Abstract
Breast implant surgery for cosmetic or reconstructive purposes is becoming increasingly common. While the devices used are regulated and approved by the US Food and Drug Administration, all patients with breast implants require continued follow-up. Many patients will seek this care from their primary care providers, especially when follow-up with their plastic surgeon is difficult. It is vital that treating clinicians are knowledgeable about the history of breast implants, routine screening guidelines, and the recent breast implant “hot topics”—breast implant–associated anaplastic large cell lymphoma (BIA-ALCL), connective tissue disease, and breast implant illness. This paper will provide the necessary information for primary care providers to appropriately counsel patients with breast implants to maintain not only their trust, but also their health.
INTRODUCTION
Breast implants have been utilized for aesthetic and reconstructive surgery after mastectomy for over 60 years.1,2 The American Society of Plastic Surgeons reports that approximately 300,000 aesthetic breast implant surgeries and another 100,000 implant-based breast cancer reconstructions were performed in 2019 in the USA alone.3 Patients with breast implants require routine long-term care to screen for breast implant–related symptoms and pathology. While patients commonly address breast implant concerns with their plastic surgeon, many may first seek care from their primary care providers, especially those who face barriers to specialty care access or who are beyond the initial postoperative period. It is crucial for primary care providers to have up-to-date knowledge of breast implant safety. In this brief review, we summarize evidence and recommendations for breast implant–associated anaplastic large cell lymphoma (BIA-ALCL), a rare long-term complication of breast implant surgery. In addition, we discuss current evidence on possible systemic effects of breast implants, specifically connective tissue disease and “breast implant illness,” the latter of which has recently garnered significant attention.
IMPLANT CHARACTERISTICS
Breast implants are among the most highly scrutinized devices approved by the US Food and Drug Administration (FDA). Since the first breast augmentation in 1962, breast implants have undergone several generations of improvement,4 including changes to filling material (silicone or saline), surface texture (smooth or textured), and shape (round or anatomic).5
Silicone- and saline-filled implants are approved for use in breast surgery. The advantages of silicone implants are less rippling (i.e., less visibility of the implant shell under the skin) and preservation of breast shape if the implant ruptures due to the cohesive, self-containing gel. Silicone implants also tend to feel more natural to patients. Saline implants, however, have the potential advantage of smaller incisions needed for implantation. Additionally, while silicone implants require regular MRIs to detect potential ruptures, saline implants do not since they deflate when ruptured. While surgeons may recommend silicone or saline implants to a patient based on anatomical features and medical history (i.e., thin breast skin or a history of radiation to the breast which may show more rippling), the choice is ultimately up to the patient. Most patients currently choose silicone implants, and several studies examining patient-reported satisfaction following breast reconstruction suggest silicone implants have superior outcomes.6,7
Both silicone and saline implants have outer silicone shells that can be textured or smooth. Textured surfaces allow for some fibrous tissue ingrowth, preventing rotation and potentially reducing capsular contracture, a form of scarring around the implant. Texturing can be used for both round and anatomic implants. Round implants have a symmetric shape, allowing for smooth and textured surfaces, and silicone versions have a flat side that is placed against the chest wall. Anatomic implants, however, are teardrop-shaped to mimic the natural shape of the breast, so these implants are textured to maintain their orientation in the breast pocket.
GENERAL BREAST IMPLANT CARE
Primary care providers should know the implant’s fill (saline or silicone) and surface type (smooth or textured). Patients can find this information on the implant card containing type and serial number that is given to them after surgery. Otherwise, the clinician can obtain surgical records to verify the exact implant style.
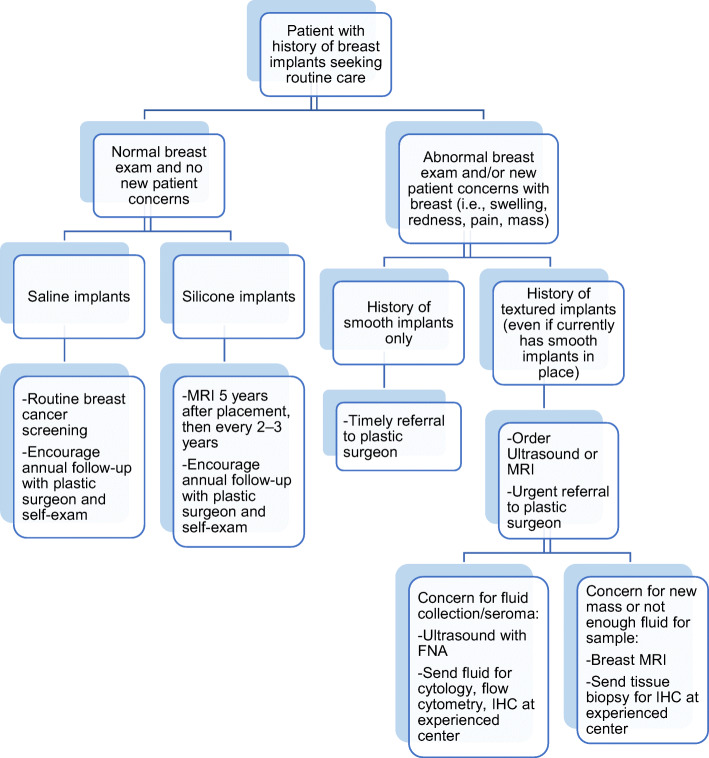
The provider should establish a baseline breast exam to recognize changes to the implant, including two major implant concerns: capsular contracture and implant rupture. Approximately 10% of patients with breast implants will experience capsular contracture, causing pain and implant shape distortion.8 Exam findings include a tight breast, pain with a palpable capsule, tethering, or skin indentation. Additionally, while normal breast implants rotate within the pocket when palpated, implants with capsular contracture seem immobile. In terms of implant rupture, breast implants carry a rupture risk of about 1% per year.9 Ruptured saline implants deflate, which can be identified on exam and are often identified immediately by the patient. However, silicone implant ruptures are difficult to diagnose by physical exam because they maintain their shape. Some patients experience pain or signs of capsular contracture, potentially after trauma to the breast. However, many do not have symptoms, so any patient with silicone breast implants receives a breast MRI 5 years after placement and then every 2–3 years thereafter (Fig. 1).10 Both these conditions should prompt providers to refer patients to a plastic surgeon.
Figure 1.
Workflow for a patient with breast implants seeking routine primary care. BIA-ALCL, breast implant–associated anaplastic large cell lymphoma; FNA, fine needle aspiration; MRI, magnetic resonance imaging; IHC, immunohistochemistry.
Routine breast cancer screening guidelines are unchanged for women with breast augmentation. However, mammography should be performed with 4 views instead of 2, and an MRI may be needed if there are areas of poor visualization due to the implant capsule or implant position.11 Similarly, since breast reconstruction patients have received mastectomies and have no breast tissue remaining, breast cancer surveillance should follow guidelines for postmastectomy breast cancer patients.12
BREAST IMPLANT–ASSOCIATED ANAPLASTIC LARGE CELL LYMPHOMA (BIA-ALCL)
First reported in 1997, BIA-ALCL is a T-cell lymphoma associated with textured breast implants.13 The most recent FDA data shows 733 cases worldwide, making this condition rare; however, case numbers continue to increase.14 Current estimates of lifetime risk for BIA-ALCL in patients with textured implants range from 1-in-559 to 1-in-355.15,16
When screening for BIA-ALCL, the primary care provider should ask the patient about texturing of the implant, the manufacturer of the implant, and the duration of implant placement.17 On physical exam, BIA-ALCL commonly presents as new breast swelling or fluid collection 7–10 years after implant insertion, but it can also present as a new mass or capsular contracture.18 Ultrasound can then confirm the presence of a fluid collection, and breast MRI is ideal for potential new masses. If there is a fluid collection, it should be sampled using fine needle aspiration and sent for cytology (atypical, large, pleiomorphic cells), flow cytometry, and immunohistochemistry (CD30 positive, ALK negative).18 Because other forms of lymphoma may yield similar results, specimens should be specifically flagged for concern of BIA-ALCL if they necessitate analysis at a tertiary center. Patients with suspected BIA-ALCL should then also be referred to a plastic surgeon.
Patients with confirmed BIA-ALCL should seek care from an experienced team of medical and surgical oncologists to achieve the best prognosis. They should receive a PET scan for appropriate staging using the tumor–node–metastasis (TNM) solid tumor staging system. Most confirmed BIA-ALCL cases are stage I and have excellent prognoses. Treatment is almost always an “enbloc” capsulectomy, which involves removal of the implant along with the entire capsule.18 Because advanced cases are rare, there is no standardized treatment approach to chemotherapy or radiation for BIA-ALCL. The majority of BIA-ALCL patients are disease free at 3 years, largely due to a strict follow-up routine: exams every 3–6 months followed by imaging every 6 months for 2 years.18
Many patients with textured breast implants ask whether to remove their implants, so providers should have a framework for guiding discussion and decision making.19 Currently, the FDA does not recommend breast implant exchange and capsulectomy in asymptomatic patients with textured breast implants.20 There is insufficient evidence linking prophylactic implant exchange and/or capsulectomy with BIA-ALCL risk reduction, and these surgeries still carry the risk of complications.21,22 Still, the risk of developing BIA-ALCL can cause significant psychological distress for some patients, so referral to a plastic surgeon for a discussion regarding implant removal may be beneficial for these patients.19,23
BREAST IMPLANTS AND CONNECTIVE TISSUE DISEASES
Despite exhaustive investigation, data does not support an association between breast implants and connective tissue diseases.24–27 Nevertheless, misinformation propagated through the media has continued to raise concerns.28 Primary care physicians of breast implant patients presenting with concerns about connective tissue diseases should be aware that some of these fears stem from studies conducted in the 1990s, most of which have since been disputed. These investigations implicated a relationship between silicone implants and Sjogren’s, rheumatoid arthritis, systemic sclerosis, dermatomyositis/polymyositis, and other connective tissue diseases, speculating that the immunogenicity of silicone can contribute to the development of these autoimmune disorders.29,30 In response, the FDA downgraded the status of silicone breast implants to investigational in 1992.31 Since then, multiple systematic reviews and meta-analyses, including a report by the Institute of Medicine, have almost unanimously confirmed the lack of association between silicone implants and connective tissue diseases.24–27 The FDA ended its moratorium on silicone breast implants in 2006, conditional on the implementation of large post-approval studies (“Core Studies”) by the two largest implant manufacturers (Allergan and Mentor) to examine long-term safety.
Recently, the 10-year follow-up results of the Core Studies were published, confirming the safety of silicone implants; 82% (Allergan) and 91% (Mentor) of augmentation patients still had their original implants after 10 years.32,33 However, another recent study that examined these data found increased risk of Sjogren’s, scleroderma, and rheumatoid arthritis (standardized incidence ratios: 8.14, 7.00, and 5.96, respectively) for patients with silicone implants compared with national normative data, reigniting the controversy over breast implants and connective tissue disease.34 This study had significant methodological flaws in terms of loss-to-follow-up, reporting, and categorization of cases, which likely led to an overestimation of these incidence ratios.35 In addition, the study did not account for the significant overlap that exists between the population most at risk for connective tissue disease and the population most likely to undergo breast augmentation—young women in their thirties.36
Currently, the FDA does not recognize an association between silicone breast implants and connective tissue diseases.10 However, this may understandably be a source of anxiety for patients. Providers should reassure patients that breast implants do not cause connective tissue disease and do not need to be removed. They can offer referral to rheumatology for conclusive diagnosis if medically indicated, as well as to plastic surgery if the patient does desire implant removal.
BREAST IMPLANT ILLNESS
Related to the controversy over connective tissue diseases and silicone breast implants, a poorly defined collection of non-specific systemic symptoms called “breast implant illness” has gained recent media attention.28,37 Patients may present with a number of symptoms and request referral for removal of both the breast implants and peri-prosthetic capsules. These symptoms are broad-ranging and include chronic fatigue, “brain fog,” myalgias, arthralgias, hair loss, anxiety, heart palpitations, and frequent urination.38,39 Because of the number and variety of symptoms, “breast implant illness” is hard to verify or define, and some investigators have claimed that it is psychosomatic, given the recent social media attention.28,40,41 A recent propensity-matched analysis of a retrospective cohort of 22,000 women with or without breast implants has demonstrated no increased risk of “non-specific” systemic symptoms in women with breast implants, supporting the possibility that confounding factors explain these symptoms, rather than breast implantation.42 However, others propose an autoimmune etiology, potentially as a reaction to silicone itself or as an immunological response to biofilm formation on the implant.38 A recent prospective cohort study of explanted (removed) breast implants by Lee et al. has found that patients with breast implant illness symptoms were more likely to have positive bacterial cultures from their implants and associated capsules than individuals with no symptoms.39
Regardless of the ultimate etiology, these symptoms can be distressing to patients and affect quality of life, leading many to seek explantation, which has been effective for some patients. In the Lee et al. study, 84% of patients reported partial or complete resolution of symptoms after explantation, which is in line with a recent review conducted by de Boer et al. that estimated 75% of patients had improvement of symptoms.39,43 However, for individuals who have clinically diagnosed autoimmune conditions, symptoms were not likely to improve after explantation unless immunosuppressive therapy was also initiated.43
The FDA currently recognizes breast implant illness as a potential risk of breast implant surgery.[10,
CONCLUSIONS
Breast implant surgeries have been performed for decades, with hundreds of thousands of women undergoing these procedures every year. Primary care providers will encounter breast implant patients in their practices and should therefore be aware of the latest screening and treatment guidelines for long-term breast implant conditions (see Table 1 for practice takeaways). In this review, we have provided current guidelines for BIA-ALCL, a rare cancer associated with textured breast implants, as well as research updates on connective tissue diseases and breast implant illness. With this information, primary care providers can participate in discussions with patients and their surgeons and counsel patients appropriately about long-term breast implant risks.
Table 1.
Practice Takeaways for Breast Implant Concerns
| Breast implant concern | Practice takeaway |
|---|---|
| BIA-ALCL |
• BIA-ALCL occurs in patients who have or have had textured breast implants. • BIA-ALCL commonly presents as new swelling or fluid collection about 7–10 years after implant placement. • Diagnosis is made by sampling the fluid or capsule surrounding the implant. Tissue samples should be marked to specifically rule out BIA-ALCL and may often require careful analysis at experienced laboratories. • Timely referral to a plastic surgeon for removal of the implant and surrounding capsule is the treatment of choice. • BIA-ALCL has an excellent prognosis when detected early but should be managed by an experienced team of medical and surgical oncologists. • Rigorous follow-up is essential after a confirmed diagnosis of BIA-ALCL, with exams every 3–6 months and imaging every 6 months for 2 years. • The FDA currently does not recommend prophylactic implant removal. |
| Connective tissue disease |
• There is no association between silicone breast implants and risk of connective tissue diseases. Concerns about connective tissue disease stem primarily from disputed studies conducted in the 1990s. • Providers should reassure patients, counsel them about the history of these concerns, and refer them to rheumatology for conclusive diagnoses. |
| Breast implant illness |
• “Breast implant illness” is a poorly understood collection of systemic symptoms that may be linked with breast implants. • Providers should refer patients to plastic surgery for discussion of the risks and benefits of potential explantation. |
Acknowledgements
Editorial support in the preparation of this paper was provided by Dagmar Schnau and Olga Rukovets.
Abbreviations
- BIA-ALCL
Breast implant–associated anaplastic large cell lymphoma
- FDA
US Food and Drug Administration
- MRI
Magnetic resonance imaging
- TNM
Tumor, node, metastasis
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Cayla D. McKernan and Joshua Vorstenbosch are co-first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collett DJ, Rakhorst H, Lennox P, Magnusson M, Cooter R, Deva AK. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg. 2019;143:30S–40S. doi: 10.1097/PRS.0000000000005567. [DOI] [PubMed] [Google Scholar]
- 2.Rohrich RJ, Kaplan J, Dayan E. Silicone implant illness: science versus myth? Plast Reconstr Surg. 2019;144(1):98–109. doi: 10.1097/PRS.0000000000005710. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Plastic Surgeons. 2019 plastic surgery statistics report. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. https://www.plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf. Published 2020. Accessed December 22, 2020
- 4.Cronin T, Gerow F. Transactions of the Third International Congress of Plastic Surgery. Amsterdam: Experta Medical Foundation; 1963. Augmentation mammaplasty: a new “natural feel” prostheses; pp. 41–49. [Google Scholar]
- 5.Groth AK, Graf R. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthetic Plast Surg. 2020;44(1):1–12. doi: 10.1007/s00266-019-01521-3. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010;116(24):5584–5591. doi: 10.1002/cncr.25552. [DOI] [PubMed] [Google Scholar]
- 7.Macadam SA, Ho AL, Cook EF, Jr, Lennox PA, Pusic AL. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast Reconstr Surg. 2010;125(3):761–771. doi: 10.1097/PRS.0b013e3181cb5cf8. [DOI] [PubMed] [Google Scholar]
- 8.Shen Z, Chen X, Sun J, et al. A comparative assessment of three planes of implant placement in breast augmentation: a Bayesian analysis. J Plast Reconstr Aesthet Surg. 2019;72(12):1986–1995. doi: 10.1016/j.bjps.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Calobrace MB, Schwartz MR, Zeidler KR, Pittman TA, Cohen R, Stevens WG. Long-term safety of textured and smooth breast implants. Aesthet Surg J. 2017;38(1):38-48. [DOI] [PubMed]
- 10.US Food and Drug Administration. Breast implants - certain labeling recommendations to improve patient communication: guidance for industry and Food and Drug Administration staff. FDA (Food and Drug Administration). https://www.fda.gov/media/131885/download. Published 2020. Updated 9/29/2020. Accessed December 23, 2020
- 11.Tang SS, Gui GP. A review of the oncologic and surgical management of breast cancer in the augmented breast: diagnostic, surgical and surveillance challenges. Ann Surg Oncol. 2011;18(8):2173–2181. doi: 10.1245/s10434-011-1578-6. [DOI] [PubMed] [Google Scholar]
- 12.Zakhireh J, Fowble B, Esserman LJ. Application of screening principles to the reconstructed breast. J Clin Oncol. 2010;28(1):173–180. doi: 10.1200/JCO.2008.21.7588. [DOI] [PubMed] [Google Scholar]
- 13.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Medical device reports of breast implant-associated anaplastic large cell lymphoma. https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma. Published 2019. Accessed May 8, 2020.
- 15.Nelson JA, Dabic S, Mehrara BJ, et al. Breast implant-associated anaplastic large cell lymphoma incidence: determining an accurate risk. Ann Surg. 2020;272(3):403-409. [DOI] [PMC free article] [PubMed]
- 16.Cordeiro PG, Ghione P, Ni A, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. 2020;73(5):841–846. doi: 10.1016/j.bjps.2019.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCoster RC, Lynch EB, Bonaroti AR, et al. Breast implant-associated anaplastic large cell lymphoma: an evidence-based systematic review. Ann Surg. 2021;273(3):449-458. [DOI] [PubMed]
- 18.Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Supplement_1):S3-S13. [DOI] [PubMed]
- 19.Nelson JA, McCarthy C, Dabic S, et al. BIA-ALCL and textured breast implants: a systematic review of evidence supporting surgical risk management strategies. Plast Reconstr Surg. 2021;147(5S):7S–13S. doi: 10.1097/PRS.0000000000008040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Questions and answers about breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). https://www.fda.gov/medical-devices/breast-implants/questions-and-answers-about-breast-implant-associated-anaplastic-large-cell-lymphoma-bia-alcl. Published 2019. Updated 10/23/2019. Accessed December 22, 2020.
- 21.McGuire PA, Deva AK, Glicksman CA, Adams WP, Jr., Haws MJ. Management of asymptomatic patients with textured surface breast implants. Aesthet Surg J Open Forum. 2019;1(3):ojz025. [DOI] [PMC free article] [PubMed]
- 22.Calobrace MB, Mays C. An algorithm for the management of explantation surgery. Clin Plast Surg. 2021;48(1):1–16. doi: 10.1016/j.cps.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 23.McGuire PA, Haws MJ, Nahai F. Breast implant illness: how can we help? Aesthet Surg J. 2019;39(11):1260-1263. [DOI] [PubMed]
- 24.Sánchez-Guerrero J, Colditz GA, Karlson EW, Hunter DJ, Speizer FE, Liang MH. Silicone breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med. 1995;332(25):1666–1670. doi: 10.1056/NEJM199506223322502. [DOI] [PubMed] [Google Scholar]
- 25.Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342(11):781–790. doi: 10.1056/NEJM200003163421105. [DOI] [PubMed] [Google Scholar]
- 26.Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum. 2001;44(11):2477–2484. doi: 10.1002/1529-0131(200111)44:11<2477::AID-ART427>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine . Safety of silicone breast implants. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 28.Adidharma W, Latack KR, Colohan SM, Morrison SD, Cederna PS. Breast implant illness: are social media and the internet worrying patients sick? Plast Reconstr Surg. 2020;145(1):225e-227e. [DOI] [PubMed]
- 29.Sánchez-Guerrero J, Schur PH, Sergent JS, Liang MH. Silicone breast implants and rheumatic disease. Clinical, immunologic, and epidemiologic studies. Arthritis Rheum. 1994;37(2):158-168. [DOI] [PubMed]
- 30.Hennekens CH, Lee IM, Cook NR, et al. Self-reported breast implants and connective-tissue diseases in female health professionals. A retrospective cohort study. JAMA. 1996;275(8):616–621. doi: 10.1001/jama.1996.03530320040032. [DOI] [PubMed] [Google Scholar]
- 31.Kessler DA. The basis of the FDA’s decision on breast implants. N Engl J Med. 1992;326(25):1713–1715. doi: 10.1056/NEJM199206183262525. [DOI] [PubMed] [Google Scholar]
- 32.Spear SL, Murphy DK, Allergan Silicone Breast Implant US Core Clinical Study Group. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg. 2014;133(6):1354-1361. [DOI] [PMC free article] [PubMed]
- 33.Hammond DC, Canady JW, Love TR, Wixtrom RN, Caplin DA. Mentor contour profile gel implants: clinical outcomes at 10 years. Plast Reconstr Surg. 2017;140(6):1142–1150. doi: 10.1097/PRS.0000000000003846. [DOI] [PubMed] [Google Scholar]
- 34.Coroneos CJ, Selber JC, Offodile AC, II, Butler CE, Clemens MW. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269(1):30–36. doi: 10.1097/SLA.0000000000002990. [DOI] [PubMed] [Google Scholar]
- 35.Colwell AS, Mehrara B. Editorial: US FDA breast implant postapproval studies—long-term outcomes in 99,993 patients. Ann Surg. 2019;269(1):39–40. doi: 10.1097/SLA.0000000000003029. [DOI] [PubMed] [Google Scholar]
- 36.Gunnarsson R, Molberg O, Gilboe IM, Gran JT. PAHNOR1 Study Group. The prevalence and incidence of mixed connective tissue disease: a national multicentre survey of Norwegian patients. Ann Rheum Dis. 2011;70(6):1047–1051. doi: 10.1136/ard.2010.143792. [DOI] [PubMed] [Google Scholar]
- 37.Tang SYQ, Israel JS, Afifi AM. Breast implant illness: symptoms, patient concerns, and the power of social media. Plast Reconstr Surg. 2017;140(5):765e-766e. [DOI] [PubMed]
- 38.Magnusson MR, Cooter RD, Rakhorst H, McGuire PA, Adams WP Jr, Deva AK. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):74S-81S. [DOI] [PubMed]
- 39.Lee M, Ponraja G, McLeod K, Chong S. Breast implant illness: a biofilm hypothesis. Plast Reconstr Surg Glob Open. 2020;8(4):e2755. doi: 10.1097/GOX.0000000000002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dush DM. Breast implants and illness: a model of psychological factors. Ann Rheum Dis. 2001;60(7):653–657. doi: 10.1136/ard.60.7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jewell ML, Jewell HL. Breast implant-associated illness: medicine by belief, so says Dr. Google. Aesthet Surg J. 2019;39(4):NP87–NP89. doi: 10.1093/asj/sjz007. [DOI] [PubMed] [Google Scholar]
- 42.Barbosa MR, Makris UE, Mansi IA. Association of breast implants with nonspecific symptoms, connective tissue diseases, and allergic reactions: a retrospective cohort analysis. Plast Reconstr Surg. 2021;147(1):42e–49e. doi: 10.1097/PRS.0000000000007428. [DOI] [PubMed] [Google Scholar]
- 43.de Boer M, Colaris M, van der Hulst RRWJ, Cohen Tervaert JW. Is explantation of silicone breast implants useful in patients with complaints? Immunol Res. 2017;65(1):25–36. doi: 10.1007/s12026-016-8813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]