Abstract
Background
SARS-CoV-2 vaccination has started worldwide, including Japan. Although high rates of vaccine response and adverse reactions of BNT162b2 vaccine have been reported, knowledge about the relationship between sex differences and antibody response is limited. Furthermore, it is uncertain whether adverse reactions are associated with the vaccine response.
Methods
This prospective observational study included 673 Japanese participants working in a medical school and its affiliated hospital in Tokyo, Japan (UMIN000043340). Serum samples were collected before the first dose and three weeks after the second dose of BNT162b2 vaccine, and antibody titers against the receptor-binding domain of the spike protein of SARS-CoV-2 were measured. Answers to questionnaires about background characteristics and adverse reactions were obtained at the time of sample collection, and the relationship between antibody titers was analyzed.
Results
After excluding participants who did not complete receiving two doses of vaccination or two series of serum sample collection, 646 participants were analyzed. Although all participants became sero-positive after vaccination, antibody titers were highly variable among individuals (260.9–57,399.7A U/mL), with a median titer of 13478.0AU/mL. Mean titer was higher in females than in males and higher in young (≤45 years old) participants than in aged (>45 years old) participants. Participants who experienced adverse reactions demonstrated a higher antibody titer after vaccination than those without adverse reactions. Multivariable analysis demonstrated that young age, female sex, and adverse reactions after the second dose were independently related to higher antibody titers after the second dose.
Discussion
A favorable antibody response was observed after two doses of BNT162b2 vaccination among mostly healthy Japanese participants, especially among female and young participants. Although further investigation is essential, our results imply that the systemic adverse reactions (i.e., fever and general fatigue) are associated with a higher antibody response that indicates the acquisition of humoral immunity.
Keywords: SARS-CoV-2 vaccination, Systemic adverse reactions, Antibody titer
1. Introduction
The coronavirus disease (COVID-19) pandemic continues to affect the health of the global population, as well as the world economy. Vaccination is the key method to combat the pandemic. The Pfizer-BioNTech BNT162b2 mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine is one of the newly developed SARS-CoV-2 vaccines using the messenger RNA coding spike protein of SARS-CoV-2 and has demonstrated dramatic efficacy in clinical trials [1], [2] and the real-world [3], [4]. In Japan, although the BNT162b2 vaccine was approved in February 2021, the vaccination rate remains low after approval according to the limited number of vaccines and human resources, as well as a poor logistic system [5]. Therefore, only a few studies have been conducted on vaccination responses in the Japanese population. It is of considerable interest to study whether high vaccination efficacy can be obtained in the Japanese population as observed relative to other populations.
While the severity of COVID-19 is thought to be related to age, sex, and obesity [6], [7], [8], it is uncertain whether these factors are also related to vaccination responses. Furthermore, it has been reported that the rate of adverse reactions is high after SARS-CoV-2 vaccination, including BNT162b2 vaccination [9]. However, the relationship between immune responses to vaccination and adverse reactions remains to be elucidated. Oyebanji et al reported the relationship between post-vaccination reactions and high antibody titers [10], while Hwang et al reported no association [11] and Held et al demonstrated the relationship was weak [12]. Thus, larger cohort studies are required to clarify the relationship between immune responses following vaccination and the adverse effects of vaccines. As the first step to explore vaccination efficacy and adverse reactions, we focused on antibody responses in the early phase after vaccination. Vaccination efficacy is represented by the prevention rate for COVID-19, which results from humoral immunity and cellular immunity acquired by vaccination. In addition, it is possible that some adverse reactions may be caused by immune reactions related to vaccination. Antibody responses in the early phase are expected to provide suggestive information regarding efficacy and adverse reactions.
We conducted a prospective observational study to assess the factors affecting antibody responses to BNT162b2 vaccination and whether the occurrence of adverse reactions is associated with antibody responses in the Japanese population. We hypothesized that antibody responses to the BNT162b2 vaccination may be related to age, sex and adverse reactions.
2. Material and methods
2.1. Study population
From February 16, 2021, to March 9, 2021, Japanese health care workers and university staff of Keio University Shinanomachi Campus (Tokyo, Japan), who were vaccinated against SARS-CoV-2, were recruited for the present study. The campus has a university hospital with 960 beds and a medical school. Before mass vaccination, written informed consent was obtained from all participants. The study design was approved by the ethics committee of the Keio University School of Medicine (Project authorization No. 20200330). Mass vaccination was carried out using BNT162b2 vaccines (COMIRNATY® intramuscular injection, Pfizer, New York, USA), which were stored and prepared according to the package insert. Each person underwent two doses of vaccination, three weeks apart.
2.2. Sampling and measurement of antibody titers
Serial serum samples from each participant were collected, as described below. The pre-vaccination samples were collected before or on the same day as the first dose of vaccination. Post-vaccination samples were collected between April 15 and April 28, 2021, approximately 3 weeks after the second dose. Immediately after sample collection, anti-SARS-CoV-2 spike protein S1 subunit receptor-binding domain (RBD) antibody titers were measured using SARS-CoV-2 IgG II Quant reagents (Abbott Laboratories, Illinois, USA) and Alinity Analyzer i 1000SR (Abbott Laboratories, Illinois, USA) according to the manufacturer’s instructions [13]. The manufacturer’s cut-off of the reagents is 50AU/mL.
2.3. Questionnaires
Answers to questionnaires were obtained from each participant at the time of sampling. At the pre-vaccination sampling, an inquiry into age, sex, height, body weight, use of systemic steroids or other immunosuppressants, ongoing cancer chemotherapy, and history of immunodeficiency, cancers, autoimmune diseases, diabetes, COVID-19, COVID-19-like illness, and close contact with COVID-19 patients was made. At the post-vaccination sample collection, the dates of the first and second doses of BNT162b2 vaccination and adverse reactions after each vaccination were recorded. Questions about adverse reactions were as follows: 1. Have you experienced any symptoms feeling not well after vaccination? (For participants who answered “yes” to the first question); 2. Please select all the symptoms applicable for you from fever, gastrointestinal symptoms (nausea, vomiting, or diarrhea), local reactions (ex. pain, swelling, reddishness), general fatigue, and others (free description), and 3. According to the symptoms after vaccination, how much was your quality of daily living affected? (3 choices displayed: not so much affected, somehow affected (ex. efficacy of the job was reduced), and largely affected (ex. ill in bed)). At the time of the second sample collection, participants were also able to answer questionnaires on web forms a few days before sample collection. The date of vaccination was validated according to the description of BNT162b2 vaccination in participants’ medical records, and the interval between the second dose of vaccination and post-vaccination sample collection was calculated.
2.4. Statistical analysis
Participants who did not receive two series of vaccinations and those who did not cooperate with pre- and post-vaccination sample collection were excluded from the statistical analysis set.
Summary statistics of the participants were constructed using frequencies and proportions for categorical data, and mean and standard deviation (SD) for continuous variables. We compared the participant characteristics according to sex and age group using Fisher’s exact test for categorical values and Student’s t-test for continuous variables. Participants were divided into two groups according to the age of 45 years (median age of overall participants); the young group were 45 years or younger and the aged group were older than 45 years. In addition, the association between episodes of adverse reactions of the first and second doses and participant characteristics were analyzed.
To determine the factors that affected the antibody response after vaccination, antibody titers of the second sample were compared according to patient characteristics such as sex, age group, BMI, and history of COVID-19. Additionally, to investigate whether adverse reactions were related to vaccination response, antibody titers of post-vaccination samples were compared according to the episode of adverse reactions after each dose.
Finally, to identify factors independently associated with post-vaccination antibody titers, analysis of covariance (ANCOVA) was performed using the least-squares method. The model consisted of five categorical values (sex, aged or young, history of COVID-19, episode of adverse events after the first dose, and adverse events after the second dose) and three continuous values (pre-vaccination antibody titers, days after vaccination, BMI). All statistical analyses were performed using JMP, version 15 and SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set at p < 0.05.
3. Results
3.1. Study participants
Of the 673 health care workers and university staff included in the present study, 23 participants who did not cooperate with post-vaccination sample collection and four participants who did not complete two doses of BNT162b2 vaccination were excluded from the analysis. Finally, 646 participants were analyzed, of which 462 were female and 184 were male. Mean participant age was 45 years. Summary characteristics of the participants are presented in Table 1 . Nine participants reported a history of COVID-19. Antibody titers of pre-vaccination samples were 9.8 ± 106.1AU/mL (Supplement Fig. S1a).
Table 1.
Characteristics of the study participants.
| All n = 646 |
Male n = 184 |
Female n = 462 |
p value | Young (≤45 y) n = 344 |
Aged (>45 y) n = 302 |
p value | |
|---|---|---|---|---|---|---|---|
| Age, years | 44.1 ± 10.8 | 43.6 ± 11.4 | 44.2 ± 10.6 | 0.470 | – | – | – |
| Male | 184 (28.5) | – | – | – | 108 (31.4) | 76 (25.2) | 0.082 |
| Female (%) | 462 (71.5) | – | – | – | 236 (68.6) | 226 (74.8) | |
| BMI, kg/m2 | 23.4 ± 17.5 | 23.1 ± 3.0 | 21.4 ± 3.1 | <0.001 | 21.3 ± 3.0 | 22.5 ± 3.2 | <0.001 |
| Systemic steroid use | 7 (1.1) | 0 (0) | 7 (1.5) | 0.201 | 1 (0.3) | 6 (0.2) | 0.055 |
| Other immunosuppressant use | 11 (1.7) | 1 (0.5) | 10 (2.2) | 0.193 | 3 (0.9) | 8 (2.7) | 0.125 |
| Undergoing chemotherapy (%) | 0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| History of immunodeficiency (%) | 1 (0.2) | 1 (0.5) | 0 (0) | 0.285 | 1 (0.3) | 0 (0) | 1.000 |
| History of malignancy (%) | 12 (1.9) | 3 (1.6) | 9 (2.0) | 1.000 | 4 (1.2) | 8 (2.7) | 0.243 |
| History of autoimmune diseases (%) | 17 (2.6) | 1 (0.5) | 16 (0.4) | 0.052 | 6 (1.7) | 11 (3.6) | 0.147 |
| History of diabetes (%) | 4 (0.6) | 1 (0.5) | 3 (0.7) | 1.000 | 0 (0) | 4 (1.3) | 0.047 |
| History of COVID-19 (%) | 9 (1.4) | 3 (1.6) | 6 (1.3) | 0.720 | 6 (1.7) | 3 (1.0) | 0.514 |
| History of COVID-19-like illness (%) | 60 (9.3) | 16 (8.7) | 44 (9.5) | 0.881 | 39 (11.3) | 21 (7.0) | 0.059 |
| History of close contact with COVID-19 patients (%) | 18 (2.8) | 4 (2.2) | 14 (3.0) | 0.792 | 8 (2.3) | 10 (3.3) | 0.480 |
| Interval between the 2nd dose of vaccination and post-vaccination sample collection, days | 21.2 ± 1.6 | 21.4 ± 1.8 | 21.2 ± 1.6 | 0.082 | 21.3 ± 1.7 | 21.1 ± 1.5 | 0.126 |
BMI, body mass index; COVID-19, coronavirus disease
3.2. Antibody titers of post-vaccination samples
After two doses of BNT162b2 vaccination, all the analyzed participants became seropositive. The mean antibody titers were 15,463.9 ± 9,560.5 AU/mL (maximum: 57,399.7 AU/mL, minimum: 260.9 AU/mL) and median titers after the two doses were 13,478.0AU/mL (In quartile range: 8,482.8–20,560.0AU/mL).
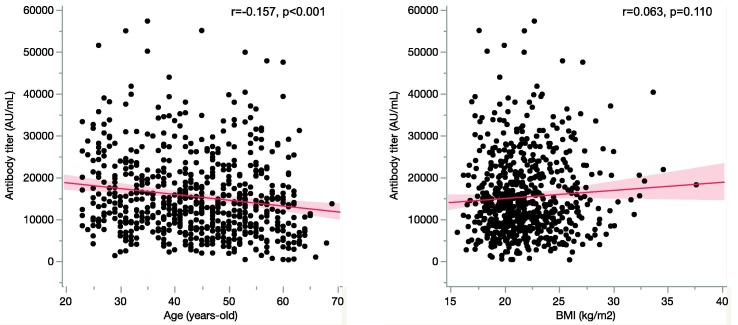
Mean antibody titers were higher in female participants than in male participants (16,272.0 ± 9,721.2AU/mL vs. 13,434.7 ± 8,849.0AU/mL, p < 0.001) and higher in young participants than in aged participants (16,562.1 ± 9,745.1 AU/mL vs. 14,212.9 ± 9,203.1 AU/mL, p = 0.002) (Supplement Fig. S1b). Correlation analysis of age and post-vaccine antibody titer also demonstrated a negative correlation (r = −0.157, p < 0.001, Pearson’s correlation coefficient). However, no significant relationship were observed between BMI and post- vaccine antibody titer (r = 0.063, p = 0.110, Pearson’s correlation coefficient) (Fig. 1 ). Participants with COVID-19 history demonstrated higher antibody titers than other participants (Table 2 ).
Fig. 1.
Relationship between age, body mass index and antibody titer after the second dose of vaccination. Scatter plots showing the correlation between age and antibody titer, and the correlation between BMI and antibody titer. The red lines and shading represent the regression lines and 95% confidence intervals, respectively. BMI, body mass index; r, Pearson’s correlation coefficient. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Relationship between participant characteristics and vaccination responses.
| Number of patients (n = 646) |
Antibody titer of post-vaccine samples, AU/mL (mean ± SD) |
|||
|---|---|---|---|---|
| Sex | ||||
| Male | 184 | 13,434.7 ± 8,849.0 | p < 0.001 | |
| Female | 462 | 16,272.0 ± 9,721.2 | ||
| Age | ||||
| Young (≤45 y) | 344 | 16,562.1 ± 9,745.1 | p = 0.002 | |
| Aged (>45 y) | 302 | 14,212.9 ± 9,203.1 | ||
| Systemic steroid use | ||||
| No | 639 | 15,535.8 ± 9,533.2 | p = 0.068 | |
| Yes | 7 | 8,899.1 ± 10,549.4 | ||
| Other immunosuppressant use | ||||
| No | 635 | 15,526.0 ± 9,459.6 | p = 0.423 | |
| Yes | 11 | 11,879.0 ± 14,417.2 | ||
| History of immunodeficiency | ||||
| No | 645 | 15,472.6 ± 9,565.3 | P = 0.555 | |
| Yes | 1 | 9,815.8 | ||
| History of malignancy | ||||
| No | 634 | 15,362.9 ± 9,377.5 | P = 0.051 | |
| Yes | 12 | 20,795.1 ± 16,356.6 | ||
| History of autoimmune diseases | ||||
| No | 629 | 15,504.9 ± 9,521.6 | P = 0.508 | |
| Yes | 17 | 13,946.3 ± 11,124.2 | ||
| History of diabetes | ||||
| No | 642 | 15,465.9 ± 9,574.9 | P = 0.938 | |
| Yes | 4 | 15,139.9 ± 7,651.7 | ||
| History of COVID-19 | ||||
| No | 636 | 15,350.8 ± 9,465.4 | P = 0.051 | |
| Yes | 9 | 24,644.5 ± 12,151.1 | ||
| History of COVID-19-like illness | ||||
| No | 585 | 15,389.6 ± 9,563.2 | P = 0.453 | |
| Yes | 60 | 16,365.9 ± 9,548.4 | ||
| History of close contact with COVID-19 patients | ||||
| No | 627 | 15,384.8 ± 9,527.5 | P = 0.181 | |
| Yes | 18 | 18,813.0 ± 10,321.5 | ||
3.3. Adverse reactions
Of the total participants, 61.9% experienced adverse reactions after the first dose, while 81.7% experienced adverse reactions after the second dose (Table 3 ). The most common adverse reactions after the first dose were local reactions, while almost the same number of participants complained of local reactions, general fatigue, and fever after the second dose. Although one participant answered that she had anaphylaxis after the first dose, she was excluded from the analysis because she could not receive the second dose according to the contraindications of the vaccine. Younger age and female participants were likely to demonstrate more adverse reactions, especially after the second dose. Participants with adverse reactions after the second dose demonstrated approximately 1.5 times higher antibody titers than those without adverse reactions (16,276.29 ± 9,802.80 AU/mL vs. 11,989.69 ± 7,891.74 AU/mL, p < 0.001). In addition, the participants who experienced systemic reactions such as fever and general fatigue were likely to demonstrate higher antibody titers (Table 4 , Fig. S2).
Table 3.
Relationship between participant characteristics and adverse reactions.
| Adverse reactions after the 1st dose |
Adverse reactions after the 2nd dose |
|||||
|---|---|---|---|---|---|---|
| (−) | (+) | P value | (−) | (+) | P value | |
| Number of participants (%) | 245 (38.1) | 398 (61.9) | 115 (18.3) | 514 (81.7) | ||
| Age, years | 44.3 ± 11.3 | 43.9 ± 10.5 | 0.619 | 46.9 ± 11.0 | 43.5 ± 10.7 | 0.001 |
| Female sex (%) | 157 (64.1) | 303 (76.1) | 0.001 | 65 (56.5) | 384 (74.7) | <0.001 |
| BMI, kg/m2 | 21.8 ± 2.9 | 21.9 ± 3.3 | 0.698 | 22.2 ± 3.2 | 21.8 ± 3.1 | 0.211 |
| Pre-vaccination antibody titer, AU/mL | 5.5 ± 34.1 | 12.6 ± 132.5 | 0.409 | 9.5 ± 51.4 | 10.2 ± 116.4 | 0.951 |
| Systemic steroid use (%) | 2 (0.8) | 5 (1.3) | 0.714 | 0 (0) | 6 (1.2) | 0.598 |
| Other immuno-suppressant use (%) | 1 (0.4) | 10 (2.5) | 0.059 | 2 (1.7) | 8 (1.6) | 1.000 |
| History of immunodeficiency (%) | 0 (0) | 1 (0.3) | 1.000 | 0 (0) | 1 (0.2) | 1.000 |
| History of malignancy (%) | 6 (2.5) | 6 (1.5) | 0.388 | 3 (2.6) | 9 (1.8) | 0.467 |
| History of autoimmune diseases (%) | 5 (2.0) | 12 (3.0) | 0.615 | 2 (1.7) | 14 (2.72) | 0.749 |
| History of diabetes (%) | 2 (0.8) | 2 (0.5) | 0.638 | 1 (0.87) | 3 (0.58) | 0.555 |
| History of COVID-19 (%) | 3 (1.2) | 6 (1.5) | 1.000 | 3 (2.6) | 6 (1.2) | 0.213 |
| History of COVID-19-like illness (%) | 20 (8.2) | 39 (9.8) | 0.574 | 14 (12.3) | 44 (8.6) | 0.213 |
| History of close contact with COVID-19 patients (%) | 12 (4.9) | 6 (1.5) | 0.014 | 4 (3.5) | 14 (2.7) | 0.550 |
BMI, body mass index; COVID-19, coronavirus disease.
Table 4.
Relationship between adverse reactions and vaccination responses.
| Number of participants | Antibody titer of post-vaccine samples, AU/mL (mean ± SD) | |||
|---|---|---|---|---|
| Adverse reactions after the first dose | ||||
| All | ||||
| No | 245 | 14,727.2 ± 8,844.6 | P = 0.119 | |
| Yes | 398 | 15,941.2 ± 9,988.4 | ||
| Local reactions | ||||
| No | 289 | 14,957.1 ± 9,150.2 | p = 0.213 | |
| Yes | 354 | 15,904.4 ± 9,909.3 | ||
| General fatigue | ||||
| No | 506 | 15,243.8 ± 9,542.3 | p = 0.233 | |
| Yes | 137 | 16,346.1 ± 9,703.3 | ||
| GI symptoms | ||||
| No | 623 | 15,527.3 ± 9,520.3 | p = 0.473 | |
| Yes | 20 | 13,963.4 ± 11,462.5 | ||
| Fever | ||||
| No | 604 | 15,187.2 ± 9,270.8 | P = 0.002 | |
| Yes | 39 | 19,992.8 ± 12,820.5 | ||
| Quality of daily living affected | ||||
| Not so much | 161 | 15,541.3 ± 10,365.4 | P = 0.035 | |
| Some how | 213 | 15,696.5 ± 9,204.0 | ||
| Largely | 23 | 21,178.2 ± 13,095.7 | ||
| Adverse reactions after the second dose | ||||
| All | ||||
| No | 115 | 11,989.7 ± 7,891.7 | P < 0.001 | |
| Yes | 514 | 16,726.3 ± 9,802.8 | ||
| Local reactions | ||||
| No | 256 | 14,764.2 ± 9,043.0 | P = 0.116 | |
| Yes | 373 | 15,992.5 ± 9,978.7 | ||
| General fatigue | ||||
| No | 240 | 13,629.5 ± 9,463.3 | P < 0.001 | |
| Yes | 389 | 16,642.0 ± 9,548.1 | ||
| GI symptoms | ||||
| No | 582 | 15,359.8 ± 9,497.4 | P = 0.224 | |
| Yes | 47 | 17,136.8 ± 11,013.7 | ||
| Fever | ||||
| No | 342 | 12,957.9 ± 8,285.6 | P < 0.001 | |
| Yes | 287 | 18,513.0 ± 10,244.3 | ||
| Quality of daily living affected | ||||
| Not so much | 87 | 13,154.9 ± 8,424.5 | P < 0.001 | |
| Some how | 228 | 15,328.2 ± 9,138.4 | ||
| Largely | 199 | 18,727.2 ± 10,538.1 | ||
GI, gastrointestinal.
3.4. Relation between age, sex, adverse reactions, and antibody response
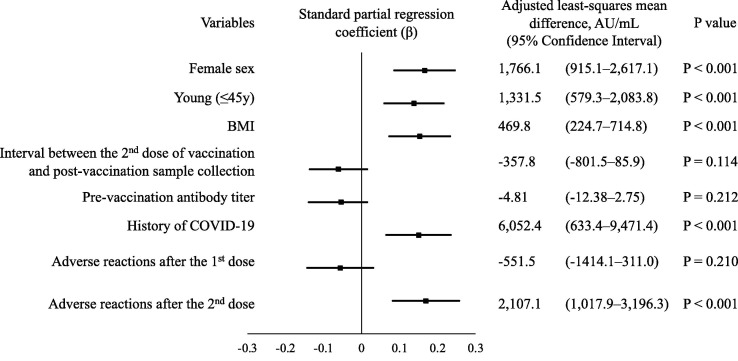
The ANCOVA model of eight variables demonstrated that female sex (Adjusted least-squares mean difference 1,766.1 AU/mL (95% CI; 915.1–2,617.1 AU/mL)), young age (1,331.5 AU/mL (95% CI; 579.3–2,083.8 AU/mL)), BMI (469.8 AU/mL (95% CI; 224.7–714.8 AU/mL)), history of COVID-19 (6,052.4 AU/mL (95% CI; 633.4–9,471.4 AU/mL)), and adverse reactions after the second dose (2,107.1 AU/mL (95% CI;1,017.9–3,196.3 AU/mL),were independently related to a high antibody titer after two doses of vaccination (Fig. 2 ). Pre-vaccination antibody titer, and adverse events after the first dose did not have statistically significant relationships.
Fig. 2.
Covariate analysis of antibody titer after the second dose of vaccination in relation to the participant background and adverse reactions. The ANCOVA model of eight variables was demonstrated. A dot and bar represented standardized partial regression coefficient β and 95% confidence interval for β. BMI, body mass index; COVID-19, coronavirus disease.
4. Discussion
The messenger RNA vaccine was put to practical use first in SARS-CoV-2 vaccination, which demonstrated a high vaccination response compared to other conventional forms of inactivated vaccines [2]. The robust response to BNT162b2 vaccine was also observed in our study: All the participants became seropositive after two doses of the vaccination, and the mean titer of the antibodies was higher than the highest titer of pre-vaccination samples collected from the participants with COVID-19 histories (2,606.5 AU/mL). Therefore, according to our study results, the BNT162b2 vaccine was demonstrated to have sufficient immunogenicity among mostly healthy Japanese populations.
In this study, participants with a history of COVID-19 had higher antibody responses after vaccination than those without a history of COVID-19, as was found in a previous study [14]. In addition, younger age and female sex were associated with higher antibody titers after vaccination compared to older aged and male participants, respectively. Although it is unclear whether the antibody titer is directly related to protective effects against SARS-CoV-2 infections because the antibody we measured did not guarantee neutralization activity, it is reasonable to consider that a higher humoral response after exposure to the spike protein of SARS-CoV-2 could occur in young and female populations. This is compatible with the findings of a previous study demonstrating that aging decreased antibody response among COVID-19 patients and the fact that aged people demonstrated weaker immunologic responses in COVID-19 vaccine trials [2], [15]. Although reports about sex differences according to vaccine response were limited, Takahashi et al. demonstrated that more robust T cell activation was observed in females than in males, which implies that a stronger immune response could occur in females after exposure to SARS-CoV-2 [16]. Additionally, Fink et al. demonstrated greater TLR7 activation and antibody production in female mice after influenza vaccination [17], which led to the understanding of higher antibody responses in females after vaccination. Bauernfeind et al. reported that antibody responses were higher in males, especially among those with severe adverse reactions [18]. However, the number of analyzed vaccinee was limited after matching, which might lead to the contrary results to our study.
Adverse reactions were observed in approximately 80% of participants in our study, while severe allergic reactions were observed in only one participant with anaphylaxis who was eliminated from the analysis. Young and female participants were likely to complain of adverse reactions more frequently, which is consistent with the findings of previous reports [2], [19]. Similar to previous reports [20], a higher frequency of adverse reactions, except for anaphylaxis, was observed after the second dose than after the first dose, supporting the hypothesis that adverse reactions are partially related to acquired immunity after the first dose. The fact that systemic reactions such as fever and general fatigue were more common after the second dose than after the first dose supports our hypothesis, although information on the antibody titer immediately before the second dose is required to confirm our hypothesis.
Thus, sex differences in the frequency of adverse reactions might not be apparent after the first dose, but sex differences after the second dose, according to a stronger immune response after vaccination among females.
Interestingly, our study results demonstrated higher antibody titers among the participants with adverse reactions after the second dose, which was independently statistically significant after multivariate analysis including age, sex, and BMI. This fact might be useful information for those who had adverse reactions after the BNT162b2 vaccine, because the adverse reactions after the second dose might be a sign of a better response to vaccination. According to our hypothesis about adverse reactions and acquired immunity by the BNT162b2 vaccination, it is easy to explain why the relation to the antibody titer was not observed with the adverse event after the first dose but was observed after the second dose. Although further studies are essential to make a firm conclusion, our new findings showing the association of higher antibody responses with adverse reactions after the second dose suggest the possibility that some adverse reactions such as fever and general fatigue after vaccination may reflect acquisition of the immunity against SARS-CoV-2. Therefore, the results might aid in promoting COVID-19 vaccination to people even if they fear experiencing the adverse reactions.
The present study had several limitations. First, the antibody titers measured was not neutralizing antibodies, which are directly related to protection against SARS-CoV-2 infections. However, the epitope of the antibody our quantitative reagents measured were the receptor-binding domain of the S1 subunit of the spike protein; therefore, we might expect that our results could be similar to that of the neutralizing antibody. Second, the use of questionnaires could not avoid recall bias, although the questionnaires were obtained within a few weeks after vaccination. Additionally, we did not use objective definition (for example, defining fever as a body temperature higher than 37.5 °C) of the symptoms of adverse reactions for participants when collecting the questionnaire data, therefore the answer might be subjective. Third, data on cellular immunity are lacking, which have important roles in natural infections [21]. Fourth, our participants were university campus staff, and most of them were younger than 65 years old, which is the retirement age of the university. Therefore, it is uncertain whether people older than 65 years demonstrate the same trends as those of our study. Fifth, most of the participants in this study were Japanese. According to the homogenous nature of the study population, the generalizability of the results might be limited due to the potential racial or geographical differences in immune reactions. Finally, our observations were limited to the antibody response after three weeks of vaccination, which might be the peak of the antibody response after exposure to SARS-CoV-2 components [22]. To overcome these limitations, a longitudinal observation of the cohort including cellular immunity and neutralization antibody is essential, which are now ongoing in our study groups in addition to these preliminary results.
5. Conclusions
In conclusion, an observational study of over 600 healthy Japanese cohorts revealed sufficient antibody response after two doses of BNT162b2 vaccination, which were related to younger age, female sex, and adverse reactions after the second dose, suggesting that adverse reactions after the second dose might reflect acquisition of the immunity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank the staff of Keio University Shinanomachi Campus for participating in the study.
Funding
This study was funded by the Research Funds of the Keio University School of Medicine and Grant from Public Foundation of the Vaccination Research Center, Japan.
Author contribution
YU conceived and designed the study. YU, AS, AT, TA, AO, WY, and MW recruited the participants. TK, YT, AS, AT, YY, MN, TA, and AO collected the data. YU, YS, MW, and MM analyzed and interpreted the data; YU wrote the manuscript. YS, HY, SU, TN, NH, HS, MW, and MM discussed the data and critically reviewed and revised the manuscript. All authors approved the final version of the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.01.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White E.M., Yang X., Blackman C., Feifer R.A., Gravenstein S., Mor V. Incident SARS-CoV-2 Infection among mRNA-Vaccinated and Unvaccinated Nursing Home Residents. N Engl J Med. 2021;385(5):474–476. doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japan-Cabinet-Public-Relations-Office. Novel Coronavirus Vaccines.
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapin-Bardales J., Gee J., Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA. 2021;325(21):2201. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 10.Oyebanji O.A., Wilson B., Keresztesy D., Carias L., Wilk D., Payne M., et al. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin Exp Res. 2021;33(11):3151–3160. doi: 10.1007/s40520-021-01987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang Y.H., Song K.-H., Choi Y., Go S., Choi S.-J., Jung J., et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med. 2021;36(6):1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Held J., Esse J., Tascilar K., Steininger P., Schober K., Irrgang P., et al. Reactogenicity correlates only weakly with humoral immunogenicity after COVID-19 vaccination with BNT162b2 mRNA (Comirnaty(®)) Vaccines (Basel) 2021;9(10):1063. doi: 10.3390/vaccines9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instructions for Use SARS-CoV-2 IgG II Quant Reagent for Abbott Alinity i.
- 14.Anichini G., Terrosi C., Gandolfo C., Gori Savellini G., Fabrizi S., Miceli G.B., et al. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N Engl J Med. 2021;385(1):90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink A.L., Engle K., Ursin R.L., Tang W.-Y., Klein S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A. 2018;115(49):12477–12482. doi: 10.1073/pnas.1805268115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauernfeind S., Salzberger B., Hitzenbichler F., Scigala K., Einhauser S., Wagner R., et al. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines (Basel) 2021;9(10):1089. doi: 10.3390/vaccines9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae S., Lee Y.W., Lim S.Y., Lee J.-H., Lim J.S., Lee S., et al. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J Korean Med Sci. 2021;36(17) doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir Med. 2021;9(11):1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamayoshi S., Yasuhara A., Ito M., Akasaka O., Nakamura M., Nakachi I., et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32:100734. doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.