Abstract
Objectives
The aim was to outline the challenges of implementing outcomes-based contracts (OBCs) in Europe.
Methods
A scoping review was conducted, building on the searches of a previous systematic review and updating them for December 2017 until May 2021. The combined results were screened, based on inclusion and exclusion criteria. All identified studies published in the English language that described specific OBC schemes for medicines in European countries were included. Insights into the challenges of OBCs were extracted and analysed to develop a conceptual framework.
Results
Ten articles from the previous systematic review matched our inclusion criteria, along with 14 articles from electronic searches. Analysis of these 24 articles and classification of the challenges revealed that there are multiple barriers that must be overcome if OBCs that benefit all stakeholders are going to be adopted widely across Europe. These challenges were grouped according to five key themes: negotiation framework; outcomes; data; administration and implementation; and laws and regulation.
Conclusions
If the promise of OBCs is to be fully realised in Europe, there remain major challenges that need to be overcome by all stakeholders working in partnership. The overlapping and interconnected nature of these challenges highlights the complexity of OBC arrangements.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01070-1.
Key Points for Decision Makers
| An analysis of the literature and classification of the challenges concludes there are multiple barriers that must be addressed if outcomes-based contracts (OBCs) that benefit all stakeholders are going to be adopted widely across Europe. These challenges can be grouped according to five key themes: negotiation framework; outcomes; data; administration and implementation; and laws and regulation. |
| The overlapping and interconnected nature of these challenges highlights the complexity of OBC arrangements. Acknowledging this complexity is the first step to moving forward; parties need to develop a fundamentally different approach to problem solving to progress from there. |
Introduction
The development of personalised therapies for increasingly smaller subsets of patients and potentially curative interventions for genetic diseases has provided a challenge to healthcare systems and the pharmaceutical industry. Smaller populations can result in insufficient evidence for formal reimbursement decisions as well as challenging threshold limitations, with tension in achieving access to innovative therapies that is both sustainable and incentivises innovation. In Europe, this tension is being considered through different mechanisms, including new licensing and access pathways. There is concern that this may introduce methodological complexity into health technology assessments (HTAs) [1]. This has renewed the focus on how best to pursue a value-based healthcare approach, with the aim of providing the best possible outcomes that matter to patients at the same or reduced cost.
As part of this approach, the pharmaceutical industry and payers have considered contracting agreements designed to achieve value for patients by linking reimbursement to outcomes achieved (including clinical outcomes and/or patient-reported outcomes [PROs]). Outcomes-based contracts (OBCs) have the potential to offer an opportunity to pursue a more effective allocation of resources, maximise patient health outcomes, promote the use of medicines best fit for patients, and incentivise the generation of additional evidence [2, 3]. However, uptake across Europe has been mixed, with higher uptake in Italy and Spain [4, 5], but a trend to simple discounting in the UK and elsewhere [6]. This may provide short-term relief to resource-constrained systems, but does not lead to a sustainable innovative ecosystem.
This review was performed to assess the challenges of developing OBCs in Europe with a view to understanding their complexity and barriers to implementation. This is the first review specifically focused on the challenges associated with implementing OBCs across European health systems, therapeutic areas and stakeholders, and addresses the question, ‘What are the common challenges in implementing OBCs experienced by European health stakeholders?’
Methods
A scoping review based on Arksey and O’Malley’s (2005) framework [7] was used to address the research question. This approach gives an overview of an area of research that is heterogeneous and rapidly evolving. It involves identifying the research question, identifying and selecting studies, then extracting and analysing the data, and reporting results.
Relevant studies were identified through a literature search. The search terms were based on a research paper by Cole et al. [8], which was produced in partnership with leading European health institutions and includes interviews, patient surveys and case studies, as well as multiple systematic literature reviews (SLRs). It included a systematic search of studies that reference specific OBC examples, but it did not fully consider challenges in implementation, looked at a broader scope of countries, and has not been updated since 2018. As this review aimed to appraise OBC implementation challenges, which are highly contextual, the narrower scope of European countries was used. The original search by Cole et al. ran between January 2007 and January 2018 (the search terms are presented in the electronic supplementary material). The search was updated for the current paper to May 2021. The updated searches were run in PubMed, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Web of Science and EconLit, as was the case in Cole et al. [8]. The reference lists of SLRs eligible for inclusion were checked, and the search was extended by discussion with co-authors to identify any publications not published in peer-reviewed scientific papers. It became clear that saturation had been reached as no new subthemes were identified. After screening of titles and abstracts, full texts were reviewed independently according to the inclusion and exclusion criteria by two experienced reviewers. Data were extracted by independent reviewers using Microsoft Word and analysed using thematic analysis. Following familiarisation with the literature, passages of text describing specific challenges were extracted from included articles. These were coded in Microsoft Word, so that conceptual patterns could be identified. These were discussed and iterated by the reviewers to form higher order themes and subthemes, based on weight from the literature and consensus by the reviewers. This resulted in an inductively developed conceptual framework with a two-level hierarchy of broad themes and detailed subthemes. Following review of the included papers, the co-authors assessed the conceptual framework for clarity, comprehensiveness and credibility based on their experience. The framework was refined by integrating the interpretations of all authors until it was concluded that the data were fully contextualised.
Results
Database searches identified 390 records. Of those, 24 were shortlisted for full-text assessment, of which 12 met the inclusion criteria. From Cole et al. [8], ten studies met the inclusion criteria, and two additional studies were identified from the SLRs eligible for inclusion (Fig. 1). The main reason for exclusion was being a theoretical record, without reference to European OBCs (N = 11).
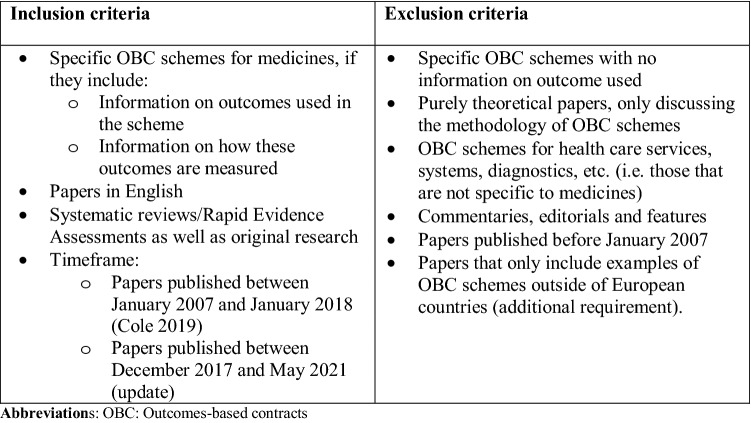
Fig. 1.
Inclusion and exclusion criteria for the literature search. OBCs outcomes-based contracts
The final sample included 24 studies (Table 1). Some studies were evaluations of a specific OBC case study, whilst others included a broader approach, with multiple OBC examples analysed in the same paper.
Table 1.
Studies included in the scoping review
| Author, year | Aim | Method | Core findings |
|---|---|---|---|
| Adamski et al., 2010 [16] | Analyse existing examples of 'risk-sharing' schemes for pharmaceuticals and related considerations | Literature review | A large number of ‘risk-sharing' schemes with pharmaceuticals are in existence incorporating both financial-based models and performance-based/outcomes-based models. There are a number of challenges associated with these schemes and a lack of publicly available evaluations |
| Antonanzas et al., 2019 [3] | Summarise RSAs from conceptual, theoretical and empirical perspectives, and determine stakeholder perceptions | Literature review | There are copious examples of how RSAs have been implemented, and stakeholders’ perceptions are usually favourable. However, theoretical modelling and empirical evidence of their value and economic impact are scarcer. A lack of transparency and aggregated registries are the main obstacles to understand whether these agreements have delivered the desired results for the healthcare systems |
| Bouvy et al., 2018 [15] | Evaluate MEAs for adaptative pathways products in Europe and explore how they can be enabled | Literature review, interview study and qualitative analysis | Outcomes-based MEAs were seldom used for technologies with a conditional marketing authorisation or authorised under exceptional circumstances, during the period of analysis (2006–2016). The main enablers for the uptake of outcomes-based MEAs are multi-country data collection, improved existing data infrastructures, flexible pricing models and support from HTA bodies and payers |
| Clopes et al., 2017 [9] | Estimate the financial consequences of the payment-by-results reimbursement model and determine the perception of the stakeholders involved in the agreement | Quantitative and qualitative analysis | Tangible and intangible benefits were identified with respect to the interests of the parties involved. This led to the incorporation of innovation for patients under acceptable conditions |
| Dabbous et al., 2020 [17] | Describe how MEAs are being implemented in Europe, and the main obstacles to their use | Literature review | MEAs currently used are predominantly finance-based and performance-based agreements, although the complexity associated with the latter deters their widespread use. CEDs are particularly useful to negotiate pricing and reimbursement when faced with uncertainty, facilitating market access |
| Darbà and Ascanio, 2019 [4] | Identify, characterise and analyse current publicly available agreement reports signed by the Catalan Health Service and different pharmaceutical companies evaluating the current market access scene for new drugs in Catalonia | Quantitative and qualitative analysis | MEAs are gaining popularity and are viewed as positive schemes by stakeholders, payers and health services, leading to a general increase of accords during recent years. However, there are hardly any studies regarding the impact of RSA post-implementation, a field of great relevance regarding health policies |
| Zampirolli Dias et al., 2020 [2] | Describe MEAs and understand the main concerns among payers and their advisers | Literature review | Most MEAs involved medicines for oncology. Financial-based schemes are more prevalent than outcome-based schemes, which is partially explained by the complexities associated with implementing and assessing the latter |
| Edo-Solsona et al., 2020 [27] | Describe a risk-sharing programme’s implementation and results on enzyme replacement therapy for lysosomal diseases | Observational study | The implanted risk-sharing programme is Spain’s first published event of paying for clinical results using orphan drugs. Economic impact has been limited, and programme implementation has gone through a complex process of formulation and management. However, the greatest achievement has been to reduce the knowledge gap between efficacy and effectiveness, stating that the therapies administered have shown the optimal benefits for which the funder is willing to pay |
| Garattini and Casadei, 2011 [5] | Understand lessons from the Italian implementation of performance-based arrangements | Qualitative analysis | There are a number of challenges associated with these schemes and a lack of publicly available evaluations |
| García-Collado et al., 2021 [21] | Assess the economic impact of an RSA on certolizumab pegol for rheumatoid arthritis and estimate the potential impact of an alternative agreement | Quantitative analysis | The RSA resulted in improved efficiency and savings in the hospital’s pharmacy service. The savings generated by the RSA would allow payment for the treatment of approximately 20% more patients |
| Garrison et al., 2013 [10] | Set out the standards that should be applied to good practices in the use of a PBRSA, encompassing questions around the desirability, design, implementation and evaluation of such an arrangement | Qualitative analysis | Additional evidence collection is costly, and there are numerous barriers to establishing viable and cost-effective PBRSAs: negotiation, monitoring and evaluation costs can be substantial |
| Jarosławski and Toumi, 2011 [22] | Analyse the differences between P4P and CED pharmaceutical reimbursement agreements and the motivations behind their creation | Qualitative analysis | While commercial agreements and P4P have the potential to reduce payers’ expenditure on costly drugs while maintaining a high list price, CED address initial uncertainty related to assessing the real-life value of new drugs and enable a final HTA recommendation or reimbursement and pricing decisions |
| Kim et al., 2020 [40] | Evaluate how PBRSAs for drug reimbursement have been used in different healthcare models | Literature review | PBRSAs can be used by payers, providers and manufacturers to cope with uncertainty surrounding technologies. However, the technology price should be scaled appropriately based on value |
| Lorente et al., 2019 [20] | Analyse how risk-sharing agreements have been implemented in Spain | Survey and quantitative analysis | Although risk-sharing agreements can promote budget control and drug management, as well as increase patients’ access to drugs, they pose specific challenges, such as infrastructure and managerial challenges. Pharmacists responding to a survey cited treatment efficacy and uncertainty as a key consideration when establishing pay-for-performance agreements. The authors suggest the creation of a national data registry as a starting point to improve data collection and record management |
| Makady et al., 2019 [23] | Evaluate the CF framework, focusing on HTA procedures | Qualitative and quantitative analysis | Theoretically, conditional financing provided an option for quick but conditional access to drugs. However, numerous aspects related to the design and implementation of CF negatively affected its value in practice. Future CED schemes should aim to incorporate learnings from the CF example to increase their impact in healthcare practice |
| Michelsen et al., 2020 [14] | Describe the implementation of outcome-based spread payments in Europe, focusing on existing barriers and ways of overcoming them | Literature review | Spread payments pose specific challenges, namely having to consider 12-month budget cycles, which hinders financial agreements and may violate international accounting rules. The need for additional data collection, absence of clear governance structures and administrative burden and costs pose additional challenges to outcome correction of payments |
| Navarria et al., 2015 [18] | Analyse the strategies currently approved in Italy and propose a novel model called ‘success fee’ to improve payment-by-result schemes and to guarantee patients rapid access to novel therapies | Quantitative and qualitative analysis | ‘Success fee’ represents an effective strategy to promote value-based pricing, making available to patients rapid access to innovative and expensive therapies, with an affordable impact on drug expenditure and, simultaneously, ensuring third-party payers to share with manufacturers the risk deriving from uncertain safety and effectiveness |
| Neumann et al., 2011 [11] | Analyse the potential opportunities and challenges associated with implementing risk-sharing agreements for pharmaceuticals in the United States | Qualitative analysis | Risk-sharing arrangements could gain traction in the United States as payers and product manufacturers acquire experience with the concept and as measurement techniques and information systems improve. For the foreseeable future, they are likely to remain the exception as drug companies pursue payment models unconnected to data collection or performance assessment |
| Persson et al., 2010 [41] | Describe actual ex-ante VBP decision-making process in Sweden, using the case of rimonabant in the treatment of obesity | Quantitative and qualitative analysis |
The decision to provide reimbursement coverage for pharmaceutical products is difficult. In Sweden, the HTA body responsible (LFN) must consider need and solidarity, cost-effectiveness and marginal utility, and they must identify the correct comparators, indications and appropriate disease severities The alternative is to delay the reimbursement approval until satisfactory evidence is available or until the manufacturer has reduced the price to the level at which the expected benefits of approval exceed the opportunity cost of approval |
| Pickin et al., 2009 [42] | Assess the feasibility of risk-sharing schemes, looking at long-term clinical outcomes, to determine the price at which high-cost treatments would be acceptable to the NHS | Case study | Successful recruitment, follow-up and early data analysis suggest that risk-sharing schemes should be able to deliver their objectives. However, important issues of analysis and political and commercial conflicts of interest still need to be addressed |
| Ronco et al., 2021 [43] | Investigate the processes for pricing and reimbursement of ATMPs in five European countries | Literature review | Pricing and reimbursement of ATMPs partially followed expert recommendations, and outcome-based MEAs have been increasingly used for ATMPs. However, annuity payments are still limited and perspectives in cost considerations are still narrow |
| Vreman et al., 2020 [13] | Assess the feasibility of financial and outcome-based MEAs, considering how they could be combined and variations in their implementation in regard to level, setting and therapeutic areas | Literature review | Most MEAs can be combined and applied across different settings. Reimbursement mechanisms can be tailored by decision makers to better suit the specificities of the therapy and the demands of the healthcare system |
| Wenzl and Chapman, 2019 [12] | Review MEAs in OECD countries and EU member states (2018/19) | Survey and interviews; quantitative and qualitative analysis | Lack of formal assessment and confidentiality agreements limit to what extent the impact of MEAs can be analysed. Although payment-by-results are progressively used, aggregated data are not always collected due to costs and administrative burden, with limited evidence on product performance. Overcoming these limitations can be achieved by defining a strategy on how to use performance-based MEAs, identifying uncertainties in each coverage decision, implementing a transparent governance framework and limiting confidentiality |
| Willis et al., 2010 [19] | To describe VBP reimbursement decision-making using CED in actual practice in Sweden | Case study | Despite reviewing the initial trial-based, ‘piggy-back’ economic analysis, TLV was uncertain of the cost-effectiveness in actual practice and deferred a final decision until observational data from the DAPHNE study became available. Second, acceptance of economic modelling and use of temporary reimbursement conditional on additional evidence development provide a mechanism for risk sharing between TLV and manufacturers, which enabled patient access to a drug with proven clinical benefit while necessary evidence to support claims of cost-effectiveness could be generated |
ATMP advanced therapy medicinal products, CED coverage with evidence development, CF conditional financing, HTA health technology assessment, MEAs managed entry agreements, NHS National Health Service, OECD Organisation for Economic Co-operation and Development, PBRSA Performance Based Risk Sharing Agreement, P4P Pay for Performance; RSA Risk Sharing Agreement, TLV Swedish Dental and Pharmaceutical Benefits Agency (Tandvårds- och läkemedelsförmånsverket), VBP value-based pricing
The key themes that were identified when analysing the 24 records were as follows: negotiation framework; outcomes; data; administration and implementation; and laws and regulation. Each of these themes was further detailed into sub-themes (Table 2). Sample quotes are provided that incapsulate the essence of each subtheme and illustrate the way in which passages of text were used to identify the theme.
Table 2.
Themes and subthemes of the conceptual framework
| Themes | Subthemes | Example quote |
|---|---|---|
| Negotiation framework | Terminology: variation in the terminology and taxonomy used for outcomes-based schemes | ‘Numerous terms have been used to describe such schemes, including risk-sharing arrangements, risk-sharing schemes, MEAs, managed entry schemes, performance-based risk sharing schemes, performance-based risk-sharing arrangements, outcome-based MEAs, outcome-based contracting and patient access schemes’ [2] |
| Trust: collaborative and joint partnership between the industry, payers and patients | ‘[A] lack of trust between payers and manufacturers might be one of the key hurdles to more extensive use of outcomes-based arrangements.’ [15] | |
| Alignment: coordination of value, attitude to risk and objectives for the negotiation among the different stakeholders | ‘[Payment-by-result] could lead to risk selection in the patient population treated. If firms are only paid for treatments that result in a positive outcome, there is a financial incentive, and therefore a risk, that firms could encourage providers to only use the product in selected patient sub-groups in which treatment success is more likely.’ [12] | |
| Desirability: understanding to what extent the outcomes-based scheme represents the best reimbursement option | ‘[K]ey information on performance-based MEAs is often confidential or not readily available to third parties, including information on the health outcome measures used, details on the analyses of product performance, and the decisions made as a result of these analyses. This makes it very difficult for third parties to evaluate whether MEAs achieve their objectives.’ [12] | |
| Risk: burden of uncertainty regarding the affordability, real-world efficacy and cost-effectiveness of innovative medicines | ‘Another limitation of CEDs is that although evidence is being developed, payers still pay full price for the drug, and if the evidence does not support the agreed upon price, they cannot recuperate the expenses.’ [17] | |
| Governance: procurement processes, encompassing price, patient eligibility, outcomes, timeline and withdrawing mechanism, among others | ‘[Governance] framework should entail a clear structure to initiate payments, specify the data collection process with attention to ownership of the data and foreseeing regular data audits, establish a defined management framework, define the funding arrangements of the agreement and clearly state the opportunities for appeal when requirements are not met (…) the governance procedures should indicate the standardised decision-making criteria that are used to support the adjustment of payments based on the outcomes achieved.’ [14] | |
| Complexity: variation in the structure, components and implementation of outcomes-based schemes | ‘Responders perceived that the widespread adoption of risk-sharing agreements would lead to more complex protocols and clinical management of patients.’ [20] | |
| Outcomes | Treatment efficacy and safety: determination of treatment effects | ‘[P]roven validated surrogate endpoints may not exist for all disease areas which complicates agreement between payer and developer on the chosen outcome.’ [14] |
| Patient outcomes: measurement of outcomes that matter for the patients | ‘Measuring the drugs’ effects has proved difficult because of the varying course of the disease and because the measurement is subject to interrater variability.’ [11] | |
| Time horizon: balance between optimum timescale for the condition and the scheme | ‘[P]ayers are faced with the fact that effects of possibly curative therapies might only appear in the long-term while actionable outcomes are restricted to those measured in the short-term which is similar to the experienced difficulties of the United Kingdom MS scheme to provide conclusive answers on long-term functional outcomes.’ [14] | |
| Data | Data infrastructure and integrity: capacity of the data systems to centralise and collect outcome measures | ‘Availability of adequate data systems to measure outcomes and monitor accountability and the involvement of healthcare professionals were acknowledged as crucial.’ [9] |
| Data privacy: legal and clinical considerations surrounding data, including data ownership and intellectual property rights | ‘[U]se of data collection systems to collect personal data will require compliance with data privacy regulations such as the General Data Protection Regulation (GDPR) to protect personal data during OBAs. (…) [C]ontractual terms of OBAs may require sharing of identifiable personal data to allow for adjustment of payments. Therefore, additional risk management may be needed to ensure that personal data protection remains guaranteed (…) especially for agreements based on individual patient data where the need for identifiable patient data is higher than agreements dependent on aggregated population-level data.’ [14] | |
| Administration and implementation | Human resources: need for wide-ranging roles, including leaders and negotiators, as well as healthcare professionals, IT staff and administrative personnel | ‘[B]etter trained personnel are needed in the preliminary phases of the negotiation as well as in the pharmacy and clinical analysis areas of the hospitals’ [3] |
| Administration and data collections costs: requirement for development of data collection protocols, negotiation of arrangements, assessment of product performance, policemen of contractual arrangements, and design of procedures to adjudicate disputes | ‘[D]ata on savings under a particular agreement addressing a specific technology, they were rather small when compared to the administrative burden imposed by the contract.’ [3] | |
| Claims management: challenges associated with patients’ tracking and claims’ refunding | ‘[N]eed for an improved information system for managing the agreements and for follow-up of patients and clinical results.’ [3] | |
| Laws and regulation | Variation in legal context: disparities in laws and regulations between countries and regions | ‘[T]he way in which healthcare is organized and funded and the way decisions are made differ between countries and may impact the feasibility of some strategies’ [13] |
| Fiscal: discrepancy between payment cycles and taxes calendar | ‘Spreading payments over multiple years may conflict with the standard 12-months financial cycles of both the payer and manufacturer. (…) Payer’s yearly budgets may not be equipped to implement spread payments over time since the complete cost of the one-shot therapy will have to be budgeted in the year of administration (…) a change in accounting standards is required to budget treatments over multiple years.’ [14] |
CED coverage with evidence development, MEAs managed entry agreements, MS multiple sclerosis, OBAs outcome-based agreements
Example OBCs are referenced in the following sections and summarised in Table 3.
Table 3.
Examples of OBCs referenced in main body text
| Programme | Partners | Year | Example of which challenge | References |
|---|---|---|---|---|
| Risedronate for osteoporosis | Warner Chilcott/Health Alliance (USA) | 2008 | Data infrastructure | Neumann et al., 2011 [11] |
| Certolizumab pegol for rheumatoid arthritis | UCB Pharma (Catalonia, Spain) | 2017 | Complexity | García-Collado et al., 2021 [21] |
| Oxaliplatin for treatment of stage III colon cancer | The Netherlands | (Not reported) |
Patient outcomes Data infrastructure |
Bouvy et al., 2018 [15] |
| Zoledronic acid for osteoporosis | Novartis/German Sickness Fund | (Not reported) | Patient outcomes | Kim et al., 2020 [40] |
| Tisagenlecleucel for B-cell acute lymphoblastic leukaemia and Diffuse large B-cell lymphoma | Haute Autorité de Sante (France) | (Not reported) |
Human resources Administration and data collections costs |
Ronco et al., 2021 [43] |
| Acetylcholinesterase inhibitor for Alzheimer’s disease | Italian NHS (Italy) | (Not reported) | Treatment efficacy |
Adamski et al., 2010 [16] Zampirolli Dias et al., 2020 [2] |
| Beta-interferon for multiple sclerosis | NHS (UK) | 2003 | Overly long time horizons, unpredictable time horizons |
Adamski et al., 2010 [16] Neumann et al., 2011 [11] Garrison et al., 2013 [10] |
| Continuous intraduodenal infusion of levodopa/carbidopa for the treatment of advanced Parkinson’s disease | Neopharma (Sweden) | 2005 | Time consuming process for gathering evidence and negotiating reimbursement | Willis et al., 2010 [19] |
|
Sitagliptin/sitagliptin with metformin for diabetes |
Merck/Cigna (USA) | 2009 | Effective incentive alignment | Neumann et al., 2011 [11] |
| Ranibizumab for macular degeneration | Novartis/ NHS (UK) | 2008 | Administrative burden and compliance | Neumann et al., 2011 [11] |
| Nilotinib or dasatinib for chronic myeloid leukaemia | (Not reported) | (Not reported) | Overly short time horizons | Garattini and Casadei, 2011 [5] |
| Bortezomib in multiple myeloma | Johnson & Johnson/NHS (UK) | 2006 | Administrative burden of tracking patient outcomes | Neumann et al., 2011 [11] |
NHS National Health Service, OBAs outcome-based agreements
Negotiation Framework
Terminology
There is variation in the terminology and taxonomy used for OBCs. They may also be called outcomes-based agreements, pay-for-performance agreements, risk-sharing agreements, cost-sharing agreements, coverage with evidence development (CED), access with evidence development, patient access schemes, conditional licensing, managed entry schemes, performance-based risk-sharing agreements and payment-by-result arrangements [2, 4, 5, 9–13]. Effective communication is the foundation of effective negotiation, and thus differences in terminology may be a barrier to a positive outcome.
Trust
An underlying lack of trust can undermine the establishment and implementation of OBCs [14, 15]. Whilst there are examples of collaborations and partnerships working between industry and payers with respect to OBCs [11], for instance, the programme for bortezomib in multiple myeloma established between Johnson & Johnson and the National Health Service (NHS) (UK), these are not the norm. Payers often suspect these schemes are extensions of marketing activities [16]. Payers also have concerns the Marketing Authorisation Holders (MAH) will overprice medicines with limited data at market entry, anticipating reduced revenues as the evidence base grows [10]. Likewise, payers expressed concerns that temporary coverages can become permanent [11], as reversing coverage decisions is a complex process [17].
OBCs require payers to trust that any refunds will be received. This process can be administratively complex and relies on the participation of stakeholders who are not incentivised to correctly implement the process [10, 18]. An alternative refund model proposed in the literature is the use of bonus payments for the MAH, also known as success fee, when outcomes are achieved [18]. The main feature of this model is that payment is provided to the MAH after efficacy has been evaluated, which precludes costs for non-responders.
Loss aversion amongst patients complicates negotiations. Patients can be resistant to losing access to medicines, even if new evidence emerges that they are not cost-effective. Some OBCs are structured such that a medicine is reimbursed temporarily, whilst additional real-world evidence is collected [19]. For these agreements to be effective, payers need to be confident that they can withdraw a medicine when it is not found to be cost-effective, without mass resistance from patients [11]. If this is not the case, a payer may prefer to delay market access, until further evidence is gathered upfront [19].
Alignment
A range of stakeholders will be involved in developing OBCs, and their assessment of value, attitude to risk, and objectives for the negotiation will vary [15]. The OBC objectives will also differ by country and healthcare system.
Establishing a shared purpose in a multi-stakeholder negotiation requires building trust, respect and an understanding of the different motivations, expectations and time frames of each party. The literature includes descriptions of a successful OBC implementation because of the ‘alignment of payer and manufacturer incentives to support better outcomes’ [11]. Techniques like horizon scanning can also be used to inform the contract and its conditions [14]. However, early and explicit objective setting is not standard practice in OBC negotiations [10], and this lack of a clear guidance framework may be a drawback to implementing them [20].
Desirability
When faced with considerable uncertainty about whether a product or service is cost-effective, payers have four options: adopt or partially adopt with the option to revisit the decision if more information becomes available; refuse to adopt until better information is supplied; demand or mandate a lower price; or enter into an OBC [10].
Whether an OBC represents the best reimbursement option is a significant decision for all stakeholders. It requires detailed exchange of information and data, as well as challenging questions regarding data collection, data privacy and scheme structure to be addressed upfront. Short-term static benefits such as whether the new intervention is prescribed to the appropriate population are easier to measure than long-term, dynamic efficiency benefits that result from aligning incentives in a way that promotes high-quality research and evidence generation [7]. Typically only the former are considered explicitly, yet the latter are fundamental to evaluating the benefit of an OBC [10].
Assessing the desirability of different OBCs is made more challenging due to the limited evaluation of existing schemes in the public domain [21]. Key information such as the health outcomes measures used and the analyses performed are seldom publicly available [12], and economic modelling is uncommon [3]. Furthermore, learning from other country examples is often prevented because information about the effectiveness of the current schemes is rarely available [5].
Risk
The anatomy of an agreement comprises three basic components: the expected return; upside potential; and downside risk. The objective of all parties in an OBC is to have a fair expected return, positive upside, and limited downside. The risk for each party associated with an OBC will vary for different products and populations [16]. One of the key challenges for the negotiation is balancing risk and reward for each party, taking account of their overall attitude to risk.
OBCs provide a mechanism to share the burden of uncertainty regarding the affordability, real-world efficacy and cost-effectiveness of innovative medicines between payers and the MAH [18]. However, in many cases, OBCs have been used to transfer risk from payers to the MAH, particularly in cases where the agreement on reimbursement has not been reached using the normal decision-making framework. Neumann et al. [11] raises the further question as to whether the MAH can obtain additional gains if medicines offer unexpected benefits. Conversely, for schemes like CED, it may happen that while evidence is being developed, payers still pay full price for the technology, with no possibility of recuperating those expenses if the evidence does not support the agreed upon price [17].
Agreeing which party bears the burden of proof is challenging and doing so can be complex and costly. In practice, the burden of proof sits with the beneficiary of the adjustment, which will depend on whether this is a bonus or a discount. The way in which the responsibility for establishing burden of proof is defined within an OBC has significant implications for the level of risk borne by each party.
Governance
It is good practice to establish clear governance structures for OBCs. Such structures should contemplate how data are collected, including who owns the data and how it will be audited; when payments are initiated; how funding is activated; and when appeals can be launched in the case of outcomes not being achieved [14]. Such schemes should be open to all eligible organisations, irrespective of size, geographical location, or product portfolio. This builds on the established procurement processes and could be in the form of ‘requests for OBC’ schemes amongst competing MAH in given disease areas or patient populations [16].
The cost of outcome data collection can be substantial, and ensuring the integrity and validity of the process is crucial [11, 16]. From a legal and ethical perspective, patients who are having information about their health collected should be informed as to whether the payer or MAH is providing funding [16].
For an OBC to function effectively, there needs to be a clear and pre-agreed process for revising the price or the eligible patient population. Garrison et al. [10] makes a distinction between schemes that specify the way in which additional evidence will affect pricing and those with a pre-specified review date at which a new price will be negotiated. If this process and the associated timelines are not clearly defined, there is a risk that access and price remain constant due to inertia, even if new evidence becomes available [11]. Neumann et al. [11] cites the example of beta-interferons in the UK which were not found to be cost-effective at the 2009 review date, yet prices had not been adjusted 2 years later.
The risk that new therapies may not be cost-effective for an individual patient or group of patients is inherent to OBCs. Prior to commencement, there needs to be an agreement in place for withdrawing the medicine, and—where applicable—transferring patients to an alternative [22]. Adamski et al. [16] concluded that clear ‘exit strategies’ need to be planned in advance, to respond to situations in which treatments do not achieve the specified outcomes. For patients who are benefiting, appropriate ‘grandfathering’ mechanisms may need to be in place. Willis et al. [19] described the situation in which the intestinal gel levodopa/carbidopa was found not to be cost-effective, yet discontinuation would require burdensome surgery, making delisting impractical.
Complexity
OBCs are inherently more complex than standard discounts both to evaluate and to implement. This is perhaps the most commonly cited deterrent for healthcare payers [13, 15, 17, 20].
Complexity can result from variation of established standard of care between healthcare providers, making selection of a baseline or comparator more difficult. Agreeing the comparator and ensuring it has widespread use is required ahead of any OBC commencing.
An additional source of complexity is that the patients initially prescribed the medicine will not always be those most likely to benefit [16]. The clinical trial cohort of patients may not always reflect the external validity of a more heterogeneous real-world patient population. This uncertainty has significant implications for the cost-effectiveness of the medicine, and consequently the reimbursement that is linked to patient outcomes. There are some ways to mitigate this risk. Navarria et al. [18] points to the Italian example of Italian Medicines Agency (Agenzia Italiana del Farmaco) ‘AIFA Notes’, which limit the reimbursement of the medicine to the agreed population subgroups.
Outcomes
Treatment Efficacy and Safety
OBCs require stakeholders to have explicit conversations about when a therapy ‘works’ [11]. A major challenge lies in the specification and determination of treatment effects in non-randomised settings, where only certain types of outcome may prove suitable [11, 23]. Changes in clinical practice over time also constrain the reliable measurement of a treatment’s effectiveness [13].
Ideally, outcomes should be objective, clearly defined, reproducible and difficult to manipulate [11]. Successful OBCs have had clearly defined clinical events such as osteoporosis fractures confirmed with x-ray or well-established biomarkers such as reduction in serum M protein in multiple myeloma [11].
OBCs take place in non-randomised settings and can be affected by factors beyond the control of stakeholders. Health systems, clinical pathways, treatment adherence, socioeconomic status and behavioural factors can all influence outcome collection and may not be considered during negotiations [11].
Patient Outcomes
The outcomes that matter most to patients were not explored comprehensively in the literature. Proven validated surrogate outcomes may not exist for all therapeutic areas [14]. These domains may be explored through patient-reported outcome measures (PROMs) that capture health-related quality of life, symptom burden, and/or function, and can be paired with clinical outcomes to better understand the patient experience of treatment [24]. Though Neumann et al. [11] made mention of the risk of failing to measure outcomes that matter most to patients, such as fatigue in multiple sclerosis, they cited a New York Times article that stated ‘measuring improvements in the quality of life is an imprecise science at best’ [25]. Both articles demonstrate a gap in understanding of the rigorous science behind patient outcomes research.
Time Horizon
The selection of treatments suitable for an OBC must consider the outcome timescale for the condition. Timeframes must be long enough to allow for a reliable clinical assessment and adequate data collection but not so long that they become difficult to enforce or execute [5, 14, 23]. For example, only a small proportion of chronic myeloid leukaemia patients resistant to nilotinib or dasatinib can be detected within 4 weeks [5]. Therefore, assessing haematological and cytogenic tests over a longer period—such as 3–6 months—might be a more sensible ‘threshold’ to distinguish non-responders from patients who are more likely to benefit from this treatment [5].
On the other hand, long timelines have other risks [17, 23]. Technological advancements can result in changed clinical practice that make the OBC obsolete. Difficulties in timelines can arise due to slow patient recruitment [5] or challenges capturing and accessing data [9].
Data
Data Infrastructure and Integrity
Decentralised healthcare systems often have their own data infrastructure, making it technically challenging to share patient information between systems [5, 9, 20]. Even in the UK’s ‘national’ health system, the data infrastructure is fractured, and linkage is difficult.
One of the challenges of ensuring the reliability and validity of OBC data is that it is managed on a per patient basis, where response is based on individual trajectory and is not aggregated to inform evidence-based reimbursement decisions [12, 16, 22]. It is also challenging to guard against bias in the selection of patients. In a review of 19 OBCs, Jarosławski and Toumi [22] did not identify any process to ensure an unbiased selection of patients in Italy.
Ethical considerations are important when stakeholders are considering OBCs [16]. A lack of transparent or established procurement and monitoring processes could lead to preferential treatment, bias or gaming [16]. These concerns could be addressed through the utilisation of a trusted third party to undertake data collection or analysis within common data formats [14, 15].
The evaluation of proposed arrangements must also adhere to high ethical standards, including the declaration of any contacts and conflicts of interest between experts and MAH that could potentially influence evaluations [16].
Data Privacy
Legal and clinical governance considerations must be fully addressed when proposing and developing future OBCs [2, 16]. This includes data ownership, intellectual property rights and opportunities for appeal [16]. Ensuring privacy and security of data is paramount to gain support from patients and other data owners. In Europe, MAH are bound by the General Data Protection Regulation (GDPR) and Code of Conduct on Data [26], and collection of personal data requires compliance with that regulation [14]. However, complying with data privacy regulations can add further burden to the analyses [2].
Furthermore, participation in an OBC should be clearly explained to patients and consideration should be given as to how this may complicate the principle of informed consent. These regulatory issues, and the expertise required to meet them, received limited discussion within the academic literature [12]. This may in part be due to the pace at which this area is evolving.
Administration and Implementation
Human Resources
For many payers, OBCs are a new paradigm, different to the tools and techniques used in previous assessment of treatments. Designing and implementing OBCs is complex, time consuming and requires strong leadership. Clopes et al. identified leadership as the most important organisational aspect of implementation of OBCs [9].
There also need to be appropriately trained professionals in place to evaluate the proposed schemes, from the preliminary stages of negotiation to the pharmacy and clinical contexts [3]. These include healthcare professionals, pharmacology, IT and economic experts [16]. Michelsen et al. [14] highlighted how low compliance with data input from healthcare professionals resulted in low-quality and insufficient data, during the implementation of CED in the Netherlands and outcome-based agreements (OBAs) in Italy. The burden of data collection for healthcare and other professionals involved, as well as patients, should be anticipated [2, 3, 13].
To meet the expanded responsibilities of OBC negotiation, implementation and management, the mandates and processes of regulatory, HTA and funding organisations may need to be revised.
Administration and Data Collection Costs
OBCs generate additional costs and require additional resources, responsibility for which remains unclear. These include developing data collection protocols, negotiating arrangements, assessing product performance, policing contractual arrangements and designing procedures to adjudicate disputes. For instance, one OBC for a multiple sclerosis medicine in the UK required 120 additional nurses in 70 centres to implement the agreement [11]. Long-term funding is required alongside the technical infrastructure to capture and analyse the data. The complexity of implementing a risk-sharing agreement for enzyme replacement therapy in lysosomal storage diseases at a national level led the Spanish authorities to instead establish regional or even hospital-level agreements [27]. For certain technologies, the administrative burden of these schemes may offset the benefits [2, 3, 13, 14].
Variation between the administrative requirements of different schemes adds further complexity. For example, ranibizumab involved clear criteria—a dose cap at 14 injections—which resulted in a relatively low administrative burden. In contrast, the multiple sclerosis arrangement involved a longer timeframe, difficult to gauge outcomes and higher administrative costs [11].
Claims Management
Response-based schemes pose challenges for tracking patients and ensuring that refunds are claimed [3], creating an additional administrative burden [20]. A survey of oncology pharmacists in 31 UK NHS hospitals found that between 2007 and 2009, 47% of eligible manufacturer paybacks from OBCs were not recovered by Primary Care Trusts [22]. Experience is similar in Italy, where Navarria et al. [18] suggest there is no incentive for healthcare professionals to update the registries, close the patients’ files and submit a refund claim on a regular basis, possibly because the money to be refunded does not go to the prescribing cost centre, rather to the hospital general budget. Therefore, an ‘incentivisation gap’ exists between the stakeholder who receives the funding and the individual in charge of the reimbursement procedures [3, 18].
Laws and Regulation
Variation in Legal Context
A solid legal framework is essential to every OBC, [9] yet this can be complex to establish [11]. The laws and regulations that govern OBCs in each country and region are highly variable [12, 13]. For instance, in France and the UK, price negotiations occur at a national level [10], whereas in Spain they occur at a regional level [9]. Garrison et al. [10] note that France were considering a law that would fine MAH who did not provide evidence in a timely manner to disincentivise them from holding back unfavourable results.
Navigating the diverse and evolving legal landscape is a challenge to manufacturers pursuing OBCs in multiple countries. Michelsen et al. [14] suggested how scientific advice should be sought from the European Medicines Agency (EMA) and the different HTA bodies on how to enable collaborations between the regulatory agency and multiple national payers.
Fiscal
As discussed earlier, some OBCs have long timelines, and this results in a delay in determining whether the agreed patient outcomes have been achieved. Thus, the MAH may receive payment—or payers rebates—long after the medicine is dispensed. Reimbursement may occur in a different tax year, creating fiscal and accrual challenges for all stakeholders [14], including government treasury departments [18].
Discussion
This scoping review is the first to examine the challenges of implementing OBCs across European healthcare systems, and to develop a comprehensive framework. Some of the challenges that have been highlighted are familiar; concerns around the complexity and cost of implementation, particularly in relation to data infrastructure for recording patient outcomes, have been discussed elsewhere [3, 12, 15]. This analysis of the published literature identified several less-recognised areas for further research on challenges associated with OBCs, which are discussed below.
This review did not assess the impact of specific OBC schemes because, as has been discussed, the necessary information is generally not made available publicly. Further, the review stops short of making detailed recommendations for how to overcome the challenges discussed. As the review is limited to considering challenges within Europe, it does not include those relating to OBC examples in other healthcare systems, such as the United States (US).
There was little discussion in the literature of the role that patients and healthcare providers play in OBC measurement frameworks, negotiation and implementation, despite coverage of multiple issues relevant to them. Examples include incentives to participate in outcomes data collection and monitoring; the prioritisation and relevance of selected outcomes; privacy concerns surrounding data ownership, security and sharing; uncertainty around treatments being de-listed as the result of ex-post review; and the existence of subsequent treatment plans or changing treatment pathways. In our view, a critical examination of the implication of OBCs for patients and healthcare providers is vital to ensure better outcomes for all parties.
Relatedly, there is minimal discussion of outcomes that matter most to patients and how this is integral to understanding the value of a treatment [21]. The literature focuses on clinical outcomes with the apparent perception they are more objective, unbiased and quantifiable. However, quantifying the patient experience with robust methodology and analysis includes direct patient insights, disease-specific conceptual frameworks and measurement with valid, reliable and sensitive instruments used in an appropriate context [28, 29]. Evaluating these complex, and at times interdependent, variables impacting treatment outcomes creates an opportunity to enhance shared patient–clinician decision making [30] and system resource allocation, [31, 32] and may guide development of outcome measurement frameworks informing OBCs in the future. This has particular potential in the UK, where there is a history of using PROMs for comparisons of providers’ performance [33]. Further research is critical to establish patient outcomes research methodology as a tool to transform value and access conversations.
Challenges of OBCs in the US have received coverage elsewhere, and the context for implementing OBCs differs [34–36]. This paper examines patterns in the challenges discussed in the European literature. Some of the challenges included in this study are specific to the EU, or to particular countries within the EU; others are more general. Regulatory compliance is highly contextual, with different considerations at the regional, national and international level. Clopes et al. [9] highlight the importance of Catalonian oncology policy for OBCs in that region. Complying with the GDPR and ensuring data security whilst harnessing the increasing capability to capture and analyse real-time data is a key consideration across EU health systems [26]. Research in the US context focuses less on data governance and more on other areas of legislation, such as the Medicaid Drug Rebate Program’s (MDRP’s) ‘best price requirement’ [36]. Co-ordinated pursuit of value for money via HTA is less of a dominant paradigm within the US system [37]. Although the UK has left the EU, much of the shared regulatory framework remains in place, and there is substantial commonality between health systems.
Distrust between payers and the pharmaceutical industry is a recurring theme and a characterisation of zero-sum price focused negotiations. This is one area in which the authors believe that there has been progress towards a more collaborative relationship that recognises the shared desire to achieve improved outcomes that matter most to patients. That process is based on a rigorous assessment of value throughout the whole care pathway and not simply through good negotiation skills. There is an appetite on all sides to explore greater use of OBCs, but there does need to be greater shared understanding of the challenges faced by both industry and payers in implementing them. Trust is easily lost; without sensitivity as to where OBCs can put greater pressure on healthcare systems, it is easy to assume that the reluctance to implement them is due to other motives. This review is instrumental in highlighting the areas of potential misunderstanding so that they can be considered and addressed in advance of OBC design.
Progress has also been made towards technical OBC solutions, such as third-party platforms for facilitating data collection, analysis and payments [38]. This has the potential to build the trust needed for OBCs to function effectively and must include transparent protocols to meet data governance regulation.
There are two factors that drive the gaps that have been identified in the literature: limited transparent evaluation of existing schemes and a lag between developments within a rapidly evolving policy area and the academic literature. Future research may attempt to address these gaps by drawing on alternative sources, such as interviews, in addition to the published literature. A European register of existing schemes, with sensitive financial information removed, would enhance collective understanding.
Historically, OBCs were used as a vehicle of risk sharing, facilitating reimbursement of the MAH and reducing the risk faced by the payers. OBCs have also been used as a ‘last-resort’ when traditional reimbursement structures were ineffective at achieving agreement, but this is changing. For example, new medicines, including curative therapies and advanced therapy medicinal products (ATMPs) present a significant value potential, but also a cost containment challenge [39]. OBCs present a mechanism for providing reimbursement at a level proportional to the value they create, by linking the level of reimbursement, and the timing, to the outcomes achieved for patients, thus, achieving a mutually beneficial arrangement across all stakeholders. These opportunities mean they are likely to have a significant role in how the life sciences industry is reimbursed in the future.
Conclusion
Although shared information, knowledge and technical understanding are important for OBC implementation, they are not enough. These agreements are complex structures, which require partnership, collaboration and learning by a range of stakeholders with complementary expertise.
An OBC that worked in one place at one time will not necessarily work somewhere else or even in the same place at another time, even with the challenge appearing superficially to be the same. Acknowledging this complexity through dialogue is the first step to moving forward; parties may need to develop a fundamentally different approach to problem solving to progress from there. As European healthcare systems evolve their regulatory and reimbursement landscape, and adopt ways of contracting for innovative therapeutics that balance sustainability and innovation, appreciating the challenges highlighted by this review will be key to constructive dialogue.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Vasileios Kontogiannis and Corinna Bull for their contribution to this study.
Declarations
Author Contributions
SB, FGJ and AD led the development of the thematic analysis, by identifying relevant topics and subtopics. NB, JRB and HL contributed with comments to the thematic analysis. SB, FGJ, DGB and AD were involved in the literature review and data extraction. All authors contributed to the writing of this article and reviewed and approved the manuscript.
Funding
This study was partially funded by Pfizer Limited.
Conflict of interest
NB and JRB are employees of Pfizer Limited. HL is employed by Swansea University, which has received funding from Pfizer Limited. SB, FGJ, DGB and AD are employees of Symmetron Limited, who received funding from Pfizer Limited to complete this work.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data available on request.
Code availability
Not applicable.
References
- 1.Catchpole P, Barrett V. Keeping pace with pharmaceutical innovation: the importance of the NICE methods review. Pharmacoeconomics. 2020;38(9):901–903. doi: 10.1007/s40273-020-00918-2. [DOI] [PubMed] [Google Scholar]
- 2.Zampirolli Dias C, et al. Integrative review of managed entry agreements: chances and limitations. Pharmacoeconomics. 2020;38(11):1165–1185. doi: 10.1007/s40273-020-00943-1. [DOI] [PubMed] [Google Scholar]
- 3.Antonanzas F, et al. The use of risk-sharing contracts in healthcare: theoretical and empirical assessments. Pharmacoeconomics. 2019;37(12):1469–1483. doi: 10.1007/s40273-019-00838-w. [DOI] [PubMed] [Google Scholar]
- 4.Darbà J, Ascanio M. The current performance-linked and risk sharing agreement scene in the Spanish region of Catalonia. Expert Rev Pharmacoecon Outcomes Res. 2019;19(6):743–748. doi: 10.1080/14737167.2019.1587296. [DOI] [PubMed] [Google Scholar]
- 5.Garattini L, Casadei G. Risk sharing agreements: what lessons from Italy? Int J Technol Assess Health Care. 2011;27(2):169–172. doi: 10.1017/S0266462311000079. [DOI] [PubMed] [Google Scholar]
- 6.Carlson JJ, Chen S, Garrison LP. Performance-based risk-sharing arrangements: an updated international review. Pharmacoeconomics. 2017;10(35):1063–1072. doi: 10.1007/s40273-017-0535-z. [DOI] [PubMed] [Google Scholar]
- 7.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 8.Cole A, et al. Making Outcome-Based Payment a Reality in the NHS. 2019. 10.1007/s40271-020-00430-x.
- 9.Clopes A, et al. Financial consequences of a payment-by-results scheme in Catalonia: gefitinib in advanced EGFR-mutation positive non-small-cell lung cancer. J Med Econ. 2017;20(1):1–7. doi: 10.1080/13696998.2016.1215991. [DOI] [PubMed] [Google Scholar]
- 10.Garrison LP, Jr, et al. Performance-based risk-sharing arrangements; good practices for design, implementation, and evaluation: report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value Health. 2013;16(5):703–719. doi: 10.1016/j.jval.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Neumann PJ, et al. Risk-sharing arrangements that link payment for drugs to health outcomes are proving hard to implement. Health Aff. 2011;30(12):2329–2337. doi: 10.1377/hlthaff.2010.1147. [DOI] [PubMed] [Google Scholar]
- 12.Wenzl M, Chapman S. Performance-based managed entry agreements for new medicines in OECD countries and EU member states: how they work and possible improvements going forward, in Health Working Papers OECD. 2019.
- 13.Vreman RA, et al. Application of managed entry agreements for innovative therapies in different settings and combinations: a feasibility analysis. Int J Environ Res Public Health. 2020;17(22):8309. doi: 10.3390/ijerph17228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelsen S, et al. Barriers and opportunities for implementation of outcome-based spread payments for high-cost, one-shot curative therapies. Front Pharmacol. 1914;2020(11):5. doi: 10.3389/fphar.2020.594446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouvy JC, Sapede C, Garner S. Managed entry agreements for pharmaceuticals in the context of adaptive pathways in Europe. Front Pharmacol. 2018;9:280. doi: 10.3389/fphar.2018.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamski J, et al. Risk sharing arrangements for pharmaceuticals: potential considerations and recommendations for European payers. BMC Health Serv Res. 2010;10:153–153. doi: 10.1186/1472-6963-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabbous M, et al. Managed entry agreements: policy analysis from the European perspective. Value Health. 2020;23(4):425–433. doi: 10.1016/j.jval.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Navarria A, et al. Do the current performance-based schemes in Italy really work? “Success fee”: a novel measure for cost-containment of drug expenditure. Value Health. 2015;18(1):131–136. doi: 10.1016/j.jval.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Willis M, et al. Reducing uncertainty in value-based pricing using evidence development agreements. Appl Health Econ Health Policy. 2010;8(6):377–386. doi: 10.2165/11531160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Lorente R, Antonanzas F, Rodriguez-Ibeas R. Implementation of risk-sharing contracts as perceived by Spanish hospital pharmacists. Heal Econ Rev. 2019;9(1):25. doi: 10.1186/s13561-019-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Collado CG, et al. Impact of a risk-sharing agreement in rheumatoid arthritis in Spain. Health Policy. 2021;125(3):335–340. doi: 10.1016/j.healthpol.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Jarosławski S, Toumi M. Market access agreements for pharmaceuticals in Europe: diversity of approaches and underlying concepts. BMC Health Serv Res. 2011;11(1):259. doi: 10.1186/1472-6963-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makady A, et al. Implementing managed entry agreements in practice: the Dutch reality check. Health Policy. 2019;123(3):267–274. doi: 10.1016/j.healthpol.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 24.US Food & Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Accessed 2020.
- 25.Pollack A. Pricing pills by the results. New York Times. 2007. https://www.nytimes.com/2007/07/14/business/14drugprice.html. Accessed 14 Jul 2007.
- 26.Department of Health & Social Care. Code of conduct for data-driven health and care technology. 2019. https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology. Accessed 2020.
- 27.Edo-Solsona MD, Vitoria-Minana I, Poveda-Andres JL. Implementation and results of a risk-sharing scheme for enzyme replacement therapy in lysosomal storage diseases. Farm Hosp. 2020;44(1):10–15. doi: 10.7399/fh.11262. [DOI] [PubMed] [Google Scholar]
- 28.Deshpande PR, et al. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137–144. doi: 10.4103/2229-3485.86879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The European Patients' Academy on Therapeutic Innovation (EUPATI). Patient-reported outcomes (PROs) assessment. 2020. https://toolbox.eupati.eu/resources/patient-reported-outcomes-pros-assessment/. Accessed 2020.
- 30.National Insitute for Health and Care Excellence (NICE). Shared decision making. 2021. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/shared-decision-making. Accessed 2021. [PubMed]
- 31.Basch E, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;34(30):4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 32.Guindo LA, et al. From efficacy to equity: Literature review of decision criteria for resource allocation and healthcare decisionmaking. Cost Effective Resourc Alloc. 2012;10(1):9. doi: 10.1186/1478-7547-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black N. Patient reported outcome measures could help transform healthcare. Br Med J. 2013;346:167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 34.Brown JD, et al. Payer and pharmaceutical manufacturer considerations for outcomes-based agreements in the United States. Value Health. 2018;21(1):33–40. doi: 10.1016/j.jval.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Seeley E, Kesselheim AS. Outcomes-based pharmaceutical contracts: an answer to high US Drug Spending? 2017. https://www.commonwealthfund.org/publications/issue-briefs/2017/sep/outcomes-based-pharmaceutical-contracts-answer-high-us-drug. Accessed 2021. [PubMed]
- 36.Mytelka D, et al. Managing uncertainty in drug value: outcomes-based contracting supports value-based pricing. Health Affairs Blog. 2020. https://www.healthaffairs.org/do/10.1377/hblog20200128.542919/full/. Accessed 30 Jan 2020.
- 37.Mulligan K, et al. Health technology assessment for the US Healthcare system. 2020. https://healthpolicy.usc.edu/wp-content/uploads/2020/02/Health-Technology-Assessment-for-the-U.S.-Healthcare-System_White-Paper.pdf.
- 38.Kent S, et al. Common problems, common data model solutions: evidence generation for health technology assessment. Pharmacoeconomics. 2021;39:275–285. doi: 10.1007/s40273-020-00981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heppolette W, Rouse J. Pharmaceutical industry memorandum of understanding. 2017. https://www.gmhsc.org.uk/wp-content/uploads/2018/04/05-Pharma-Industry-MoU-Cover-Sheet-FINAL.pdf.
- 40.Kim AE, et al. Performance-Based risk-sharing arrangements (PBRSA): is it a solution to increase bang for the buck for pharmaceutical reimbursement strategy for our nation and around the world? Clin Drug Investig. 2020;40(12):1107–1113. doi: 10.1007/s40261-020-00972-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persson U, Willis M, Odegaard K. A case study of ex ante, value-based price and reimbursement decision-making: TLV and rimonabant in Sweden. Eur J Health Econ. 2010;11(2):195–203. doi: 10.1007/s10198-009-0166-1. [DOI] [PubMed] [Google Scholar]
- 42.Pickin M, et al. The multiple sclerosis risk sharing scheme monitoring study–early results and lessons for the future. BMC Neurol. 2009;9:1–1. doi: 10.1186/1471-2377-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronco V, et al. Price and reimbursement of advanced therapeutic medicinal products in Europe: are assessment and appraisal diverging from expert recommendations? J Pharmaceut Policy Pract. 2021;14(1):30. doi: 10.1186/s40545-021-00311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.