Abstract
The Triage parasite panel (BIOSITE Diagnostics, San Diego, Calif.) is a new qualitative enzyme immunoassay (EIA) panel for the detection of Giardia lamblia, Entamoeba histolytica/E. dispar, and Cryptosporidium parvum in fresh or fresh, frozen, unfixed human fecal specimens. By using specific antibodies, antigens specific for these organisms are captured and immobilized on a membrane. Panel performance was evaluated with known positive and negative stool specimens (a total of 444 specimens) that were tested by the standard ova and parasite (O&P) examination as the “gold standard,” including staining with both trichrome and modified acid-fast stains. Specimens with discrepant results between the reference and Triage methods were retested by a different method, either EIA or immunofluorescence. A number of samples with discrepant results with the Triage device were confirmed to be true positives. After resolution of discrepant results, the number of positive specimens and the sensitivity and specificity results were as follows: for G. lamblia, 170, 95.9%, and 97.4%, respectively; for E. histolytica/E. dispar, 99, 96.0%, and 99.1%, respectively; and for C. parvum, 60, 98.3%, and 99.7%, respectively. There was no cross-reactivity with other parasites found in stool specimens, including eight different protozoa (128 challenges) and three different helminths (83 challenges). The ability to perform the complete O&P examination should remain an option for those patients with negative parasite panel results but who are still symptomatic.
With the increasing interest in rapid diagnostic testing, potential waterborne outbreak situations, fewer well-trained microscopists, and confirmation that Giardia lamblia, Entamoeba histolytica/E. dispar, and Cryptosporidium parvum can cause severe symptoms in humans, laboratories are reviewing their options with regard to immunoassay kits that can be incorporated into their routine testing protocols (2, 4–6, 15, 17–21, 24–27, 32). Not only must these methods be acceptable in terms of sensitivity and specificity but they must provide clinically relevant, cost-effective, rapid results, particularly in a potential waterborne outbreak situation (1, 3, 11, 23).
It is well known that protozoan cysts, in particular, Giardia cysts, are not shed in the stool on a consistent basis and that their numbers vary from day to day; this is also true of coccidian oocysts. Examination of stool specimens collected on consecutive days or even within the recommended 10-day time frame may not confirm infection with Giardia, E. histolytica/E. dispar, or C. parvum (13). In patients who are infected with one or more of these parasites, the use of routine diagnostic methods such as concentration and trichrome and modified acid-fast staining may be insufficient to demonstrate the presence of these organisms (16, 33). Renewed awareness of potential waterborne transmission of these parasites is based on the number of well-documented outbreaks during the past few years and the publicity surrounding water regulations and testing.
Among patients with cryptosporidiosis, the majority of immunocompetent patients have initially been symptomatic, with large numbers of oocysts present in their stools. In this situation, a number of diagnostic procedures would be acceptable (8, 12, 13). However, as the acute infection resolves and the patient becomes asymptomatic, the number of oocysts dramatically decreases. Also, the number of oocysts passed by patients, including those with AIDS, varies from day to day and week to week. It has also been established that the infective dose of Cryptosporidium oocysts in humans can be relatively low (7, 10).
Antigen detection assays for G. lamblia, E. histolytica/E. dispar, and C. parvum have proved to be very useful in the diagnosis of these infections (4–6, 9, 14–22, 28–31). The advantages of these assays include labor, time, and batching efficiencies that may lead to cost reductions. Certainly, these reagents offer alternative methods to the routine ova and parasite (O&P) examination method and provide the added sensitivity required to confirm infections in patients with low parasite numbers.
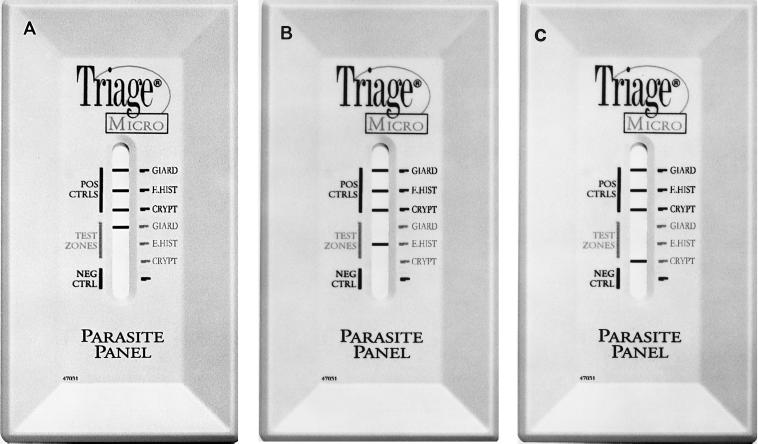
On the basis of the need for improved diagnostic procedures, a rapid immunoassay device for the detection of Giardia, E. histolytica/E. dispar, and Cryptosporidium antigens has been developed (Fig. 1). This BIOSITE Diagnostics (San Diego, Calif.) Triage rapid qualitative enzyme immunoassay (EIA) can be performed in approximately 15 min with fresh or fresh, frozen, unfixed human fecal specimens. This device was tested against known positive and negative fecal specimens on the basis of the results of the O&P examination for the detection of G. lamblia and E. histolytica/E. dispar and on the basis of the results of modified acid-fast staining for the detection of C. parvum. Specimens with discrepant results were retested by EIA or fluorescent-antibody methods.
FIG. 1.
BIOSITE EIA Triage parasite panel demonstrating positive results. (A) Positive and negative controls and positive test zone for G. lamblia (GIARD); (B) positive and negative controls and positive test zone for E. histolytica/E. dispar (E. HIST); (C) right, positive and negative controls and positive test zone for C. parvum (CRYPT).
MATERIALS AND METHODS
Specimens.
Fresh, unpreserved stool specimens were used according to the manufacturer's directions for testing the Triage parasite panel. Specimens (n = 444) were collected in clean, leak-proof containers and were frozen and maintained at −20°C or colder prior to testing. A total of 444 specimens were tested by the reference methods and with the Triage parasite panel.
Routine O&P examination, modified acid-fast staining examination.
Immediately after collection and prior to freezing, a portion of each stool specimen was placed into a vial with 10% formalin and a vial with polyvinyl alcohol. The O&P examination (formalin-ethyl acetate [FeAc] concentration, trichrome staining) and modified acid-fast staining (FeAc concentration, modified acid-fast staining) were considered the reference methods (12, 13). The modified acid-fast stain was prepared from the FeAc concentration sediment (centrifugation at 500 × g for 10 min) (12, 13). Of the 444 specimens examined, a certain number were positive for the following parasites on the basis of the results of the reference methods: Giardia, n = 142 specimens; E. histolytica/E. dispar, n = 42 specimens; and Cryptosporidium, n = 58 specimens. Different parasites (eight protozoa and three helminths; 211 challenges) were also found among the 444 specimens. Specific organisms included Blastocystis hominis (n = 71), Chilomastix mesnili (n = 2), Dientamoeba fragilis (n = 2), Trichomonas hominis (n = 2), Endolimax nana (n = 27), Iodamoeba bütschlii (n = 16), Entamoeba coli (n = 2), Entamoeba hartmanni (n = 6), hookworm eggs (n = 2), Ascaris lumbricoides (n = 74), and Trichuris trichiura eggs (n = 7). Many specimens had multiple parasites, while some were negative for all parasites. Although the results of the O&P examinations were known, the specimens were coded and tested blind when the Triage parasite panel was used.
Specimen preparation for EIA methods.
All EIA kits were used with fresh or fresh, frozen stool specimens.
Triage parasite panel.
The following immunoassay diagnostic kit was used according to the manufacturer's directions: Triage parasite panel (BIOSITE Diagnostics). Using specific antibodies, antigens specific for Giardia, E. histolytica/E. dispar, and Cryptosporidium are captured and immobilized on a membrane. The assay procedure involves the addition of 4.5 ml of specimen diluent to the specimen tube. Sample (0.5 ml) is added, and the mixture is vortexed for at least 10 s. This diluted, mixed sample is centrifuged at 1,500 × g for at least 5 min. The sample supernatant is poured into the sample filter device and is filtered into the filtrate tube. The filtered sample (0.5 ml) is then added to the center of the test device with a transfer pipette. Enzyme conjugate (140 μl) is added to the center of the membrane. Six drops of wash solution is added to the membrane; this step is repeated once. Then, four drops of the substrate is added to the membrane, followed by a 5-min incubation at 15 to 25°C. The device is then read and the results are interpreted. Positive results are visualized as purple-black lines in the appropriate position in the results window. The tubes, pipettes, devices, and all reagents are provided with the kit. Positive and negative controls are included in the device, and the total time is approximately 15 min.
Testing for resolution of discrepant results.
Specimens with discrepant results for G. lamblia and C. parvum were retested by the Alexon-Trend ProSpecT microplate EIA for Giardia and the Meridian Diagnostics Merifluor combination Cryptosporidium-Giardia reagent for G. lamblia and C. parvum. The Alexon-Trend ProSpectT microplate assay for E. histolytica/E. dispar was used to test specimens with discrepant results for this group of organisms.
RESULTS
EIA for Giardia.
On the basis of the results of the O&P examination reference method, known positive specimens (G. lamblia, n = 142) and negative samples (n = 302) were tested by use of the Triage parasite panel. Additional positive specimens (n = 28) were identified by using the Triage parasite panel. All specimens with discrepant results with the Triage parasite panel were retested by the immunoassay (IA) method designated for discrepancy resolution. If positive by any two methods, the specimen was considered truly positive. After resolution, the total number of positive specimens was 170, the sensitivity was 95.9%, the specificity was 97.4%, and the negative predictive value (NPV) was 97.4% (Tables 1 and 2).
TABLE 1.
Comparison of results prior to and after testing of specimens with discrepant resultsa
| Organism | Result (no. of specimens) | No. of specimens with the indicated results
|
|||||
|---|---|---|---|---|---|---|---|
| O&P examination, permanent stains (reference methods)
|
Triage parasite panel
|
After EIA or FA to resolve discrepancies
|
|||||
| Pos | Neg | Pos | Neg | Pos | Neg | ||
| G. lamblia | Pos (170) | 142 | 302 | 170 | 274 | 170 | 274 |
| Neg (274) | |||||||
| Total (444) | |||||||
| E. histolytica/E. dispar | Pos (99) | 42 | 401 | 98 | 345 | 99 | 344 |
| Neg (344) | |||||||
| Total (443) | |||||||
| C. parvum | Pos (60) | 58 | 386 | 60 | 384 | 60 | 384 |
| Neg (384) | |||||||
| Total (444) | |||||||
Abbreviations: FA, fluorescent antibody; Pos, positive; Neg, negative.
TABLE 2.
Sensitivity, specificity, and NPV data compared with data for true-positive and true-negative specimens
| Method |
G. lamblia
|
E. histolytica/E. dispar
|
C. parvum
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | NPV (%) | Sensitivity (%) | Specificity (%) | NPV (%) | Sensitivity (%) | Specificity (%) | NPV (%) | |
| O&P examination, permanent stains (reference methods) | 79.4 | 97.4 | 88.4 | 38.4 | 98.8 | 84.8 | 88.3 | 98.7 | 98.2 |
| Triage parasite panel | 95.9 | 97.4 | 97.4 | 96.0 | 99.1 | 98.8 | 98.3 | 99.7 | 99.7 |
EIA for E. histolytica/E. dispar.
On the basis of the results of the O&P examination reference method, positive specimens (E. histolytica/E. dispar, n = 42) and negative samples (n = 401) were tested with the Triage parasite panel; 1 specimen could not be tested; thus, the total was 443. Additional positive specimens (n = 56) were identified with the Triage parasite panel, and one specimen with a false-negative result was seen. All specimens with discrepant results with the Triage parasite panel were retested by the EIA method designated for discrepancy resolution. If positive by any two methods, the specimen was considered truly positive. After resolution, the total number of positive specimens was 99, the sensitivity was 96.0%, the specificity was 99.1%, and the NPV was 98.8% (Tables 1 and 2).
EIA for Cryptosporidium.
On the basis of the results of modified acid-fast staining, positive specimens (C. parvum, n = 58) and negative samples (n = 386) were tested with the Triage parasite panel. Additional positive specimens (n = 2) were identified with the Triage parasite panel. All specimens with discrepant results with the Triage parasite panel were retested by immunofluorescence (Tables 1 and 2). If positive by any two methods, the specimen was considered truly positive. After resolution, the number of positive specimens was 60, the sensitivity was 98.3%, the specificity was 99.7%, and the NPV was 99.7%.
DISCUSSION
The selection of a particular diagnostic kit and approach for incorporation into the work flow should be the responsibility of each laboratory. These decisions are based on a number of factors, including clinical relevance, cost-containment, anticipated workload, ease of kit performance, number of trained staff, single-sample versus batched-sample testing, physician clients, physician ordering patterns, size and configuration of client base, laboratory size, availability of equipment, ease with which a new procedure fits into the routine laboratory work flow, turnaround time for achieving a result, reporting limitations (computer system), and the necessity for staff training and client in-service information distribution.
The rapid immunoassays do not replace routine O&P examinations, but they are very useful when trying to confirm Giardia and Cryptosporidium infections (12). Some laboratories have included both the O&P examination and a Giardia or Cryptosporidium screen in their test menus; both are separate, orderable tests. On the basis of the results of the O&P examination with trichrome stain and the modified acid-fast stain and commercial EIA and immunofluorescence kits for testing of specimens with discrepant results, it is clear that the routine microscopy methods used in this study do not reveal as many positive specimens as the more rapid, newer immunoassay reagents. With the need for strict requirements for specimen collection and fixation, plus the availability of fewer well-trained microscopists who can recognize the subtle differences between organisms for organism differentiation, additional more rapid tests will serve as excellent adjunct methods to the O&P examination, provided that the pros and cons of each approach are clearly recognized. Fecal specimen panels and potential modifications in laboratory test menus should be reviewed in light of these and other published results (2, 4–6, 15, 17–21, 24–33).
It has been reported that the Giardia EIA can detect Giardia in at least 30% more specimens than the microscopic examination (31), and it has been reported to have a sensitivity and specificity of 98 and 100%, respectively (4). In another study, the sensitivity and specificity of ColorPAC (Becton Dickinson) for Giardia detection were 100 and 100%, respectively (15), while an earlier study reported an EIA sensitivity of 97% and a specificity of 96% (30). Other studies reported a range in sensitivity from 91.4 to 100% and a range in specificity from 97.8 to 100% (6). Sensitivities and specificities in studies for the detection of C. parvum have ranged from 66.3 to 100% and 93 to 100%, respectively, with the sensitivities and specificities in the majority of studies ranging from 93 to 100% and 98 to 100%, respectively (2, 15, 16). Various studies looking at antigen detection in stool specimens for the detection of E. histolytica/E. dispar have reported sensitivities and specificities that range from 68.3 to 95% and 97 to 99%, respectively (18, 19, 25, 27). Stool antigen studies for pathogenic E. histolytica provide sensitivities and specificities that range from 87 to 97.6% and 92.6 to 98%, respectively (5, 17, 20, 21).
Although the sensitivities and specificities reported for all of the available immunoassay kits are similar, some formats are more time-consuming and labor-intensive. The ability to concurrently detect and distinguish between G. lamblia, E. histolytica/E. dispar, and C. parvum antigens in fresh or fresh, frozen fecal specimens with a 15-min qualitative EIA panel provides the laboratorian with another very useful diagnostic tool, and this can be accomplished with the BIOSITE Triage parasite panel. The Triage parasite panel procedure is simple to perform, requires minimal training, and can be used for single-specimen or batch-testing approaches. The Triage parasite panel will provide diagnostic laboratories with a simple, convenient, alternative method for performing simultaneous, discrete detection of Giardia-, Cryptosporidium-, and E. histolytica/E. dispar-specific antigens in patient fecal specimens.
REFERENCES
- 1.Addis D G, David J P, Roberts J M, Mast E E. Epidemiology of Giardiasis in Wisconsin: increasing incidence of reported cases and unexplained season trends. Am J Trop Med Hyg. 1992;47:13–19. doi: 10.4269/ajtmh.1992.47.13. [DOI] [PubMed] [Google Scholar]
- 2.Arrowood M J. Diagnosis. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 43–64. [Google Scholar]
- 3.Atherton F, Newman C P, Casemore D P. An outbreak of waterborne cryptosporidiosis associated with a public water supply in the UK. Epidemiol Infect. 1995;115:123–131. doi: 10.1017/s0950268800058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr M A, Kokoskin E, Gyorkos T W, Cédilotte L, Faubert G M, MacLean J D. Laboratory diagnosis for Giardia lamblia infection: a comparison of microscopy, coprodiagnosis and serology. Can J Infect Dis. 1997;8:33–38. doi: 10.1155/1997/270179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskar S, Singh S, Sharma M. A single-step immunochromatographic test for the detection of Entamoeba histolytica antigen in stool samples. J Immunol Methods. 1996;196:193–198. doi: 10.1016/0022-1759(96)00125-1. [DOI] [PubMed] [Google Scholar]
- 6.Boone J H, Wilkins T D, Nash T E, Brandon J E, Macias E A, Jerris R C, Lyerly D M. TechLab and Alexon Giardia enzyme-linked immunosorbent assay kits detect cyst wall protein 1. J Clin Microbiol. 1999;37:611–614. doi: 10.1128/jcm.37.3.611-614.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell C L, Okhuysen P C, Sterling C R, Wang C, Jakubowski W, DuPont H L. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg. 1999;60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 8.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;3:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doing K M, Hamm J L, Jellison J A, Marquis J A, Kingsbury C. False-positive results obtained with the Alexon ProSpecT Cryptosporidium enzyme immunoassay. J Clin Microbiol. 1999;37:1582–1583. doi: 10.1128/jcm.37.5.1582-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 11.Fayer R, Trout J M, Jenkins M C. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. J Parasitol. 1998;84:1165–1169. [PubMed] [Google Scholar]
- 12.Garcia L S. Practical guide to diagnostic parasitology. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 13.Garcia L S, Bruckner D A. Diagnostic medical parasitology. 3rd ed. Washington, D.C.: ASM Press; 1997. [Google Scholar]
- 14.Garcia L S, Shimizu R Y. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for the detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997;35:1526–1529. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia L S, Shimizu R Y. Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay. J Clin Microbiol. 2000;38:1267–1268. doi: 10.1128/jcm.38.3.1267-1268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia L S, Shum A C, Bruckner D A. Evaluation of a new monoclonal antibody combination reagent for direct fluorescent detection of Giardia cysts and Cryptosporidium oocysts in human fecal specimens. J Clin Microbiol. 1992;30:3255–3257. doi: 10.1128/jcm.30.12.3255-3257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Ruiz A, Haque R, Rehman T, Aguirre A, Hall A, Guhl F, Warhurst D C, Miles M A. Diagnosis of amebic dysentery by detection of Entamoeba histolytica fecal antigen by an invasive strain-specific, monoclonal antibody-based enzyme-linked immunosorbent assay. J Clin Microbiol. 1994;32:964–970. doi: 10.1128/jcm.32.4.964-970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque R, Ali I K, Akther S, Petri W A., Jr Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haque R, Neville L M, Hahn P, Petri W A., Jr Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol. 1995;33:2558–2561. doi: 10.1128/jcm.33.10.2558-2561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque R, Neville L M, Wood S, Petri W A., Jr Short report: detection of Entamoeba histolytica and E. dispar directly in stool. Am J Trop Med Hyg. 1994;50:595–596. doi: 10.4269/ajtmh.1994.50.595. [DOI] [PubMed] [Google Scholar]
- 21.Jelinek T, Peyerl G, Loscher T, Nothdurft H D. Evaluation of an antigen-capture enzyme immunoassay for detection of Entamoeba histolytica in stool samples. Eur J Clin Microbiol Infect Dis. 1996;15:752–755. doi: 10.1007/BF01691966. [DOI] [PubMed] [Google Scholar]
- 22.Kehl K C, Cicirello H, Havens P L. Comparison of four different methods for the detection of Cryptosporidium species. J Clin Microbiol. 1995;33:416–418. doi: 10.1128/jcm.33.2.416-418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie W R, Hoxie N J, Proctor M E. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 24.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong S J, Cheng M Y, Liu K H, Horng C B. Use of the ProSpecT microplate enzyme immunoassay for the detection of pathogenic and non-pathogenic Entamoeba histolytica in faecal specimens. Trans R Soc Trop Med Hyg. 1996;90:248–249. doi: 10.1016/s0035-9203(96)90234-5. [DOI] [PubMed] [Google Scholar]
- 26.Pieniazek N J, Bornay-Llinares F J, Slemenda S B, da Silva A J, Moura I N S, Arrowood M J, Ditrich O, Addis D G. New Cryptosporidium genotypes in HIV-infected persons. Emerg Infect Dis. 1999;5:444–449. doi: 10.3201/eid0503.990318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai D R, Kain K C. Immunochromatographic strip-based detection of Entamoeba histolytica-E. dispar and Giardia lamblia coproantigen. J Clin Microbiol. 1999;37:3017–3019. doi: 10.1128/jcm.37.9.3017-3019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priest J W, Kwon J P, Moss D M, Roberts J M, Arrowood M J, Dworkin M S, Juranek D D, Lammie P J. Detection of enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–1392. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenblatt J E, Sloan L M. Evaluation of an enzyme-linked immunosorbent assay for detection of Cryptosporidium spp. in stool specimens. J Clin Microbiol. 1993;31:1468–1471. doi: 10.1128/jcm.31.6.1468-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenblatt J E, Sloan L M, Schneider S K. Evaluation of an enzyme-linked immunosorbent assay for the detection of Giardia lamblia in stool specimens. Diagn Microbiol Infect Dis. 1993;16:337–341. doi: 10.1016/0732-8893(93)90086-m. [DOI] [PubMed] [Google Scholar]
- 31.Rosoff J D, Sanders C A, Sonnad S S, De Lay P R, Hadley W K, Vincenzi F F, Yajko D M, O'Hanley P D. Stool diagnosis of giardiasis using a commercially available enzyme immunoassay to detect Giardia-specific antigen 65 (GSA 65) J Clin Microbiol. 1989;27:1997–2002. doi: 10.1128/jcm.27.9.1997-2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe M S. Giardiasis. Clin Microbiol Rev. 1992;5:93–100. doi: 10.1128/cmr.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerman S K, Needham C A. Comparison of conventional stool concentration and preserved-smear methods with Merifluor Cryptosporidium/Giardia direct immunofluorescence assay and ProSpecT Giardia EZ microplate assay for detection of Giardia lamblia. J Clin Microbiol. 1995;33:1942–1943. doi: 10.1128/jcm.33.7.1942-1943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]