Abstract
White–Sutton syndrome (WHSUS) is a neurodevelopmental disorder caused by heterozygous loss-of-function variants in POGZ. Through the Deciphering Developmental Disorders study and clinical testing, we identified 12 individuals from 10 families with pathogenic or likely pathogenic variants in POGZ (eight de novo and two inherited). Most individuals had delayed development and/or intellectual disability. We analyzed the clinical findings in our series and combined it with data from 89 previously reported individuals. The results demonstrate WHSUS is associated with variable developmental delay or intellectual disability, increased risk of obesity, visual defects, craniofacial dysmorphism, sensorineural hearing loss, feeding problems, seizures, and structural brain malformations. Our series includes further individuals with rod-cone dystrophy, cleft lip and palate, congenital diaphragmatic hernia, and duplicated renal drainage system, suggesting these are rare complications of WHSUS. In addition, we describe an individual with a novel, de novo missense variant in POGZ and features of WHSUS. Our work further delineates the phenotypic spectrum of WHSUS highlighting the variable severity of this disorder and the observation of familial pathogenic POGZ variants.
Subject terms: ADHD, Medical genomics, Genetic testing
Introduction
White–Sutton syndrome (WHSUS, OMIM 616364) is a rare neurodevelopmental disorder caused by heterozygous variants in the POGZ gene. A range of clinical features have been described in WHSUS patients including delayed development (DD), intellectual disability (ID), dysmorphic facial features, microcephaly, behavioural problems, visual difficulties, sensorineural hearing loss, increased incidence of obesity, and a variety of brain anomalies [1–12]. Almost 90 individuals with WHSUS have been described in the literature to date. The relatively non-specific phenotypic features of WHSUS make it difficult to diagnose clinically. Therefore, most reported individuals have been diagnosed by exome or whole-genome sequencing. Some individuals with mild features may be considered to have non-syndromic ID. Here we use WHSUS to refer to the entire spectrum of POGZ-related ID.
The POGZ (POGO transposable element with ZNF domain) protein is expressed in many tissues, including the foetal and adult brain [1]. It has been proposed that POGZ is a chromatin regulator involved in chromosomal segregation and mitotic progression [13]. Through chromatin remodelling POGZ regulates the transcriptional networks controlling neuronal developmental gene expression [14]. POGZ is a highly constrained gene with fewer than expected missense and protein-truncating variants (PTVs) in the general population (gnomAD pLI = 1 and missense Z-score = 3.51) [15]. POGZ missense variants have been reported in patients with autistic spectrum disorder (ASD) and schizophrenia [14, 16–20] while PTVs are the predominant mutation type reported in WHSUS [1–11].
Here, we describe a series of previously unreported individuals with heterozygous POGZ variants identified through exome or gene panel testing. We analyze the range of phenotypes and variants found in our series and compare them with individuals previously reported in the literature.
Subjects and methods
Seven probands (individuals 2, 3, 7, 9 and 11–13) underwent trio-based whole-exome sequencing (WES) as part of the Deciphering Developmental Disorders (DDD) study [21]. Limited information about individuals 7, 9 and 12 was included in previous DDD publications [7, 22]. The remaining four probands (individuals 1, 4, 8, and 10) were identified through clinical testing using either trio-based WES or a next-generation sequencing ID gene panel. All individuals have been submitted to the DECIPHER database (IDs: individual 1, 430757; individual 2, 277002; individual 3, 282229; individuals 4–6, 430735; individual 7, 270842; individual 8, 430545; individual 9, 264054; individual 10, 379029; individual 11, 273462; individual 12, 260897; individual 13, 296434) [23]. Individuals 5 and 6 (the brother and mother of individual 4) had targeted Sanger sequencing to confirm the presence of the familial POGZ variant. All subjects (apart from individuals 5 and 6), had array comparative genomic hybridisation testing in addition to other single gene and panel tests, without an alternative molecular cause being found.
A deep phenotyping questionnaire was devised based on the literature. Broad subheadings in the questionnaire included growth, development, learning, vision and hearing, neurobehavioral, skeletal, cardiac, gastrointestinal, immune and other features. Detailed phenotyping was undertaken by local clinicians. All individuals or their parents/guardians gave consent for publication. Three families gave consent for publication of photographs. Individuals who had evaluation or analysis beyond routine clinical care were part of research studies approved by the Cambridge South Research Ethics Committee (10/H0305/83). Variant positions are based on POGZ transcript NM_015100.3, genome assembly GRCh37 (hg19) and genomic reference file NC_000001.10. POGZ variants were classified using American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP) guidelines and the 2020 Association for Clinical Genomic Science (ACGS) guidelines [24, 25].
Results
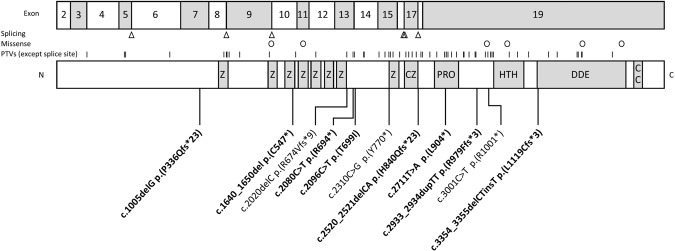
We ascertained 12 individuals (six males, six females) with PTVs in POGZ that were classified as pathogenic or likely pathogenic. The clinical features of the 12 individuals are summarised in Table 1. Detailed descriptions are provided in Supplementary Table 1. The age range of the individuals was 11 months to 34 years. As with previous reports the variants were spread throughout the gene (Fig. 1). Seven variants were have not been previously published. Two variants were inherited (one novel and one recurrent). The novel c.2080 C > T p.(Arg694*) variant was found in individual 4, his brother individual 5, and their mildly affected mother, individual 6. Testing found similar, but slightly skewed allele ratios in blood samples from all three family members (158 reference reads/121 variant reads, 125/116 and 138/108 respectively). Sanger sequencing confirmed the variant was also present in the mother’s urine sediment and a buccal swab. Thus, reducing the likelihood of mosaicism. Testing of other family members was not possible. The other inherited variant, c.2020delC p.(Arg674Valfs*9), was found in individual 3. The variant was inherited from individual 3’s father who was described as having mild ID (only limited information about him was available). The c.2020delC p.(Arg674Valfs*9) variant was previously observed de novo in two unrelated individuals with ID/DD and ASD [1, 5].
Table 1.
Clinical and molecular features in 12 new individuals with WHSUS.
| Individual | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Age | 9 y | 10 y | 10 y | 4 y | 3 y | 34 y | 11 y | 2 y | 2 y | 11 m | 7 y | 4 y |
| Sex | Male | Female | Male | Male | Male | Female | Female | Female | Female | Male | Male | Female |
| Mutation (NM_015100.3) | c.1005delG p.(Pro336Glnfs*23) | c.1640_1650 del p.(Cys547*) | c.2020delC p.(Arg674Valfs*9) | c.2080 C > T p.(Arg694*) | c.2080 C > T p.(Arg694*) | c.2080 C > T p.(Arg694*) | c.2310 C > G p.(Tyr770*) | c.2520_2521delCA p.(His 840Glnfs*23) | c.2711 T > A p.(Leu904*) | c.2933_2934dupTT, p.(Arg979Phefs*3) | c.3001 C > T p.(Arg1001*) | c.3354_3355delCTinsT p.(Leu 1119Cysfs*3) |
| ACMG/ACGS criteria | PVS1, PS2_sup, PM2 | PVS1, PS2_sup, PM2 | PVS1, PS4_sup, PM2 | PVS1, PM2 | PVS1, PM2 | PVS1, PM2 | PVS1, PS2_sup, PM2 | PVS1, PS2_sup, PM2 | PVS1_strong, PS2_sup, PS4_ sup, PM2 | PVS1_strong, PS2_sup, PM2 | PVS1_strong, PS2_sup, PS4_mod, PM2 | PVS1_strong, PS2_sup, PS4_sup, PM2 |
| Inheritance | De novo | De novo | Paternal | Maternal | Maternal | Unknown | De novo | De novo | De novo | De novo | De novo | De novo |
| Novel | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| Microcephaly | − | + | − | + | + | + | − | + | + | + | − | + |
| DD or ID | Mild | Moderate | Moderate | Mild | DD | nr | Moderate | Mod-Severe | Severe | DD | Severe | Mild |
| Severe Speech delay | − | + | + | + | + | nr | − | + | + | n/a | + | − |
| Brain MRI | Normal | nr | nd | nd | nd | nd | nd | CC hypoplasia | Prominent CSF spaces | CC hypoplasia, prominent CSF spaces | Normal | nr |
| Hypotonia | nr | nr | nr | + | nr | nr | nr | + | + | + | nr | nr |
| Feeding | Initial delay in solids | Slow eater | Normal | Oesophageal dysmotility | Slow eater | Normal | Normal | Initial delay in solids | Initial delay in solids | Unsafe swallow | Initial delay in solids | Fussy eater |
| Obesity | + | − | + | − | − | + | + | − | − | − | − | − |
| Hearing | Mixed loss | Normal | Normal | Normal | Glue ear | Normal | Glue ear/ Grommets | Normal (ASSR) | SNHL | SNHL | Glue ear/ Grommets | Normal |
| Vision | Ptosis | Strabismus, ptosis | Myopia | HM | HM | HM, macular drusen and pigment displacement | HM | Bilateral retinal coloboma | Cone-rod dystrophy, high HM | Prominent right eye with strabismus | Myopia | Left iris coloboma, normal vision |
| Other features | Talipes equinovarus, CALs, mild scoliosis/lordosis sandal gap, widely spaced upper incisors | Sparse hair, brachydactyly | Small genitalia, sandal gap, irregular teeth, cleft lip and palate. | Intermittent unprovoked vomiting, hypermobile, | Early teeth eruption, hypermobile, | Asthma, hyperthyroidism, hypermobile | CALs | Diaphragmatic hernia, unilateral duplicated collecting system | Initial raised nuchal translucency | Cystic hygroma, atrial septal defect, pyloric stenosis, bilateral cryptorchidism | Atopy. gum hypertrophy | Atopy, asthma, splenic cyst |
Individuals 9 and 12 were reported in previous DDD publications but with limited phenotype information.
Age d(ays), m(onths), y(ears), ASSR auditory steady-state response, CALs café au lait macules, CC corpus callosum, CSF cerebrospinal fluid, DD developmental delay, HM hypermetropia, ID intellectual disability, n/a not applicable, nd not done, nr not reported, OFC occipitofrontal circumference, SNHL sensorineural hearing loss, sz seizures.
Fig. 1. Exon and protein models of POGZ showing location of the variants.
On top is an exonic model of POGZ (the first exon is non-coding). Below is the POGZ protein which contains a series of zinc finger domains (Z), a chromobox 5 binding region which contains a further zinc finger domain (CZ), proline-rich domain (PRO), helix-turn-helix centromere protein-B-like DNA-binding domain (HTH), a DDE superfamily endonuclease domain (DDE) and a coiled coil domain (CC). Below the protein model are variants reported in this series. Novel variants are bold. Above the protein model are lines indicating previously reported PTVs (except splice spite), circles representing missense variants in individuals reported to have WHSUS and triangles for variants predicted to affect splicing.
We analysed the frequency of clinical findings in the 12 individuals then combined the data with information from 89 previously published individuals with WHSUS. The frequency of features in our series and the combined cohort is given in Table 2.
Table 2.
Frequency of clinical features in our series and combined with all published WHSUS patients.
| Feature | Our series (n = 12) | All WHSUS (n = 101) |
|---|---|---|
| Severe ID | 3/10 | 26/75 (34%) |
| Moderate ID | 3/10 | 11/75 (15%) |
| Mild ID | 3/10 | 38/75 (51%) |
| Speech delay | 10/10 | 80/83 (96%) |
| Significant speech delay | 6/10 | 27/83 (33%) |
| Autism spectrum disorder/traits | 4/10 | 39/61 (64%) |
| Hypotonia | 4/4 | 26/35 (74%) |
| Abnormal brain MRI | 3/5 | 34/57 (60%) |
| Microcephaly | 8/12 | 41/74 (55%) |
| Sensorineural hearing loss | 3/12 | 15/52 (29%) |
| Feeding problems | 7/11 | 30/51 (59%) |
| Obesity | 4/12 | 38/68 (56%) |
| Short stature (first decile) | 4/12 | 14/39 (36%) |
| Intrauterine growth restriction | 2/11 | 8/38 (21%) |
| Brachydactyly | 1/8 | 8/37 (22%) |
| Broad thumb/hallux | 1/8 | 4/11 (36%) |
| Obstructive sleep apnoea | 1/12 | 7/20 (35%) |
| Strabismus | 2/12 | 18/38 (47%) |
| Hypermetropia | 4/12 | 16/51 (31%) |
| Myopia | 2/12 | 8/51 (16%) |
Rare features (our series + previous individuals):
Rod-cone dystrophy 1 + 2, optic nerve hypoplasia 0 + 2, macular drusen 1 + 0, coloboma 2 + 1, keratitis 0 + 1, Horner syndrome 0 + 1, ptosis 2 + 3, high palate 1 + 5, bifid uvula 0 + 2, cleft lip and/or palate 1 + 2, dextrocardia 0 + 1, atrial septal defect 1 + 6, patent ductus arteriosus 0 + 1, congenital diaphragmatic hernia 1 + 4, pyloric stenosis 1 + 0, intussusception 0 + 1, malrotation 0 + 3, duplicated renal drainage system 1 + 2, pelviureteric junction obstruction with dysplastic kidney 0 + 2, male genital abnormalities 2 + 7.
Neurodevelopment
DD or ID was evident in all but one of the individuals with PTVs in our series. Where severity could be assessed (10/12) the DD/ID in subjects with PTVs was severe in two, moderate-to-severe in one, moderate in three and mild in three. Individual 6 did not require additional educational support and was in full-time employment. However, she had not undergone a formal cognitive assessment. Speech was severely affected in six out of ten individuals. Two patients were diagnosed with ASD. Two more were noted to have autistic traits. Two individuals had a history of at least one seizure. No individuals in our series were noted to have ataxia. Among previously reported individuals there were two with ataxia. MRI brain scans were normal in two subjects and showed minor abnormalities in three others (hypoplasia of the corpus callosum and/or prominent CSF spaces).
Growth and feeding
Birth occipitofrontal circumference (OFC) was recorded in four individuals. Congenital microcephaly (OFC ≤ −2 standard deviations (SD) below the mean for age and sex) was present in one (the other three had birth OFCs below −1.5 SD). Two individuals had a history of intrauterine growth restriction. Birthweight for gestational age ranged from −2.3 SD to +1.1 with two individuals < −2 SD). Early feeding problems were common (7/11). Individual 10 had an unsafe swallow and required nasogastric feeding. He also developed pyloric stenosis. Individual 4 had oesophageal dysmotility. Four individuals were obese (4/5 over the age of 10 years). Last recorded weights in our series ranged from −1.9 SD to +2.4 SD. Height ranged from −1.9 to +2.2 SD. Four of the 12 individuals had a height in the first decile (<−1.28 SD) for age, while only two had an above-average height. Microcephaly was present in 8/12 individuals.
Hearing and vision
Consistent with previous reports, visual problems were a common finding. These included refractory errors (6/12), strabismus (2/12), ptosis (2/12), colobomata (2/12, unilateral iris coloboma in individual 12 and bilateral retinal colobomata in individual 8), and rod-cone dystrophy (1/12). Two previous individuals with WHSUS and rod-cone dystrophy have been reported. Individual 6 had drusen and pigment displacement. Six individuals had hearing loss (sensorineural in three and conductive in three).
Dysmorphic features
There was a spectrum of dysmorphic features in our series (Fig. 2 and Supplementary Table 1). Common facial features included midface hypoplasia, hypertelorism, downslanting palpebral fissures, flat nasal tip, thin upper lip, short philtrum and downturned mouth. There was wide variability and some patients only had subtle facial features. Individual 3 had a cleft lip and palate. Individual 10 had a high palate.
Fig. 2. Photographs of three individuals with POGZ variants.
Individual 1 (A, D) at 9 years of age, individual 12 (B, E) at 4 years of age and individual 13 (C, F) at 7 years of age. Individual 1 and 12 have POGZ PTVs and individual 13 has a de novo missense variant in POGZ c.2096 C > T p.(Thr699Ile). The three individuals demonstrate the variable facial features of WHSUS, with 12 and 13 showing more typical features, including downslanting palpebral fissures, midface hypoplasia, smooth philtrum and a thin upper lip.
Other systemic features and congenital anomalies
A range of other congenital anomalies were reported including minor male genital abnormalities in two individuals (small genitalia and cryptorchidism). Individual 9 had an initial raised nuchal translucency and individual 10 had a cystic hygroma. Individual 8 had a congenital diaphragmatic hernia, an abnormality that has been reported in four previous individuals with POGZ variants [2, 10, 11, 26]. One of these individuals was part of a series of patients with congenital diaphragmatic hernia and further phenotypic information is limited [26]. Individual 8 had a duplicated renal collecting system, a feature that has been reported in two previous individuals with WHSUS [2, 11].
Combined analysis with previous WHSUS individuals
We combined the clinical observations in our series with the findings for 89 previously reported individuals with WHSUS (Table 2, Supplementary Table 2). Across the combined cohort, 49% had moderate or severe learning difficulties. Speech delay was present in all but three individuals. It was reported to be severe in 33%. Hypotonia (74%), brain malformations (60%), ASD/autistic features (64%), early feeding problems (59%), microcephaly (55%), obesity (56%), and sensorineural hearing loss (29%) were also commonly seen.
Novel missense variant in POGZ
In addition to the individuals with POGZ PTVs, we identified a novel de novo missense variant c.2096 C > T p.(Thr699Ile) in a 7-year-old male (individual 13) with mild ID, epilepsy, sleep apnoea and facial features similar to WHSUS patients (Fig. 2C, F). A detailed case report is given in the Supplementary Material. The formal ACMG-AMP/ACGS classification of the missense was as a variant of uncertain clinical significance (VUS). Individual 13 also had compound heterozygous pathogenic variants in POLG. It is therefore unclear how much the POGZ variant contributed to the individual 13’s phenotype or if the POLG variants were the sole cause of his clinical features. Predictions from in silico algorithms were conflicting with meta-predictor tools such as REVEL and ClinPred predicting the variant was benign (Supplementary Table 3). However, the variant is in a highly constrained region of POGZ (Supplementary Fig. 1).
Discussion
We have presented detailed clinical and genetic findings from 12 new individuals with POGZ PTVs and one individual with a novel de novo missense variant. Two of the variants were inherited from mildly affected parents. Inherited PTVs variants (c.3591_3592delTG p.(Ser1197Argfs*4) and c.1524-3 C > G) in POGZ causing WHSUS have previously been reported in two other families [10, 11]. In both families, the parent was mildly affected. The intronic variant described by Garde et al. was predicted to affect splicing and was also inherited by an affected sibling [11]. This highlights that inherited POGZ variants should not be overlooked during variant filtering and interpretation of trio-based exome or genome data.
We identified a person (individual 13) with a de novo missense variant and features of WHSUS. Missense variants in POGZ are less frequently reported in patients with features of WHSUS, but rare/de novo missense variants are enriched in patients with ASD and schizophrenia [16–20]. Missense variants may have milder deleterious effects than PTVs but still contribute to neurodevelopmental disorders (potentially in an oligo- or polygenic manner). However, the variable and relatively non-specific clinical features of WHSUS make it difficult to be certain about the clinical significance of specific missense variants. Of the previously reported individuals with WHSUS to date, including in this series of 12 individuals, only 6 are reported to have missense variants. Of these, only four have commented on the level of ID (3 individuals reported to have mild ID and 1 individual reported to have severe ID [7, 8, 11, 17]. Further evidence from epidemiological or functional studies will be needed to fully establish the contribution of missense POGZ variants to syndromic and non-syndromic ID.
Our series includes further individuals with WHSUS and rod-cone dystrophy, cleft lip and palate, congenital diaphragmatic hernia, and/or a duplicated renal drainage system. These appear to be rare complications of WHSUS alongside the more common and more widely reported phenotype of variable DD or ID, obesity, other visual defects, craniofacial dysmorphism, sensorineural hearing loss, feeding problems, seizures and structural brain malformations. Based on these findings, a reasonable approach to management following diagnosis would include a thorough neurological and physical examination, hearing assessment and ophthalmological review. Individuals with WHSUS are likely to benefit from continued neurodevelopmental and nutritional review and support.
Variants that cause WHSUS are spread throughout the gene. Four PTVs in our series were in the large final exon of POGZ. The final exon encodes 553 amino acids (residues 857–1410, around 39% of the total protein) and contains over half of the pathogenic PTVs reported to date. PTVs in the final exon may escape nonsense-mediated decay (NMD) and produce truncated products lacking one or more of the four C-terminal domains (Fig. 1). PTVs in the small penultimate exon 18 (which encodes 7 amino acids) would also be predicted to escape NMD. Batzir et al. suggested that patients with 3′ truncating variants predicted to escape NMD were more likely to have severe speech delay [10]. The hypothesis being that PTVs which escape NMD may cause dominant-negative effects rather than simply acting through haploinsufficiency. In our combined analysis we found slightly more individuals with a PTV in the last two exons had severe speech delay (18/39, 46%) compared with individuals with a PTV in or before exon 17 (8/34, 34%) (Supplementary Table 2). However, this difference was not quite statistically significant (Fisher’s exact test, P = 0.053). Similarly, it has been proposed that although there is variability, individuals with PTVs that escape NMD might have more severe neurodevelopmental problems [10]. In our combined analysis we found individuals with a PTV in the last two exons had a higher rate of moderate or severe ID (21/39, 53%) compared with individuals with a PTV in or before exon 17 (12/33, 36%). Again, this difference was not statistically significant (Fisher’s exact test, P = 0.16). Therefore, we cannot confirm a clear genotype-phenotype correlation between PTV position and ID severity.
Another previous report which performed analysis with phenotyping software (DeepGestalt) suggested that variants in the C-terminal DDE domain were generally associated with less typical WHSUS facial appearance which predominately affected the lower facial portion [9]. Individual 12 in our series had a PTV in the DDE domain and, in our opinion, had typical facial features of WHSUS, including midface hypoplasia (Fig. 2).
We recognise the limitations of our series and the combined analysis with previous individuals. The subjects were recruited and assessed by different clinicians in different centres. Data for some individuals was missing or not available. Phenotypic reporting is likely variable between different series, with rare but significant features only mentioned when present rather than absent. It is possible that mild or subtle features such as brachydactyly or minor male genital anomalies may be underreported. Reported individuals with WHSUS are generally young at the time of phenotyping, with the oldest being 34 years old (individual 6). This may be because younger patients with ID are more likely to have genetic testing. We expect there are many older individuals with WHSUS who do not have a genetic diagnosis. From a phenotypic perspective, younger patients may not yet have manifested key features (e.g. obesity). Therefore, further work analysing the phenotype of older individuals with WHSUS is needed.
POGZ has been shown to interact with HP1-α (Heterochromatin protein 1-alpha, CBX5; OMIM 604478) [13]. HP1-α has an essential role in heterochromatin formation and mitotic progression through interaction with several cell cycle proteins [27, 28]. POGZ knockdown in human 293 cells disrupts mitotic HP1-α localisation, and causes mitotic defects, abnormal chromosome segregation and nuclear fragmentation [13]. Another protein reported to interact with both HP1-α and POGZ is CHAMP1 [29]. Like POGZ, loss-of-function variants in CHAMP1 have been reported to cause ASD/ID and there are several phenotypic similarities between the conditions [29, 30]. Individuals with CHAMP1-related ID are commonly reported to have severe speech delay, hypotonia, high palate, hypermetropia, strabismus and sleep apnoea [29, 30]. Like WHSUS, the facial features of CHAMP1-related ID are variable, but there are some shared features, including hypertelorism, midface hypoplasia and a thin upper lip, which can also be more prominent centrally. These similarities raise the possibility that disturbed HP1-α function is a pathogenic mechanism of both WHSUS and CHAMP1-related ID.
In summary, we have reported 12 new individuals with pathogenic or likely pathogenic variants in POGZ. We combined clinical data from these individuals with 89 previously reported individuals. Our analysis demonstrated that WHSUS is a neurodevelopmental disorder associated with increased risk of obesity, visual defects, craniofacial dysmorphism, sensorineural hearing loss, feeding problems, seizures and structural brain malformations. The findings demonstrate the variable effects and severity of WHSUS. This work highlights several rare complications of WHSUS; the potential role of deleterious missense variants in POGZ; and the observation of further inherited pathogenic variants in POGZ.
Supplementary information
Acknowledgements
We would like to thank the patients, families and clinicians who have contributed to this report. AEF was supported by the Wales Gene Park. Wales Gene Park is a Health and Care Research Wales funded infrastructure support group. The DDD study presents independent research commissioned by the Health Innovation Challenge Fund [grant number HICF-1 009-003]. This study makes use of DECIPHER (http://decipher.sanger.ac.uk), which is funded by Wellcome. See Nature PMID: 25533962 or www.ddduk.org/access.html for full acknowledgement. Clinical testing and analysis were performed by The Department of Medical Genetics, Telemark Hospital Trust, Skien, Norway and the Exeter Genomics Laboratory, Royal Devon & Exeter NHS Foundation Trust, UK.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Individuals who had evaluation or analysis beyond routine clinical care were part of research studies approved by the Cambridge South Research Ethics Committee (10/H0305/83).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-021-00961-3.
References
- 1.Stessman HAF, Willemsen MH, Fenckova M, Penn O, Hoischen A, Xiong B, et al. Disruption of POGZ is associated with intellectual disability and autism spectrum disorders. Am J Hum Genet. 2016;98:541–52. doi: 10.1016/j.ajhg.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White J, Beck CR, Harel T, Posey JE, Jhangiani SN, Tang S, et al. POGZ truncating alleles cause syndromic intellectual disability. Genome Med. 2016;8:3. doi: 10.1186/s13073-015-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dentici ML, Niceta M, Pantaleoni F, Barresi S, Bencivenga P, Dallapiccola B, et al. Expanding the phenotypic spectrum of truncating POGZ mutations: association with CNS malformations, skeletal abnormalities, and distinctive facial dysmorphism. Am J Med Genet A. 2017;173:1965–9. doi: 10.1002/ajmg.a.38255. [DOI] [PubMed] [Google Scholar]
- 4.Gilissen C, Hehir-Kwa JY, Thung DT, van de Vorst M, van Bon BWM, Willemsen MH, et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–7. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 5.Lelieveld SH, Reijnders MRF, Pfundt R, Yntema HG, Kamsteeg E-J, de Vries P, et al. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat Neurosci. 2016;19:1194–6. doi: 10.1038/nn.4352. [DOI] [PubMed] [Google Scholar]
- 6.Ferretti A, Barresi S, Trivisano M, Ciolfi A, Dentici ML, Radio FC, et al. POGZ-related epilepsy: case report and review of the literature. Am J Med Genet A. 2019;179:1631–6. doi: 10.1002/ajmg.a.61206. [DOI] [PubMed] [Google Scholar]
- 7.Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–8. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samanta D, Ramakrishnaiah R, Schaefer B. The neurological aspects related to POGZ mutation: case report and review of CNS malformations and epilepsy. Acta Neurol Belg. 2019;120:447–50. [DOI] [PubMed]
- 9.Pascolini G, Agolini E, Fleischer N, Gulotta E, Cesario C, D’Elia G, et al. A novel patient with White-Sutton syndrome refines the mutational and clinical repertoire of the POGZ-related phenotype and suggests further observations. Am J Med Genet A. 2020;182:1791–5. doi: 10.1002/ajmg.a.61605. [DOI] [PubMed] [Google Scholar]
- 10.Assia Batzir N, Posey JE, Song X, Akdemir ZC, Rosenfeld JA, Brown CW, et al. Phenotypic expansion of POGZ-related intellectual disability syndrome (White-Sutton syndrome) Am J Med Genet A. 2020;182:38–52. doi: 10.1002/ajmg.a.61380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garde A, Cornaton J, Sorlin A, Moutton S, Nicolas C, Juif C, et al. Neuropsychological study in 19 French patients with White-Sutton syndrome and POGZ mutations. Clin Genet. 2021;99:407–17. doi: 10.1111/cge.13894. [DOI] [PubMed] [Google Scholar]
- 12.Dal S, Hopper B, du Chattel MVR, Goel H A case of White-Sutton syndrome with previously described loss-of-function variant in DDE domain of POGZ (p.Arg1211*) and Kartagener syndrome. Am J Med Genet A. 2020;185:1006–7. [DOI] [PubMed]
- 13.Nozawa R-S, Nagao K, Masuda H-T, Iwasaki O, Hirota T, Nozaki N, et al. Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nat Cell Biol. 2010;12:719–27. doi: 10.1038/ncb2075. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura K, Seiriki K, Okada S, Nagase M, Ayabe S, Yamada I, et al. Pathogenic POGZ mutation causes impaired cortical development and reversible autism-like phenotypes. Nat Commun. 2020;11:859. doi: 10.1038/s41467-020-14697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukai R, Hiraki Y, Yofune H, Tsurusaki Y, Nakashima M, Saitsu H, et al. A case of autism spectrum disorder arising from a de novo missense mutation in POGZ. J Hum Genet. 2015;60:277–9. doi: 10.1038/jhg.2015.13. [DOI] [PubMed] [Google Scholar]
- 18.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–99. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional, and chromatin genes disrupted in autism. Nature. 2014;515:209–15. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–8. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright CF, Fitzgerald TW, Jones WD, Clayton S, McRae JF, van Kogelenberg M, et al. Genetic diagnosis of developmental disorders in the DDD study: a scalable analysis of genome-wide research data. Lancet Lond Engl. 2015;385:1305–14. doi: 10.1016/S0140-6736(14)61705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D, et al. DECIPHER: database of chromosomal imbalance and phenotype in humans using ensembl resources. Am J Hum Genet. 2009;84:524–33. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med J Am Coll Med Genet. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellard S Best Practice Guidelines. The Association for Clinical Genomic Science. 2020. https://www.acgs.uk.com/quality/best-practice-guidelines/
- 26.Longoni M, High FA, Qi H, Joy MP, Hila R, Coletti CM, et al. Genome-wide enrichment of damaging de novo variants in patients with isolated and complex Congenital Diaphragmatic Hernia. Hum Genet. 2017;136:679–91. doi: 10.1007/s00439-017-1774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheutin T, McNairn AJ, Jenuwein T, Gilbert DM, Singh PB, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–5. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- 28.Motamedi MR, Hong E-JE, Li X, Gerber S, Denison C, Gygi S, et al. HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol Cell. 2008;32:778–90. doi: 10.1016/j.molcel.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isidor B, Küry S, Rosenfeld JA, Besnard T, Schmitt S, Joss S, et al. De Novo Truncating Mutations in the Kinetochore-Microtubules Attachment Gene CHAMP1 Cause Syndromic Intellectual Disability. Hum Mutat. 2016;37:354–8. doi: 10.1002/humu.22952. [DOI] [PubMed] [Google Scholar]
- 30.Hempel M, Cremer K, Ockeloen CW, Lichtenbelt KD, Herkert JC, Denecke J, et al. De Novo Mutations in CHAMP1 Cause Intellectual Disability with Severe Speech Impairment. Am J Hum Genet. 2015;97:493–500. doi: 10.1016/j.ajhg.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.