Abstract
Sex differences in the nervous system have gained recent academic interest. While the prominent differences are observed in mood and anxiety disorders, growing number of evidences also suggest sex difference in pain perception. This review focuses on estrogen as the key molecule underlying such difference, because estrogen plays many functions in the nervous system, including modulation of transient receptor potential vanilloid 1 (TRPV1) and P2X purinoceptor 3 (P2X3), two important nociceptive receptors. Estrogen was shown in various studies to up-regulate TRPV1 expression through two distinct pathways, resulting in pro-nociceptive effect. However, estrogen alleviated pain in other studies, by down-regulating nerve growth factor (NGF)–activated pathways and TRPV1. Estrogen may also attenuate nociception by inhibiting P2X3 receptors and ATP-signaling. Understanding the mechanism underlying the pro- and anti-nociceptive effect of estrogen might be crucial to understand pathophysiology of the burning mouth syndrome (BMS), a common chronic orofacial pain disorder in menopausal women. The involvement of TRPV1 is strongly suspected because of burning sensation. Reduced estrogen level of the BMS patient might have caused increased activity of P2X3 receptors. Interestingly, the increased expression of TRPV1 and P2X3 in oral mucosa of BMS patients was reported. The combinational impact of differential modulation of TRPV1/P2X3 during menopause might be an important contributing factor of etiology of BMS. Understanding the estrogen-dependent regulation of nociceptive receptors may provide a valuable insight toward the peripheral mechanism of sex-difference in pain perception.
Keywords: Burning mouth syndrome, Estrogen, P2X3, Nociception, Sex characteristics, TRPV1
Introduction
Sex differences in pain perception have gained growing academic interest. Several clinical reports suggested that women are more sensitive to pain than men, leading to a high percentage of women among patients diagnosed with a pain syndrome.1, 2, 3, 4, 5 Moreover, common chronic pain disorders, such as neuropathic pain, fibromyalgia, osteoarthritis, or migraine, are generally more prevalent in women.6, 7, 8 A large cohort study measuring pressure, mechanical, and thermal pain sensitivity observed greater orofacial pain sensitivity in women.9 Despite these reports, the mechanism underlying the sex difference in pain perception remains unclear.1, 2, 3, 4, 5, 6, 7, 8, 9
Pain arises from the activation of specialized primary afferent neurons called nociceptors.10 The nociceptive information is transmitted to the central nervous system and ultimately perceived as pain.11 Nociceptors detect noxious stimuli through specific receptors, which have been the subject of extensive research for the past several decades. The two most studied families of nociceptive receptors are transient receptor potential (TRP) receptors and purinergic receptors.
The transient receptor potential vanilloid 1 (TRPV1), a representative member of the TRP family, is a Ca2+-permeable ion channel activated by multiple sensory stimuli, such as heat (>43 °C), protons (pH < 6.0), and vanilloids.12, 13, 14 Interestingly, TRPV1 activation is modulated by inflammatory mediators, such as prostaglandins, extracellular ATP, bradykinin, glutamate, and the nerve growth factor (NGF), suggesting its central role as a pain mediator.15, 16, 17, 18 Compelling amounts of evidence demonstrate the contribution of TRPV1 in the mechanism underlying chronic pain.19 According to immunohistochemical studies in various peripheral neuropathy models, subsets of sensory neurons within the dorsal root ganglion (DRG) and trigeminal ganglia (TG) express high TRPV1 levels.18,20,21 While many studies have demonstrated the robust expression and functional contribution of TRPV1 in chronic pain induced by inflammation or nerve injuries of these ganglia, recent findings showing TRPV1 expression in the central nervous system were perplexing and interesting.14,22
P2X3 is a purinergic receptor with a well-characterized role in nociception. It is expressed in small-to medium-sized DRG or TG neurons23,24 and activated by extracellular ATP released from damaged tissue. P2X3 activation generates electrical impulses that are transmitted to the brain and perceived as pain. In particular, the fast desensitization and slow recovery of P2X3 make it a central element in chronic pain conditions.25
There are several clinical and animal studies that have suggested that steroid hormones, including sex hormones, influence neuronal survival, neurogenesis, receptor expression, neurotransmitter synthesis, and neuronal excitability.26, 27, 28 In particular, estrogen mainly exerts excitatory actions in both a rapid and delayed mode26 and play a key role in altering neural function through genomic and non-genomic pathways. Estrogen may play an essential role in chronic pain pathologies.29, 30, 31 Thus, estrogen might alter the functional expression of nociceptive receptors, thereby contributing to the sex difference in pain perception. This review summarizes the current understanding of potential estrogen effects regarding TRPV1/P2X3 receptor function to elaborate the sex difference in nociception mechanisms. Furthermore, we discuss an orofacial pain disorder with extreme prevalence in middle-aged females called burning mouth syndrome (BMS) as a possible result of such difference.
Upregulation of TRPV1 by estrogen
Recent experimental data revealed that estrogen regulates TRPV1 and P2X3 activity and expression.22,32 Upregulation of those nociceptive receptors by estrogen can provide important insights into the neuronal mechanisms of pain and sex differences. To date, many studies have suggested that estrogen has a pro-nociceptive effect on TRPV1, while it has the opposite effect on P2X3.28,32, 33, 34, 35, 36, 37 According to the studies related to TRPV1, estrogen upregulates TRPV1 through two different pathways: a classical genomic pathway and a non-classical pathway.
Estrogen is a lipid-soluble hormone that activates the classic nuclear estrogen receptors (ERα and ERβ), affecting the transcription of target genes and resulting in the classical long-term effect.38 The first attempt to demonstrate the relationship between TRPV1 and estrogen showed that ERα and ERβ knockout mice had significantly lower TRPV1 expression levels than wild-type mice.32 Another group revealed that 17β-estradiol, the most potent estrogen, binds to the estrogen receptor located in the cytoplasm of DRG neurons. Then, the estrogen receptor translocates to the nucleus, where it binds to the estrogen response elements located in the promoter region of the TRPV1 gene.28 This results in the transcription of the TRPV1 gene, increasing its expression in the DRG and promoting pain. Besides the DRG, immunohistochemistry experiments showed that high estrogen doses increased TRPV1 mRNA expression in the TG.33
A behavioral test using ovariectomized rats supported a similar pro-nociceptive estrogen effect. It showed that ovariectomy completely reversed the enhanced sensitivity to the capsaicin-induced nocifensive response.34 Besides, ovariectomized rats treated with high estrogen doses for 2 days showed greater capsaicin-evoked nocifensive behavior than ovariectomized rats treated with low estrogen doses.33 In addition, observing rats at different estrous cycle phases has helped to prove the hormonal effect. Consistent with the previous studies, rats in the proestrus phase (where estrogen levels rise) display a higher capsaicin-induced response than rats in other phases.34
By contrast, TRPV1 can be upregulated through a non-classical pathway involving the membrane-bound G-protein-coupled receptor 30 (GPR30). GPR30 is an important non-genomic receptor modulating pain perception expressed in neurons of the primary sensory ganglia.39,40 In female rats, ovariectomy downregulates GPR30 expression in DRG neurons; estrogen replacement can help recover GPR30 expression.41 Moreover, estrogen and GPR30 agonists injected into rats induced mechanical hyperalgesia, whereas GPR30 antagonists inhibited it.42,43 A recent study suggested a protein kinase C epsilon (PKCε)-dependent mechanism, independent from classical estrogen receptors, to explain the GPR30-mediated pain pathway. It observed that estrogen acts through GPR30, triggering an intracellular signaling pathway that activates PKCε, which phosphorylates TRPV1.44 Rats with TRPV1 phosphorylated by PKCε in their DRG were hypersensitive to capsaicin, heat, and acid.45,46 This indicates that estrogen is a downstream signaling factor of PKCε-induced TRPV1 sensitization.
Downregulation of TRPV1 and P2X3 by estrogen
In contrast to the above findings, accumulating evidence suggests that estrogen alleviates pain. For example, prolonged elevation of estrogen levels during pregnancy elevates the pain threshold.47 Another study showed that female rats tested in diestrus, proestrus, or estrus endured a hot plate significantly longer than others.48 Recent clinical researches suggest that, besides the endogenous ones, exogenous hormones can lower pain in postmenopausal women. Although this so-called estrogen replacement therapy still has an unclear, non-generalizable mechanism, it has been widely used for postmenopausal diseases, such as osteoporosis, pelvic pain, or temporomandibular joint pain.30 Animal studies also support estrogen replacement therapy inhibiting pain by an estrogen receptor-mediated process.49,50
With regard to TRPV1, downregulation of NGF by estrogen was reported as a mechanism responsible for its pain reduction effects. A recent time-course study showed that NGF could sensitize TRPV1, cause the acute-to-chronic transition, and maintain pain.51 A major effect of NGF was to translocate TRPV1 to the cell surface membrane through tropomyosin-related kinase A (TrkA) signaling.52,53 Estrogen significantly reduced NGF mRNA and protein expression levels in rat chondrocytes.54 This is similar to previous studies showing that estrogen reduced NGF levels in rat hippocampus and sympathetic neurons.55,56 Moreover, long-term estrogen replacement decreased TrkA mRNA levels in the DRG, which can alter NGF response.57
Laboratory experiments also point out that estrogen may attenuate pain by altering nociceptive signaling via P2X3. Prolonged exposure to 17β-estradiol (0.1–1 μM) had an anti-nociceptive effect on DRG neurons, inhibiting ATP-induced intracellular Ca2+ influx in the DRG.58,59 The involvement of P2X3 receptors in the anti-nociceptive effect of estrogen has been recently discussed.60 P2X3 activation in the rat DRG, which promoted ATP-induced peripheral hyperalgesia, may be reversed by estrogen replacement (30 μg/kg, 0.4 mL/day).61 Another study also showed that estrogen replacement (30 μg/kg, 0.4 mL/day) reversed the ovariectomy-induced increase in P2X3 mRNA and protein expression levels in the DRG. By contrast, using progesterone on female rats or estrogen on male rats had no effect.62,63 In the TG, estrogen treatment (30 μg/kg, 0.4 mL/day) also decreased P2X3 mRNA and protein expression levels. The estrogen-induced trigeminal P2X3 mRNA and protein expression decrease correlate with reduced facial mechanical pain. These results suggest that estrogen inhibits P2X3 receptors through a genomic mechanism.
The estrogen effect on nociceptors is most likely concentration-dependent. The inhibitory effect of 17β-estradiol on ATP-mediated responses occurs at 100 nM but not at 10 nM.63 Applying 30 μg/kg of 17β-estradiol on mice for 6 weeks decreased P2X3 mRNA expression,36,60,61,63 but applying 0.1 μg/kg for the same duration had no effect.64 Moreover, different estrogen concentrations have anti-nociceptive effects on TRPV1. A report showed that exposure of DRG neurons to 17β-estradiol (10–100 nM) overnight inhibited TRPV1 activation.37 Overnight incubation with 1 nM of 17β-estradiol or short (10 min) incubation did not remarkably alter TRPV1 expression.65 Considering that a 1-day exposure to 100 pM 17β-estradiol rather significantly increased TRPV1 mRNA expression,65 the anti-nociceptive effects of estrogen on TRPV1 may require a relatively high concentration or long exposure duration.
It is conceivable that estrogen has both pro-nociceptive and anti-nociceptive effects, depending on the nervous system region, the exposure conditions, and the pathway it affects. Moreover, studies on estrogen-related pain mechanisms should be considered according to the estrogen concentration and duration applied to come up with a consensus.
Burning mouth syndrome
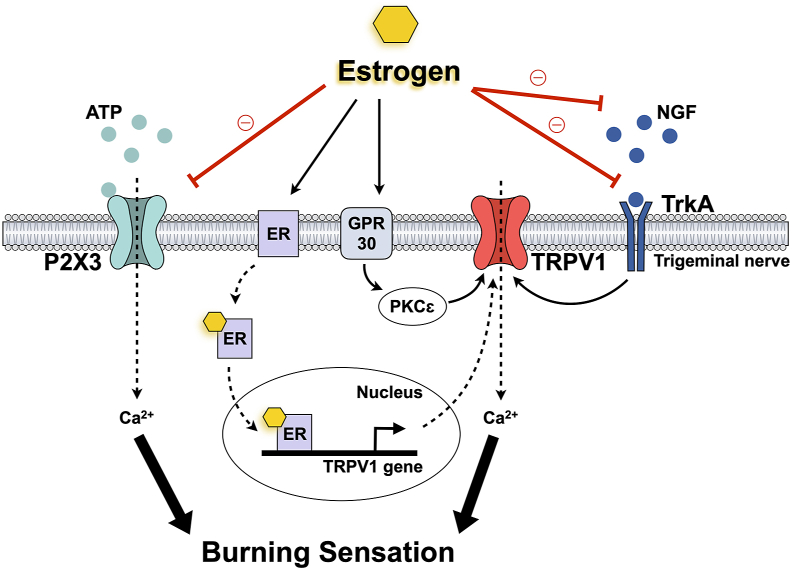
Burning mouth syndrome (BMS) is an idiopathic chronic and intractable oral pain condition characterized by lowered sensory and pain thresholds in the absence of detectable oral mucosa changes.66 Many clinical and laboratory studies have searched the causes of this syndrome, such as psychiatric, local, and systemic factors, yet the underlying mechanism remains unclear. Although its etiology is quite multifactorial and poorly understood, BMS is remarkably more prevalent in women with a sex ratio of 7:1.67 In particular, most BMS patients are middle-aged or older women during peri- or post-menopause.68 Estrogen replacement therapy has been suggested for BMS patients, revealing that most patients who responded to this therapy had an increased estrogen receptor expression.69 This strongly implies that estrogen plays an essential role in BMS. Moreover, BMS is related to a small-fiber idiopathic neuropathy of the trigeminal system affecting oral sensation.70 This indicates that key ion channels involved in pain perception at the peripheral level, TRPV1 and P2X3, should also be investigated together to understand the role of estrogen on these nociceptive ion channels and neuropathic pain pathways of BMS (Fig. 1).
Figure 1.
Estrogen regulation on TRPV1 and P2X3 with burning mouth syndrome. Abbreviations: ATP, adenosine triphosphate; ER, estrogen receptor; GPR30; G-protein-coupled receptor 30; NGF, nerve growth factor; P2X3, P2X purinoceptor 3; PKCε, protein kinase C epsilon; trkA, tropomyosin-related kinase A; TRPV1, transient receptor potential vanilloid 1.
The TRPV1 channel mediates pain perception and may participate in BMS symptoms. BMS patients have shown a low tolerance to noxious heat stimulation, including capsaicin.71 The burning sensation comes from a small-fiber sensory neuropathy affecting the tongue's epithelial and sub-papillary nerve fibers.72 The expression of TRPV1 and its regulator NGF was significantly increased in the epithelial fibers of BMS patients, and this expression correlated with their capsaicin pain score.70 While a high TRPV1 expression in trigeminal afferents may be associated with hypersensitivity in BMS, a high NGF concentration plays a crucial role in enhanced intracellular signaling. Considering that estrogen can reduce NGF/TrkA signals to TRPV1, increased NGF concentrations might increase pain sensitivity in postmenopausal women.
Furthermore, a recent study has found a significant correlation between P2X3 and BMS. It found an increased P2X3 immunoreactivity in the trigeminal sensory system in the lingual mucosa of patients with BMS.73 P2X3 in the trigeminal sensory system may play a pivotal role, along with TRPV1, in developing and maintaining BMS symptoms. With regard to the anti-nociceptive effect of estrogen, 17β-estradiol inhibits ATP-induced intracellular Ca2+ influxes in ganglia neurons.59 Considering that P2X3 receptors are ATP-dependent Ca2+-permeable channels, estrogen might also regulate P2X3 expression by attenuating ATP-induced Ca2+ influxes. In fact, many studies have suggested that estrogen inhibits P2X3. Estrogen decreased P2X3 mRNA and protein expression levels in primary afferent neurons.35,36 Also, estrogen replacement reversed ovariectomy-induced increased P2X3 expression in rats.63 These results suggest that estrogen inhibits P2X3-mediated peripheral pain transduction.
Despite extensive research on the role of TRPV1 and P2X3 receptors on pain perception, knowledge about the interaction of these receptors in sensory ganglion activity remains sparse. Previous studies have reported colocalization of TRPV1 and P2X3 on peripheral afferent fibers23,74 and a high co-expression percentage of these receptors in pain conditions, including BMS.32 However, those channels certainly respond differently to various inflammatory factors.75 Understanding the complex peripheral mechanism of BMS would require further examination of the interaction of co-expressed TRPV1 and P2X3 receptors. Moreover, 17β-estradiol modulates both channels. Considering the inconsistent estrogen effect on pain perception, the combined effect of estrogen on those nociceptive receptors together should also be addressed. In conclusion, to explain the sex differences in BMS observed in clinical practice and explore the therapeutic role of estrogen, further studies need to confirm the interaction between TRPV1 and P2X3.
Discussion
In summary, it is evident that estrogen regulates nociceptive receptors, TRPV1 and P2X, through genomic and non-genomic pathways, potentially contributing to the sex difference in pain. Many studies reported the activation of TRPV1 and inhibition of P2X3 by 17β-estradiol in TG and DRG, although a few studies with varying concentration of 17β-estradiol, or with the different pain models reported inconsistent results.
BMS is one of the representative diseases where sex is a strong predisposing factor. The strong prevalence of BMS in post-menopausal woman suggests reduced estrogen as a triggering factor. The estrogen-deficient status of the postmenopausal women might cause the enhanced NGF signaling and TrkA, thereby increasing TRPV1 activity in the peripheral nerves. Decreased estrogen level can also increase pain sensitivity by reducing estrogen-dependent modulation of P2X3 in the sensory neurons. These results suggest that reversing the activity of NGF, TrkA or P2X3 could be a proposing therapeutic strategy of BMS. Studies that test the inhibitors of NGF, TrkA or P2X3 might provide valuable new insights on the etiology or treatment of BMS, especially of peripheral nerve origin.
In conclusion, the interaction between P2X3, TRPV1, and estrogen in sensory neurons may represent a novel mechanism that can explain gender-driven discrepancies in pain perception, and uncovering it could help figure out appropriate therapeutic targets and developing treatments.
Declaration of competing interest
The authors have no conflict of interest relevant to this article.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant (2018R1D1A1B07049067) funded by the Korea government (Ministry of Education).
References
- 1.Unruh A.M. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 2.Riley J.L., Robinson M.E., Wise E.A., Myers C.D., Fillingim R.B. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 3.Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., Rahim-Williams B., Riley J.L. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogil J.S. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 5.Sorge R.E., Totsch S.K. Sex differences in pain. J Neurosci Res. 2017;95:1271–1281. doi: 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 6.Rollman G.B., Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon M.M. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatr. 2004;65:5–9. [PubMed] [Google Scholar]
- 8.Gazerani P., Andersen O.K., Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–163. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Ostrom C., Bair E., Maixner W., et al. Demographic predictors of pain sensitivity: results from the OPPERA study. J Pain. 2017;18:295–307. doi: 10.1016/j.jpain.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterina M.J., Leffler A., Malmberg A.B., et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 11.Dubin A.E., Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 13.Tominaga M., Caterina M.J., Malmberg A.B., et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 14.Jara-Oseguera A., Simon S., Rosenbaum T. TRPV1: on the road to pain relief. Curr Mol Pharmacol. 2010;1:255–269. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Premkumar L.S., Ahern G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 16.Chuang H.H., Prescott E.D., Kong H., et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira J., Da Silva G.L., Calixto J.B. Contribution of vanilloid receptors to the overt nociception induced by B 2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immke DC, Gavva NR. The TRPV1 receptor and nociception. Semin Cell Dev Biol;17:582-591. [DOI] [PubMed]
- 19.Palazzo E., Luongo L., de Novellis V., Rossi F., Marabese I., Maione S. Transient receptor potential vanilloid type 1 and pain development. Curr Opin Pharmacol. 2012;12:9–17. doi: 10.1016/j.coph.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Novakovic S.D., Kassotakis L.C., Oglesby I.B., et al. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 21.Hwang S.J., Oh J.M., Valtschanoff J.G. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Ho K.W., Ward N.J., Calkins D.J. TRPV1: a stress response protein in the central nervous system. Am J Neurodegener Dis. 2012;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa H., Sugimoto T. The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res. 2004;998:130–135. doi: 10.1016/j.brainres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Kanazawa T., Matsumoto S. Expression of transient receptor potential vanilloid 1 and anoctamin 1 in rat trigeminal ganglion neurons innervating the tongue. Brain Res Bull. 2014;106:17–20. doi: 10.1016/j.brainresbull.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Wirkner K., Sperlagh B., Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36:165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- 26.Joëls M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 27.Gintzler A.R., Liu N.J. The maternal spinal cord: biochemical and physiological correlates of steroid-activated antinociceptive processes. Prog Brain Res. 2001;133:83–97. doi: 10.1016/s0079-6123(01)33007-8. [DOI] [PubMed] [Google Scholar]
- 28.Méndez-Reséndiz K.A., Enciso-Pablo Ó, González-Ramírez R., Juárez-Contreras R., Rosenbaum T., Morales-Lázaro S.L. Steroids and TRP channels: a close relationship. Int J Mol Sci. 2020;21:1–36. doi: 10.3390/ijms21113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hapidou E.G., Rollman G.B. Menstrual cycle modulation of tender points. Pain. 1998;77:151–161. doi: 10.1016/S0304-3959(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 30.Riley J.L., 3rd, Robinson M.E., Wise E.A., Price D. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 31.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 32.Cho T., Chaban V.V. Expression of P2X3 and TRPV1 receptors in primary sensory neurons from estrogen receptors-α and estrogen receptor-β knockout mice. Neuroreport. 2012;23:530–534. doi: 10.1097/WNR.0b013e328353fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamagata K., Sugimura M., Yoshida M., et al. Estrogens exacerbate nociceptive pain via up-regulation of TRPV1 and ANO1 in trigeminal primary neurons of female rats. Endocrinology. 2016;157:4309–4317. doi: 10.1210/en.2016-1218. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y.C., Chen C.W., Wang S.Y., Wu F.S. 17Β-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Therapeut. 2009;331:1104–1110. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y., Jiang Q., Yu L., et al. 17β-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERα and GPR30. Endocrinology. 2013;154:2421–2433. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- 36.Yu L., Li N., Liu C.Y., Ma B. Estrogen altered facial mechanical pain threshold and trigeminal P2X3 receptor expression. Neuroendocrinol Lett. 2011;32:811–815. [PubMed] [Google Scholar]
- 37.Xu S., Cheng Y., Keast J.R., Osborne P.B. 17Β-estradiol activates estrogen receptor Β-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149:5540–5548. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deroo B.J., Korach K.S. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craft R.M. Modulation of pain by estrogens. Pain. 2007;132 doi: 10.1016/j.pain.2007.09.028. S3-S. [DOI] [PubMed] [Google Scholar]
- 40.Dennis M.K., Burai R., Ramesh C., et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takanami K., Sakamoto H., Matsuda K.I., et al. Expression of G protein-coupled receptor 30 in the spinal somatosensory system. Brain Res. 2010;1310:17–28. doi: 10.1016/j.brainres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn J., Dina O.A., Goswami C., Suckow V., Levine J.D., Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez P., Bogen O., Levine J.D. Role of nociceptor estrogen receptor GPR30 in a rat model of endometriosis pain. Pain. 2014;155:2680–2686. doi: 10.1016/j.pain.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goswami C., Kuhn J., Dina O.A., et al. Estrogen destabilizes microtubules through an ion-conductivity-independent TRPV1 pathway. J Neurochem. 2011;117:995–1008. doi: 10.1111/j.1471-4159.2011.07270.x. [DOI] [PubMed] [Google Scholar]
- 45.Mandadi S., Tominaga T., Numazaki M., et al. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCε-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Wang S., Joseph J., Ro J.Y., Chung M.-K. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain. 2015;156:931–941. doi: 10.1097/j.pain.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawson-Basoa M., Gintzler A.R. Gestational and ovarian sex steroid antinociception: synergy between spinal κ and δ opioid systems. Brain Res. 1998;794:61–67. doi: 10.1016/s0006-8993(98)00192-9. [DOI] [PubMed] [Google Scholar]
- 48.Stoffel E.C., Ulibarri C.M., Folk J.E., Rice K.C., Craft R.M. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradshaw H.B., Berkley K.J. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41:157–165. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 50.Mannino C.A., South S.M., Quinones-Jenab V., Inturrisi C.E. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Barker P.A., Mantyh P., Arendt-Nielsen L., Viktrup L., Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res. 2020;13:1223–1241. doi: 10.2147/JPR.S247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X., Huang J., McNaughton P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji R.R., Samad T.A., Jin S.X., Schmoll R., Woolf C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 54.Shang X., Zhang L., Jin R., Yang H., Tao H. Estrogen regulation of the expression of pain factor NGF in rat chondrocytes. J Pain Res. 2021;14:931–940. doi: 10.2147/JPR.S297442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaur G., Janik J., Isaacson L.G., Callahan P. Estrogen regulation of neurotrophin expression in sympathetic neurons and vascular targets. Brain Res. 2007;1139:6–14. doi: 10.1016/j.brainres.2006.12.084. [DOI] [PubMed] [Google Scholar]
- 56.Gibbs R.B., Wu D., Hersh L.B., Pfaff D.W. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 57.Liuzzi F.J., Scoville S.A., Bufton S.M. Long-term estrogen replacement coordinately decreases trkA and β-PPT mRNA levels in dorsal root ganglion neurons. Exp Neurol. 1999;155:260–267. doi: 10.1006/exnr.1998.6999. [DOI] [PubMed] [Google Scholar]
- 58.Lee D.Y., Chai Y.G., Lee E.B., et al. 17β-Estradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 2002;70:2047–2059. doi: 10.1016/s0024-3205(01)01534-x. [DOI] [PubMed] [Google Scholar]
- 59.Chaban V.V., Mayer E.A., Ennes H.S., Micevych P.E. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 60.Rosyidi R.M., Priyanto B., Wardhana D.P.W., et al. P2X3 receptor expression in dorsal horn of spinal cord and pain threshold after estrogen therapy for prevention therapy in neuropathic pain. Ann Med Surg. 2020;60:389–395. doi: 10.1016/j.amsu.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Q., Li W., Sun J., et al. Inhibitory effect of estrogen receptor beta on P2X3 receptors during inflammation in rats. Purinergic Signal. 2017;13:105–117. doi: 10.1007/s11302-016-9540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma B., Yu L., Fan J., et al. Estrogen modulation of peripheral pain signal transduction: involvement of P2X3 receptors. Purinergic Signal. 2011;7:73–83. doi: 10.1007/s11302-010-9212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan J., Yu L., Zhang Y., Ni X., Ma B., Burnstock G. Estrogen altered visceromotor reflex and P2X3 mRNA expression in a rat model of colitis. Steroids. 2009;74:956–962. doi: 10.1016/j.steroids.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Carley M.E., Cliby W.A., Spelsberg T.C. P2X3 receptor subunit messenger RNA expression in the female mouse bladder after oophorectomy with or without estrogen replacement. Am J Obstet Gynecol. 2002;187:103–106. doi: 10.1067/mob.2002.125705. [DOI] [PubMed] [Google Scholar]
- 65.Payrits M., Sághy É., Cseko K., et al. Estradiol sensitizes the transient receptor potential vanilloid 1 receptor in pain responses. Endocrinology. 2017;158:3249–3258. doi: 10.1210/en.2017-00101. [DOI] [PubMed] [Google Scholar]
- 66.Ship J.A., Grushka M., Lipton J.A., Mott A.E., Sessle B.J., Dionne R.A. Burning mouth syndrome: an update. J Am Dent Assoc. 1995;126:842–853. doi: 10.14219/jada.archive.1995.0305. [DOI] [PubMed] [Google Scholar]
- 67.Kolkka-Palomaa M., Jääskeläinen S.K., Laine M.A., Teerijoki-Oksa T., Sandell M., Forssell H. Pathophysiology of primary burning mouth syndrome with special focus on taste dysfunction: a review. Oral Dis. 2015;21:937–948. doi: 10.1111/odi.12345. [DOI] [PubMed] [Google Scholar]
- 68.Sun A., Wu K.M., Wang Y.P., Lin H.P., Chen H.M., Chiang C.P. Burning mouth syndrome: a review and update. J Oral Pathol Med. 2013;42:649–655. doi: 10.1111/jop.12101. [DOI] [PubMed] [Google Scholar]
- 69.Forabosco A., Criscuolo M., Coukos G., et al. Efficacy of hormone replacement therapy in postmenopausal women with oral discomfort. Oral Surg Oral Med Oral Pathol. 1992;73:570–574. doi: 10.1016/0030-4220(92)90100-5. [DOI] [PubMed] [Google Scholar]
- 70.Yilmaz Z., Renton T., Yiangou Y., et al. Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007;14:864–871. doi: 10.1016/j.jocn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Imamura Y., Shinozaki T., Okada-Ogawa A., et al. An updated review on pathophysiology and management of burning mouth syndrome with endocrinological, psychological and neuropathic perspectives. J Oral Rehabil. 2019;46:574–587. doi: 10.1111/joor.12795. [DOI] [PubMed] [Google Scholar]
- 72.Lauria G., Majorana A., Borgna M., et al. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005;115:332–337. doi: 10.1016/j.pain.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 73.Beneng K., Yilmaz Z., Yiangou Y., McParland H., Anand P., Renton T. Sensory purinergic receptor P2X3 is elevated in burning mouth syndrome. Int J Oral Maxillofac Surg. 2010;39:815–819. doi: 10.1016/j.ijom.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Hermes S.M., Andresen M.C., Aicher S.A. Localization of TRPV1 and P2X3 in unmyelinated and myelinated vagal afferents in the rat. J Chem Neuroanat. 2016;72:1–7. doi: 10.1016/j.jchemneu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu M., Huang W., Wu D., Priestley J.V. TRPV1, but not P2X3, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci. 2006;24:1–6. doi: 10.1111/j.1460-9568.2006.04889.x. [DOI] [PubMed] [Google Scholar]