Abstract
Following CARMENA and SURTIME, patients with metastatic renal cell carcinoma (mRCC) and International Metastatic RCC Database Consortium (IMDC) intermediate and poor risk receive systemic therapy with the primary tumour (primary) in place, with the option of deferred cytoreductive nephrectomy (CN) in responding patients. We retrospectively analysed the safety and efficacy of first-line nivolumab/ipilimumab in 71 primary mRCC patients (42.3% IMDC poor risk; 43.6% with more than three metastatic sites). The baseline mean primary diameter was 9.3 cm and median follow-up was 11.5 mo. Of 69 patients with at least one follow-up computed tomography scan, 23 (33.3 %) had a partial response (PR) of the primary after a median of 4.8 mo, which was associated with a 91.3% overall response rate at metastatic sites (MSs) and absence of progressive disease, irrespective of the IMDC risk. The complete response (CR) rate at MSs (n = 7 [10.1%]) is similar to the CR rate in CheckMate 214. Thirteen deferred CNs were performed (18.8%) after a median of 13 mo, rendering four patients disease free. Only 4.3% of primaries progressed; grade 3–4 immune-related adverse events occurred in 31.9%. Irrespective of the IMDC risk, patients with a PR in the primary had a 1-yr overall survival rate of 89% versus 67% in those without (p = 0.012).
Patient summary
Patients with metastatic kidney cancer receiving immunotherapy with nivolumab and ipilimumab had superior response at metastatic sites and better survival irrespective of International Metastatic RCC Database Consortium (IMDC) risk.
Keywords: Kidney cancer, Metastatic, Nivolumab, Ipilimumab, Primary tumour in place, Immune checkpoint inhibitor combination therapy, Primary metastatic renal cell carcinoma, Synchronous
Based on the results of CARMENA and SURTIME [1], [2], European guidelines recommended that upfront cytoreductive nephrectomy (CN) is no longer the standard of care in patients requiring systemic therapy [3], [4]. Instead, patients received upfront vascular endothelial growth factor receptor–targeted therapy with the primary tumour (primary) in place and the option of undergoing deferred CN in case of a response at metastatic sites or local symptoms. Meanwhile, immune checkpoint inhibitor (ICI)-based combination therapy has become the standard first-line treatment for International Metastatic RCC Database Consortium (IMDC) intermediate- and poor-risk patients [5]. Consequently, the recent evidence and recommendations from CARMENA and SURTIME have been superseded, and up to 30% of metastatic renal cell carcinoma (mRCC) patients were treated with their primary in place in the pivotal ICI combination therapy trials [6]. With up to 16% of complete response (CR) rates at metastatic sites [7], patients are increasingly being offered deferred CN to achieve surgical complete remissions. In a retrospective analysis involving 20 patients who underwent deferred CN following ICI therapy, 10% had a complete pathological response in the primary [8], and currently two randomised controlled trials investigate the role of deferred CN in this setting [6]. We retrospectively analysed, within the context of a clinical audit, safety and oncological outcome data from three European referral centres of patients with treatment-naïve mRCC who received first-line nivolumab and ipilimumab with the primary in place (Supplementary material).
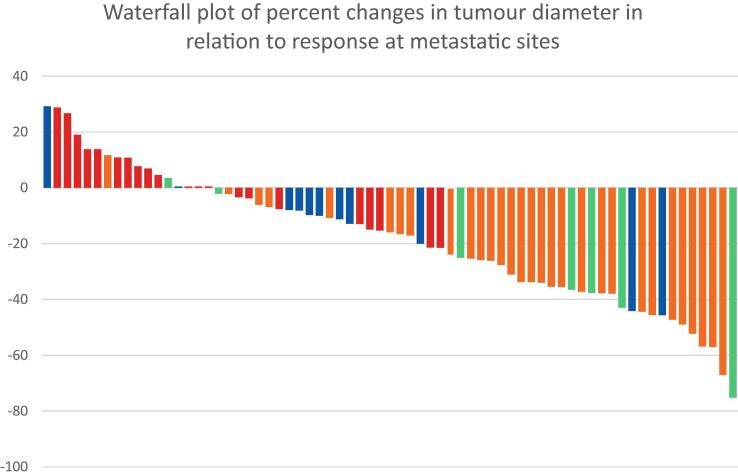
Of 71 patients treated between April 2019 and April 2021 (Table 1), 69 had at least one follow-up cross-sectional imaging result available for response assessment by radiology review at each centre. The median follow-up was 11.5 (interquartile range [IQR] 6.9–15.8) mo. Adverse events (AEs) were similar to those previously reported, with 31.9% grade 3–4 immune-related AEs (Supplementary Table 1). Five patients (7%) developed macroscopic haematuria, requiring embolisation in two (2.8%). The overall response rate (ORR) was 33.3% (23/69; 95% confidence interval [CI] 0.233–0.45) with no patients achieving a CR. Analysing ORR for primary and metastatic sites separately, all 23 patients (33.3%) had a RECIST 1.1 partial response (PR) in the primary (mean baseline diameter of 10.14 cm [range 2.9–15.3 cm]; Fig. 1 and Supplementary Table 2), with a median time to response of 4.8 (IQR 2.5–6) mo. Of these patients, 91.3% (20/23) achieved responses at metastatic sites, with 17.3% (4/23) achieving a CR. No patient had progressive disease (PD) at the time of first response assessment. Only 21.7% (5/23) patients progressed following a response or stable disease at metastatic sites, and 8.7% (2/23) died of disease. This compares favourably with the 66.7% (46/69) of patients who had no confirmed objective response in their primary. The ORR at metastatic sites in these patients was only 34.8% (16/46), and 45.6% (21/46) had PD as the best response. A total of 69.6% (32/46) progressed during follow-up and 30.4% (14/46) died of disease (Supplementary Table 2). A response in the primary tumour discriminated responders better than median tumour downsizing (Supplementary Table 3). Time to response in the primary was not associated with the outcome (Supplementary Table 4 and Supplementary Fig. 2).
Table 1.
Patient and tumour characteristics
| Characteristics of patients treated with their primary tumour in place (n = 71) | |
|---|---|
| Age (yr), median (range) | 64 (40–82) |
| Gender, n (%) | |
| Male | 57 (80.3) |
| Female | 14 (19.7) |
| IMDC risk, n (%) | |
| Intermediate | 41 (57.7) |
| Poor | 30 (42.3) |
| ECOG performance score, n (%) | |
| 0 | 22 (30.9) |
| 1 | 36 (50.7) |
| ≥2 | 13 (18.3) |
| Subtype, n (%) | |
| Clear cell | 68 (95.7) |
| Papillary type 2 | 2 (2.8) |
| NOS | 1 (1.4) |
| Duration of systemic therapy with nivolumab and ipilimumab (d), median (range) | 151 (1–696) |
| Primary tumour diameter (cm), mean (range) | 9.25 (2.5–16.1) |
| Number of metastatic sites, n (%) | |
| 1 | 16 (22.5) |
| 2 | 24 (33.8) |
| ≥3 | 31 (43.6) |
| Metastatic sites, n (%) | |
| Lung | 46 (76.7) |
| Lymph nodes | 36 (60) |
| Bone | 34 (56.7) |
| Liver | 10 (16.7) |
| Adrenal | 10 (16.7) |
| Pleura | 6 (10) |
| Brain | 6 (10) |
ECOG = Eastern Cooperative Oncology Group; IMDC = International Metastatic RCC Database Consortium; NOS = not otherwise specified; RCC = renal cell carcinoma.
Fig. 1.
Waterfall plot of percentage changes in tumour diameter in relation to RECIST 1.1 response at metastatic sites. The green colour indicates complete response, yellow partial response, blue stable disease, and red progressive disease.
Overall, only 4.4% (3/69) patients had RECIST 1.1 PD of the primary, none of whom developed local symptoms, whereas 10.1% (7/69) patients had a CR at metastatic sites (Fig. 1).
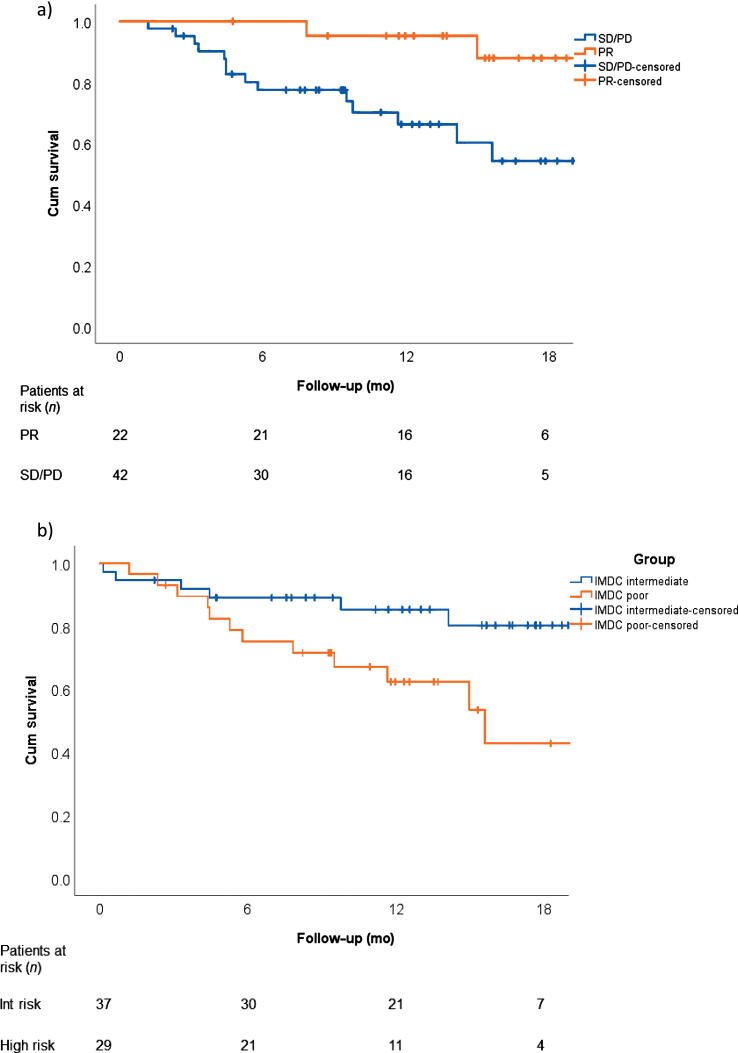
Irrespective of the IMDC risk, patients with a PR in the primary had a 1-yr overall survival (OS) rate of 89% versus 67% in patients without (p = 0.012; Fig. 2A). The median OS has been reached only for IMDC poor-risk patients (14.7 mo [95% CI 10–19.4]; Fig. 2B) who overall had a poorer outcome (Supplementary Table 5 and Supplementary Fig. 3). The median progression-free survival (PFS) was 10.1 mo (95% CI 4.84–15.4; Supplementary Fig. 4).
Fig. 2.
Overall survival (A) for patients with a partial response in the primary tumour (PR) and those without (SD/PD) and (B) for IMDC intermediate- and poor-risk patients. CR = complete response; Cum = cumulative; IMDC = International Metastatic RCC Database Consortium; Int = intermediate PD = progressive disease; PR = partial response; RCC = renal cell carcinoma; SD = stable disease.
A total of 13/69 (18.8%) deferred CNs were performed after a median time to surgery of 13 (IQR 10–14.9) mo, the majority (62%) being in IMDC intermediate-risk patients (Supplementary Table 5). The predominant reason for deferred CN was a response at metastatic sites (n = 12; four CRs and eight PRs). In three patients, deferred CN was performed to control the increase of the primary after initial downsizing (n = 3 with CR, PR, and stable disease at metastatic sites), and in one patient because the primary was the only site of PD (increase by 53.2%) after initial downsizing by 5.3%. One patient with a CR at metastatic sites had a complete pathological response; all others had remaining vital tumour after CN with various degrees of necrosis.
These real-world data are comparable with those of a subgroup of 55 patients without prior nephrectomy from the CheckMate 214 trial [9]. Following nivolumab and ipilimumab, the median PFS and OS were 8.1 (95% CI 5.5–20.9) and 26.1 (95% CI 13.9–25.4] mo, respectively. The ORR was 34%, with none of the patients achieving a CR as the primary was included in the RECIST target lesions. Assessing response in the primary and metastatic sites separately, our CR rate at metastatic sites is comparable with the rate reported in the CheckMate 214 trial. Similar data have been reported for the combination of avelumab and axitinib in 55 patients without prior nephrectomy from the Javelin 101 trial [10]. A PR in the primary occurred in 34.5% after a median of 4.4 mo. The agreement rate between patients with a PR in the primary and an ORR in all target lesions was 83.6%. In some of these patients, deferred CN may result in no evidence of disease (NED). The majority of patients with deferred CN in our series had a CR or a near CR at metastatic sites. A study of 111 patients without nephrectomy treated with nivolumab in second (63%) and third lines revealed only a 6% PR rate in the primary [11]. Acknowledging that these patients had previous lines of therapy, this suggests that combination therapies may be more effective in downsizing the primary.
In summary, these real-world data demonstrate that, similar to the tyrosine kinase inhibitor era, irrespective of the IMDC risk, patients with a RECIST response in their primary have better outcome in terms of progression and disease-related death. In addition, deferred CN leads to NED in those with a CR at metastatic sites. As a legacy of CARMENA and SURTIME [4], two phase 3 randomised controlled trials are investigating deferred CN versus no CN after ICI combination therapy (NORDICSUN [NCT03977571] and PROBE trial [NCT04510597]) [6]. Finally, treatment with the primary tumour in place seems to be safe. Only two patients required embolisation to control haematuria.
Limitations include retrospective design, a small number of patients, and multi-institutional inclusion.
Author contributions: Axel Bex had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bex, Meerveld-Eggink, Graafland, Haanen, Powles.
Acquisition of data: Bex, Graafland, Meerveld-Eggink, Wilgenhof, Van Thienen, Szabados, Boleti, Grant.
Analysis and interpretation of data: Lalezari, Bex, Blank, Powles, Meerveld-Eggink, Kuusk, Abu-Ghanem, Haanen.
Drafting of the manuscript: Bex, Meerveld-Eggink, Kuusk.
Critical revision of the manuscript for important intellectual content: Blank, Kuusk, Powles, Graafland.
Statistical analysis: Meerveld-Eggink, Bex, Abu-Ghanem.
Obtaining funding: None.
Administrative, technical, or material support: Lalezari, Kuusk, Abu-Ghanem, Meerveld-Eggink.
Supervision: Bex, Haanen, Powles.
Other: None.
Financial disclosures: Axel Bex certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Thomas Powles reports research funding from Merck Serono, Merck Sharp & Dohme, Roche, Bristol Myers Squibb (BMS), Astra Zeneca, Astellas, Novartis, Johnson and Johnson, Seattle Genetics, Pfizer, Exelixis and Eisai, and honoraria from Merck Serono, Merck Sharp & Dohme (MSD), Roche, BMS, Astra Zeneca, Astellas, Novartis, Johnson and Johnson, Seattle Genetics, Pfizer, Exelixis, and Eisai. Axel Bex reports restricted educational grant for an investigator-initiated trial of neoadjuvant therapy in high risk renal cancer from Pfizer; being a steering committee member and local investigator in an adjuvant trial for BMS, a steering committee member and PI in an adjuvant trial for Roche/Genentech, a medical steering committee member to advise the patient advocacy group on medical topics and strategy for the International Kidney Cancer Coalition, and a medical steering committee member to advise the patient advocacy group on medical topics and strategy for the Kidney Cancer Association. John Haanen reports research funding from Bristol Myers Squibb (BMS), and honoraria from Merck Serono, Merck Sharp & Dohme (MSD), Roche, BMS, Astra Zeneca, Novartis, and Pfizer. Christian U. Blank reports honoraria from Merck Serono, Merck Sharp & Dohme (MSD), Roche, BMS, Novartis, and Pfizer. All other authors report no conflicts of interest.
Funding/Support and role of the sponsor: None.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2021.11.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mejean A., Ravaud A., Thezenas S., et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379:417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 2.Bex A., Mulders P., Jewett M., et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5:164–170. doi: 10.1001/jamaoncol.2018.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escudier B., Porta C., Schmidinger M., et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B., Albiges L., Abu-Ghanem Y., et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Albiges L., Powles T., Staehler M., et al. Updated European Association of Urology guidelines on renal cell carcinoma: immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol. 2019;76:151–156. doi: 10.1016/j.eururo.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Kuusk T., Abu-Ghanem Y., Mumtaz F., Powles T., Bex A. Perioperative therapy in renal cancer in the era of immune checkpoint inhibitor therapy. Curr Opin Urol. 2021;31:262–269. doi: 10.1097/MOU.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 7.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 8.Singla N., Hutchinson R.C., Ghandour R.A., et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: an analysis of the National Cancer Database. Urol Oncol. 2020;38:604.e609–604.e617. doi: 10.1016/j.urolonc.2020.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albiges L., Tannir N., Burotto M., et al. 711P Nivolumab + ipilimumab (N+I) vs sunitinib (S) for first-line treatment of advanced renal cell carcinoma (aRCC) in CheckMate 214: 4-year follow-up and subgroup analysis of patients (pts) without nephrectomy. Ann Oncol. 2020;31:S559–S560. [Google Scholar]
- 10.Albiges L., Rini B.I., Haanen J.B.A.G., et al. Primary renal tumour shrinkage in patients (pts) who did not undergo upfront cytoreductive nephrectomy (uCN): subgroup analysis from the phase III JAVELIN Renal 101 trial of first-line avelumab + axitinib (A + Ax) vs sunitinib (S) for advanced renal cell carcinoma (aRCC) Ann Oncol. 2019;30:v359–v360. [Google Scholar]
- 11.Courcier J., Dalban C., Laguerre B., et al. Primary renal tumour response in patients treated with nivolumab for metastatic renal cell carcinoma: results from the GETUG-AFU 26 NIVOREN trial. Eur Urol. 2021;80:325–329. doi: 10.1016/j.eururo.2021.05.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.