Abstract
Background/purpose
Baicalin, a natural bioactive flavonoid extracted from Scutellaria baicalensis Georgi, mediates bone metabolism, and recent studies have revealed that it has cell signaling properties. However, its biological functions in cementoblasts still remain unclear. This study therefore aimed to investigate the effects of baicalin on bone resorption markers, including osteoprotegerin (OPG) and receptor activator of nuclear factor-κβ ligand (RANKL), in human cementoblast-lineage cells, as well as their proliferation ability.
Materials and methods
Human cementoblast cell line (HCEM) cells were cultured and treated with 0, 0.01, 0.1, or 1 μM of baicalin. The proliferative capacity of cultured HCEM cells was analyzed using bromodeoxyuridine immunoassay and cell counting. The baicalin effect on OPG and RANKL expression was determined using quantitative polymerase chain reaction (qPCR) and western blotting. Furthermore, OPG expression was measured in 1 μM baicalin-treated HCEM cells in the presence or absence of the Wnt signaling pathway inhibitor, Dickkopf (Dkk)-1, using qPCR and western blotting.
Results
The addition of 0.01, 0.1, and 1 μM of baicalin did not significantly change the proliferative capacity of cultured HCEM cells. Compared with the non-supplemented group, baicalin increased and suppressed OPG and RANKL gene and protein expression, respectively, in a concentration-dependent manner. OPG mRNA and protein expression levels were increased by 1 μM baicalin, which was suppressed by Dkk-1 addition.
Conclusion
Baicalin enhanced OPG expression in HCEM cells through the Wnt/beta-catenin signaling pathway, which could contribute to periodontal tissue regeneration.
Keywords: Cell proliferation, Dental cementum, OPG, RANKL
Introduction
Periodontitis is the second most common dental disease worldwide and affects approximately 538 million people.1 It is characterized by progressive alveolar bone destruction and loss of connective tissue attachment.2 Conventional periodontal treatment strategies can effectively promote infection control and eliminate inflammation. However, these therapies cannot correct defects resulting from disease progression.3 Therefore, functional and esthetic sequelae are common among patients with a history of periodontitis. Periodontitis-induced tooth reduces not only masticatory function and quality of life, but also contribute toward various systemic diseases, including arteriosclerosis and rheumatoid arthritis.4 Therefore, periodontitis treatment is crucial for preventing systemic diseases. Regenerative periodontal therapy is designed to restore periodontal tissue structure and function. Consequently, this promotes the formation of new cementum, alveolar bone, and functional-oriented periodontal ligament. Furthermore, there are several methods for promoting periodontal regeneration.5 There have been recent studies on the facilitation of predictable periodontal regeneration using tissue engineering.6 These methods include bone transplantation; guided tissue regeneration; stem cell therapy; cell sheet engineering; laser therapy; enamel matrix protein derivatives; and the use of growth factors, including fibroblast growth factor-2, bone morphogenetic protein-2, and brain-derived neurotrophic factor.7 Clinical and basic research on these methods has demonstrated good therapeutic effects; however, issues such as safety and long-term stability remain, thus a need for further studies.7 Combined periodontal treatment and pharmacotherapy is necessary for patients with severe periodontal disease and comorbid systemic or bone metabolic disease. There have been clinical trials on whether nonsteroidal anti-inflammatory drugs, enzyme inhibitors, and receptor antagonists can prevent periodontal tissue inflammation and bone destruction.8 However, long-term administration of antimicrobials and drugs for improving bone metabolism negatively affects renal and hepatic metabolism, as well as causes adverse effects.8 Therefore, there is a need to develop and assess therapies using complementary drug discovery with fewer side effects and utility in periodontal and bone repair. There has been a recent increase in attention on herbs and herbal medicines as an alternative medicine with fewer side effects and pharmacological activity in periodontal therapy.9
Herbal medicine comprises several natural medicines, including natural plants and minerals. There are numerous types of Chinese medicines, with Sho-saiko-to being the most often clinically prescribed. The dry raw root of Scutellaria baicalensis Georgi (wogon) is among the seven herbal medicines that comprise Sho-saiko-to and contains the flavonoid compounds, baicalin, and baicalein. Sho-saiko-to exerts antimicrobial and anti-inflammatory effects and is clinically prescribed for hepatitis, gastritis, cold, asthma, etc.10 Baicalin (C21H18O11, 7-glucuronic acid, 5, 6-dihydroxy flavone) is the marker compound for quality control of the dry raw root of S. baicalensis.10 Furthermore, baicalein (C15H10O5, 5, 6, 7-trihydroxyflavone), which is an aglycone and baicalin metabolite, is hydrolyzed from baicalin by intestinal microflora.11 Their pharmacological effects are discussed mutually as a set of active compounds.
Baicalin has several activities, including anti-inflammatory effects,12 anti-tumor action,13 antibacterial effect,14 and anti-viral activities,15 and there have been studies on its effect on osteoporosis via the osteogenesis promotion action. Baicalin ingestion in osteoporotic model rats causes bone tissue restoration similar to that observed with alendronate, an osteoporotic drug.16 Moreover, baicalin increases alveolar bone mineral density and height in a miniature swine model of periodontitis.17 Given this background, we focused on the bioactive effects of baicalin and its effects on periodontal tissues. We previously reported that baicalin enhances the osteogenic differentiation of human cementoblast-lineage cells through the Wnt/beta (Wnt/β)-catenin signaling pathway in vitro.18 Furthermore, baicalin ingestion during experimental tooth movement in rats increases osteoprotegerin (OPG), decreases the expression of receptor activator of nuclear factor-kB ligand (RANKL), and suppresses root resorption.19 Consequently, it is assumed that baicalin may affect metabolic regulation by bone resorption markers in human cementoblast-lineage cells. However, no detailed studies have been conducted to determine the effects of baicalin on bone resorption markers and cell proliferation in vitro. Hence, to clarify these unclear points, the present study was designed to verify the proliferative effect and the involvement of baicalin in bone metabolism using human cementoblast-lineage cells. To this end, this study investigated baicalin effects on bone resorption markers, including OPG and RANKL, in human cementoblast-lineage cells, as well as their cell proliferation ability. This study is the first to investigate the effect of baicalin on bone resorption markers using a human cementoblast cell line (HCEM) with differing properties from those of osteoblasts and periodontal ligament fibroblasts.
Materials and methods
Cell culture
Human-extracted root surface cementitious material was treated as previously described20 and then immobilized with human telomerase reverse transcriptase to establish a HCEM. HCEM cells were cultured in α-minimum essential medium (α-MEM) (Sigma Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS) (Daiichi Chemical, Tokyo, Japan) and supplemented with 240 ng/mL kanamycin (Meiji Seika, Tokyo, Japan), 1 mg/mL amphotericin B (ICN Biomedicals, Costa Mesa, CA, USA), and 500 ng/mL penicillin (Sigma Aldrich) at 37 °C in a humidified atmosphere of 5% CO2 and used for subsequent experiments.
Baicalin adjustment
Baicalin (purity ≥ 98%) (Wako, Osaka, Japan) was dissolved in methanol (Wako) and added into the medium to a final concentration of 0, 0.01, 0.1, and 1 μM. To determine the optimal concentration for obtaining the optimal effect of baicalin and the toxicity of baicalin to HCEM cells, different concentrations (0.01, 0.1, and 1 μM) of baicalin were used.
Baicalin effect on HCEM cell proliferation
Daily changes in the HCEM cell number with baicalin addition
To investigate the baicalin effect on the growth potential of HCEM cells, HCEM cells (1.0 × 104) were seeded in 24-well plates (FALCON, Franklin Lakes, NJ, USA), with 0–1 μM baicalin being added the next day. Cells were harvested in phosphate-buffered saline (PBS) containing 0.25% trypsin (Nacalai Tesque, Kyoto, Japan) and 0.25% ethylenediaminetetraacetic acid (EDTA) (Wako) at 0, 3, and 5 days after baicalin addition. Moreover, the number of live cells was counted using trypan blue solution.
Analysis of cell proliferation activity using 5-bromo-2-deoxyuridine immunoassay
HCEM cells (2.0 × 103 cells per well) were seeded in 96-well plates (FALCON). After reaching 60% confluence, there was a stepwise reduction of FBS (Daiichi Chemical) levels in the medium to 0%. Subsequently, 0–1 μM baicalin (Wako) was added and after 24 h, the cellular proliferative potential was assessed using the Cell proliferation ELISA 5-brom-2-deoxyuridine (BrdU) Kit (Roche Diagnostics, Basel, Switzerland). Each well was supplemented with BrdU (Roche Diagnostics) and cultured for 2 h at 37 °C, 5% CO2 gas-phase conditions. Next, each well was washed and treated with the anti-BrdU antibody (Roche Diagnostics). Moreover, we measured the absorbance of the developed color using a microplate reader (Varioscan Flash; Thermo Fisher Scientific, Waltham, MA, USA) at 370 nm.
Baicalin effect on the expression of bone resorption markers in HCEM cells
Quantitative polymerase chain reaction (qPCR)
HCEM cells (1.0 × 105 cells per well) were seeded in 6-well plates (FALCON). After reaching confluence, the FBS (Daiichi Chemical) concentration in the medium underwent stepwise reduction to 0%. Subsequently, 0–1 μM baicalin (Wako) was added, and after 48 h, the cells were harvested with trypsin (Nacalai Tesque)/EDTA (Wako) treatment. Furthermore, total ribonucleic acid (RNA) was harvested using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) and quantified by measuring the absorbance at 260 nm using a spectrophotometer BioSpec-nano (Shimadzu Corporation, Kyoto, Japan). Next, 1 μg of purified total RNA was reverse-transcribed into complementary deoxyribonucleic acid (cDNA) using a ReverTra Ace first-strand cDNA synthesis kit (Toyobo, Osaka, Japan). Furthermore, we analyzed OPG and RANKL gene expression levels using Light Cycler 480® (Roche Diagnostics) and SYBR Green Real-time PCR Master Mix (Toyobo). Table 1 shows the primer sequences.
Table 1.
Primer sequence.
| Gene | Sequence (5′→3′) |
| OPG | Forward: CTG CTG AAG CTG TGG AAA Reverse: GAT TTG CAG GTC TTT CTC G |
| RANKL | Forward: CAC ACC TCA CCA TCA ATG Reverse: AGT CTG TAG GTA CGC TTC C |
| GAPDH | Forward: ATG GCC TTC CGT GTT CCT Reverse: CCC AAG ATG CCC TTC AGT |
Western blot analysis for detection of bone resorption markers
HCEM cells (1.0 × 105 cells per well) were seeded in 6-well-plates and cultured in 10% FBS (Daiichi Chemical)-containing α-MEM (Sigma Aldrich). After reaching confluency, 0–1 μM baicalin (Wako) was added with subsequent medium changes at 2-day intervals. Seven days after the addition of baicalin (Wako), the cells were harvested using a cell scraper for protein extraction. Protein extraction and Western blot analysis were performed as follows: proteins were recovered using Triton lysis buffer [50 mM Tris–HCL (pH 7.4), 250 mM NaCl, 0.1% Triton-X buffer (Roche Diagnostics), 1 mM EDTA (Wako), 50 mM NaF, and protease inhibitor (Takara Bio, Shiga, Japan)]; subsequently, they were rapidly frozen in liquid nitrogen. Next, the samples were kept on ice and centrifuged at 4 °C at 13,000 rpm for 20 min for collection of supernatants. We measured protein levels using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA); furthermore, 40 μg protein was mixed with 10% 2-mercaptoethanol and 0.01% bromophenol blue and denatured at 100 °C for 3 min. Next, the proteins were electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel (ATTO, Tokyo, Japan) and transferred onto a polyvinylidene fluoride membrane using iblot® gel transfer system (Thermo Fisher Scientific). This membrane was then immersed in a blocking agent for 30 min at room temperature; subsequently, the primary antibody diluted in CanGet Signal® Solution 1 (Toyobo) was added and left for 12 h at 4 °C. Next, the membrane was washed using a wash buffer (Tween-20 containing PBS) followed by the addition of the secondary antibody diluted to a concentration of 1:10,000 using CanGet Signal® Solution 2 (Toyobo) for 30 min at room temperature. Subsequently, the membrane was thoroughly washed with wash buffer followed by target antigen detection using a near-infrared fluorescence detection device Odyssey® Imaging System (LI-COR Bioscience, Lincoln, NB, USA). The primary antibodies were anti-OPG antibody [ab73400, (Abcam Cambridge, UK), 1:1000] and anti-RANKL antibody (NB100-56512, (Novus Biologicals, Centennial, CO, USA), 1:500). Anti-β-actin antibody (011–24554, Wako, 1: 2000) was used as the endogenous control.
Baicalin effect in the presence of Wnt/β-Catenin signaling pathway inhibitor on RANKL expression in HCEM cells
qPCR analysis
HCEM cells (1.0 × 105 cells per well) were seeded in 6-well plates (FALCON). After reaching confluence, FBS (Daiichi Chemical) levels were stepwise reduced in the medium to 0% as aforementioned, and then 100 ng/mL human recombinant Dickkopf (Dkk)-1, a Wnt signaling pathway antagonist (R&D system, Minneapolis, MN, USA) was added for 1 h before addition of 0–1 μM baicalin (Wako). Forty-eight hours after baicalin addition, we performed qPCR analysis as aforementioned.
Western blot analysis
HCEM cells (1.0 × 105 cells per well) were seeded in 6-well plates and cultured in 10% FBS (Daiichi Chemical)-containing α-MEM (Sigma Aldrich). After reaching confluency, Dkk-1 (R&D system) and baicalin (Wako) were added at 100 ng/mL and 0–1 μM, respectively. Protein extraction and Western blot analysis were performed 7 days after baicalin addition as mentioned earlier, using the same blocking agents and antibodies.
Statistical analysis
Results were expressed as mean ± standard deviation. Between and among-group comparisons were performed using the Mann–Whitney's U–test and Steel–Dwass method, respectively. Statistical significance was set at P < 0.05 or P < 0.01.
Results
Baicalin effect on the cell proliferative potential of HCEM cells
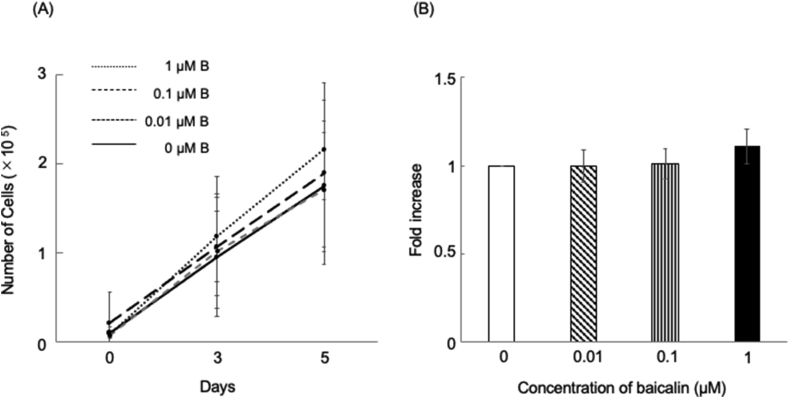
There was an increase in the HCEM cell number over time by day 5 of culture; however, there was no significant between-group difference (Fig. 1A). The BrdU immunoassay revealed no significant between-group difference in the DNA-synthesizing ability of HCEM cells (Fig. 1B).
Figure 1.
Baicalin effect on HCEM cell proliferation. (A) Daily changes in the quantification of the HCEM cell number with the addition of baicalin. HCEM cells were seeded at a density of 1.0 × 104 cells in 24-well plates with the addition of 0–1 μM baicalin on the next day. The number of live cells was counted using trypan blue solution at 0, 3, and 5 days after baicalin addition. There was an increase in the HCEM cell number over time by day 5 of culture. However, there was no significant difference between the baicalin-supplemented and non-supplemented groups at 3 and 5 days after plating (Fig. 1A). ∗∗p < 0.01, ∗p < 0.05, n = 3. (B) Analysis of cell proliferation activity using BrdU immunoassay. Compared with the control group, treatment with 0.01, 0.1, or 1 μM baicalin did not significantly alter HCEM cell proliferative activity (Fig. 1B). ∗∗p < 0.01, ∗p < 0.05, n = 16.
Baicalin effect on gene expression of bone resorption markers in HCEM cells
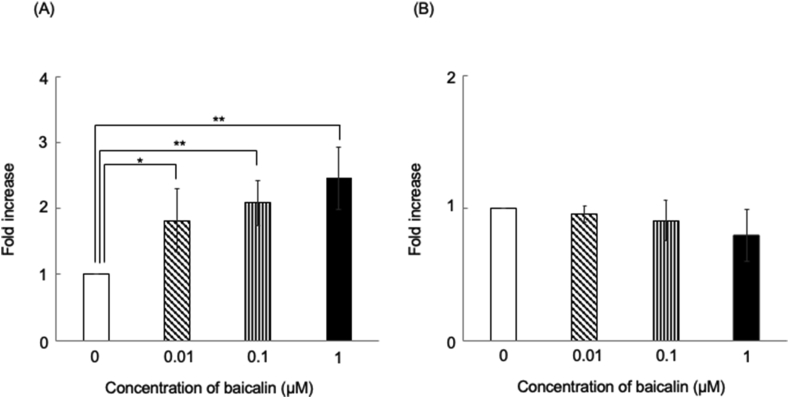
There was a significant concentration-dependent increase and decrease in OPG and RANKL gene expression, respectively, in HCEM cells in the 0, 0.01, 0.1, and 1 μM baicalin-treated groups compared with the non-treated group (Fig. 2A and B).
Figure 2.
Baicalin effects on OPG and RANKL gene expression levels in HCEM cells. HCEM cells were cultured after reaching confluence; subsequently, medium FBS concentration was reduced stepwise to 0%. Next, 0–1 μM baicalin was added with mRNA extraction after 48 h. OPG and RANKL gene expression was analyzed using Light Cycler 480®. OPG gene expression in HCEM cells was significantly enhanced by 0, 0.01, 0.1, and 1 μM baicalin compared with the non-treated group (Fig. 2A). Furthermore, there was a concentration-dependent suppression of RANKL gene expression (Fig. 2B). ∗∗p < 0.01, ∗p < 0.05, vs control, n = 3.
Baicalin effect on protein expression of bone resorption markers in HCEM cells
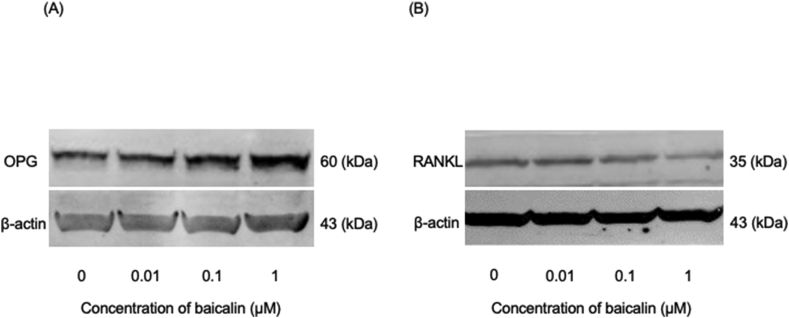
Baicalin addition induced a concentration-dependent increase and decrease in OPG and RANKL protein expression respectively, in HCEM cells (Fig. 3A and B).
Figure 3.
Baicalin effects on OPG and RANKL protein expression in HCEM cells. HCEM cells were cultured in 10% FBS-containing α-MEM. After reaching confluency, 0–1 μM baicalin was added with medium changes being performed at 2-day intervals. After 7 days, the sample was extracted. Western blotting was performed using the Odyssey® Imaging System. Addition of 0.01, 0.1, and 1 μM baicalin enhanced and decreased OPG and RANKL protein expression, respectively, in HCEM cells in a concentration-dependent manner compared with the non-treated group (Fig. 3A and 3B). The lower bands show the β-actin amount, n = 3.
Effects of baicalin supplementation in the presence of Dkk-1 on OPG expression in HCEM cells
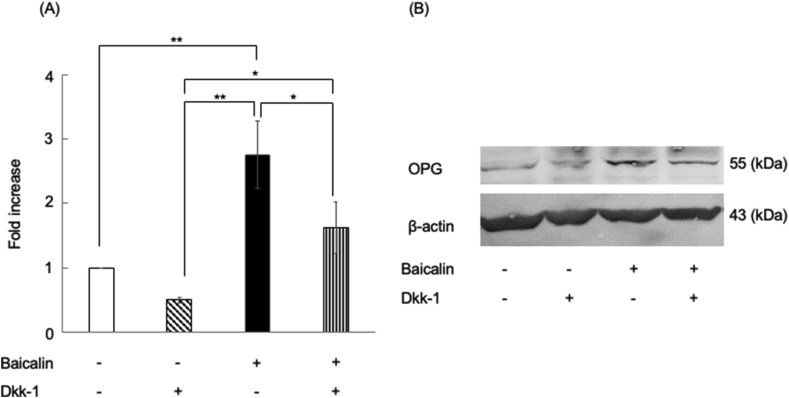
Baicalin supplementation significantly enhanced OPG gene and protein expression (Fig. 4A), which was significantly suppressed by the addition of Dkk-1, a Wnt signaling pathway inhibitor (Fig. 4B).
Figure 4.
Effects of the Wnt signaling pathway inhibitor on OPG mRNA and protein expression in HCEM cells treated with baicalin. HCEM cells were treated with 1 μM baicalin in the presence or absence of 100 ng/mL Dkk-1 followed by 48-h culturing. Gene expression was evaluated using qPCR. ∗∗p < 0.01, ∗p < 0.05, vs control n = 3. Baicalin treatment significantly enhanced OPG gene expression in the baicalin-treated group, which was significantly suppressed by adding Dkk-1, a Wnt signaling pathway inhibitor (Fig. 4A). HCEM cells were seeded and cultured in α-MEM containing 10% FBS. When 80% confluence was reached, the cells were treated with 1 μM baicalin in the presence or absence of 100 ng/mL Dkk-1. After 7 days, the cells were collected and evaluated using western blotting. OPG protein levels were increased by baicalin, which was suppressed after inhibition of Wnt signaling using Dkk-1 (Fig. 4B). The lower bands show the amount of β-actin, n = 3.
Discussion
Cementum, which comprises the thin mineralized tissue layer covering the tooth-root surface,20 is critical for the development and restoration of healthy periodontal tissue and is degenerated in patients with periodontal diseases.21 Cementogenesis, which begins after root dentin formation, is mediated by interactions between Hertwig's epithelial root sheath and dental follicle mesenchymal cells.22 Notably, there are distinct structural and functional differences between cementum. Further, although both tissues have similar internal composition, cementum has a relatively limited remodeling potential.23 Specifically, progenitor cells migrate into the perivascular area of the periodontal ligament tissue from bone marrow spaces; subsequently, they differentiate into osteoblasts and cementoblasts at the bone and tooth-root surface, respectively.24 Previous studies have reported similar characteristics between cementoblasts and osteoblasts, including similar molecular properties and mineralization ability, as well as expression of mineral-associated genes, including bone sialoprotein (BSP) and osteocalcin, which reflect mature cementoblast differentiation.25,26 Accordingly, we focused on the baicalin effect on human cementoblast-lineage cell metabolism.
We found that the addition of 0.01, 0.1, and 1 μM baicalin to HCEM cells did not significantly change the number of viable cells on days 3 and 5 of culture, which was confirmed by the BrdU immunoassay. In a previous study, addition of 0–10 μM baicalein, which is a glycoside of baicalin with similar structure, slightly but not significantly, suppressed proliferation of human cultured periodontal cells (hPDLCs) in a concentration-dependent manner.27 Furthermore, the addition of 0–200 μg/mL baicalin to cervical cancer HeLa cells and culturing for 24 h was found to significantly inhibit cell growth at a concentration of ≥25 μg/mL.28 Furthermore, Pei et al.29 reported that adding 0–1 μg/mL baicalin to hPDLCs did not affect cell proliferative capacity. The results of these studies are consistent with our findings.
RANKL, which is a member of the tumor necrosis factor (TNF) family, is a 317-amino-acid peptide found on the surface of osteoclast precursors that stimulates preosteoclast fusion, attachment of osteoclasts to bone, osteoclast activation, and enhanced resorption.30 RANKL expression, which is induced by inflammatory mediators and causes alveolar bone loss, has been reported in the periodontal tissue and ligament cells.31 OPG is a peptide consisting of 380-amino acids and a member of the TNF receptor superfamily. It acts as a decoy RANKL receptor, which suppresses osteoclast differentiation.32 OPG is another key factor in periodontal disease pathogenesis. The RANKL-OPG system is a crucial regulator of osteoclastogenesis and bone resorption in both physiological and pathological conditions.33 Baicalin is a flavonoid compound with estrogenic effects, which suggests that it may affect OPG and RANKL regulation in human cementoblast-lineage cells.
We found that baicalin enhanced OPG gene and protein expression levels in a dose-dependent manner compared with the non-supplemented control. Baicalin enhances gene expression of differentiation markers, including type Ⅰ collagen, osteocalcin, runt-related transcription factor (Runx) 2, and OPG, in osteoblasts.34 Furthermore, baicalin addition to hPDLCs increases OPG protein and gene expression.29 The results of these studies are consistent with our findings.
Furthermore, the addition of baicalin slightly, but not significantly, suppresses RANKL gene and protein expression. However, baicalin did not significantly affect RANKL gene expression levels.34 Baicalin inhibits RANKL mRNA expression by suppressing cyclooxygenase-2 expression induced by interleukin-1 beta.35 Pei et al.29 reported that 0.01 μg/mL baicalin increases and decreases OPG and RANKL expression, respectively. Furthermore, baicalin could regulate RANKL and OPG expression in a dose-dependent manner under physiological conditions in hPDLCs.29 This study showed that the addition of baicalin slightly, but not significantly, suppressed RANKL gene and protein expression. Although our findings are consistent with those of previous studies, future studies should elucidate the differences in the mechanisms of action using different baicalin concentrations.
Although the detailed mechanism for the pro-osteogenic baicalin effects remains unclear, baicalin-induced osteoblastogenesis has been shown to be dependent on the Wnt/β-catenin signaling pathway.34 Regarding abnormalities in the Wnt/β-catenin signaling pathway and bone diseases, mutations in the Wnt-signal receptor cause juvenile osteoporosis.36
Therefore, osteometabolic therapeutics for improving the functions of the Wnt/β-catenin signaling pathway are valuable for osteoporotic treatment.37 Furthermore, the Wnt/β-catenin signaling pathway is relevant for oral regions and promotes cementum formation in cementoblasts.38 Moreover, the interruption of the Wnt/β-catenin signal pathway suppresses cementoblast differentiation and prevents murine root formation.39 There is an inferred association between the Wnt/β-catenin signaling pathway and cementoblasts. Therefore, we applied Dkk-1, a specific Wnt/β-catenin signaling pathway inhibitor, and assessed the regulatory effects of baicalin on bone resorption using human cementoblast-lineage cells. We found that 1 μM baicalin increased OPG mRNA and protein expression, which was reduced by Dkk-1.
Baicalein increases osteogenic markers and Alizarin red staining in periodontal ligament cells; this increase is inhibited by Dkk-1.27 We previously reported that baicalin enhances osteogenic differentiation of human cementoblast-lineage cells through the Wnt/β-catenin signaling pathway, which is consistent with the present findings. These findings suggest that baicalin may affect OPG regulation via Wnt/β-catenin signaling in HCEM cells.
However, the mechanism of baicalin-induced OPG/RANKL regulation remains unclear. Baicalin activity is not only mediated by Wnt/β-catenin signaling but also by the nuclear factor-kappa beta, mitogen-activated protein kinase/extracellular signal-regulated kinase, and transforming growth factor-β/Smad signaling pathways.27,40 Thus, there is a need for further studies to elucidate the precise mechanism of baicalin activity in periodontal tissue components. This study is novel because it used a HCEM with differing properties from those of osteoblasts and periodontal ligament fibroblasts and showed that baicalin addition does not affect cell growth but increases the expression of OPG, a bone resorption metabolic marker, which may be mediated by the Wnt signaling pathway.
In conclusion, baicalin increased OPG expression in human cementoblast lineage cells via the Wnt/β-catenin signaling pathway, indicating that baicalin could be applied in future complementary drug discovery for periodontal regeneration.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
We are grateful to Dr. M Motokawa for providing advice pertaining to this study. This study was performed at the Research Center for Molecular Medicine, Hiroshima University and was supported by the Japan Society for the Promotion of Science, Tokyo, Japan [KAKENHI grant numbers 16K11790, 17K17330, and 19K19270].
References
- 1.GBD 2015 disease and injury incidence and prevalence collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;vol. 388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dentino A., Lee S., Mailhot J., Hefti A.F. Principles of periodontology. Periodontol. 2000;2013(61):16–53. doi: 10.1111/j.1600-0757.2011.00397.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramseier C.A., Rasperini G., Batia S., Giannobile W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontol. 2000;2012(59):185–202. doi: 10.1111/j.1600-0757.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teeuw W.J., Gerdes V.E.A., Loos B.G. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sculean A., Nikolidakis D., Schwarz F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials–biological foundation and preclinical evidence: a systematic review. J Clin Periodontol. 2008;35:106–116. doi: 10.1111/j.1600-051X.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds M.A., Kao R.T., Camargo P.M., et al. Periodontal regeneration–intrabony defects: a consensus report from the AAP Regeneration Workshop. J Periodontol. 2015;86:105–107. doi: 10.1902/jop.2015.140378. [DOI] [PubMed] [Google Scholar]
- 7.Izumi Y., Aoki A., Yamada Y., et al. Current and future periodontal tissue engineering. Periodontol. 2000;2011(56):166–187. doi: 10.1111/j.1600-0757.2010.00366.x. [DOI] [PubMed] [Google Scholar]
- 8.Seymour R.A. Effects of medications on the periodontal tissues in health and disease. Periodontol. 2000;2006(40):120–129. doi: 10.1111/j.1600-0757.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 9.Martίnez C.C., Diaz Gόmez M., Oh M.S. Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: a review. Pharm Biol. 2017;55:1992–1998. doi: 10.1080/13880209.2017.1347188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K.L., Sheu S.J. Determination of flavonoids and alkaloids in the scute-coptis herb couple by capillary electrophoresis. Anal Chim Acta. 1995;313:113–120. [Google Scholar]
- 11.Aryal P., Kim K., Park P.H., Ham S., Cho J., Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014;281:4644–4658. doi: 10.1111/febs.12969. [DOI] [PubMed] [Google Scholar]
- 12.Lee W., Ku S.K., Bae J.S. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation. 2015;38:110–125. doi: 10.1007/s10753-014-0013-0. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y., Liang J., Zheng X., et al. Lung-targeting drug delivery system of baicalin-loaded nanoliposomes: development, biodistribution in rabbits, and pharmacodynamics in nude mice bearing orthotopic human lung cancer. Int J Nanomed. 2016;12:251–261. doi: 10.2147/IJN.S119895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J., Dong B., Wang K., et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PloS One. 2017;12 doi: 10.1371/journal.pone.0176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghaddam E., Teoh B.T., Sam S.S., et al. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G., Li C., Niu Y., Yu Q., Chen Y., Liu E. Osteoprotective effect of Radix Scutellariae in female hindlimb-suspended Sprague-Dawley rats and the osteogenic differentiation effect of its major constituent. Molecules. 2017;22:1044. doi: 10.3390/molecules22071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng H., Li F., Wei H., Shi J.F., Rao G.Z., Gou J.Z. Preliminary study of the dual release baicalin and rhBMP-2 system to improve periodontal tissue regeneration in minipigs. Shang Hai Kou Qiang Yi Xue. 2013;22:126–131. [PubMed] [Google Scholar]
- 18.Kimura A., Kunimatsu R., Yoshimi Y., et al. Baicalin promotes osteogenic differentiation of human cementoblast lineage cells via the wnt/β catenin signaling pathway. Curr Pharmaceut Des. 2018;24:3980–3987. doi: 10.2174/1381612824666181116103514. [DOI] [PubMed] [Google Scholar]
- 19.Kunimatsu R., Kimura A., Tsuka Y., et al. Baicalin inhibits root resorption during tooth movement in a rodent model. Arch Oral Biol. 2020;116:104770. doi: 10.1016/j.archoralbio.2020.104770. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa M., Tahara H., Kitagawa S., et al. Characterization of established cementoblast-like cell lines from human cementum-lining cells in vitro and in vivo. Bone. 2006;39:1035–1042. doi: 10.1016/j.bone.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Nemoto E., Koshikawa Y., Kanaya S., et al. Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone. 2009;44:805–812. doi: 10.1016/j.bone.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Diercke K., Kӧnig A., Kohl A., Lux C.J., Erber R. Human primary cementoblasts respond to combined IL-1β stimulation and compression with an impaired BSP and CEMP-1 expression. Eur J Cell Biol. 2012;91:402–412. doi: 10.1016/j.ejcb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Hammarstrӧm L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 24.McCulloch C.A., Melcher A.H. Cell density and cell generation in the periodontal ligament of mice. Am J Anat. 1983;167:43–58. doi: 10.1002/aja.1001670105. [DOI] [PubMed] [Google Scholar]
- 25.Matias M.A., Li H., Young W.G., Bartold P.M. Immunohistochemical localisation of extracellular matrix proteins in the periodontium during cementogenesis in the rat molar. Arch Oral Biol. 2003;48:709–716. doi: 10.1016/s0003-9969(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 26.Somerman M.J., Shroff B., Agraves W.S., et al. Expression of attachment proteins during cementogenesis. J Biol Buccale. 1990;18:207–214. [PubMed] [Google Scholar]
- 27.Chen C., Zhang C., Cai L., et al. Baicalin suppresses IL-1β-induced expression of inflammatory cytokines via blocking NF-κB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models. Int Immunopharm. 2017;52:218–226. doi: 10.1016/j.intimp.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Peng Y., Fu Z.Z., Guo C.S., et al. Effects and mechanism of baicalin on apoptosis of cervical cancer HeLa cells in-vitro. Iran J Pharm Res (IJPR) 2015;14:251–261. [PMC free article] [PubMed] [Google Scholar]
- 29.Pei Z., Wang B., Zhang F., et al. Response of human periodontal ligament cells to baicalin. J Periodontol. 2014;85:1283–1290. doi: 10.1902/jop.2014.130635. [DOI] [PubMed] [Google Scholar]
- 30.Jiang L., Song J., Hu X., et al. The proteasome inhibitor bortezomib inhibits inflammatory response of periodontal ligament cells and ameliorates experimental periodontitis in rats. J Periodontol. 2017;88:473–483. doi: 10.1902/jop.2016.160396. [DOI] [PubMed] [Google Scholar]
- 31.Nukaga J., Kobayashi M., Shinki T., Song H. Regulatory effects of interleukin-1β and prostaglandin E2 on expression of receptor activator of nuclear factor- κB ligand in human periodontal ligament cells. J Periodontol. 2004;75:249–259. doi: 10.1902/jop.2004.75.2.249. [DOI] [PubMed] [Google Scholar]
- 32.Kurinami H., Shimamura M., Nakagami H., et al. A novel therapeutic peptide as a partial agonist of RANKL in ischemic stroke. Sci Rep. 2016;6:38062. doi: 10.1038/srep38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belibasakis G.N., Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 34.Guo A.J.Y., Choi R.C.Y., Cheung A.W.H., et al. Baicalin, a flavone, induces the differentiation of cultured osteoblasts: an action via the Wnt/β-catenin signaling pathway. J Biol Chem. 2011;286:27882–27893. doi: 10.1074/jbc.M111.236281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G.F., Wu Z.F., Wan L., Wang Q.T., Chen F.M. Influence of baicalin on the expression of receptor activator of nuclear factor-κB ligand in cultured human periodontal ligament cells. Pharmacology. 2006;77:71–77. doi: 10.1159/000092853. [DOI] [PubMed] [Google Scholar]
- 36.Ai M., Heeger S., Bartels C.F., Schelling D.K. Osteoporosis-Pseudoglioma Collaborative Group. Clinical and molecular findings in osteoporosis-pseudoglioma syndrome. Am J Hum Genet. 2005;77:741–753. doi: 10.1086/497706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauman W.A., Cardozo C.P. Osteoporosis in Individuals with spinal cord injury. Pharm Manag PM R. 2015;7:188–201. doi: 10.1016/j.pmrj.2014.08.948. [DOI] [PubMed] [Google Scholar]
- 38.Kim T.H., Lee J.Y., Baek J.A., Lee J.C., Yang X., Taketo M.M. Constitutive stabilization of ß-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011;412:549–555. doi: 10.1016/j.bbrc.2011.07.116. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R., Yang G., Wu X., Xie J., Yang X., Li T. Disruption of Wnt/β-catenin signaling in odontoblasts and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci. 2013;9:228–236. doi: 10.7150/ijbs.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J., Cheng J., Zhu S., et al. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int Immunopharm. 2019;73:193–200. doi: 10.1016/j.intimp.2019.04.052. [DOI] [PubMed] [Google Scholar]