INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) encompasses simple steatosis, steatohepatitis, advanced fibrosis, and cirrhosis. Transient elastography has emerged as an accurate noninvasive diagnostic tool for assessing hepatic fibrosis and steatosis as compared to biopsy.1, 2 Diabetes is a predictor of adverse clinical outcomes such as advanced hepatic fibrosis and mortality in patients with NAFLD.3 Therefore, we characterize the current prevalence of NAFLD and hepatic fibrosis by controlled attenuation parameter (CAP)–enhanced transient elastography among adults with normal glucose tolerance, prediabetes, and diabetes in the USA.
METHODS
We analyzed the 2017–2018 National Health and Nutrition Examination Survey (NHANES) data. Participants were evaluated by the CAP score and liver stiffness measurements documented in the NHANES dataset.4 We used two cut-offs of CAP score and liver stiffness measurements for suspected fibrosis and cirrhosis (Figs. 1 and 2).2, 5, 6 We defined diabetes as having either diagnosed diabetes, treatment with anti-diabetic medication, or HbA1c ≥6.5%, and prediabetes as any participant who neither had diagnosed diabetes nor was taking anti-diabetic medication but who had HbA1c level of 5.7–6.4% (n=4,702). For those with fasting results (≥9 h, n=2,073), we added criteria of fasting glucose ≥126 mg/dL for diabetes and fasting glucose 100–125 mg/dl for prediabetes. We used appropriate sample weights to reconstitute representative US population. We calculated age-standardized prevalence estimates by using direct standardization with the 2000 US Census.
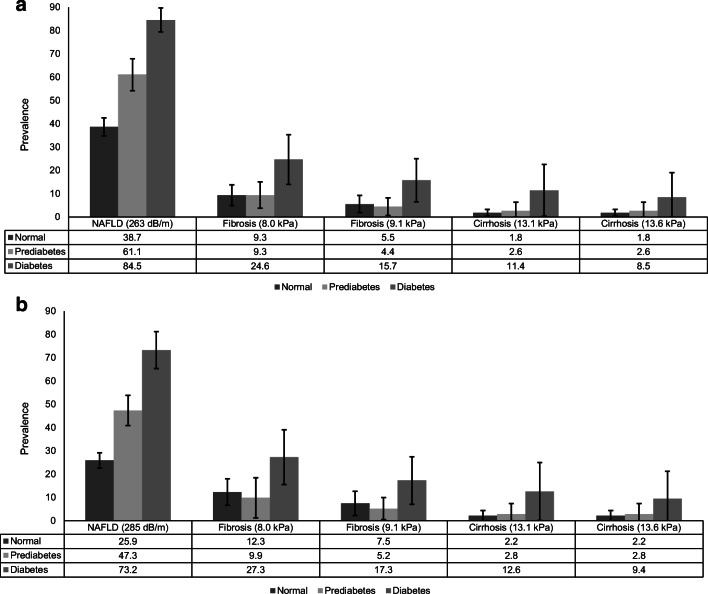
Figure 1.
Age-standardized prevalence of suspected nonalcoholic fatty liver disease (NAFLD) and suspected fibrosis and cirrhosis by normal glucose tolerance, prediabetes, and diabetes in the USA, 2017–2018 (n=4,207). a Suspected NAFLD defined as controlled attenuation parameter (CAP) score ≥263 dB/m (cut-off values for 90% sensitivity) and suspected fibrosis (≥F2, liver stiffness measurement [LSM] ≥8.0 kPa or >9.1 kPa) and suspected cirrhosis (F4, liver stiffness measurement [LSM] ≥13.1 kPa or >13.6 kPa) among individuals with NAFLD. b Suspected NAFLD defined as controlled attenuation parameter (CAP) score ≥285 dB/m (cut-off values optimizing sensitivity and specificity) and suspected fibrosis (≥F2, liver stiffness measurement [LSM] ≥8.0 kPa or >9.1 kPa) and suspected cirrhosis (F4, liver stiffness measurement [LSM] ≥13.1 kPa or >13.6 kPa) among individuals with NAFLD.
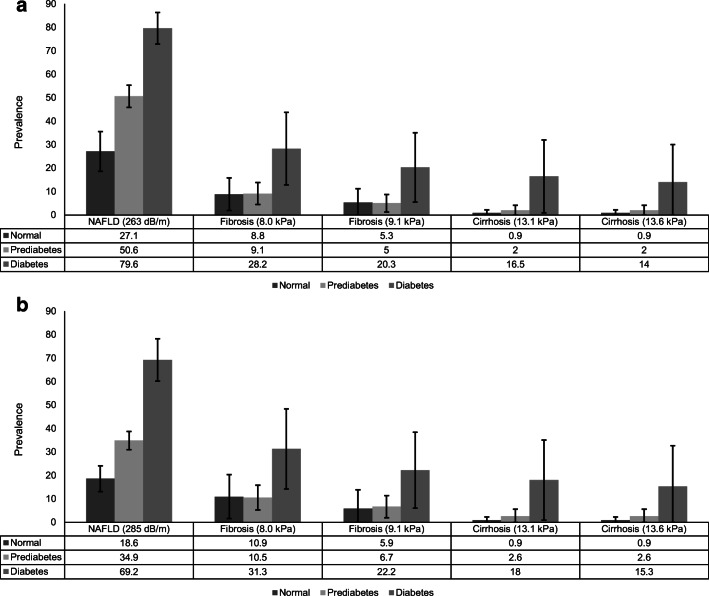
Figure 2.
Age-standardized prevalence of suspected nonalcoholic fatty liver disease (NAFLD) and suspected fibrosis and cirrhosis by normal glucose tolerance, prediabetes, and diabetes in the USA, 2017–2018 (fasting sample, n =2,073). a Suspected NAFLD defined as controlled attenuation parameter (CAP) score ≥263 dB/m (cut-off values for 90% sensitivity) and suspected fibrosis (≥F2, liver stiffness measurement [LSM] ≥8.0 kPa or >9.1 kPa) and suspected cirrhosis (F4, liver stiffness measurement [LSM] ≥13.1 kPa or >13.6 kPa) among individuals with NAFLD. b Suspected NAFLD defined as controlled attenuation parameter (CAP) score ≥285 dB/m (cut-off values optimizing sensitivity and specificity) and suspected fibrosis (≥F2, liver stiffness measurement [LSM] ≥8.0 kPa or >9.1 kPa) and suspected cirrhosis (F4, liver stiffness measurement [LSM] ≥13.1 kPa or >13.6 kPa) among individuals with NAFLD.
RESULTS
Of adults (≥18 years) who underwent an examination (n=5,533), we excluded participants with significant alcohol consumption (>28 g/day [women] and >42 g/day [men] by a 24-h dietary recall, n=438) and viral hepatitis (n=72), those who were exposed to steatogenic medications (amiodarone, methotrexate, valproate, corticosteroid, and tamoxifen, n=91), and those for whom transient elastography data were incomplete (<10 complete stiffness measures or liver stiffness interquartile range/median ≥30%, or fasted <3 h, n=725). Four thousand two hundred seven participants were finally included in the study (extra-large [XL] probe: 26.5%). The mean age was 47.1 years (95% confidence interval [CI] 45.7–48.5), with 48.1% men. The age-standardized weighted prevalence of prediabetes and diabetes was 21.1% (95% CI 19.0–23.4) and 13.6% (95% CI 12.3–15.0), respectively. As shown in Figure 1 a, the age-standardized prevalence of NAFLD (≥263 dB/m; sensitivity fixed at 90%) was substantially higher in diabetes (84.5%, 95% CI 79.4–89.7), followed by prediabetes (61.1%, 95% CI 54.2–67.9), and normal glucose tolerance (38.7%, 95% CI 34.8–42.5). When we used cut-off values optimizing sensitivity and specificity (≥285 dB/m),2 the age-standardized prevalence of NAFLD was 25.9% (22.6–29.2), 47.3% (40.9–53.8), and 73.2% (66.3–81.2) among individuals with normal glucose tolerance, prediabetes, and diabetes, respectively (Fig. 1b). Among individuals with NAFLD (Fig. 1a), the age-standardized prevalence of suspected fibrosis (≥F2) was highest among individuals with diabetes (15.7–24.6%). Likewise, the age-standardized prevalence of suspected cirrhosis was highest (8.5–11.4%) among individuals with diabetes. When we used cut-off values of ≥285 dB/m to define NAFLD, prevalence of suspected fibrosis and cirrhosis was identical (Fig. 1b). In participants who fasted (≥9 h, n=2,073), the prevalence of NAFLD was substantially higher in diabetes (73.2–79.6%), followed by prediabetes (34.9–50.6%), and normal glucose tolerance (Fig. 2). The prevalence of suspected fibrosis and cirrhosis was similar to analyses using total population despite large confidence intervals.
DISCUSSION
We found that the prevalence of NAFLD by CAP measurement was high in individuals with diabetes (range: 70.7–82.1%) and prediabetes (38.5–52.9%). Fibrosis and cirrhosis were found in approximately one-fourth of patients with diabetes and one-sixth of those with prediabetes among individuals with NAFLD. A previous meta-analysis estimated the global prevalence of NAFLD in patients with diabetes to be 55.5% (95% CI 47.3–63.7), with a regional prevalence of 51.8% in the US.3 The prevalence of advanced fibrosis based on liver biopsy among 439 patients with NAFLD and diabetes was 17.0% (95% CI 7.3–34.9).3 Compared with other studies, our data are unique in using the CAP-enhanced elastography with a nationally representative sample. Limitations of our study include lack of universal cut-off guideline for CAP score and liver stiffness; we employed cut-off points from previous landmark studies.1, 2, 5, 6 We used 24-h dietary recall for alcohol use, which may lead to misclassification and under-estimation. Our findings support the existing notion that patients with diabetes and prediabetes are at-risk population and can be screened for NAFLD and NAFLD-related fibrosis.
Abbreviations
- CAP
Controlled attenuation parameter
- NHANES
National Health and Nutrition Examination Survey
- CI
Confidence interval
- NAFLD
Nonalcoholic fatty liver disease
Author Contribution
Donghee Kim was involved in study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. George Cholankeril and Rohit Loomba were involved in the interpretation of data and critical revision of the manuscript. Aijaz Ahmed was involved in study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior Presentations
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Donghee Kim, Email: messmd@chol.com.
George Cholankeril, Email: georgetc@stanford.edu.
Rohit Loomba, Email: roloomba@ucsd.edu.
Aijaz Ahmed, Email: aijazahmed@stanford.edu.
References
- 1.Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(5):1264–81. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17(1):156–63. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 4.National Health and Nutrition Examination Survey: Liver Ultrasound Transient Elastography Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2018_Liver_Ultrasound_Elastography_Procedures_Manual.pdf. Accessed at Apr 2020.
- 5.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(6):1717–30. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Serra-Burriel M, Graupera I, Toran P, Thiele M, Roulot D, Wai-Sun Wong V, et al. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71(6):1141–51. doi: 10.1016/j.jhep.2019.08.019. [DOI] [PubMed] [Google Scholar]