Take Home Message
Increasing age, pre- and postoperative hydronephrosis, impaired baseline kidney function, and adjuvant chemotherapy contribute to significant kidney function decline following radical cystectomy. Nearly one-third of patients develop advanced chronic kidney disease within a year of radical cystectomy.
Keywords: CKD, Chronic Kidney Disease; VHA, Veterans Health Administration; eGFR, estimated Glomerular Filtration Rate
Keywords: Chronic kidney disease, Cystectomy, Overall survival, Renal decline
Abstract
Background
Patients with chronic kidney disease (CKD) are poor candidates for standard treatments for muscle-invasive bladder cancer (MIBC) and may be more likely to experience adverse outcomes when diagnosed with MIBC.
Objective
To investigate factors associated with the development of advanced CKD following radical cystectomy.
Design, setting, and participants
Using national Veterans Health Administration utilization files, we identified 3360 patients who underwent radical cystectomy for MIBC between 2004 and 2018.
Outcome measurements and statistical analysis
We examined factors associated with the development of advanced CKD (estimated glomerular filtration rate [eGFR] of <30 ml/min/1.73 m2) after radical cystectomy using multivariable logistic and proportional hazard regression, with and without consideration of competing risks. We examined survival using Kaplan-Meier product limit estimates and proportional hazard regression.
Results and limitations
The median age at surgery was 67 yr and the mean preoperative eGFR was 69.1 ± 20.3 ml/min/1.73 m2. Approximately three out of ten patients (n = 962, 29%) progressed to advanced CKD within 12 mo. Older age (hazard ratio [HR] per 5-yr increase 1.15, 95% confidence interval [CI] 1.10–1.20), preoperative hydronephrosis (HR 1.50, 95% CI 1.29–1.76), adjuvant chemotherapy (HR 1.19, 95% CI 1.00–1.41), higher comorbidity index (HR 1.13, 95% CI 1.11–1.16 per point), and lower baseline kidney function (HR 0.75, 95% CI 0.73–0.78) were associated with the development of advanced CKD. Baseline kidney function at the time of surgery was associated with survival. Generalizability is limited due to the predominantly male cohort.
Conclusions
Impaired kidney function at baseline is associated with progression to advanced CKD and mortality after radical cystectomy. Preoperative kidney function should be incorporated into risk stratification algorithms for patients undergoing radical cystectomy.
Patient summary
Impaired kidney function at baseline is associated with progression to advanced chronic kidney disease and mortality after radical cystectomy.
1. Introduction
Bladder cancer is among the top ten most common cancers with an estimated 570 000 cases diagnosed worldwide in 2020 [1]. Patients diagnosed with bladder cancer are typically older and have a high burden of comorbid conditions, including chronic kidney disease (CKD) [2], [3], [4]. CKD has been associated with increased risks of cardiovascular events, hospitalization, and mortality [4], [5]. Patients with bladder cancer and CKD are more likely to experience adverse outcomes, including worse oncologic outcomes, related to bladder cancer treatment [6], [7], [8].
Numerous studies have demonstrated that kidney function typically declines following radical cystectomy and requisite urinary diversion [4], [9]. Previously described factors associated with this decline include preoperative hydronephrosis, pyelonephritis, nephrotoxic chemotherapy, and other age-related changes [10], [11], [12], [13], while the type of urinary diversion (eg, ileal conduit or orthotopic neobladder) has not been associated with postoperative kidney function [14], [15], [16]. The most prominent decline in kidney function occurs in the first 2 yr after cystectomy; however, the impact on the timing of contributing risk factors has not been explored fully [9], [17].
We set out to evaluate patient-, cancer-, and treatment-related factors associated with the development of advanced CKD following radical cystectomy. We used data from the Veterans Health Administration (VHA), the largest national integrated health care system in the USA. Patients treated in the VHA are more likely to be older and have a higher number of comorbidities than patients treated in the community and, as a result, may be more sensitive to changes in kidney function following radical cystectomy [18], [19], [20]. We hypothesized that patients with bladder cancer treated with cystectomy would have a high rate of progression to advanced CKD and that specific clinical characteristics would help stratify the risk of renal morbidity in this population.
2. Patients and methods
We identified patients diagnosed with bladder cancer who underwent radical cystectomy in the VHA between 2004 and 2018 across 170 medical centers and 1074 outpatient sites of care. We abstracted clinical data from the VA Corporate Data Warehouse (CDW) including patient age, sex, self-reported race/ethnicity, year of surgery, receipt of neoadjuvant or adjuvant chemotherapy, and presence of pre- and/or postoperative hydronephrosis [21]. Information on drug prescription and administration was used to identify chemotherapy administration, and an agent was included for analysis if found to be an active and filled prescription in a patient’s health record during the specified time period. We quantified patient comorbidity using the Deyo-Romano modification of the Charlson Comorbidity Index in the 2 yr prior to the date of surgery. We abstracted tumor-specific data from the CDW oncology database using the 2019 version of the American Joint Committee on Cancer (AJCC) TNM cancer staging [22].
2.1. Kidney function assessment
We obtained all serum creatinine concentration measurements from the VHA Managerial Cost Accounting (MCA) database. For each patient who underwent cystectomy, we estimated baseline kidney function by averaging all outpatient serum creatinine measurements in the 6 mo prior to the date of surgery. We calculated the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation as originally defined, including a term for self-reported race (black vs nonblack). We estimated postoperative kidney function using outpatient serum creatinine concentrations starting 30 d after surgery to minimize the transient effect of surgical complications or acute kidney injury following cystectomy. For each patient, we calculated the slope of the postoperative 1/serum creatinine and eGFR using linear regression.
2.2. Primary and secondary outcomes
We defined the primary outcome as the time to develop advanced CKD (eGFR of <30 ml/min/1.73 m2) after radical cystectomy [23]. For patients with normal or near-normal preoperative kidney function (eGFR >60 ml/min/1.73 m2), we determined whether patients developed an eGFR of <45 or <60 ml/min/1.73 m2 in the 12 mo following cystectomy. We chose this secondary outcome since moderate to advanced CKD is a relative contraindication to the use of cisplatin-based chemotherapy. We considered all-cause mortality as a secondary outcome. We obtained survival data from the VA Vital Status File, which compiles data from the VHA, the Centers for Medicare and Medicaid, the Social Security Administration, and the National Cemetery Association. We censored survival time on August 31, 2019 for patients alive after this date [24].
2.3. Statistical analyses
We fit multivariable Cox proportional hazard regression models to determine whether the type of urinary diversion, receipt of neoadjuvant or adjuvant chemotherapy, receipt of nephrotoxic chemotherapy agents (cisplatin), or presence of hydronephrosis was associated with the development of advanced CKD. We included 1/serum creatinine to adjust for baseline kidney function in order to obtain valid parameter estimates for age, sex, and self-reported race, since these variables are embedded in the estimate of the glomerular filtration rate, and because serum creatinine is not normally distributed. To account for the competing risk of death, we calculated subdistribution hazard ratios (HRs) using the Fine-Gray modification of the Cox model. We utilized logistic regression to assess for factors associated with eGFR decline in the 12 mo following cystectomy when patients may be eligible for cisplatin-based adjuvant chemotherapy. We conducted companion analyses to determine whether survival following radical cystectomy was independently associated with preoperative eGFR when controlling for relevant variables.
This study was approved by the Stanford University Institutional Review Board and the Veterans Affairs Palo Alto Health Care System. All analyses were performed within the VA Informatics and Computing Infrastructure (VINCI) platform using SAS v9.4 (SAS Institute, Cary, NC, USA). We created figures using JMP Pro 15 (SAS Institute).
3. Results
We identified 3360 patients diagnosed with bladder cancer who underwent radical cystectomy in the VHA from January 1, 2004 to September 30, 2018. Of these patients, 11% had neobladder reconstruction and 89% underwent urinary diversion with an ileal conduit. The median age was 67 yr (interquartile range [IQR] 62, 73). Of the cohort population, 99% was male and with significant comorbidity. Approximately one in four patients receiving neobladder (26%) and one in three patients receiving ileal conduit (32%) had a Charlson Comorbidity Index of ≥4. A total of 631(19%) patients had preoperative hydronephrosis and 984(29%) had postoperative hydronephrosis. Patients receiving a neobladder were significantly younger than those receiving an ileal conduit reconstruction (63.8 vs 67.5 yr, p < 0.0001) and with slightly higher preoperative kidney function (eGFR 74.6 vs 68.3 ml/min/1.73 m2, p < 0.0001). The mean (standard deviation) preoperative eGFR was 69.1 ± 20.3 ml/min/1.73 m2 (Table 1).
Table 1.
Clinical characteristics of patients who underwent radical cystectomy for bladder cancer in the VHA from 2004 through 2018 stratified by baseline kidney function.
| Baseline eGFR | eGFR <60 | eGFR ≥60 | Missing |
|---|---|---|---|
| No. of patients | 1081 | 2121 | 158 |
| Age at surgery (yr), n (%) | 70.6 ± 7.9 | 65.9 ± 7.7 | 67.1 ± 8.7 |
| ≤59 | 91 (8.4) | 429 (20.2) | 33 (20.9) |
| 60–69 | 433 (40.1) | 1,101 (51.9) | 69 (43.7) |
| 70–79 | 418 (38.7) | 503 (23.7) | 41 (25.9) |
| ≥80 | 139 (12.9) | 88 (4.1) | 15 (9.5) |
| Sex, n (%) | |||
| Female | 7 (0.6) | 21 (1.0) | 3 (1.9) |
| Male | 1074 (99.4) | 2100 (99.0) | 155 (98.1) |
| Race, n (%) | |||
| White | 937 (86.7) | 1815 (85.6) | 121 (76.6) |
| Black | 91 (8.4) | 213 (10.0) | 25 (15.8) |
| Other/unknown | 37 (3.4) | 49 (2.3) | 9 (5.7) |
| Missing | 16 (1.5) | 44 (2.1) | 3 (1.9) |
| Pathologic T stage, n (%) | |||
| T0 | 22 (2.0) | 91 (4.3) | 3 (1.9) |
| T1 | 94 (8.7) | 247 (11.6) | 23 (14.6) |
| T2 | 207 (19.1) | 510 (24.0) | 26 (16.5) |
| T3 | 273 (25.3) | 427 (20.1) | 34 (21.5) |
| T4 | 161 (14.9) | 178 (8.4) | 25 (15.8) |
| TA | 22 (2.0) | 37 (1.7) | 3 (1.9) |
| CIS | 23 (2.1) | 65 (3.1) | 4 (2.5) |
| Unknown | 229 (21.2) | 464 (21.9) | 34 (21.5) |
| Missing | 50 (4.6) | 102 (4.8) | 6 (3.8) |
| Pathologic N stage, n (%) | |||
| N0 | 547 (50.6) | 1184 (55.8) | 78 (49.4) |
| N1 | 70 (6.5) | 128 (6.0) | 13 (8.2) |
| N2 | 127 (11.7) | 181 (8.5) | 19 (12.0) |
| N3 | 14 (1.3) | 27 (1.3) | 4 (2.5) |
| Unknown | 273 (25.3) | 501 (23.6) | 38 (24.1) |
| Missing | 50 (4.6) | 100 (4.7) | 6 (3.8) |
| Pathologic M stage, n (%) | |||
| M0 | 368 (34.0) | 677 (31.9) | 61 (38.6) |
| M1 | 18 (1.7) | 17 (0.8) | 7 (4.4) |
| Unknown | 188 (17.4) | 315 (14.9) | 39 (24.7) |
| Missing | 507 (46.9) | 1112 (52.4) | 51 (32.3) |
| Charlson Comorbidity Index, n (%) | 6.3 ± 2.7 | 4.9 ± 2.5 | 5.2 ± 2.6 |
| 2 | 72 (6.7) | 408 (19.2) | 27 (17.1) |
| 3 | 80 (7.4) | 369 (17.4) | 24 (15.2) |
| 4 | 158 (14.6) | 337 (15.9) | 25 (15.8) |
| 5 | 173 (16.0) | 218 (10.3) | 22 (13.9) |
| 6+ | 598 (55.3) | 789 (37.2) | 60 (38.0) |
CIS = carcinoma in situ; eGFR = estimated glomerular filtration rate; VHA = Veterans Health Administration.
We noted that kidney function after radical cystectomy declines at a median rate of –2.07 ml/min/1.73 m2 per year for all patients (IQR –1.00, –4.30). When stratified by diversion type, rates of decline were similar; patients receiving an ileal conduit experienced a median decline of –2.10 ml/min/1.73 m2 per year (IQR –0.99, –4.47) versus –1.92 ml/min/1.73 m2 per year (IQR –1.02, –3.26) for patients receiving a neobladder reconstruction (Fig. 1). In total, 962 (29%) patients progressed to advanced CKD (<30 ml/min/1.73 m2) following cystectomy within a median of 10.9 mo (IQR 3.0, 33.4).
Fig. 1.
Contour plot illustrating 67 643 eGFR measurements in 3360 patients following radical cystectomy. Contours signify the density of postoperative eGFR measurements for patients with a neobladder diversion shown in red and with ileal conduit diversion shown in blue. Kidney function declined in the 5 yr after cystectomy, with the largest decline in the first 12 mo. Across all patients and measurements, the average decline in kidney function is shown as a line following neobladder (red) and ileal conduit (blue) diversion. eGFR = estimated glomerular filtration rate.
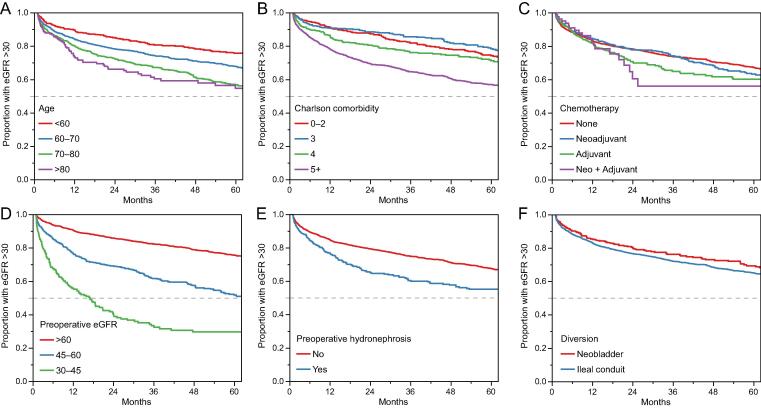
In unadjusted models, older age at surgery (HR 1.15, 95% confidence interval [CI] 1.10–1.20 per year), presence of preoperative hydronephrosis (HR 1.50, 95% CI 1.29–1.76), receipt of adjuvant chemotherapy (HR 1.19, 95% CI 1.00–1.41) but not neoadjuvant chemotherapy (HR 0.98, 95% CI 0.84–1.16), higher comorbidity index (HR 1.13 per 1 unit change, 95% CI 1.11–1.16), and baseline reciprocal serum creatinine (HR 0.75 per 0.1 unit change, 95% CI 0.73–0.78/mg/dl) were associated with progressing to advanced CKD following radical cystectomy (Fig. 2).
Fig. 2.
Kaplan-Meier plots demonstrating the unadjusted freedom from advanced CKD as a function of (A) age, (B) Charlson Comorbidity Index, (C) receipt of chemotherapy, (D) preoperative kidney function (eGFR), (E) presence of preoperative hydronephrosis, and (F) type of urinary diversion after cystectomy. CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
In fully adjusted models, better preoperative kidney function was associated with a lower hazard of development of advanced CKD (HR 0.75, 95% CI 0.73–0.78). Diversion type (HR 0.98, 95% CI 0.79, 1.20) or receipt of neoadjuvant cisplatin (HR 0.99, 95% CI 0.82–1.20) was not associated with the time to develop eGFR <30 ml/min/1.73 m2 following radical cystectomy. We confirmed these findings in the competing risk model (HR 0.79, 95% CI 0.77–0.82) wherein age was no longer associated with the hazard of developing advanced CKD (Table 2).
Table 2.
Univariable and multivariable Cox proportional hazard and competing risk models predicting the development of advanced CKD (eGFR <30 ml/min/1.73 m2) following radical cystectomy.
| Cox proportional hazard |
Competing risk model | ||
|---|---|---|---|
| Univariable, HR (95% CI) | Multivariable, HR (95% CI) | Multivariable, HR (95% CI) | |
| Age at surgery (unit = 5 yr) | 1.15 (1.10, 1.20) | 1.08 (1.03, 1.12) | 1.01 (0.97, 1.05) |
| Sex (male vs female) | 1.82 (0.82, 4.07) | 0.84 (0.35, 2.04) | 0.93 (0.38, 2.28) |
| Preop hydronephrosis (yes vs no) | 1.50 (1.29, 1.76) | 0.76 (0.64, 0.91) | 0.69 (0.57, 0.83) |
| Baseline kidney function (1/Cr; unit = 0.1) | 0.75 (0.73, 0.78) | 0.75 (0.73, 0.78) | 0.79 (0.77, 0.82) |
| Comorbidity Index | 1.13 (1.11, 1.16) | 1.08 (1.06, 1.11) | 1.04 (1.01, 1.07) |
| Neoadjuvant chemo (yes vs no) | 0.98 (0.84, 1.16) | 1.05 (0.89, 1.24) | 1.03 (0.87, 1.21) |
| Adjuvant chemo (yes vs no) | 1.19 (1.00, 1.41) | 1.14 (0.95, 1.37) | 1.03 (0.86, 1.24) |
| Diversion type | |||
| Neobladder | Ref | Ref | Ref |
| Ileal conduit | 1.20 (0.98, 1.47) | 0.98 (0.79, 1.20) | 0.92 (0.75, 1.14) |
CI = confidence interval, Cr = creatinine (mg/dl); HR = hazard ratio; Ref = reference.
Of the 3360 patients in our cohort, 1143 (34%) received neoadjuvant or adjuvant chemotherapy. Of those patients, 860 (75.2%) progressed to an eGFR of <60 ml/min/1.73 m2 and 321 (28.6%) to an eGFR of <30 ml/min/1.73 m2. Of the 743 with baseline eGFR >60 ml/min/1.73 m2, 516 (69.5%) progressed to an eGFR of <60 ml/min/1.73 m2 and 55 (7.4%) progressed to an eGFR of <45 ml/min/1.73 m2. We then examined kidney function outcomes for patients who did not receive neoadjuvant chemotherapy. Of the 2668 patients who did not get neoadjuvant chemotherapy, 779 (29.3%) reached and eGFR of <30 ml/min/1.73 m2. Of the 1656 patients with baseline eGFR >60 ml/min/1.73 m2, 1092 (65.9%) reached an eGFR of <60 ml/min/1.73 m2 and 732 (44.2%) reached an eGFR of <45 ml/min/1.73 m2 (Fig. 3). Among 664 patients with a preoperative eGFR of 45–60 ml/min/1.73 m2, 53 (8.0%) had their kidney function improved to an eGFR of >60 ml/min/1.73 m2 at 6 mo, while 71 (10.7%) had worsening of kidney function to an eGFR of <45 ml/min/1.73 m2. In fully adjusted logistic regression models to identify factors associated with reduced postoperative kidney function in the first 12 mo, we identified that older age (odds ratio [OR] 1.13, 95% CI 1.05, 1.22), receipt of adjuvant chemotherapy (OR 1.60, 95% CI 1.17, 2.19), and postoperative hydronephrosis (OR 5.51, 95% CI 3.32, 9.14) are associated with postoperative eGFR <60 ml/min/1.73 m2. When assessing the decline to eGFR <45 ml/min/1.73 m2 in the 12 mo after cystectomy, adjuvant chemotherapy (OR 1.37, 95% CI 1.04, 1.80) and postoperative hydronephrosis remained statistically significant (OR 3.55, 95% CI 2.51, 5.01; Table 3).
Fig. 3.
Sankey diagram demonstrating the flow of kidney function stages for 3360 patients from preoperative baseline to 6 mo following radical cystectomy. The width of each bar is proportional to the number of patients represented. eGFR = estimated glomerular filtration rate.
Table 3.
Multivariate logistic regression analysis to identify characteristics associated with the odds of developing an eGFR of <45 or <60 ml/min/1.73 m2 in the 12 mo following radical cystectomy in patients who did not receive neoadjuvant chemotherapy.
| eGFR <45 OR (95% CI) |
eGFR <60 OR (95% CI) |
|
|---|---|---|
| Age at surgery (unit = 5) | 1.05 (0.98, 1.12) | 1.13 (1.05, 1.22) |
| Sex (male vs female) | 0.43 (0.15, 1.22) | 0.25 (0.08, 0.82) |
| Preop hydronephrosis (yes vs no) | 0.24 (0.15, 0.39) | 0.16 (0.09, 0.29) |
| Postop hydronephrosis (yes vs no) | 3.55 (2.51, 5.01) | 5.51 (3.32, 9.14) |
| Baseline kidney function (1/Cr; unit = 0.1) | 0.78 (0.73, 0.83) | 0.69 (0.64, 0.73) |
| Charlson Comorbidity Index | 1.01 (0.97, 1.06) | 0.94 (0.90, 0.98) |
| Diversion type | ||
| Neobladder | Ref | Ref |
| Ileal conduit | 0.75 (0.56, 1.02) | 0.76 (0.54, 1.07) |
| Adjuvant chemo (yes vs no) | 1.37 (1.04, 1.80) | 1.60 (1.17, 2.19) |
CI = confidence interval; Cr = creatinine (mg/dl); eGFR = estimated glomerular filtration rate; OR = odds ratio; Ref = reference.
A total of 732 patients had a preoperative eGFR of >60 ml/min/1.73 m2 and reached an eGFR of <45 ml/min/1.73 m2 after cystectomy, while 1092 patients had a preoperative eGFR of >60 ml/min/1.73 m2and reached an eGFR of <60 ml/min/1.73 m2 after cystectomy.
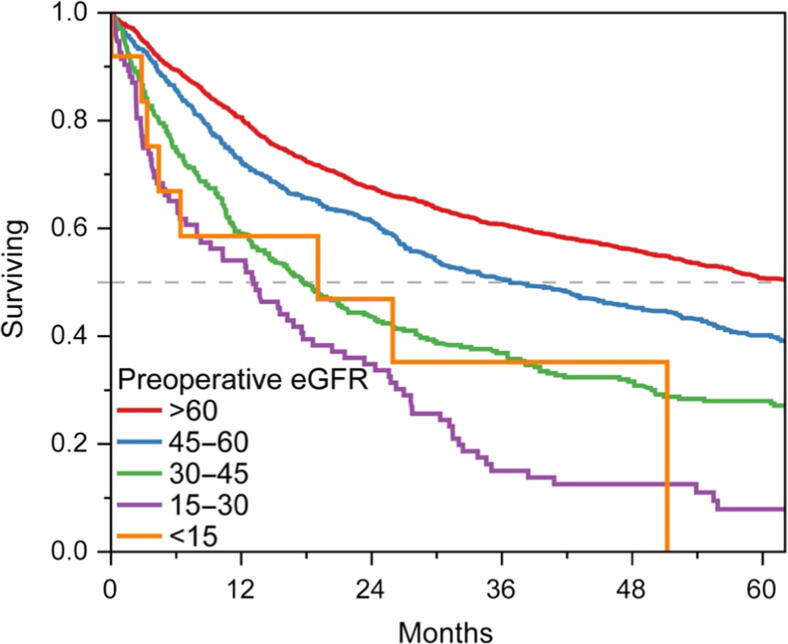
Preoperative baseline kidney function was associated with survival (Fig. 4). The median survival was 5.3, 3.1, 1.5, 1.1, and 1.6 yr for patients with baseline CKD stage 1 or 2, 3, 4, and 5, respectively. Age, pathologic stage, and comorbidity were also independently associated with survival (Table 4).
Fig. 4.
Kaplan-Meier plot demonstrating the overall survival of patients following radical cystectomy stratified by preoperative kidney function (eGFR in ml/min/1.73 m2). eGFR = estimated glomerular filtration rate.
Table 4.
Multivariable Cox proportional hazard regression model to predict mortality for patients following radical cystectomy.
| HR (95% CI) | |
|---|---|
| Age at surgery (unit = 5) | 1.11 (1.08, 1.15) |
| Sex (male vs female) | 1.46 (0.81, 2.66) |
| Preop hydronephrosis (yes vs no) | 1.05 (0.89, 1.25) |
| Postop hydronephrosis (yes vs no) | 1.07 (0.93, 1.24) |
| eGFR CKD-EPI (ml/min/1.73 m2) | |
| <15 | 1.89 (0.93, 3.84) |
| 15–<30 | 1.94 (1.53, 2.47) |
| 30–<45 | 1.41 (1.21, 1.64) |
| 45–<60 | 1.13 (1.01, 1.27) |
| ≥60 | Ref |
| Charlson Comorbidity Index | 1.05 (1.04, 1.07) |
| Neoadjuvant chemo (yes vs no) | 1.12 (0.99, 1.26) |
| Adjuvant chemo (yes vs no) | 1.07 (0.94, 1.21) |
| Diversion type | |
| Neobladder | Ref |
| Ileal conduit | 1.12 (0.97, 1.31) |
| Pathologic T stage | |
| T0 | Ref |
| TA | 2.13 (1.24, 3.65) |
| CIS | 2.00 (1.22, 3.28) |
| T1 | 2.03 (1.34, 3.06) |
| T2 | 2.39 (1.61, 3.55) |
| T3 | 4.08 (2.75, 6.05) |
| T4 | 4.32 (2.88, 6.47) |
| Unknown | 2.24 (1.44, 3.47) |
| Missing | 5.62 (0.76, 41.6) |
| Pathologic N stage | |
| N0 | Ref |
| N1 | 1.55 (1.30, 1.85) |
| N2 | 2.01 (1.73, 2.34) |
| N3 | 2.40 (1.71, 3.37) |
| Unknown | 1.27 (1.02, 1.58) |
| Missing | 0.48 (0.07, 3.46) |
| Pathologic M stage | |
| M0 | Ref |
| M1 | 1.99 (1.39, 2.85) |
| Unknown | 1.39 (1.20, 1.60) |
| Missing | 1.06 (0.95, 1.19) |
CI = confidence interval; CIS = carcinoma in situ; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; eGFR = estimated glomerular filtration rate; HR = hazard ratio; Ref = reference.
4. Discussion
We demonstrate that the development of advanced CKD is common following radical cystectomy, with one in four patients experiencing an eGFR of <30 ml/min/1.73 m2 within a year of surgery. Previous studies describing kidney function decline following radical cystectomy have primarily been single-institution series stratified by diversion type and, recently, surgical approach [4], [9], [17], [25], [26]. This report extends these findings through the evaluation of a large national cohort with longitudinal follow-up and access to long-term kidney function and survival data. We identified age, higher comorbidity index, receipt of adjuvant chemotherapy with carboplatin or cisplatin, and lower baseline kidney function as risk factors associated with progression to advanced CKD in a large national integrated health system. These data can be used to stratify risk, inform medical decision–making, and help identify patients who may benefit from nephrology care in the perioperative period.
Baseline kidney function before cystectomy was associated with both the development of clinically significant CKD and overall survival. The landmark paper by Go et al [5] demonstrated that even small differences in baseline kidney function were associated with hospitalization, cardiovascular events, and mortality among more than 1 million adults. Here, we confirm that the association between baseline kidney function and survival holds among patients with bladder cancer receiving cystectomy. The association between baseline kidney function, long-term kidney function outcomes, and survival remained significant in models after adjusting for patient age and overall comorbidity burden. This suggests that evaluating kidney function can contribute to risk stratification for patients undergoing cystectomy.
These data also confirm prior findings that the type of urinary diversion (ileal conduit vs orthotopic neobladder diversion) is not independently associated with renal morbidity after surgery. While increased reabsorption of electrolytes in patients with continent urinary diversion could theoretically worsen kidney function, multiple assessments accounting for numerous clinical factors demonstrate no difference in eGFR decline by diversion type [4], [10], [14], [16], [27], [28]. The current study should provide additional assurance to appropriately selected patients that a neobladder reconstruction would not be associated with an increased risk of progressive CKD.
The standard of care treatment for muscle-invasive bladder cancer is neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. However, many patients are ineligible for cisplatin-based neoadjuvant therapy, with CKD being the most common reason [29], [30], [31], [32], [33]. In our patient population, we noted that 18% received neoadjuvant chemotherapy, demonstrating low utilization despite level 1 evidence. While concern for developing CKD may contribute to nonutilization of neoadjuvant chemotherapy, we did not demonstrate an increased risk for patients who received cisplatin-based neoadjuvant chemotherapy. While CKD may contribute to patients being ineligible to receive cisplatin, there have been few studies investigating the impact of carboplatin-based neoadjuvant chemotherapy and/or adjuvant chemotherapy on CKD [34], [35], [36]. We focused on the 12-mo postoperative timeline as this is the setting in which patients may be recommended to receive adjuvant chemotherapy and found that the majority of patients who do not receive cisplatin in the neoadjuvant settings experience further eGFR decline postoperatively. Given that one in three patients will experience a decline in kidney function following radical cystectomy to an eGFR of <45 ml/min/1.73 m2 and two in three will experience a decline to <60 ml/min/1.73 m2, the neoadjuvant setting may be the only window of cisplatin eligibility for many patients with adverse features identified on bladder pathology.
Our study has notable strengths. We examined renal morbidity and mortality in a large cohort of patients receiving care in an integrated health care system. These data reflect “real-world” outcomes across >100 hospitals. We were able to demonstrate that age, comorbidity status, and adjuvant chemotherapy are associated with developing advanced CKD following radical cystectomy. We also demonstrated that diversion type and neoadjuvant chemotherapy do not. Furthermore, we noted that preoperative CKD is independently associated with overall survival for patients with bladder cancer undergoing radical cystectomy.
Despite these strengths, our findings should be interpreted in the context of several limitations to our study. We may not have captured all patients undergoing radical cystectomy, since we used stringent criteria to identify cases that were included in both the cancer registry and had procedure codes for cystectomy. While this may have led us to miss some cases, we are reassured that these cystectomy cases were performed as part of primary cancer treatment strategies. Additionally, while we made efforts to adjust for relevant clinical covariates, there is the potential for residual confounding in these analyses. Further, studies in the VHA include predominantly male patients, and there is a possibility that these results are not generalizable to patients in the community. While we did not demonstrate any sex-based differences in our study outcomes, these results are limited by a small number of female patients. We evaluated kidney function decline as a function of time, with specific assessments at 6 and 12 mo after surgery to evaluate CKD stage at those time periods; thus, we may have missed some patients whose kidney function declines were not sustained following these assessments. We evaluated the role of neoadjuvant and adjuvant chemotherapy in kidney function, but were not able to include granularity with respect to doses or numbers of cycles administered. While we evaluated the impact of hydronephrosis, we were unable to capture granular details of procedures used to address this in our population, and similarly, we were not able to evaluate the specific impact of ureteroenteric stricture or infections in our population, which have been demonstrated in other studies to contribute to kidney function decline.
5. Conclusions
This study evaluated the renal morbidity following radical cystectomy in patients with bladder cancer. We assessed the patient- and cancer-specific factors associated with this decline and demonstrated that kidney function declines significantly following surgery, with nearly one-third of patients experiencing severe CKD within a year of surgery. We noted that better baseline kidney function is protective against developing CKD and that only a minority of patients who undergo surgery with a marginal eGFR improve their kidney function. We explored the timing of neoadjuvant and adjuvant chemotherapy and urinary diversion, and noted that patient’s receipt of neoadjuvant cisplatin and orthotopic neobladder diversions are not associated with an increased risk of developing CKD. We further found that baseline kidney function is associated with survival. Despite changes in chemotherapy regimens and surgical techniques, the 5-yr survival for patients with muscle-invasive bladder cancer has remained near 50% for the past 20 yr [37]. Currently, there are few clinically utilized preoperative nomograms to predict survival for patients with muscle-invasive bladder cancer, and our study suggests that future models should include preoperative CKD status in these calculations and that a multidisciplinary approach that involves nephrologists may benefit the care of patients with bladder cancer.
Author contributions: Bogdana Schmidt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Velaer, Schmidt, Leppert.
Acquisition of data: Thomas, Leppert.
Analysis and interpretation of data: Schmidt, Thomas, Chertow, Leppert.
Drafting of the manuscript: Velaer, Schmidt, Leppert.
Critical revision of the manuscript for important intellectual content: Schmidt, Thomas, Ganesan, Song, Pao, Thong, Liao, Chertow, Skinner, Leppert.
Statistical analysis: Schmidt, Thomas, Leppert.
Obtaining funding: Leppert.
Administrative, technical, or material support: Leppert.
Supervision: Leppert.
Other: None.
Financial disclosures: Bogdana Schmidt certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: Department of Veterans Affairs Health Services Research and Development Service work was supported using resources and facilities at the VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government. Dr. Glenn M. Chertow was supported by NIDDK K24 DK085446.
Associate Editor: Jochen Walz
References
- 1.Richters A., Aben K.K.H., Kiemeney L.A.L.M. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams S.B., Kamat A.M., Chamie K., et al. Systematic review of comorbidity and competing-risks assessments for bladder cancer patients. Eur Urol Oncol. 2018;1:91–100. doi: 10.1016/j.euo.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams S.B., Huo J., Chamie K., et al. Underutilization of radical cystectomy among patients diagnosed with clinical stage T2 muscle-invasive bladder cancer. Eur Urol Focus. 2017;3:258–264. doi: 10.1016/j.euf.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg M.S., Thompson R.H., Frank I., et al. Long-term renal function outcomes after radical cystectomy. J Urol. 2014;191:619–625. doi: 10.1016/j.juro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto A., Nakagawa T., Kanatani A., et al. Preoperative chronic kidney disease is predictive of oncological outcome of radical cystectomy for bladder cancer. World J Urol. 2018;36:249–256. doi: 10.1007/s00345-017-2141-2. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli M., Wiebe N., Culleton B., et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 8.Malyszko J., Tesarova P., Capasso G., Capasso A. The link between kidney disease and cancer: complications and treatment. Lancet. 2020;396:277–287. doi: 10.1016/S0140-6736(20)30540-7. [DOI] [PubMed] [Google Scholar]
- 9.Rouanne M., Perreaud A., Letang N., et al. Trends in renal function after radical cystectomy and ileal conduit diversion: new insights regarding estimated glomerular filtration rate variations. Clin Genitourin Cancer. 2015;13:e139–e144. doi: 10.1016/j.clgc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Jin X.-D., Roethlisberger S., Burkhard F.C., Birkhaeuser F., Thoeny H.C., Studer U.E. Long-term renal function after urinary diversion by ileal conduit or orthotopic ileal bladder substitution. Eur Urol. 2012;61:491–497. doi: 10.1016/j.eururo.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa M., Miyake H., Yamashita M., Inoue T., Fujisawa M. Long-term changes in renal function outcomes following radical cystectomy and urinary diversion. Int J Clin Oncol. 2014;19:1105–1111. doi: 10.1007/s10147-014-0661-y. [DOI] [PubMed] [Google Scholar]
- 12.Osawa T., Shinohara N., Maruyama S., et al. Long-term renal function outcomes in bladder cancer after radical cystectomy. Urol J. 2013;10:784–789. [PubMed] [Google Scholar]
- 13.Momota M., Hatakeyama S., Tokui N., et al. The impact of preoperative severe renal insufficiency on poor postsurgical oncological prognosis in patients with urothelial carcinoma. Eur Urol Focus. 2019;5:1066–1073. doi: 10.1016/j.euf.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Hautmann R.E. Urinary diversion: ileal conduit to neobladder. J Urol. 2003;169:834–842. doi: 10.1097/01.ju.0000029010.97686.eb. [DOI] [PubMed] [Google Scholar]
- 15.Studer U.E., Varol C., Danuser H. Orthotopic ileal neobladder. BJU Int. 2004;93:183–193. doi: 10.1111/j.1464-410x.2004.04641.x. [DOI] [PubMed] [Google Scholar]
- 16.Gershman B., Eisenberg M.S., Thompson R.H., et al. Comparative impact of continent and incontinent urinary diversion on long-term renal function after radical cystectomy in patients with preoperative chronic kidney disease 2 and chronic kidney disease 3a. Int J Urol. 2015;22:651–656. doi: 10.1111/iju.12770. [DOI] [PubMed] [Google Scholar]
- 17.Makino K., Nakagawa T., Kanatani A., et al. Biphasic decline in renal function after radical cystectomy with urinary diversion. Int J Clin Oncol. 2017;22:359–365. doi: 10.1007/s10147-016-1053-2. [DOI] [PubMed] [Google Scholar]
- 18.Agha Z., Lofgren R.P., VanRuiswyk J.V., Layde P.M. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 19.Kazis L.E., Miller D.R., Skinner K.M., et al. Patient-reported measures of health: the Veterans Health Study. J Ambul Care Manage. 2004;27:70–83. doi: 10.1097/00004479-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Rogers W.H., Kazis L.E., Miller D.R., et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans’ health and medical outcomes studies. J Ambul Care Manage. 2004;27:249–262. doi: 10.1097/00004479-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Fihn S.D., Francis J., Clancy C., et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood) 2014;33:1203–1211. doi: 10.1377/hlthaff.2014.0054. [DOI] [PubMed] [Google Scholar]
- 22.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Leppert J.T., Lamberts R.W., Thomas I.-C., et al. Incident CKD after radical or partial nephrectomy. J Am Soc Nephrol. 2018;29:207–216. doi: 10.1681/ASN.2017020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohn M.-W., Arnold N., Maynard C., Hynes D.M. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lone Z., Murthy P.B., Zhang J.H., et al. Comparison of renal function after open radical cystectomy, extracorporeal robot assisted radical cystectomy, and intracorporeal robot assisted radical cystectomy. Urol Oncol. 2021;39(301):e1–e9. doi: 10.1016/j.urolonc.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert S.M., Lai J., Saigal C.S., Gore J.L. Urologic Diseases in America Project. Downstream complications following urinary diversion. J Urol. 2013;190:916–922. doi: 10.1016/j.juro.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J., Hong B., Park J.-Y., et al. Comparison of a significant decline in the glomerular filtration rate between ileal conduit and ileal neobladder urinary diversions after radical cystectomy: a propensity score-matched analysis. J Clin Med. 2020;9:2236. doi: 10.3390/jcm9072236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gore J.L., Yu H.-Y., Setodji C., et al. Urinary diversion and morbidity after radical cystectomy for bladder cancer. Cancer. 2010;116:331–339. doi: 10.1002/cncr.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrasekar T., Pugashetti N., Durbin-Johnson B., et al. Effect of neoadjuvant chemotherapy on renal function following radical cystectomy: is there a meaningful impact? Bladder Cancer. 2016;2:441–448. doi: 10.3233/BLC-160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman H.B., Speights V.O. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 31.Dash A., Galsky M.D., Vickers A.J., et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 32.Flaig T.W., Spiess P.E., Agarwal N., et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:329–354. doi: 10.6004/jnccn.2020.0011. [DOI] [PubMed] [Google Scholar]
- 33.Witjes J.A., Bruins H.M., Cathomas R., et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 34.Huo J., Ray-Zack M.D., Shan Y., et al. Discerning patterns and quality of neoadjuvant chemotherapy use among patients with muscle-invasive bladder cancer. Eur Urol Oncol. 2019;2:497–504. doi: 10.1016/j.euo.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krabbe L.M., Westerman M.E., Margulis V., et al. Changing trends in utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer. Can J Urol. 2015;22:7865–7875. [PubMed] [Google Scholar]
- 36.Chou R., Selph S.S., Buckley D.I., et al. Treatment of muscle-invasive bladder cancer: a systematic review. Cancer. 2016;122:842–851. doi: 10.1002/cncr.29843. [DOI] [PubMed] [Google Scholar]
- 37.Meeks J.J., Bellmunt J., Bochner B.H., et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]