Abstract
To validate the accuracy of rapid tests for identification of Escherichia coli, five laboratories sequentially collected 1,064 fresh, clinically significant strains with core criteria of indole-positive, oxidase-negative, nonspreading organisms on sheep blood agar plates (BAP), having typical gram-negative rod plate morphology, defined as good growth on gram-negative rod-selective media. An algorithm using beta-hemolysis on BAP, lactose reaction on eosin-methylene blue or MacConkey agar, l-pyrrolidonyl-β-naphthylamide (PYR), and 4-methylumbelliferyl-β-d-glucuronide (MUG) was evaluated. Identifications using the algorithm were compared to those obtained using commercial kit system identifications. One thousand strains were E. coli and 64 were not E. coli by kit identifications, which were supplemented with conventional biochemical testing of low probability profiles. Of the 1,064 isolates meeting the core criteria, 294 were beta-hemolytic and did not require further testing to be identified as E. coli. None of the 64 non-E. coli strains were hemolytic, although other indole-positive, lactose-negative species were found to be hemolytic when further strains were examined in a follow-up study. Of the remaining strains, 628 were identified as E. coli by a lactose-positive and PYR-negative reaction. For nonhemolytic, lactose-negative E. coli, PYR was not helpful, but a positive MUG reaction identified 65 of 78 isolates as E. coli. The remaining 13 E. coli strains required kit identifications. This scheme for E. coli identification misidentified three non-E. coli strains as E. coli, for an error rate of 0.3%. A total of 13 kit identifications, 657 PYR tests, and 113 MUG tests were needed to identify 1,000 E. coli strains with the algorithm. The use of this rapid system saves laboratory resources, provides timely identifications, and yields rare misidentifications.
In 1963, Vracko and Sherris (14) reported the use of a spot indole test for the rapid identification of members of the family Enterobacteriaceae. Since then, many studies using rapid testing have been published (1, 2, 5–9, 13), but the use of rapid methods for the identification of Escherichia coli has not been validated in a systematic manner with large numbers of strains from different geographic areas. E. coli bacteria are among the few species of lactose (LAC)-positive, oxidase-negative, gram-negative rods that are indole positive. Due to the infrequent isolation of non-E. coli strains that are indole positive, the spot indole test has been used for the rapid, presumptive identification of E. coli. Although the test is not used for LAC-negative isolates, the error rate in the clinical laboratory associated with using this one rapid test alone for the identification of LAC-positive E. coli is not known. Rapid hydrolysis of 4-methylumbelliferyl-β-d-glucuronide (MUG) is also characteristic of E. coli (5, 7, 8, 9, 11, 13). However, not all E. coli strains are MUG positive (7, 8, 10, 12), and occasionally other organisms are also MUG positive (12). E. coli does not hydrolyze l-pyrrolidonyl-β-naphthylamide (PYR), while other LAC-positive Enterobacteriaceae are PYR positive (2, 6). Using spot indole, MUG, and PYR reactions, five laboratories in different geographic areas participated in a study to differentiate E. coli from other Enterobacteriaceae by comparing results from rapid identification methods to those obtained by standard commercial multicomponent kits. The goal was to find and validate an algorithm that could be used to accurately identify this common organism in a clinically useful time frame at low cost.
(A preliminary report of this work was presented previously [M. K. York, E. J. Baron, J. E. Clarridge, R. B. Thomson, and M. P. Weinstein, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. C-447, p. 197, 1999].)
MATERIALS AND METHODS
Strains.
Each of five laboratories in different cities (San Francisco, Calif.; Stanford, Calif.; Houston, Tex.; Evanston, Ill.; and New Brunswick, N.J.) collected sequential fresh clinical isolates meeting the core criteria of indole-positive, oxidase-negative, nonspreading gram-negative rods, until each had identified at least 200 as E. coli by the commercial kit system in use in that laboratory. The selection process resulted in the collection of 1,064 strains which met the core criteria. Isolates that were considered clinically significant by the laboratory policies of each site, except those from stool cultures, were included in the study. Duplicates from the same patient were avoided; 939 isolates were from urine cultures. After the completion of the study, laboratory A collected as many clinical strains as possible that were not identified as E. coli by a commercial kit but were indole positive, oxidase negative and LAC negative, to further validate the accuracy of the initial study. These isolates had low representation in the initial study but could be confused with E. coli.
Laboratory testing.
Selection of strains meeting the core criteria, observations, and testing were done from the initial plates (blood agar with 5% sheep blood [blood agar plate (BAP)] and a gram-negative rod-selective agar) inoculated with the patient specimen, unless the presence of other flora precluded accurate testing. In the latter case, testing was performed from a subculture of the isolate such that observations could be made on both BAP and the selective medium. Determination of typical gram-negative rod plate morphology and fermentation of LAC was observed as good growth and characteristic color, respectively, on gram-negative rod-selective medium, either eosin-methylene blue (labs A and E) or MacConkey agar (labs B, C, and D). All other testing, including observation of beta-hemolysis (HEM), was performed on BAP. Any zone of clearing of the blood around the colony, but not clearing limited to that under the colony, was considered positive for HEM. Each of the 1,064 isolates was identified to species level using commercial kits in each respective laboratory: Vitek GNR (bioMerieux Vitek, Inc., Hazelwood, Mo.) (labs A and C), RapID One E (Innovative Diagnostics, Remel, Inc., Lenexa, Kans.) (lab B), API 20E (bioMerieux Vitek, Inc.) (lab D), and MicroScan Gram-Negative Breakpoint Combo Overnight panels (type 12; Dade-MicroScan, West Sacramento, Calif.) (lab E). For indole production, either 5% p-dimethylaminobenzaldehyde (14) or 1% paradimethylaminocinnamaldehyde (1) in 10% (vol/vol) concentrated HCl was poured onto filter paper. A colony >5 mm from the nearest colonies of differing morphology on a sheep BAP <24 h old was applied to the paper, and red or blue color development, respectively, was observed. For oxidase production, tetramethyl-p-phenylenediamine dihydrochloride in water was poured onto filter paper and allowed to dry. A colony from the BAP was smeared onto the paper. Development of a blue color in 10 s was considered a positive reaction. Hydrolysis of PYR was detected by inoculating a water-moistened substrate disk (Remel, Inc.) with fresh growth from the BAP. After 2 min, the developer (Remel) was added. A red color was considered a positive reaction.
For MUG testing, MUG (Sigma, St. Louis, Mo.) was dissolved in 0.05 M Sorensen's phosphate buffer (pH 7.5) to a final concentration of 31.25 μg/ml. Disks were prepared by adding 1.25 ml of MUG reagent to a vial of 50 sterile 6-mm-diameter filter paper disks (Becton Dickinson Microbiology Products, Cockeysville, Md.), to approximately 25 μl per disk. The disks were thoroughly saturated, then air dried, and stored at −20°C until use. This method yielded an approximate final concentration of 0.8 μg/disk. Disks were moistened with water and inoculated with fresh growth from the BAP. After 2 h of incubation at 35°C, the disk was observed under UV (360-nm) light. An intense blue fluorescence was considered a positive reaction. Weak reactions were interpreted as negative.
All testing was performed in the laboratory of origin of the isolate. The kit identification was considered the final identification, unless it contradicted the expected PYR or MUG result or the probability of the identification was below the accepted level for the laboratory using the kit. Identifications with low probability were confirmed by repeating the identification kit test, by using an alternative kit method, or by using standard biochemical tests (3), depending on the practice within each laboratory. If the identification was inconsistent with the rapid results, the strains were sent to laboratory A for further testing using one or more of the above methods.
Evaluation.
Test results for each isolate were recorded on separate work cards, which listed entries for LAC, HEM, indole, oxidase, MUG, PYR, the kit identification code number, and the likelihood of the identification. Space was provided for all repeat testing, for further testing, and for the final identification, as reported in the patient report. To ensure patient confidentiality, patient names were omitted, but each laboratory was asked to provide verification that the isolate did not represent a duplicate from the same patient. Obvious clerical errors and missing results were corrected after communication with the respective laboratory. If results were discrepant with the expected rapid results and the strain was available, it was retested in all five laboratories, and if all five laboratories agreed on the result, the retest was counted as the evaluable result. If the strain could not be retested because it was not available or all the laboratories did not agree on the result, the original result was used in the final evaluation.
RESULTS
Accuracy and reproducibility.
Each laboratory was sent two separate shipments of indole-positive, oxidase-negative gram-negative rods to verify that the results achieved were the same regardless of the media, reagents, and kits used by laboratories in different geographic areas. There were 10 strains in the first shipment and 12 in the second, with four E. coli strains duplicated in the second set. Strains were Morganella morganii (2 strains), Kluyvera spp., Citrobacter diversus, Proteus vulgaris, Providencia stuartii, Klebsiella oxytoca (2 strains), and E. coli (10 strains including 1 E. coli O157 strain). Oxidase, PYR, and indole results were highly reproducible, showing 100% agreement among all the laboratories. For LAC fermentation, HEM, and MUG, the results were variable, with 4, 3, and 7 of the 110 results for each test differing from the majority result, respectively. None of the discrepant results would have resulted in an erroneous identification by the rapid methods. All discrepant results were in agreement with expected variable reactions for the species. The results for LAC fermentation and HEM were in agreement at all sites for the second sample testing, after notification that the initial results had varied by site. The MUG test results continued to be variable by site in the second testing, despite the fact that all laboratories were using the same lot of MUG disks.
Consecutive sample testing.
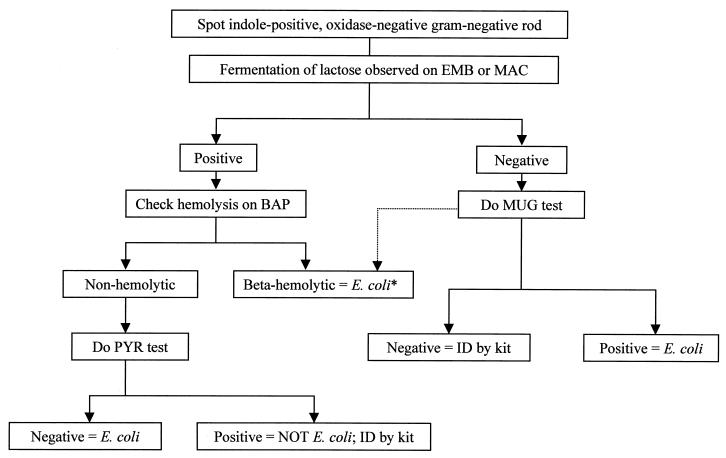
A total of 1,064 isolates of indole-positive, oxidase-negative gram-negative rods were tested; 1,000 were identified as E. coli. Key reactions are listed in Table 1. From these results, an algorithm was developed (Fig. 1), which was then applied to the strains to see if the E. coli strains could be rapidly and accurately identified. Confirmatory kit identifications to verify the identification were 237 by API, 410 by Vitek, 288 by Dade-MicroScan, and 125 by Innovative Diagnostics Systems. Kit identifications—for strains that were ultimately identified as E. coli—failed to provide an answer in 10 cases and gave an incorrect identification in 3 cases. In these latter cases, the negative PYR reaction indicated that the isolate could not be either Citrobacter or Enterobacter, as the kit identification indicated. Conventional tube biochemical testing verified the isolates as E. coli.
TABLE 1.
Organisms in study and numbers showing positive reactions
| Species | Total no. of strains | No. of positive reactions
|
|||
|---|---|---|---|---|---|
| PYR | MUG | LAC | HEM | ||
| Klebsiella oxytoca | 23 | 23 | 1a | 23 | 0 |
| Kluyvera species | 1 | 0b | 0 | 1 | 0 |
| Citrobacter species | 21 | 19a | 0 | 5 | 0 |
| Morganella morganii | 12 | 2 | 1b | 0 | 0 |
| Proteus vulgaris | 2 | 1 | 0 | 0 | 0 |
| Providencia species | 4 | 2 | 0 | 0 | 0 |
| Escherichia fergusonii | 1 | 0 | 1b | 0 | 0 |
| Escherichia coli | 1,000 | 0 | 932 | 894 | 294 |
Discordant results could not be confirmed because strains were discarded, but the accuracy of the algorithm was unaffected. Citrobacters with discordant PYR results were LAC negative.
Results represented misidentification with the algorithm.
FIG. 1.
Identification scheme for the separation of E. coli from other indole-positive, oxidase-negative gram-negative rods. See the text for an explanation of tests. EMB, eosin-methylene blue; MAC, MacConkey agar; ID, identification. ∗, LAC-negative, beta-hemolytic colonies could be M. morganii, E. tarda, or P. vulgaris. In some populations, these may all be E. coli (see text) and the MUG test is not needed, especially for isolates from noninvasion sites.
Application of the algorithm (Table 2) resulted in the correct identification of 987 of 1,000 E. coli strains without use of kits and the misidentification of 3 of the 64 non-E. coli isolates as E. coli (one strain each of Kluyvera spp., Escherichia fergusonii, and M. morganii).
TABLE 2.
Demonstration of application of algorithm to isolates in study (number of tests necessary to arrive at identification)
| Reactionc | No. of isolates
|
No. of tests required
|
No. of kits needed | ||
|---|---|---|---|---|---|
| E. coli | Non-E. coli | MUG | PYR | ||
| HEM-pos, LAC-pos | 266 | 0 | |||
| HEM-pos, LAC-neg, MUG-pos | 26 | 0 | 26b | ||
| HEM-pos, LAC-neg, MUG-neg | 2 | 0 | 2b | ||
| HEM-neg, LAC-pos, PYR-neg | 628 | 1a | 629 | ||
| HEM-neg, LAC-pos, PYR-pos | 0 | 28 | 28 | 28 | |
| HEM-neg, LAC-neg, MUG-pos | 65 | 2a | 67 | ||
| HEM-neg, LAC-neg, MUG-neg | 13 | 33 | 46 | 46 | |
| Total | 1,000 | 64 | 113 | 657 | 74 |
Resulted in misidentification as E. coli for a 0.3% error rate.
MUG testing would not be needed for these strains if beta-hemolytic M. morganii or P. vulgaris was not present in the local population.
pos, positive; neg, negative.
Although during the study no laboratory encountered a beta-hemolytic non-E. coli strain, laboratory A had previously seen beta-hemolytic M. morganii and P. vulgaris. In order to determine the extent of the presence of beta-hemolytic, indole-positive non-E. coli strains, laboratory A collected strains as follows: 24 of M. morganii (11 beta-hemolytic), 16 of P. vulgaris (2 beta-hemolytic and nonspreading), 17 of Providencia species (none were beta-hemolytic), and 8 of Edwardsiella tarda (all were beta-hemolytic). All strains were indole positive, oxidase negative, LAC negative, MUG negative, and PYR negative and would not have been called E. coli if the MUG test were performed. Laboratory B collected five strains of M. morganii, none of which were beta-hemolytic; however, two of the strains were PYR positive.
DISCUSSION
Rapid testing was analyzed to determine if indole-positive, oxidase-negative, LAC-positive isolates could be identified reliably as E. coli without further testing. The results from five laboratories indicate that the indole test is sufficient for hemolytic strains. For nonhemolytic strains, the number of misidentifications would be low (29 of 656 strains [4.4%]), representing 23 K. oxytoca strains, 5 Citrobacter sp. strains, and 1 Kluyvera sp. strain. The reproducibility studies indicated that there might be some observer variability in the ability to accurately observe colonies for hemolysis and LAC fermentation. If colony morphology could be accurately observed, E. coli strains are flat, dry, LAC-fermenting colonies on MacConkey agar and can be differentiated visually from the usual mucoid, LAC-fermenting colonies of Klebsiella species. With the use of MacConkey agar, laboratories could use colony characteristics, rather than the PYR test, to differentiate K. oxytoca from E. coli, although there would be 1% misidentifications (6 of 656 strains) representing the five Citrobacter sp. strains and the one Kluyvera sp. strain. Such an algorithm was not tested in this study but could be validated. However, even a 1% error rate might not be acceptable with isolates from blood and other normally sterile specimen sources. For these sources, the 2-min PYR test should be used with all LAC-positive, nonhemolytic strains. There was a concern that the identification of E. coli was based on a negative reaction, but in practice, the PYR test was both sensitive and reproducible.
For LAC-negative strains, 24% of the indole-positive strains were not E. coli, and one cannot rely on the indole reaction alone. However, since most of the PYR-negative microorganisms (Morganella, Providencia, and P. vulgaris) that may be misidentified are urea positive, a rapid 2-min urease test along with the PYR test could substitute for the 2-h MUG test. This option was not tested by the authors, because of the concern that other PYR-negative microorganisms (e.g., E. tarda and Shigella species) could also be called E. coli. These strains were not isolated in the study and are usually found only in stool specimens.
The rapid identification methods used in this study were compared to four different commercial systems, and it might be argued that the accepted identification of these strains was not truly accurate. However, the purpose of this work was to compare the rapid methods to the generally accepted identification methods routinely used by clinical laboratories. The use of different confirmatory methods and different media for LAC fermentation detection was deliberately chosen to access the greatest number of variables used in clinical laboratories. Because the identifications using colony morphology and spot tests were equivalent in accuracy to kit identifications, the use of the term “presumptive” for these identifications is not indicated.
Differences were seen in the phenotypic characteristics of strains recovered in different geographic areas. For example, one laboratory contributed almost no LAC-negative strains to the study. Another laboratory saw beta-hemolytic M. morganii strains, which were not found in other areas. The differences were not due to the media used in each laboratory, since each laboratory reported similar results when a series of test strains were submitted for validation of methodology. If beta-hemolytic M. morganii strains are known to be recovered by a laboratory, the MUG test should be used to avoid misidentifications.
Using the flow chart in Fig. 1, E. coli can be identified with 99.7% accuracy, which compares favorably with the identifications with commercial kits. Three species were misidentified as E. coli by rapid testing, compared to three E. coli strains that were misidentified as not E. coli using the commercial kits. Identifications by commercial kits had to be repeated because they failed to yield an identification in 10 cases. Identifications by commercial kits had to be performed in 13 cases after the rapid tests did not successfully identify the isolates. Thus, confirmatory testing was required at a similar frequency for both rapid and commercial kit methods.
The misidentification of a Kluyvera species as E. coli by the rapid method would have little or no clinical significance (4). Of concern was the misidentification of a strain of M. morganii because of a positive MUG reaction. For convenience, the MUG test was performed by the disk method in each laboratory, using the same lot of disks. The MUG disk test can be difficult to read, as evidenced from the differing results in the validation testing. Not only are tube MUG tests easier to read, but they can be performed with colonies from selective media, such as eosin-methylene blue and MacConkey agar (8). The reagent for the tube test is more concentrated than that used for the disk test (500 μg/ml) and is used by placing 2 drops of reagent in a Durham tube, which is incubated at 35°C for 2 h.
Using the algorithm described in Fig. 1, 1,000 E. coli strains were identified with a cost savings of over $3,000 in reagents, assuming that a kit identification costs $4 each or $4,256 (Table 3). This assumes that the rapid tests cost approximately $1.00 each; however, in-house-prepared reagents are much less expensive. If it is assumed that it takes 5 min to perform a commercial kit identification, the time saved with the rapid algorithm is 70 h of microbiologist time. The relative cost savings for any laboratory will depend on the utility of integrating the algorithm into that laboratory's work flow. For example, if the kit identification system used in the laboratory is combined with the susceptibility test system, the technologist time saved would not be realized by the rapid spot testing. The potential for benefit to the patient of immediate identification based on the rapid tests is unknown.
TABLE 3.
Cost of identification of 1,064 gram-negative rods by rapid-testing algorithm compared to commercial kit
| Type of test or value | Estimated cost per test of reagents in dollars (min) | No. of tests needed | Total reagent costa (dollars) | Estimated time in min (dollars)b | Total cost of reagents and labor (dollars) |
|---|---|---|---|---|---|
| PYR test | 1.00 (1) | 657 | 657 | 657 (315) | 972 |
| MUG test | 1.00 (1) | 113 + 28c | 141 | 113 + 28c (68) | 209 |
| Kits for E. coli and non-E. coli | 4.00 (5) | 77 | 308 | 385 (184) | 492 |
| Total for identification by rapid method | 1,106 | 1,183 (567) | 1,673 | ||
| Total for identification by kit method | 4.00 (5) | 1,064 | 4,256 | 5,320 (2,554) | 6,810 |
Assuming that the spot indole and oxidase tests are performed routinely regardless of identification method; thus, they are not included in the comparison.
Repeat testing was not included. Labor cost was $0.48/min based on the average of the costs in the five laboratories, including benefits.
Refer to Table 2 footnote b for an explanation of the 28 strains.
It was of interest that most of the isolates were from urine. Many of the same strains were later found in the blood of these patients, but only one isolate per patient was studied. This is not surprising, because the urinary tract is a common focus for subsequent bacteremia. While the algorithm presented can be used with great accuracy for LAC-positive isolates from urine or any other source, for LAC-negative isolates from normally sterile sites, a MUG test should always be performed (follow the dotted line in the algorithm in Fig. 1). Some Shigella species and an occasional other species have been reported to give a positive MUG reaction (12), and the MUG test is used to screen for E. coli O157 strains, which have a MUG-negative phenotype (12). Consequently, the algorithm would be misleading to use in the workup of E. coli in stool specimens.
In summary, these data validate the identification of E. coli based on the use of a small number of rapid tests (Fig. 1) with an accuracy of greater than 99%, which was equivalent to that for commercial kit identifications. Confirmatory kit testing was needed for less than 2% of isolates. Kit identifications performed on over 1,000 strains resulted in no identification for a similar number (2%) of isolates. The use of rapid testing resulted in a 75% reduction in cost of reagents and technologist time, with a decrease in time to reporting.
ACKNOWLEDGMENTS
We thank Barbara Allen, Kim Joho, Durdana Pirwan, Patricia Rodrigues-Wong, Judy Rothberg, Lynn Schwabe, and the staff of the respective microbiology laboratories for technical assistance with this work.
REFERENCES
- 1.Bale M J, McLaws S M, Matsen J M. The spot indole test for identification of swarming Proteus. Am J Clin Pathol. 1985;83:87–90. doi: 10.1093/ajcp/83.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Chagla A H, Borczyk A A, Aldom J E, Rosa S D, Cole D D. Evaluation of the l-pyrrolidonyl-β-naphthylamide hydrolysis test for the differentiation of members of the families Enterobacteriaceae and Vibrionaceae. J Clin Microbiol. 1993;31:1946–1948. doi: 10.1128/jcm.31.7.1946-1948.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer J J, III, Davis B R, Hickman-Brenner F W, McWhorter A, Huntley-Carter G P, Asbury M A, Riddle C, Wathen H G, Elias C, Fanning G R, Steigerwalt A G, O'Hara C M, Morris G K, Smith P B, Brenner D J. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21:46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer J J, III, Fanning G R, Huntley-Carter G P, Holmes B, Hickman F W, Richard C, Brenner D J. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J Clin Microbiol. 1981;13:919–933. doi: 10.1128/jcm.13.5.919-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng P C S, Hartman P A. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl Environ Microbiol. 1982;43:1320–1329. doi: 10.1128/aem.43.6.1320-1329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue K, Miki K, Tamura K, Sakazaki R. Evaluation of l-pyrrolidonyl peptidase paper strip test for differentiation of members of the family Enterobacteriaceae, particularly Salmonella spp. J Clin Microbiol. 1996;34:1811–1812. doi: 10.1128/jcm.34.7.1811-1812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iritani B, Inzana T J. Evaluation of a rapid tube assay for presumptive identification of Escherichia coli from veterinary specimens. J Clin Microbiol. 1988;26:564–566. doi: 10.1128/jcm.26.3.564-566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papasian C J, Hertlein G. Rapid identification of Escherichia coli with a fluorogenic beta-glucuronidase assay. Diagn Microbiol Infect Dis. 1987;8:255–258. doi: 10.1016/0732-8893(87)90058-7. [DOI] [PubMed] [Google Scholar]
- 9.Pérez J L, Berrocal C I, Berrocal L. Evaluation of a commercial β-glucuronidase test for rapid and economical identification of Escherichia coli. J Appl Bacteriol. 1986;61:541–545. doi: 10.1111/j.1365-2672.1986.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 10.Ratnam S, March S B, Ahmed R, Bezanson G S, Kasatiya S. Characterization of Escherichia coli serotype O157:H7. J Clin Microbiol. 1988;26:2006–2012. doi: 10.1128/jcm.26.10.2006-2012.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaller M C, Berlutti F, Dainelli B, Pezzi R. New plate medium for screening and presumptive identification of gram-negative urinary tract pathogens. J Clin Microbiol. 1988;26:791–793. doi: 10.1128/jcm.26.4.791-793.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson J S, Hodge D S, Borczyk A A. Rapid biochemical test to identify verocytotoxin-positive strains of Escherichia coli serotype O157. J Clin Microbiol. 1990;28:2165–2168. doi: 10.1128/jcm.28.10.2165-2168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trepeta R W, Edberg S C. Methylumbelliferyl-β-d-glucuronide-based medium for rapid isolation and identification of Escherichia coli. J Clin Microbiol. 1984;19:172–174. doi: 10.1128/jcm.19.2.172-174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vracko R, Sherris J C. Indole-spot test in bacteriology. Am J Clin Pathol. 1963;39:429–432. [PubMed] [Google Scholar]