Abstract
COVID-19 pandemic affected millions of people across India. COVID-19 cases are fewer in children with less severity and better outcomes than in adults. However, a small proportion develop severe illness and succumb to the disease. Clinical manifestations and optimal management of COVID-19 in immunocompromised children are not clearly known. Remdesivir was shown to be efficient in reducing the recovery time in COVID-19 patients requiring supplemental oxygen. Remdesivir is approved for use in children with severe COVID-19, but there are no guidelines in patients with risk factors like recent solid organ transplantation. We report a case of a 10-year-old kidney transplant recipient (KTR) infected with severe acute respiratory syndrome corona virus-2, 2.5 months after the transplantation. Unlike most children, he presented with high fever, cough, and vomiting. His inflammatory markers were elevated. In this case report, we discussed management and clinical outcomes of this patient. In view of recent kidney transplantation and the severity of infection with emergent oxygen requirement, we gave him remdesivir. We continued prednisolone and tacrolimus and stopped mycophenolate. He recovered completely in 7 days. We feel that severely immunosuppressed KTR children with COVID-19 will benefit with remdesivir administration. Monitoring tacrolimus trough levels is essential for maintaining adequate immunosuppression.
Keywords: Pediatric kidney transplantation, Severe acute respiratory syndrome corona virus-2, Remdesivir, High resolution computed tomography, Children
Introduction
Severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) infection affected >30 million people and resulted in around 390,000 deaths by June 21, across India, during the current COVID-19 pandemic. Cumulative incidence of COVID-19 was reported to be lower in children than in adults, and a greater number of pediatric kidney transplant recipients (KTRs) were infected during the second wave (5.9%) than during the first wave (0%) of COVID-19 in India [1, 2]. In a large series of 72,314 cases in China, fewer cases of COVID-19 in children were reported than adults and only 1% of these patients were below the age of 9 years [3]. A vaccine for children is not approved in India until recently. A new DNA vaccine has now received emergency approval in India for use in children above the age of 12 years, which may reduce the incidence of pediatric COVID-19 in the future, especially benefitting the immunocompromised subgroup. SARS-CoV-2 causes mild or moderate upper respiratory tract infection in immunocompetent children and majority of them are asymptomatic [4]. Clinical manifestations of children with COVID-19 are reported to be less severe with better outcomes than in adults [5]. A small proportion of children develop severe or critical disease requiring ventilatory support in pediatric intensive care unit and their outcomes are unfavorable [6]. Age-specific case fatality rates were 0.16 at ages 0–4 and 0.054 at ages 5–17 in South Indian children [1]. But the clinical presentation of COVID-19 in pediatric immunosuppressed patients is still unknown [6]. Marlais et al. [6] in their multinational survey of pediatric KTRs with COVID-19 described a mild course without greater severity or complications in their group of patients. There is a possibility that immunosuppression may protect them from severe form of disease [7]. But the question remains whether immunosuppressive therapy could be considered a risk factor for COVID-19 in children as a small group of them were reported to have adverse outcomes including death. This is important while making decisions regarding antiviral therapy in pediatric KTRs with SARS-CoV-2 infection.
Evidence has emerged showing the efficacy of remdesivir in shortening time to clinical recovery in adults with COVID-19 [8, 9]. Remdesivir is a nucleoside analog that inhibits viral RNA-dependent RNA polymerase, thus interfering with RNA replication. US FDA authorized the use of remdesivir as a treatment option for hospitalized COVID-19 patients and recommended dosing of remdesivir in children too [10]. However, there are no comparative clinical data evaluating efficacy or safety of remdesivir in pediatric COVID-19 patients. Children receiving remdesivir were included in several pediatric case series but the clinical outcomes were not adequately reported [11, 12]. Based on studies in adult COVID-19, severe immunocompromise and recent solid organ transplantation could be considered as a risk factor when making antiviral treatment decision. Here, we report a favorable outcome in a pediatric KTR with COVID-19, following treatment with remdesivir and modification of immunosuppression regimen.
Case Report
A 10-year-old male underwent kidney transplantation at our hospital 2 and half months ago for end-stage kidney disease due to chronic interstitial nephritis. He was detected to have PU valves and vesico-ureteric reflux at 6 months of age. He underwent resection of the PU valves and bilateral ureteric reimplantation, when he was 4 years old. He developed end-stage kidney disease by the age of 10 years. He had growth retardation and was grossly underweight. His mother, who is of the same blood group, donated her kidney to him, and he had transplantation on March 8, 2021. He did not receive any induction therapy and was on triple immunosuppression. He had uneventful course after transplantation with a serum creatinine of 0.5 mg/dL. He was started on prophylaxis for pneumocystis and cytomegalovirus (CMV). By 2 months, his tacrolimus trough level was 8 ng/mL with a dose of 1.5 mg twice daily. He was on prednisolone 5 mg per day and mycophenolate sodium 180 mg twice daily. He was on regular follow-up and had 2 cm linear growth and 4 kg weight gain after transplantation.
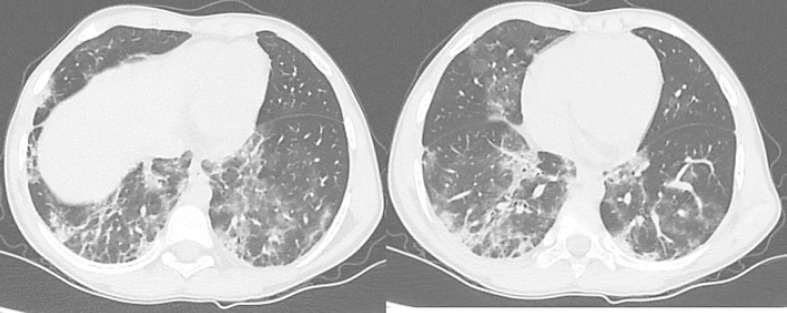
He presented to us with a history of high fever, severe cough, and vomiting for 2 days. He did not have loss of smell or taste, or diarrhea. His urine output was normal. None of his family members had symptoms of COVID-19. His weight was 22.5 kg. His temperature was 101°F, respiration 26 per min, pulse 120/min and blood pressure 120/80 mm Hg. His SpO2 was 92% (Table 1). His throat was normal. Chest was clear for auscultation. Graft kidney was nontender. Rest of the examination was unremarkable. His rapid antigen test for COVID-19 was positive. His nasopharyngeal swab was sent for SARS-CoV-2 RT-PCR analysis. High resolution computed tomography (HRCT) chest revealed CORADS-6 (COVID-19 Reporting and Data System Category 6) with a total severity score (TSS) of 18/25 (Table 1; Fig. 1).
Table 1.
Baseline profile and laboratory findings of the pediatric KTR
| Baseline profile | |
|---|---|
| Age | 9 years |
| Gender | Male |
| Clinical symptoms | |
| Body temperature | 101°F |
| Respiration | 26/min |
| Pulse | 120/min |
| Blood pressure | 120/80 mm Hg |
| SpO2 | 92% |
| HRCT chest TSS | 18/25 |
| Laboratory tests | |
| Hb | 11.3 g/dL |
| Lymphocytes | 820 cells/mm3 |
| Serum creatinine | 0.5 mg/dL |
| SGPT | 48 U/L |
| CRP | 9.3 mg/L |
| Ferritin | 203 ng/mL |
| LDH | 403 U/L |
| Procalcitonin | 0.11 ng/mL |
| D-dimer | 201 ng/mL |
This table depicts the baseline profile, and the results of various laboratory tests performed on the pediatric KTR reported in the study.
HRCT, high resolution computed tomography; TSS, Total Severity Score; Hb, hemoglobin; SGPT, serum glutamic-pyruvic transaminase; CRP, C-reactive protein; LDH, lactate dehydrogenase; KTR, kidney transplant recipients.
Fig. 1.
Figures showing HRCT chest scan images of the 10-year-old pediatric KTR taken at the time of admission. Scans show multiple confluent ground-glass opacities in bilateral lung fields (a, b), consistent with viral pneumonitis. HRCT, high resolution computed tomography; KTR, kidney transplant recipient.
He was admitted into the hospital and was further evaluated. Investigations revealed normal urinalysis, Hb 11.3 g/dL, absolute lymphocyte count 820 cells/mm3, serum creatinine 0.5 mg/dL, serum glutamic-pyruvic transaminase 48 U/L, C-reactive protein (CRP) 9.3 mg/L, ferritin 203 ng/mL, lactate dehydrogenase 403 U/L, procalcitonin 0.11 ng/mL, and D-dimer 201 ng/mL (Table 1). Ultrasound scan and doppler study of the graft kidney were normal. Blood and throat swabs were sent for bacterial cultures. Tacrolimus trough levels and qt-PCR for CMV and BK virus were analyzed.
He was given adequate hydration and antipyretics. He was started on piperacillin-tazobactam and continued till blood cultures were reported. His RT-PCR for SARS-CoV-2 was reported positive by the next day. He was continued on tacrolimus 1.5 mg twice daily and prednisolone 5 mg daily. Mycophenolate was stopped.
He was administered with remdesivir (lyophilized) 5 mg/kg intravenously loading dose on day 1 followed by 2.5 mg/kg intravenously every 24 h for the next 4 days. He became afebrile by sixth day but continued to have mild cough. His oxygen saturations were normal and urine output was normal. His creatinine was stable at 0.5 mg/dL and his liver functions were normal. PCR for CMV and BK virus was negative. Blood and throat swab cultures were sterile. Tacrolimus level was 8 ng/mL.
He was discharged on the eighth day and was advised home-quarantine. Telehealth follow-up was continued. He came for review on the fifteenth day and was asymptomatic. His renal functions, lymphocyte count, and CRP were normal. HRCT chest revealed a TSS of 3/25. He was restarted on mycophenolate sodium 180 mg daily and was continued on telehealth follow-up. His father tested positive while his mother and elder sibling were negative for COVID-19.
Discussion
Optimal management for COVID-19 in children is still uncertain and available therapeutic approaches lack firm evidence. Indication for antiviral treatment in immunocompetent and immunosuppressive children is not well established.
We described here the presentation in clinical course and management of a 10-year-old KTR who acquired COVID-19 from community exposure, 2.5 months after the transplantation. He was on triple immunosuppression with normal kidney functions and his blood pressure was under good control with 1 medication. He presented with high fever, incessant cough, and vomiting, unlike majority of the children with COVID-19 who are asymptomatic. Though clinical presentation of COVID-19 in immunosuppressed children is largely unknown, a survey that included pediatric KTRs with COVID-19 in 11 countries and surveillance of immunosuppressive children in UK showed that the clinical course in these children is mild and without complications [6, 13].
His HRCT chest revealed a TSS of 18/25 which is considered to be severe [14]. Requirement of respiratory support was favored as a better objective and relevant measure than radiographical criteria for deciding the severity of illness [15]. However, considering the possible rapid deterioration of clinical status and high mortality in adult KTRs with COVID-19 who are on triple immunosuppression at the time of infection, we hospitalized the patient [16].
He had lymphopenia with reduction of absolute lymphocyte count. Several studies reported association between lymphopenia, adult respiratory distress syndrome, and fatal outcome in COVID-19 patients [17]. Lymphocytes are primary targets of SARS-CoV-2 and further get affected by immunosuppressive therapy. A strong association was shown between high CRP levels and unfavorable outcomes including death in COVID-19 adult patients. This patient had high CRP levels, indicating severe disease. D-dimer levels were elevated in our patient and several reports showed an association between severe symptoms, disseminated intravascular coagulation, and increased D-dimer levels [18]. We did not find any evidence of deep vein thrombosis on doppler study. Procalcitonin level was 0.01 ng/mL indicating absence of bacterial sepsis. He had no other common viral infections of KTRs, as evidenced by negative qt-PCR tests for CMV and BK virus.
His HRCT chest was suggestive of COVID-19 infection with a severity score of 18/25. Though chest X-ray is employed as a first-line investigation, we found HRCT chest to be a useful tool for assessing severity and progression of COVID-19 associated pneumonia [19].
We stopped mycophenolate and continued prednisolone and tacrolimus, keeping tacrolimus trough level at 8 ng/mL. Managing immunosuppression in KTRs with COVID-19 is a challenge. An association exists between immunosuppression load and susceptibility to severe infection, ICU admission, or death [16]. Maintaining immunosuppression may impair viral clearance and facilitate progression of disease. So, 50% dose reduction or complete cessation of antimetabolite drugs is appropriate to limit the viral replication. On the other hand, Cleto-Yamane et al., [7] hypothesized that immunosuppression may act as a protection for severe forms of COVID-19. Complete withdrawal of immunosuppression may result in episodes of allograft rejection and renal failure. This may be one of the reasons for high incidence of renal failure in these patients in some earlier studies [18, 20].
Acute kidney injury and renal replacement therapy lead to worse outcomes and increased mortality in adult COVID-19 general population. However, the data on AKI in pediatric KTRs with COVID-19 are very sparse. Our patient had normal kidney function throughout his illness.
We administered our patient with 1 loading dose and 4 doses of remdesivir, as per the schedule already detailed. We considered him as severe immunocompromised as he had solid organ transplantation 2.5 months ago. The child had high fever, severe pneumonia, and emergent oxygen requirement. Sudden deterioration with requirement of supplemental oxygen therapy in pediatric intensive care unit may result in renal failure and dismal outcome in such patients. In India, even if the patient survives, second kidney transplantation is a rarity. We decided to administer remdesivir as his severe immunosuppressive state was a risk factor.
Optimal treatment for COVID-19 in children <10 years old is uncertain and no specific treatment or best practice guidelines exist in this regard [21]. In view of rapidly advancing evidence regarding the efficacy of remdesivir, FDA approved it for “Emergency Use Authorisation” [10]. It was suggested for use in severe and critically ill COVID-19 children [22]. But there is paucity of pediatric-specific data and indication for antiviral therapy is uncertain in these specific groups like KTRs. Decisions are to be made on case-by-case basis especially as in our pediatric KTR with COVID-19. Limitations of this study are short duration of follow-up and this being a single case report, generalizing the indications for remdesivir therapy in COVID-19 considering kidney transplantation and immunosuppression as risk factors may be difficult.
Conclusion
We reported the clinical course and management of a male child aged 10 years who was infected with SARS-CoV-2, two and half months after his kidney transplantation. We believe that therapy with remdesivir helped in early resolution of COVID-19 pneumonia and achieving uneventful recovery in this immunocompromised child. Stopping mycophenolate and monitoring trough levels of tacrolimus are important in preserving graft kidney function. Large case series of pediatric KTRs with COVID-19 are required to establish guidelines for antiviral therapy in these patients.
Statement of Ethics
This study was approved by the Ethics Committee of Apollo hospitals, Visakhapatnam on July 1, 2021. Written informed consent was obtained from the patient's parents.
Conflict of Interest Statement
The authors of this manuscript declare no conflicts of interest to be disclosed as described by the Case Reports in Nephrology and Dialysis.
Funding Sources
This study was not funded by any organization.
Author Contributions
Ravi Raju Tatapudi contributed to study design, data collection and Analysis, methodology, writing original draft and editing, verified the underlying data. Venkateswara Rao Kopparti contributed to study design, data collection, and formal analysis. Anusha Poosapati contributed to writing original draft, data collection and analysis, literature search, and verified the underlying data. Srinivas Metta contributed to data collection and analysis. Vedita Palli contributed to data analysis and collection, and investigation. Balakrishna Vedulla contributed to data collection and analysis.
Data Availability Statement
The data that support the findings of this study are described in the results section. No new data are generated or analyzed in this study.
References
- 1.Laxminarayan R, Wahl B, Dudala SR, Gopal K, Mohan BC, Neelima S, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020 Nov 6;370((6517)):691. doi: 10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kute VB, Meshram HS, Navadiya VV, Chauhan S, Patel DD, Desai SN, et al. Consequences of the first and second COVID-19 wave on kidney transplant recipients at a large Indian transplant centre. Nephrology. 2021 Aug 11;:10.1111/nep.13961. doi: 10.1111/nep.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 Apr 7;323((13)):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Assaker R, Colas AE, Julien-Marsollier F, Bruneau B, Marsac L, Greff B, et al. Presenting symptoms of COVID-19 in children: a meta-analysis of published studies. Br J Anaesth. 2020 Sep;125((3)):e330–2. doi: 10.1016/j.bja.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020 Jun 1;145((6)):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 6.Marlais M, Wlodkowski T, Vivarelli M, Pape L, Tönshoff B, Schaefer F, et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. 2020 Jul;44((7)):e17–e18. doi: 10.1016/S2352-4642(20)30145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleto-Yamane TL, Rodrigues-Santos G, de Magalhães-Barbosa MC, Moura PG, Vasconcelos RD, Gouveia JLS, et al. Screening of COVID-19 in outpatient children with cancer or solid organ transplantation: preliminary report. Eur J Pediatr. 2021;180:3237–41. doi: 10.1007/s00431-021-04044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020 May 27;6((5)):672–83. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshram HS, Kute VB, Patel H, Banerjee S, Navadiya V, Desai S, et al. Feasibility and safety of remdesivir in SARS-CoV2 infected renal transplant recipients: a retrospective cohort from a developing nation. Transpl Infect Dis. 2021 Apr 29;23:e13629. doi: 10.1111/tid.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration . Fact sheet for healthcare providers emergency use authorization (EUA) of veklury® (remdesivir) for hospitalized pediatric patients weighing 3.5 kg to less than 40 kg or hospitalized pediatric patients less than 12 years of age weighing at least 3.5 kg. NETEC Resource Library; [Cited 2021 Jun 9]. Available from: https://www.fda.gov/media/137566/download. [Google Scholar]
- 11.Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. Journal Pediatr. 2020;223:14–9. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Carducci FIC, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4((9)):653–61. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaunak M, Patel R, Driessens C, Mills L, Leahy A, Gbesemete D, et al. COVID-19 symptom surveillance in immunocompromised children and young people in the UK: a prospective observational cohort study. BMJ Open. 2021 Mar 17;11((3)):e044899. doi: 10.1136/bmjopen-2020-044899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19: definition and evaluation. Radiology. 2020 Apr 27;296((2)):E97–104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020 May 1;55((5)):1169–74. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Columbia University Kidney Transplant Program. Husain A, Chang J, David C, Geoffrey D, Hilda F, et al. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020 Jun;31((6)):1150. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhadedy MA, Marie Y, Halawa A. COVID-19 in renal transplant recipients: case series and a brief review of current evidence. Nephron. 2021;145((2)):192–8. doi: 10.1159/000512329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020 Jun;97((6)):1083–8. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hare SS, Rodrigues JCL, Nair A, Jacob J, Upile S, Johnstone A, et al. The continuing evolution of COVID-19 imaging pathways in the UK: a British Society of Thoracic Imaging expert reference group update. Clin Radiol. 2020 Jun;75((6)):399–404. doi: 10.1016/j.crad.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kute VB, Bhalla AK, Guleria S, Ray DS, Bahadur MM, Shingare A, et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a Multicenter Cohort Study from India. Transplantation. 2021;105((4)):851–60. doi: 10.1097/TP.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Health . Special considerations in children. National Institute of Health; 2021. Apr, [Cited 2021 Jun 9]. Available from: https://www.covid19treatmentguidelines.nih.gov/special-populations/children/ [Google Scholar]
- 22.Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatr Infect Dis Soc. 2021 Jan 1;10((1)):34–48. doi: 10.1093/jpids/piaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are described in the results section. No new data are generated or analyzed in this study.