Abstract
Background
By combining up-to-date medical knowledge and steadily increasing patient data, a new level of medical care can emerge.
Summary and Key Messages
Clinical decision support systems (CDSSs) are an arising solution to handling rich data and providing them to health care providers in order to improve diagnosis and treatment. However, despite promising examples in many areas, substantial evidence for a thorough benefit of these support solutions is lacking. This may be due to a lack of general frameworks and diverse health systems around the globe. We therefore summarize the current status of CDSSs in medicine but also discuss potential limitations that need to be overcome in order to further foster future development and acceptance.
Keywords: Clinical decision support systems, Artificial intelligence, Knowledge-based system, Gastroenterology, Hepatology, Endoscopy, Digital medicine
Background
The application and benefits of clinical decision support systems (CDSSs) are diverse. In the last few years, the amount of collected and documented patient data has increased continuously, be it through the continuous, electronic recording of vital parameters, laboratory parameters increasingly available in bulk, or teleradiological ubiquitous imaging. At the same time, we have also witnessed significant improvements in knowledge resources in the medical field. Bringing this medical knowledge and various patient data together, a new level of medical care can emerge. CDSSs can play a key role in merging and managing these diverse pieces of information.
Available Systems: Knowledge Based versus AI/Machine Learning Based
In principle, knowledge-based and artificial intelligence-based systems are available to support clinical decision. Knowledge-based systems use predefined rules that have to be created beforehand, for example, digital implementation of guidelines. Defined rules can be literature based, practice related, or patient related. Depending on the patient specifics, the respective rules are then called up in order to issue appropriate therapy recommendations [1, 2, 3]. The disadvantage of these knowledge-based CDSSs is mostly relatively rigid algorithms that allow only limited individualized tailoring.
Nonknowledge-based systems require a sufficient data source in order to use machine learning and statistical pattern recognition, which currently drive a strong artificial intelligence movement, to develop recommendations [4, 5, 6]. These algorithms have the great advantage that, as learning systems, they can improve their recommendations as the volume of data increases. It is very important that an understanding is developed of how and with what these algorithms work in order to avoid a “black box” and to be able to critically appraise recommendations given by the CDSSs [7, 8, 9].
Support in Diagnosis
Early on, the idea of supporting diagnostic processes was appealing to be supported by CDSSs [10, 11]. Entering symptoms into a data repository and querying potential diagnosis may certainly be helpful in difficult but also rare cases. However, the quality of the diagnosis clearly depends on the quality and standardization of the recorded symptoms and patient information.
With the increasing amount of data and medical literature available as well as (electronic) patient information, we have seen a rapid increase in the amount of data in the last few decades [12, 13]. Outside of the increasingly small specialty of many health care providers, this is quickly confusing and very difficult to keep up to date, in particular with regard to a critical discussion of the available literature and data from latest meetings of specific disciplines. A major advantage of the diagnostic support provided by a CDSS can be the inclusion of rare diseases in the differential diagnostic considerations. In addition, CDSS can suggest complementary and alternative tests that further confirm or rule out rare diseases. This can significantly reduce the possibility of a rare disease being overlooked in routine diagnostics [14, 15].
Another prominent example of a high benefit from CDSS is molecular tumor boards, for which the introduction of high-throughput analysis has opened up completely new perspectives. With the flood of information from genome-wide sequencing but also high-throughput drug screening, IT support for the preselection of suitable substances, studies, and therapeutic options has become indispensable [16]. Examples of databases that hold structured genomic information and knowledge about various cancers are the COSMIC database [17] or OncoKB [18] also offering links to potential therapeutic options. Recently, a European expert group reported the development of the Molecular Tumor Board Portal, a CDSS that unifies the analysis of sequencing results across 7 European comprehensive cancer centers under the umbrella of the Cancer Core Europe (CCE) network [19]. The portal may be used to select candidates for clinical studies with active recruitment across CCE sites. Even if these databases are currently still set up as standalone solutions, they already offer considerable help in the selection of essential mutations and their possible therapeutic options.
Interesting options in diagnostics are increasingly emerging in the area of imaging, be it radiological or endoscopic. However, CDSSs supporting medical imaging are mostly driven by deep learning as a very successful class of artificial intelligence approaches. For example, those deep learning strategies are regularly applied in endoscopy. Additional use of AI technology significantly increased the adenoma detection rate and the average number of adenomas found per patient [20]. This may particularly help with smaller adenomas. Furthermore, accuracy of polyp detection was estimated to be as high as 96.4% in other reports [21]. However, larger multicenter randomized studies are still needed.
With respect to tumor pathology, Kather et al. [22] illustrated that artificial neural network approaches may be used to determine the instability of microsatellites just from conventional histology of a tumor, which is important with respect to (immuno-)therapeutic options. Finally, for the visual assessment of radiological images, AI may help to objectify the qualitative assessment and reduce the intra-/interobserver variability [23, 24, 25].
In addition to this improvement in diagnostics within the hospital, support from a CDSS knowledge base can also be used to support remote diagnostics and, if necessary, to monitor continuously or at least regularly. One of the examples is smartphone APPs that evaluate skin lesions and identify possible malignant changes. Even if the possible additional use of these APPs is currently still controversial, a further development in this field and the associated opportunities and increasing autonomy are certainly foreseeable [26].
Last, even training of health care providers could benefit from CDSSs. Various studies have shown that a larger proportion of residents were overconfident with regard to their diagnosis and did not seek any further help in making the diagnosis. From this aspect, too, regular interaction of the training assistants working in a clinic with a CDSS would be helpful and would increase the quality of the diagnostics [27].
Those advantages outlined above were mostly based on knowledge-based CDSSs. A successful development and implementation of efficient AI algorithms and subsequent integration in CDSS models can result in even further improvement in diagnostics. For example, AI models for evaluating and weighting diverse combinations of serum markers, blood tests, and clinical parameters would certainly be extremely helpful for making a diagnosis. Those systems could also help to point out missing pieces of the diagnostic puzzle, which are crucial for a definite (differential) diagnosis [28].
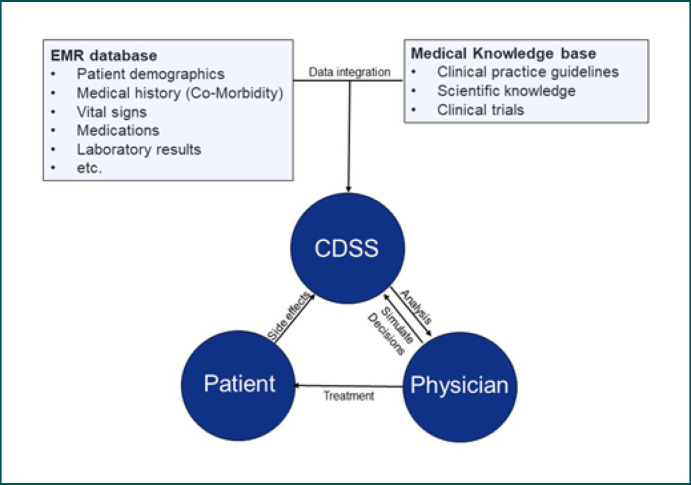
Finally, CDSSs can be very helpful in getting the diagnosis a personalized interpretation of the test and reference range in terms of age, gender, or disease entity. For example, Wilson's disease mostly diagnosed before the age of 40, a fact that could be included in a ranking of the probability in the differential diagnostic of, for example, elevated liver values [29]. In summary, a CDSS-supported diagnosis can be very helpful in many areas, especially the processing of big data, the automated detection of pathological changes, a future possible autonomous point-of-care remote diagnosis, the training, but also the combination of complex digital and analog markers (Fig. 1).
Fig. 1.
Fully developed CDSS may have key roles in data integration but also supporting physicians and patients in selecting the best possible treatment options. CDSS, clinical decision support system.
Improving Clinical Care
An improvement in clinical care through CDSSs can already be efficiently implemented with the available technologies. One of the essential aspects is the accumulation and retention of medical knowledge, as it is stored in clinical practice guidelines in particular. Continuous training for health care providers outside of their increasingly narrow specialist area is absolutely necessary but can for most health care providers not be taken for granted − even if this would be desirable. In visceral medicine, there are a number of very diverse subspecializations such as hepatology, chronic inflammatory bowel diseases, GI oncology, or transplant medicine. These develop in short-term time intervals, and, especially, after large, international congresses, there is an extensive amount of new clinical and basic scientific data that must be evaluated and entered into the existing algorithms. A claim to be up to date in all areas is very difficult to meet in day-to-day practice. As a result, continuous consultation of the guideline recommendations is very difficult to implement in clinical routine.
However, the evolution of supporting CDSSs is also difficult. A major issue certainly is that developed CDSSs fulfill different tasks and lack a general CDSS framework. Thus, the outline of CDSSs may be very diverse and are currently mostly set up as standalone solutions dependent on a local health care framework, clinical recommendations, but also insurance issues. This hinders the validation and/or comparison of these systems. Thus, defining the goal of a respective CDSS application seems to be extremely important.
A recent publication by Pawloski et al. [38] on the efficiency of CDSSs in clinical oncology summarized 24 studies and tools on this issue. CDSSs improved adherence to clinical treatment guidelines [30, 31, 32, 33, 34, 35, 36, 37] but (in individual cases) were also associated with an improved outcome and reduced hospital stay [33]. Key features that seem to be associated with a positive outcome were the conveyance of real-time information and point-of-care action. However, the authors cannot bring themselves to a clear, advantageous presentation of the benefits of CDSSs and refer to the necessity of a rigorous evaluation and validation of the CDSSs used. Furthermore, there was no significant difference between electronic CDSSs and traditional paper-based decision aids [32].
An obvious benefit is patient follow-up screening for enrollment opportunities and reminder functions, for example, the identification of necessary follow-up examinations and treatments such as pneumococcal, Haemophilus influenza, or meningococcus vaccination after a splenectomy. Despite being extremely important, respective vaccinations were not documented in 71% of the patient files [39] but can be remedied by automated reminder functions [40].
With regard to the recommended standard vaccinations, such as HPV vaccination, individual publications have shown an improvement in adherence. Although most health care providers themselves were of the opinion that they had not significantly changed their behavior with regard to awareness and prescribing if supported by an appropriate alarm system, the vaccination rates in the study by Dickson et al. [41] increased. In contrast to this, however, other studies that specifically examined reminder functions for the necessary second and third HPV vaccination did not report a statistically significant improvement in the rate of second and third vaccinations against HPV [40]. In addition to this, a study on influenza vaccination with already high vaccination levels also showed no further improvement using CDSSs [42].
Minimizing Medication Errors
Another important and effective topic that can be supported by CDSSs is the recording and avoidance of medication errors. These are fairly common. An Institute of Medicine report estimated that 44,000–98,000 people die in US hospitals each year from errors [43]. Medication errors can in principle occur in every phase of the prescription and medication process [44, 45]. A frequent source of error is the conversion of the home medication to a supposedly identical inpatient medication if a patient is referred to a hospital. Such medication errors are assumed to be rather common and occur in up to 65% of inpatients [46]. However, the correct dosage of the corresponding (alternative) medication also harbors considerable sources of error. Bates et al. [6, 43] found that almost a third of the ADEs were in principle preventable [44].
Here, CDSSs can provide significant support in avoiding medication errors. This applies to the area of prescribing, dispensing, and taking drugs as well as avoiding drug-drug interactions with potentially adverse effects [47]. Finally, certain protective measures, for example, important safety information, can be stored in CDSS, information that is often not permanently available to many colleagues.
A positive example is the study by Pruszydlo et al. [48], investigating the switch of medication when patients are hospitalized. Support by CDSSs achieved implementation without errors in over 90% of medications [48], which should go hand in hand with supposedly improved security and reduced workload and probably also reduced costs.
Further development stages of such CDSSs are in principle conceivable and in development. Automated dispensing systems for medication and barcodes using point-of-care systems for processing medication could certainly achieve significant improvements. This could be further developed up to a closed loop system in which administered medication and identifying by means of barcodes and cross-checking with the available patient information on the prescription is linked [49].
Despite such promising examples, there is little hard evidence for effectiveness with regard to patient outcome. In a review of the available literature, Jia et al. [44] showed that warnings or reminders regarding the medication lead to an improvement in the corresponding processes, but that these changes are only “most likely” to have an influence on the survival of the patients. Translated, there is currently no clear evidence of survival benefits from CDSSs [44]. This may particularly be due to the fact that only a small proportion of the studies on medication and CDSSs had safety and outcome as endpoints for the patients. The majority of the studies are also small and/or the follow-up period is too short. Finally, practically no studies describe any other potentially negative effects or problems of a CDSS with regard to drug prescription.
Improving Cost Efficiency
CDSS can make a significant contribution to cost savings in hospitals and practices. For example, cheaper alternative drugs can be suggested. An intelligent switch algorithm for the switch of medications in which a CDSS exercises a control function with regard to the changeover can also be very helpful, since side effects may require further treatments or longer hospital stays, which in turn cost money. Without CDSSs, it is assumed that 1 in 5 changes is incorrect, whereas with CDSSs, the substitution is adequate in over 90% of patients [48]. Organization and timing of examinations, procedures, and at the same time a reduction in duplicate examinations can also lead to considerable savings. This has been shown repeatedly, for example, by limiting blood counts, serology, and diagnostic laboratory panels, which have led to considerable savings without increasing the length of stay or mortality. Shortening the inpatient length of stay and optimal documentation considering billing-relevant aspects for the health insurance companies can also be significantly supported by a CDSS. Last but not least, administrative tasks can then be prepared and processed automatically, for example, the clinical and diagnostic ordering of organizational orders, such as laboratory diagnostics. The care algorithms of an inpatient process and the associated optimization of the billing-relevant ICD codes and billing modalities can also improve the accuracy of billing, which in turn can offer financial advantages. An increased speed of the encryption of patient files is certainly also given here [50, 51].
As a positive example, the work of Algaze et al. [52] from a pediatric intensive care unit in the USA can be cited, where a CDSS was used to control certain laboratory values, in particular blood counts, serological results, or coagulation parameters, every 24 h. The authors reported a considerable cost savings of USD 717,538 per year without worsening the hospital stay or the mortality of the patients [52].
A study by Anchala et al. [53] examined the cost effectiveness of EMR-based CDSS in adults with diabetes. Using a simulation cohort with over 1,000 patients with diabetes, the conclusion was that the widespread introduction of highly developed EMR-based CDSS has the potential to slightly improve the quality of care for patients with chronic diseases without significantly increasing costs for the health system [54].
Cost-effective examples of CDSS use have also been published for the treatment of hypertension. A cost efficiency ratio of USD 96.01 versus USD 36.57 per mm Hg systolic blood pressure reduction and thus good cost efficiency was found in a total of 1,628 patients [53].
In contrast, an intervention study in 511 patients found that CDSS-assisted therapy produced moderate improvements in long-term health outcomes. With regard to the investment and costs per QALY, the authors concluded that the use of CDSSs must become considerably more efficient or cheaper in order to support the treatment of patients with type 2 diabetes as a sensible, cost-effective option [55].
Also, in other medical areas such as the gynecological care in rural areas with supposedly poor medical infrastructure in Tanzania or Ghana, an improvement of the processes (by CDSS support) did not yet lead a higher cost efficiency [56, 57]. In summary, the current study situation is heterogeneous, and CDSS may not always be clearly cost effective. Diseases to be diagnosed or treated and health policy framework conditions seem to have a considerable influence on the assessment of cost efficiency.
Patient Engagement
The involvement of patients even within the framework of CDSS-based decision-making processes appears to have a positive influence on them. The patients felt better informed and felt clearer about their treatment wishes and goals, which probably results in a more active role in decision-making and a more precise risk perception [58]. However, whether this also applies to population groups with a lower level of education or even a lower level of literacy remains to be investigated [58]. In any case, patients with CDSS-supported therapy report a higher level of satisfaction [59].
Potential Disadvantages of CDSS
With regard to possible disadvantages of a CDSS, the disruption of clinical processes comes into question. A particular problem is that most of the solutions for the previously addressed support options are currently still implemented as standalone solutions and are not integrated into the clinic information systems [60, 61]. For everyday clinical practice, this means that a regular change between the information systems is necessary, which on the one hand can be time consuming and on the other hand can lead to prioritization conflicts.
A very important problem when dealing with CDSSs is the rapid alarm fatigue. As is often the case in intensive care or monitoring wards, the alarms set for seriously ill patients are often so low that they trigger frequently. This leads to a low alarm threshold, which is why the alarms are increasingly disregarded over time. Up to 95% of the CDSS warnings are assessed as clinically irrelevant, which means that doctors often contradict the warnings or distrust them, and in the medium term, this leads to the alarms being of poor value [62, 63, 64].
Nevertheless, in the medium term, the independence and critical faculties of the medical staff are maintained. An automatization as a result of which health care providers no longer master the basics of diagnostics and therapy but only rely on the algorithms needs to be avoided. Therefore, practitioners need technical competence, which is currently neither taught in medical school nor in further medical training [65].
A major problem is the continuous maintenance of a CDSS which is often neglected. However, the database foundation of the CDSSs is a crucial essence for the decision tree of the CSS. In particular, keeping the databases and knowledge algorithms regularly up to date is a lot of work and is often not included in the price when purchasing the software. The quality control, especially of external data sources, is also critical and has by no means been standardized to date. This leads to the fact that even the medical staff, if they do not trust the CDSS, establish workarounds, which are then associated with a deterioration in the benefit and can possibly also be identified as sources of danger [49].
For AI-based CDSS, the black box problem was repeatedly addressed. As the decision-making processes of an AI network may often remain opaque for different reasons [66], system programmers and operators may often not be able to explain how the algorithms work [9]. However, although other things in medicine may also be accepted to be opaque, this raises significant issues for the role out of those technologies, generally referred to as the black box problem. Validation and (its) regulation may therefore be of high importance. A 3-step validation was suggested by Price [67], ensuring high quality development (code and data), validation on an independently created data set, and a continued validation even after approval by continuous tracking ultimately leading to a learning health care system. An early deployment of a well-defined regulatory framework will also be necessary to guarantee technological standards for patients and developers [9, 66, 67].
In this context, a relevant problem for automated processing of clinical data is the lack of categorized and annotated data but also language diversity. The increasing use of electronic health records may provide progress streamlining clinical documentation as discussed in a separate review in this issue of visceral medicine. Particularly, standardization of clinical health terminologies, for example, using the SNOMED CT reference terminology, would resolve diverse problems associated with clinical data management [68]. The next step was recently initiated by implementing clinical rules which would work across different electronic health record systems and institutions using the GASTON framework [69]. Finally, a major disadvantage of CDSS is often that they are very expensive to purchase, so clinics do without them, especially in difficult economic times.
Conclusion
CDSSs are available in many areas of diagnosis, imaging, and medication. Despite promising examples in many areas, substantial evidence for a thorough benefit of these support solutions is lacking. This may be due to a lack of general frameworks and diverse health systems around the globe. Missing integration of CDSS in clinical information systems and alarm fatigue need to be overcome in order to increase efficacy and acceptance of CDSSs.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
A.T. received funding from the State Ministry of Baden-Wuerttemberg for Sciences, Research and Arts supporting the Clinical Cooperation Unit Healthy Metabolism at the Center for Preventive Medicine and Digital Health, and the Baden-Wuerttemberg Center for Digital Early Disease Detection and Prevention (BW-ZDFP). A.T. also received grants from the Sino-German Center for Research Promotion (GZ-1546 and C-0012).
Author Contributions
Writing the manuscript and proofreading were done by A.T. and H.B.
References
- 1.Yu PP. Knowledge bases, clinical decision support systems, and rapid learning in oncology. J Oncol Pract. 2015 Mar;11((2)):e206–11. doi: 10.1200/JOP.2014.000620. [DOI] [PubMed] [Google Scholar]
- 2.Usman OA, Oshiro C, Chambers JG, Tu SW, Martins S, Robinson A, et al. Selecting test cases from the electronic health record for software testing of knowledge-based clinical decision support systems. AMIA Annu Symp Proc. 2018;2018:1046–55. [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Vander Stichele R, Bates DW, Björklund J, Alexander S, Andersson ML, et al. Guiding principles for the use of knowledge bases and real-world data in clinical decision support systems: report by an international expert workshop at Karolinska Institutet. Expert Rev Clin Pharmacol. 2020 Sep;13((9)):925–34. doi: 10.1080/17512433.2020.1805314. [DOI] [PubMed] [Google Scholar]
- 4.Beilstein DP, Hawkins ES. Pedal manifestations of systemic lupus erythematosus. Clin Podiatr Med Surg. 1988 Jan;5((1)):37–56. [PubMed] [Google Scholar]
- 5.Xu F, Sepúlveda MJ, Jiang Z, Wang H, Li J, Liu Z, et al. Effect of an artificial intelligence clinical decision support system on treatment decisions for complex breast cancer. JCO Clin Cancer Inform. 2020 Sep;4:824–38. doi: 10.1200/CCI.20.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates DW, Levine D, Syrowatka A, Kuznetsova M, Craig KJT, Rui A, et al. The potential of artificial intelligence to improve patient safety: a scoping review. NPJ Digit Med. 2021 Mar 19;4((1)):54. doi: 10.1038/s41746-021-00423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzinger A, Langs G, Denk H, Zatloukal K, Müller H. Causability and explainability of artificial intelligence in medicine. Wiley Interdiscip Rev Data Min Knowl Discov. 2019 Jul;9((4)):e1312. doi: 10.1002/widm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan M. In AI we trust: ethics, artificial intelligence, and reliability. Sci Eng Ethics. 2020 Oct;26((5)):2749–67. doi: 10.1007/s11948-020-00228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felder RM. Coming to terms with the black box problem: how to justify AI systems in health care. Hastings Cent Rep. 2021 Apr 6; doi: 10.1002/hast.1248. [DOI] [PubMed] [Google Scholar]
- 10.Gifford DR, Mittman BS, Vickrey BG. Diagnostic reasoning in neurology. Neurol Clin. 1996 Feb;14((1)):223–38. doi: 10.1016/s0733-8619(05)70251-6. [DOI] [PubMed] [Google Scholar]
- 11.Malchow-Moller A, Bjerregaard B, Hilden J. Computer-assisted diagnosis in gastroenterology. Scand J Gastroenterol Suppl. 1996;216:225–33. doi: 10.3109/00365529609094577. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal R, Prabakaran S. Big data in digital healthcare: lessons learnt and recommendations for general practice. Heredity. 2020 Apr;124((4)):525–34. doi: 10.1038/s41437-020-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adibuzzaman M, DeLaurentis P, Hill J, Benneyworth BD. Big data in healthcare − the promises, challenges and opportunities from a research perspective: a case study with a model database. AMIA Annu Symp Proc. 2017;2017:384–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Dragusin R, Petcu P, Lioma C, Larsen B, Jørgensen HL, Cox IJ, et al. FindZebra: a search engine for rare diseases. Int J Med Inform. 2013 Jun;82((6)):528–38. doi: 10.1016/j.ijmedinf.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Muller T, Jerrentrup A, Schafer JR. Computer-assisted diagnosis of rare diseases. Internist. 2018 Apr;59((4)):391–400. doi: 10.1007/s00108-017-0218-z. [DOI] [PubMed] [Google Scholar]
- 16.Tamborero D, Dienstmann R, Rachid MH, Boekel J, Baird R, Braña I, et al. Support systems to guide clinical decision-making in precision oncology: the cancer core Europe molecular tumor board portal. Nat Med. 2020 Jul;26((7)):992–4. doi: 10.1038/s41591-020-0969-2. [DOI] [PubMed] [Google Scholar]
- 17.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, et al. The catalogue of somatic mutations in cancer (COSMIC) Curr Protoc Hum Genet. 2008 Apr;57((1)):Unit 10 11. doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakravarty D, Gao J, Phillips S, Kundra R, Zhang H, Wang J, et al. OncoKB: a precision oncology knowledge base. JCO Precision Oncology. 2017 Jul;((1)):1. doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggermont AMM, Apolone G, Baumann M, Caldas C, Celis JE, de Lorenzo F, et al. Cancer core Europe: a translational research infrastructure for a European mission on cancer. Mol Oncol. 2019 Mar;13((3)):521–7. doi: 10.1002/1878-0261.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019 Oct;68((10)):1813–9. doi: 10.1136/gutjnl-2018-317500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urban G, Tripathi P, Alkayali T, Mittal M, Jalali F, Karnes W, et al. Deep learning localizes and identifies polyps in real time with 96% accuracy in screening colonoscopy. Gastroenterology. 2018 Oct;155((4)):1069–e8. doi: 10.1053/j.gastro.2018.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kather JN, Pearson AT, Halama N, Jäger D, Krause J, Loosen SH, et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med. 2019 Jul;25((7)):1054–6. doi: 10.1038/s41591-019-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambinder EP. A history of the shift toward full computerization of medicine. J Oncol Pract. 2005 Jul;1((2)):54–6. doi: 10.1200/jop.2005.1.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman M, Osicka T. Reader variability: what we can learn from computer-aided detection experiments. J Am Coll Radiol. 2006 Jun;3((6)):446–55. doi: 10.1016/j.jacr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Zhou LQ, Wang JY, Yu SY, Wu GG, Wei Q, Deng YB, et al. Artificial intelligence in medical imaging of the liver. World J Gastroenterol. 2019 Feb 14;25((6)):672–82. doi: 10.3748/wjg.v25.i6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rat C, Hild S, Rault Sérandour J, Gaultier A, Quereux G, Dreno B, et al. Use of smartphones for early detection of melanoma: systematic review. J Med Internet Res. 2018 Apr 13;20((4)):e135. doi: 10.2196/jmir.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman MJ, Hoffer EP, Barnett GO, Kim RJ, Famiglietti KT, Chueh HC. Impact of a computer-based diagnostic decision support tool on the differential diagnoses of medicine residents. J Grad Med Educ. 2012 Jun;4((2)):227–31. doi: 10.4300/JGME-D-11-00180.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller DD, Brown EW. Artificial intelligence in medical practice: the question to the answer? Am J Med. 2018 Feb;131((2)):129–33. doi: 10.1016/j.amjmed.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Weitzman E, Pappo O, Weiss P, Frydman M, Haviv-Yadid Y, Ben Ari Z. Late onset fulminant Wilson's disease: a case report and review of the literature. World J Gastroenterol. 2014 Dec 14;20((46)):17656–60. doi: 10.3748/wjg.v20.i46.17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beriwal S, Rajagopalan MS, Flickinger JC, Rakfal SM, Rodgers E, Heron DE. How effective are clinical pathways with and without online peer-review? An analysis of bone metastases pathway in a large, integrated national cancer institute-designated comprehensive cancer center network. Int J Radiat Oncol Biol Phys. 2012 Jul 15;83((4)):1246–51. doi: 10.1016/j.ijrobp.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 31.Bertsche T, Askoxylakis V, Habl G, Laidig F, Kaltschmidt J, Schmitt SP, et al. Multidisciplinary pain management based on a computerized clinical decision support system in cancer pain patients. Pain. 2009 Dec 15;147((1–3)):20–8. doi: 10.1016/j.pain.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Chang PL, Li YC, Lee SH. The differences in health outcomes between Web-based and paper-based implementation of a clinical pathway for radical nephrectomy. BJU Int. 2002 Oct;90((6)):522–8. doi: 10.1046/j.1464-410x.2002.02980.x. [DOI] [PubMed] [Google Scholar]
- 33.Hsu YC, Tsui KH, Chen CL, Lee SH, Wu YS, Chang PL. Web-based clinical pathway for reducing practice variations in radical prostatectomy. Chang Gung Med J. 2008 Nov;31((6)):567–75. [PubMed] [Google Scholar]
- 34.Patkar V, Acosta D, Davidson T, Jones A, Fox J, Keshtgar M. Using computerised decision support to improve compliance of cancer multidisciplinary meetings with evidence-based guidance. BMJ Open. 2012;2((3)) doi: 10.1136/bmjopen-2011-000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Erps J, Aapro M, MacDonald K, Soubeyran P, Turner M, Warrinnier H, et al. Promoting evidence-based management of anemia in cancer patients: concurrent and discriminant validity of RESPOND, a web-based clinical guidance system based on the EORTC guidelines for supportive care in cancer. Support Care Cancer. 2010 Jul;18((7)):847–58. doi: 10.1007/s00520-009-0718-z. [DOI] [PubMed] [Google Scholar]
- 36.Bouaud J, Séroussi B, Antoine EC, Zelek L, Spielmann M. A before-after study using OncoDoc, a guideline-based decision support-system on breast cancer management: impact upon physician prescribing behaviour. Stud Health Technol Inform. 2001;84((Pt 1)):420–4. [PubMed] [Google Scholar]
- 37.Bouaud J, Spano JP, Lefranc JP, Cojean-Zelek I, Blaszka-Jaulerry B, Zelek L, et al. Physicians' attitudes towards the advice of a guideline-based decision support system: a case study with OncoDoc2 in the management of breast cancer patients. Stud Health Technol Inform. 2015;216:264–9. [PubMed] [Google Scholar]
- 38.Pawloski PA, Brooks GA, Nielsen ME, Olson-Bullis BA. A systematic review of clinical decision support systems for clinical oncology practice. J Natl Compr Canc Netw. 2019 Apr 1;17((4)):331–8. doi: 10.6004/jnccn.2018.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEvoy D, Gandhi TK, Turchin A, Wright A. Enhancing problem list documentation in electronic health records using two methods: the example of prior splenectomy. BMJ Qual Saf. 2018 Jan;27((1)):40–7. doi: 10.1136/bmjqs-2017-006707. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson TA, Dixon BE, Xiao S, Tu W, Lindsay B, Sheley M, et al. Physician clinical decision support system prompts and administration of subsequent doses of HPV vaccine: a randomized clinical trial. Vaccine. 2019 Jul 18;37((31)):4414–8. doi: 10.1016/j.vaccine.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Dixon BE, Kasting ML, Wilson S, Kulkarni A, Zimet GD, Downs SM. Health care providers' perceptions of use and influence of clinical decision support reminders: qualitative study following a randomized trial to improve HPV vaccination rates. BMC Med Inform Decis Mak. 2017 Aug 10;17((1)):119. doi: 10.1186/s12911-017-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerard MN, Trick WE, Das K, Charles-Damte M, Murphy GA, Benson IM. Use of clinical decision support to increase influenza vaccination: multi-year evolution of the system. J Am Med Inform Assoc. 2008 Nov;15((6)):776–9. doi: 10.1197/jamia.M2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bates DW, Cohen M, Leape LL, Overhage JM, Shabot MM, Sheridan T. Reducing the frequency of errors in medicine using information technology. J Am Med Inform Assoc. 2001 Jul;8((4)):299–308. doi: 10.1136/jamia.2001.0080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia P, Zhang L, Chen J, Zhao P, Zhang M. The effects of clinical decision support systems on medication safety: an overview. PLoS One. 2016;11((12)):e0167683. doi: 10.1371/journal.pone.0167683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown J, Farquhar C. Endometriosis: an overview of cochrane reviews. Cochrane Database Syst Rev. 2014 Mar 10;((3)):CD009590. doi: 10.1002/14651858.CD009590.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vonbach P, Dubied A, Krähenbühl S, Beer JH. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur J Intern Med. 2008 Oct;19((6)):413–20. doi: 10.1016/j.ejim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Carling CL, Kirkehei I, Dalsbø TK, Paulsen E. Risks to patient safety associated with implementation of electronic applications for medication management in ambulatory care: a systematic review. BMC Med Inform Decis Mak. 2013 Dec 5;13:133. doi: 10.1186/1472-6947-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pruszydlo MG, Walk-Fritz SU, Hoppe-Tichy T, Kaltschmidt J, Haefeli WE. Development and evaluation of a computerised clinical decision support system for switching drugs at the interface between primary and tertiary care. BMC Med Inform Decis Mak. 2012 Nov 27;12:137. doi: 10.1186/1472-6947-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. doi: 10.1038/s41746-020-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell CM, Jalali A, Mensah E. A decision support tool for using an ICD-10 anatomographer to address admission coding inaccuracies: a commentary. Online J Public Health Inform. 2013;5((2)):222. doi: 10.5210/ojphi.v5i2.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haberman S, Feldman J, Merhi ZO, Markenson G, Cohen W, Minkoff H. Effect of clinical-decision support on documentation compliance in an electronic medical record. Obstet Gynecol. 2009 Aug;114((2 Pt 1)):311–7. doi: 10.1097/AOG.0b013e3181af2cb0. [DOI] [PubMed] [Google Scholar]
- 52.Algaze CA, Wood M, Pageler NM, Sharek PJ, Longhurst CA, Shin AY. Use of a checklist and clinical decision support tool reduces laboratory use and improves cost. Pediatrics. 2016 Jan;137((1)):e20143019. doi: 10.1542/peds.2014-3019. [DOI] [PubMed] [Google Scholar]
- 53.Gilmer TP, O'Connor PJ, Sperl-Hillen JM, Rush WA, Johnson PE, Amundson GH, et al. Cost-effectiveness of an electronic medical record based clinical decision support system. Health Serv Res. 2012 Dec;47((6)):2137–58. doi: 10.1111/j.1475-6773.2012.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anchala R, Kaptoge S, Pant H, Di Angelantonio E, Franco OH, Prabhakaran D. Evaluation of effectiveness and cost-effectiveness of a clinical decision support system in managing hypertension in resource constrained primary health care settings: results from a cluster randomized trial. J Am Heart Assoc. 2015 Jan 5;4((1)):e001213. doi: 10.1161/JAHA.114.001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Reilly D, Holbrook A, Blackhouse G, Troyan S, Goeree R. Cost-effectiveness of a shared computerized decision support system for diabetes linked to electronic medical records. J Am Med Inform Assoc. 2012 May;19((3)):341–5. doi: 10.1136/amiajnl-2011-000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalaba MA, Akweongo P, Aborigo RA, Saronga HP, Williams J, Blank A, et al. Cost-effectiveness of clinical decision support system in improving maternal health care in Ghana. PLoS One. 2015;10((5)):e0125920. doi: 10.1371/journal.pone.0125920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saronga HP, Duysburgh E, Massawe S, Dalaba MA, Wangwe P, Sukums F, et al. Cost-effectiveness of an electronic clinical decision support system for improving quality of antenatal and childbirth care in rural Tanzania: an intervention study. BMC Health Serv Res. 2017 Aug 7;17((1)):537. doi: 10.1186/s12913-017-2457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017 Apr 12;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molenaar S, Sprangers MA, Rutgers EJ, Luiten EJ, Mulder J, Bossuyt PM, et al. Decision support for patients with early-stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life. J Clin Oncol. 2001 Mar 15;19((6)):1676–87. doi: 10.1200/JCO.2001.19.6.1676. [DOI] [PubMed] [Google Scholar]
- 60.Marcos M, Maldonado JA, Martínez-Salvador B, Boscá D, Robles M. Interoperability of clinical decision-support systems and electronic health records using archetypes: a case study in clinical trial eligibility. J Biomed Inform. 2013 Aug;46((4)):676–89. doi: 10.1016/j.jbi.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Kopanitsa G. Integration of hospital information and clinical decision support systems to enable the reuse of electronic health record data. Methods Inf Med. 2017 May 18;56((3)):238–47. doi: 10.3414/ME16-01-0057. [DOI] [PubMed] [Google Scholar]
- 62.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006 Mar;13((2)):138–47. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham KC, Cvach M. Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010 Jan;19((1)):28–35. doi: 10.4037/ajcc2010651. [DOI] [PubMed] [Google Scholar]
- 64.Rayo MF, Moffatt-Bruce SD. Alarm system management: evidence-based guidance encouraging direct measurement of informativeness to improve alarm response. BMJ Qual Saf. 2015 Apr;24((4)):282–6. doi: 10.1136/bmjqs-2014-003373. [DOI] [PubMed] [Google Scholar]
- 65.Valenta AL, Meagher EA, Tachinardi U, Starren J. Core informatics competencies for clinical and translational scientists: what do our customers and collaborators need to know? J Am Med Inform Assoc. 2016 Jul;23((4)):835–9. doi: 10.1093/jamia/ocw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wadden JJ. Defining the undefinable: the black box problem in healthcare artificial intelligence. J Med Ethics. 2021 Jul 21; doi: 10.1136/medethics-2021-107529. [DOI] [PubMed] [Google Scholar]
- 67.Price WN. Big data and black-box medical algorithms. Sci Transl Med. 2018 Dec 12;10((471)):10. doi: 10.1126/scitranslmed.aao5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willett DL, Kannan V, Chu L, Buchanan JR, Velasco FT, Clark JD, et al. SNOMED CT concept hierarchies for sharing definitions of clinical conditions using electronic health record data. Appl Clin Inform. 2018 Jul;9((3)):667–82. doi: 10.1055/s-0038-1668090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Clercq PA, Hasman A, Blom JA, Korsten HH. Design and implementation of a framework to support the development of clinical guidelines. Int J Med Inform. 2001 Dec;64((2–3)):285–318. doi: 10.1016/s1386-5056(01)00189-7. [DOI] [PubMed] [Google Scholar]