur SM, Anderson RL, Campbell PT, et al. (2017) Associations of coffee drinking and cancer mortality in the cancer prevention study-II. Cancer Epidemiol Biomarkers Prev. 26: 1477–86. [DOI] [PubMed] [Google Scholar]
- 17.Lukic M, Nilsson LM, Skeie G, Lindahl B, Braaten T. (2018) Coffee consumption and risk of rare cancers in Scandinavian countries. Eur J Epidemiol. 33: 287–302. [DOI] [PubMed] [Google Scholar]
- 18.Park S-Y, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. (2018) Prospective study of coffee consumption and cancer incidence in non-white populations. Cancer Epidemiol Biomarkers Prev. 27: 928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee J, Loftfield E, Freedman ND, Liao LM, Sinha R, Purdue MP. (2021) Coffee consumption and risk of renal cell carcinoma in the NIH-AARP Diet and Health Study. Int J Epidemiol. dyab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Tugwell P, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 15. 2021
- 22.Greenland S, Longnecker MP. (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 135: 1301–9. [DOI] [PubMed] [Google Scholar]
- 23.Orsini N, Bellocco R, Greenland S. (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J. 6: 40–57. [Google Scholar]
- 24.DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials. 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 25.Jackson D, White IR, Thompson SG. (2010) Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 29: 1282–97. [DOI] [PubMed] [Google Scholar]
- 26.Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. (2014) Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 180: 763–75. [DOI] [PubMed] [Google Scholar]
- 27.Washio M, Mori M, Sakauchi F, et al. (2005) Risk factors for kidney cancer in a Japanese population: findings from the JACC Study. J Epidemiol. 15: S203–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JE, Hunter DJ, Spiegelman D, et al. (2007) Intakes of coffee, tea, milk, soda and juice and renal cell cancer in a pooled analysis of 13 prospective studies. Int J Cancer. 121: 2246–53. [DOI] [PubMed] [Google Scholar]
- 29.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 175: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stensvold I, Jacobsen BK. (1994) Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 5: 401–8. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson LM, Johansson I, Lenner P, Lindahl B, Van Guelpen B. (2010) Consumption of filtered and boiled coffee and the risk of incident cancer: a prospective cohort study. Cancer Causes Control. 21: 1533–44. [DOI] [PubMed] [Google Scholar]
- 32.Allen N, Balkwill A, Beral V, Green J, Reeves G. (2011) Fluid intake and incidence of renal cell carcinoma in UK women. Br J Cancer. 104: 1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashibe M, Galeone C, Buys SS, et al. (2015) Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br J Cancer. 113: 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger M, Smith GD, Schneider M, Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ. 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. (2002) Quantifying heterogeneity in a meta-analysis. Stat Med. 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 36.Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw. 36: 1–48. [Google Scholar]
- 37.Crippa A, Orsini N. (2016) Multivariate dose-response meta-analysis: the dosresmeta R package. J Stat Softw. 72: 1–15. [Google Scholar]
- 38.Team RC. (2013) R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- 39.Hsieh JJ, Purdue MP, Signoretti S, et al. (2017) Renal cell carcinoma. Nature reviews. Disease primers. 3: 17009-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Bao Z, Zou J, Dong J. (2011) Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson JA, Lee JW, Hopp JW. (1994) Caffeine and nicotine: a review of their joint use and possible interactive effects in tobacco withdrawal. Addict Behav. 19: 229–56. [DOI] [PubMed] [Google Scholar]
- 42.Choi MJ, Park EJ, Oh JH, et al. (2011) Cafestol, a coffee-specific diterpene, induces apoptosis in renal carcinoma Caki cells through down-regulation of anti-apoptotic proteins and Akt phosphorylation. Chem Biol Interact. 190: 102–8. [DOI] [PubMed] [Google Scholar]
- 43.Jhee JH, Nam KH, An SY, et al. (2018) Effects of coffee intake on incident chronic kidney disease: a community-based prospective cohort study. Am J Med. 131: 1482–90. [DOI] [PubMed] [Google Scholar]
- 44.Hu EA, Selvin E, Grams ME, Steffen LM, Coresh J, Rebholz CM. (2018) Coffee consumption and incident kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 72: 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigotte Vieira M, Magriço R, Viegas Dias C, Leitão L, Neves JS. (2019) Caffeine consumption and mortality in chronic kidney disease: a nationally representative analysis. Nephrol Dial Transplant. 34: 974–80. [DOI] [PubMed] [Google Scholar]
- 46.Herber-Gast G-CM, Essen Hv, Verschuren WM, et al. (2016) Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem Cohort Study. Am J Clin Nutr. 103: 1370–7. [DOI] [PubMed] [Google Scholar]
- 47.Van Dam RM, Feskens EJ. (2002) Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 360: 1477–8. [DOI] [PubMed] [Google Scholar]
- 48.Poole R, Ewings S, Parkes J, Fallowfield JA, Roderick P. (2019) Misclassification of coffee consumption data and the development of a standardised coffee unit measure. BMJ Nutr Prev Health. bmjnph-2018–000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this manuscript are found in the literature as cited in the text.


