Abstract
Objective:
Reviews on child outcomes following in utero antidepressant exposure have focused on short-term outcomes. However, several recent individual studies reported on adverse physical, neurodevelopmental, and psychiatric outcomes beyond infancy and early childhood. The objective of this systematic review was to establish the long-term effects of prenatal antidepressant exposure on physical, neurodevelopmental, and psychiatric outcomes in individuals aged 4 years and older.
Data Sources:
Embase, MEDLINE Ovid, Web of Science, Cochrane Central, and Google Scholar were systematically searched for all relevant articles, written in English and published prior to November 8, 2018, using terms describing antidepressants, pregnancy, and developmental outcomes.
Study Selection:
All original research articles on long-term outcomes of prenatal antidepressant exposure were eligible for inclusion. After screening and removal of duplicates, a total of 34 studies were identified.
Data Extraction:
Included articles were qualitatively analyzed to determine inconsistency, indirectness, imprecision, and study bias.
Results:
The identified studies demonstrated statistically significant associations between prenatal antidepressant exposure and a range of physical, neurodevelopmental, and psychiatric outcomes. Yet, the risk of confounding by indication was high. When controlling for confounders, 5 studies investigating physical outcomes (asthma, cancer, body mass index [BMI], epilepsy) found no association except conflicting outcomes for BMI. Eighteen studies examining neurodevelopmental outcomes (cognition, behavior, IQ, motor development, speech, language, and scholastic outcomes) found no consistent associations with antidepressant exposure after taking confounders into account. Eleven studies investigated psychiatric outcomes. After adjusting for confounders, prenatal antidepressant exposure was associated with affective disorders but not with childhood psychiatric outcomes (eg, autism spectrum disorders, attention-deficit/hyperactivity disorder).
Conclusions:
Reported associations between in utero exposure to antidepressants and physical, neurodevelopmental, and psychiatric outcomes, in large part, seem to be driven by the underlying maternal disorder. When limiting confounding by indication, prenatal exposure to antidepressants was significantly associated only with offspring BMI and affective disorders.
Depression and anxiety are highly prevalent mental disorders and the leading cause of disability worldwide.1 Perinatal depression, defined as depression during pregnancy and after delivery, affects approximately 11.5% of new mothers annually in the United States.2 If left untreated, perinatal depression puts mothers at increased risk of experiencing negative mental and physical health outcomes.3,4 Moreover, perinatal depression has been linked to poor child outcomes, including adverse birth outcomes, poorer long-term cognitive and social development, and the risk of future psychopathology.5 Antidepressants are usually given as first-line treatment for depression and anxiety,6,7 and many patients continue to take their antidepressants for long periods of time as maintenance treatment to prevent relapse.8 As a result, antidepressant use during pregnancy is common, with estimated prevalence rates ranging between 2% and 13%.9–12
However, since antidepressants cross the placenta, as well as the blood-brain barrier,13 concern is growing about sequelae of in utero antidepressant exposure in the unborn child. Many antidepressants target the serotonergic system, and modifications of serotonergic signals are thought to influence brain development and subsequent functioning.14 Moreover, the serotonergic system is involved in mechanisms besides brain functioning, including motor control, food intake, and body weight regulation.15,16 Consequently, approximately 50% of women decide to discontinue their antidepressants either before or during pregnancy.17
A substantial number of observational studies have examined the use of antidepressants during pregnancy, with inconsistent results. Observational studies always carry the risk of confounding, particularly confounding by indication. Some of these studies found associations between in utero exposure to antidepressants and adverse neonatal outcomes, including low birth weight, preterm birth, persistent pulmonary hypertension of the neonate, and poor neonatal adaptation,18–25 as well as associations between prenatal antidepressant exposure and poorer neurodevelopmental and neurobehavioral outcomes in early childhood.26–28 While these short-term outcomes have frequently been investigated and subsequently consolidated in systematic reviews (eg, references29–32), very few long-term child outcomes were included in these reviews. Recently, several individual studies have reported on adverse long-term outcomes beyond infancy and early childhood, including studies on intellectual disability at age 8 years,33 as well as childhood cancer61 and psychiatric disorders34 over 15- and 17-year follow-up periods, respectively.
Our aim was to systematically evaluate the literature, examining the long-term effects of in utero exposure to antidepressants on child outcomes including and beyond the age of 4 years and untangling the results from confounding factors, such as confounding by indication, socioeconomic status, untreated mood/anxiety symptoms, and exposures to other substances. We chose to focus on outcomes from age 4 onward because the short-term effects of in utero exposure to antidepressants have been well established in a plethora of reviews focusing on infancy and very early childhood. Yet, it is paramount to also understand the long-term health effects of prenatal antidepressant exposure. Furthermore, many children start preschool at age 4, and measurements of childhood psychiatric disorders, learning disabilities, and cognitive development become more reliable at that age and afterward. Due to the nonspecific action of antidepressants, we included all investigated developmental outcomes, including physical, neurodevelopmental, and psychiatric outcomes.
METHODS
Literature Search and Data Sources
The systematic electronic literature search was performed by a medical information specialist (Wichor Bramer, MSc; Erasmus MC Medical Library; Rotterdam, the Netherlands) on November 8, 2018. All large databases, including Embase, MEDLINE Ovid, Web of Science, Cochrane Central, and Google Scholar, were searched, using search terms describing types of antidepressants (eg, selective serotonin reuptake inhibitor, tricyclic antidepressant), the target population (eg, pregnancy, pregnant women, maternal exposure), and outcome measurement timing (eg, childhood, child development, adolescent). A complete overview of the search terms used is included in Supplementary Appendix 1. This systematic review was registered in PROSPERO (no. CRD42019116981).
Selection Criteria
The selection procedure was conducted according to the guidelines described in the PRISMA statement.35 Studies were eligible for inclusion if they were peer-reviewed and written in English and if they described a population of women using any antidepressants, including serotonin reuptake inhibitors (SRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and other antidepressants during pregnancy. We defined long-term outcomes as any child outcome from the age of 4 years onward, not restricting our search to a maximum follow-up period. All original research articles were eligible for inclusion.
Study Selection and Data Extraction
Duplicate articles were screened and removed with the citation manager EndNote. Two reviewers (N.M.M. and Rachel Cohen, BSc; Icahn School of Medicine at Mount Sinai; New York, New York) independently screened the titles and abstracts and assessed the full text of potentially eligible studies. Disagreements between reviewers’ selection were resolved by discussion among the reviewers and authors. When multiple articles reported on the same cohort and the same outcome measurement, the article with the highest level of detail was included. Data were extracted using a standardized data extraction form by two reviewers (N.M.M., A.-S.R.; see Supplementary Appendix 2). Two reviewers (N.M.M., A.-S.R.) independently assessed the quality of the studies using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Institutes of Health). The overall quality of evidence per outcome was summarized according to the GRADE guidelines36 only for those outcomes reported in 5 or more studies.
Data Synthesis
Here, we report a narrative synthesis of the evidence and present extracted adjusted relative risks and 95% confidence intervals (CIs) when possible. We did not perform a meta-analysis of the results because the physical health and neurodevelopment outcomes assessed in the included studies were too varied and biased to be pooled. Moreover, a meta-analysis of the psychiatric outcomes was futile because not enough research has investigated psychiatric disorders manifesting in adolescence and adulthood and childhood psychiatric outcomes have been meta-analyzed extensively.
RESULTS
Identified and Included Articles
Thirty-four studies met the predefined inclusion criteria and were included in this review (Figure 1, Supplementary Appendix 3). Interrater reliability was high (raw interrater agreement = 98.9%). Studies reported on physical health outcomes (5), neurodevelopmental outcomes (18), and psychiatric disorders (11). Fifteen studies (44.1%) were prospectively designed. Sample size per study ranged between 36 and 1,580,629 children. All studies included SSRIs, while some also focused on SNRIs (19), TCAs (13), and MAOIs (10). Detailed characteristics of all studies, including the quality assessment per study, are provided in Table 1.
Figure 1.
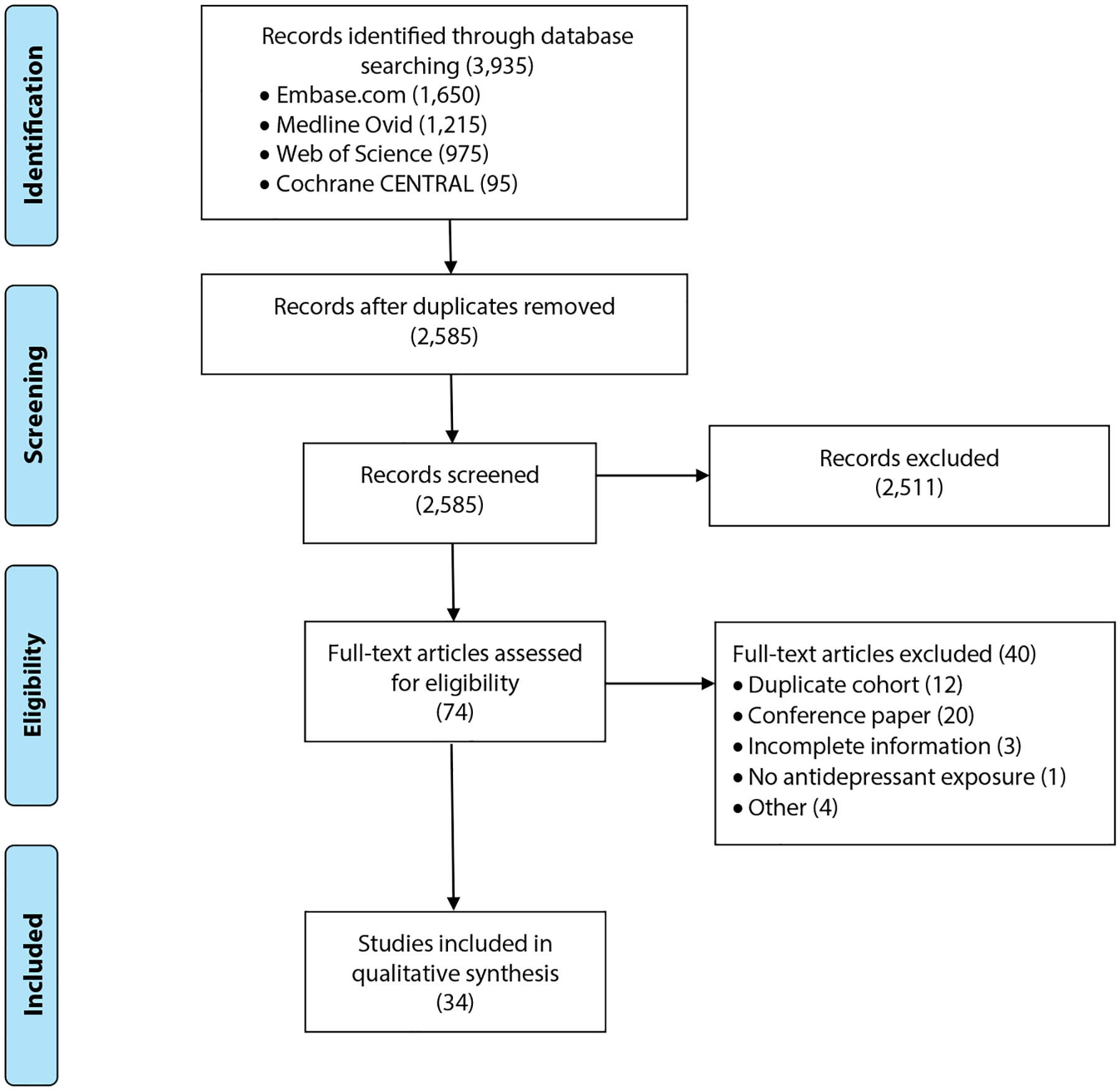
PRISMA Flow Chart of Included and Excluded Studies
Table 1.
Characteristics of Included Studies Measuring Child Outcomes Beyond the Age of 4 After in Utero Antidepressant Exposure
| Study | Study Design 1 | Study Design 2 | Antidepressants Studied | Data Source, Country (Period) | Quality Rating |
|---|---|---|---|---|---|
| Boukhris et al 201637 | R | Cohort | SSRIs, TCAs, MAOIs, SNRIs, other | Quebec Pregnancy Children’s cohort, Canada (1998–2009) | Good |
| Boukhris et al 201738 | R | Cohort | SSRIs, TCAs, MAOIs, SNRIs, other | Quebec Pregnancy Children’s cohort, Canada (1998–2009) | Good |
| Brown et al 201639 | R | Cohort | SSRIs | National Registers, Finland (1996–2010) | Good |
| Brown et al 201740 | R | Cohort | SSRIs, SNRIs | Administrative databases, Ontario, Canada (2002–2010) | Good |
| Castro et al 201641 | R | Case-control | SSRIs, TCAs, dual-action antidepressants | Electronic Health Records, Massachusetts (1997–2010) | Good |
| El Marroun et al 201442 | P | Cohort | SSRIs | Generation R study, the Netherlands (2002–2006) | Fair |
| El Marroun et al 201743 | P | Cohort | SSRIs | Generation R study, the Netherlands (2002–2006) | Fair |
| Figueroa 201044 | R | Cohort | SSRIs, bupropion | MarketScan data, United States (1997–2002) | Poor |
| Galbally et al 201545 | P | Case-control | SSRIs, SNRIs, TCAs, NaSSA | Victorian Psychotropic Registry, Australia (2004) | Poor |
| Grzeskowiak et al 201246 | R | Cohort | SSRIs | Women’s and Children’s Health Network, Australia (2000–2005) | Good |
| Grzeskowiak et al 201347 | R | Cohort | SSRIs | Danish National Birth Cohort, Denmark (1996–2002) | Fair |
| Grzeskowiak et al 201648 | R | Cohort | All antidepressants (ATC code N06A) | Danish National Birth Cohort, Denmark (1996–2002) | Fair |
| Hanley et al 201549 | P | Cohort | SSRIs, SNRIs | Primary maternity care providers BC, Vancouver, Canada (unknown) | Poor |
| Harrington et al 201450 | P | Case-control | SSRIs | Childhood Autism Risks from Genetics and the Environment study, United States (2003–2010) | Poor |
| Hermansen et al 201651 | P | Cohort | SSRIs | Mother and Child Cohort Study, Norway (2008–2009) | Poor |
| Hermansen et al 201752 | P | Cohort | SSRIs | Mother and Child Cohort Study, Norway (2008–2009) | Poor |
| Johnson et al 201628 | P | Cohort | SSRIs, SNRIs | Emory Women’s Mental Health Program, United States (2010–2012) | Fair |
| Kragholm et al 201853 | R | Cohort | SSRIs | Danish National Birth Cohort, Denmark (2005–2008) | Good |
| Laugesen et al 201354 | R | Cohort | All antidepressants (ATC code N06A) | Danish National Birth Cohort, Denmark (1996–2009) | Good |
| Liu et al 201555 | R | Cohort | SSRIs, TCAs, antidepressants with ATC code N06AX | Danish National Birth Cohort, Denmark (1996–2007) | Good |
| Liu et al 201734 | R | Cohort | All antidepressants (ATC code N06A) | Danish National Birth Cohort, Denmark (1998–2012) | Good |
| Lupattelli et al 201856 | P | Cohort | SSRIs | Mother and Child Cohort Study, Norway (1999–2008) | Good |
| Malm et al 201657 | R | Cohort | SSRIs | National Registers, Finland (1996–2010) | Good |
| Man et al 201758 | R | Cohort | All antidepressants (BNF 4.3) | Clinical Data Analysis & Reporting System, Hong Kong (2001–2009) | Good |
| Mao et al 201659 | R | Cohort | All antidepressants (ATC code N06A) | Danish National Birth Cohort, Denmark (1997–2008) | Good |
| Misri et al 200660 | P | Cohort | SSRIs | British Columbia Women’s Hospital, Vancouver, Canada (1997–1999) | Poor |
| Momen et al 201861 | R | Cohort | All antidepressants (ATC code N06A) | Danish National Birth Cohort, Denmark (1998–2012) | Good |
| Nulman et al 201264 | P | Cohort | SSRIs, venlafaxine | Motherisk Program, Toronto, Canada (2001–2006) | Fair |
| Nulman et al 201563 | P | Cohort | SSRIs, SNRIs | Motherisk Program, Toronto, Canada (2005–2008) | Fair |
| Oberlander et al 200765 | P | Cohort | SSRIs | British Columbia Women’s Hospital, Vancouver, Canada (1997–1999) | Poor |
| Partridge et al 201666 | P | Cohort | SSRIs | Infants Hospital Rhode Island, United States (unknown) | Poor |
| Sujan et al 201767 | R | Cohort | All antidepressants (ATC code N06A) | National administrative registers, Sweden (1996–2012) | Good |
| Viktorin et al 201733 | R | Cohort | All antidepressants (ATC code N06A) | National administrative registers, Sweden (2006–2007) | Good |
| Weikum et al 201362 | P | Cohort | SRIs | British Columbia Women’s Hospital, Vancouver, Canada (unknown) | Poor |
Abbreviations: ATC = anatomical therapeutic chemical, BNF = British National Formulary, MAOI = monoamine oxidase inhibitor, NaSSA = noradrenergic and specific serotonergic antidepressant, P = prospective, R = retrospective, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor, TCA = tricyclic antidepressant.
Physical Health Outcomes
Four retrospective studies reporting on physical health outcomes were identified and their quality rated fair to good (Table 2). Two studies by Grzeskowiak et al46,47 focused on childhood overweight, classified as a BMI > 85th percentile. In an Australian cohort of 6,560 women,46 an association between SSRI exposure and childhood overweight at the age of 4 to 5 years was found for female but not male offspring. In contrast, in a Danish cohort of 36,185 pregnancies,47 an association between SSRIs and childhood overweight was found for male but not female offspring at the age of 7. The authors assign the inconsistent results to the differences in study methods and indicate a higher accuracy in the Danish study.
Table 2.
Results for Child Physical Health Outcomesa
| Study | Child Age at Testing | Outcome | Comparison Groups | Adjusted Outcome | 95% CI | Covariates |
|---|---|---|---|---|---|---|
| Grzeskowiak et al 201246 | 4–5 y | BMI > 85th percentile (females only) | SSRIs (n = 33) vs unexposed (n = 3,072) | 0.27 | 0.07 to 0.99 | Maternal age, BMI, race, socioeconomic status, parity, smoking status, appropriateness of fetal growth (SGA/LGA), breastfeeding at discharge from hospital |
| SSRIs (n = 33) vs unmedicated psychiatric illness (n = 106) | 0.23 | 0.05 to 0.98 | ||||
| BMI > 85th percentile (males only) | SSRIs (n = 38) vs unexposed (n = 3,213) | 0.93 | 0.52 to 1.67 | |||
| SSRIs (n = 38) vs unmedicated psychiatric illness (n = 98) | 1.17 | 0.54 to 2.51 | ||||
| Grzeskowiak et al 201347 | 7 y | BMI > 85th percentile | SSRIs (n = 127) vs unexposed (n = 35,568) | 1.39 | 0.94 to 2.05 | Pre-pregnancy maternal BMI, weight gain during pregnancy, paternal BMI, employment status, smoking status, prenatal distress, maternal postnatal distress score, neonatal birth weight, breastfeeding |
| SSRIs (n = 127) vs unmedicated psychiatric illness (n=490) | 1.12 | 0.71 to 1.77 | ||||
| BMI > 85th percentile (females only) | SSRIs (n = 63) vs unexposed (n = 17,320) | 0.99 | 0.43 to 2.26 | |||
| SSRIs (n = 63) vs unmedicated psychiatric illness (n = 239) | 0.86 | 0.37 to 1.99 | ||||
| BMI > 85th percentile (males only) | SSRIs (n = 64) vs unexposed (n = 18,248) | 1.54 | 1.01 to 2.37 | |||
| SSRIs (n = 64) vs unmedicated psychiatric illness (n = 251) | 1.78 | 1.01 to 3.12 | ||||
| Liu et al 201555 | > 3 y | Asthma (medication and/or hospital contact) | Antidepressants (n = 8,895) vs unexposed (n = 712,314) | 1.25 | 1.18 to 1.33 | Maternal country of origin, parity, age at delivery, maternal social status at birth, smoking during pregnancy, maternal history of asthma, sex of the child, calendar year of birth |
| Antidepressants (n = 8,895) vs unmedicated prenatal depression (n = 12,476) | 1.00 | 0.93 to 1.08 | ||||
| Mao et al 201659 | < 13 y | Epilepsy (ICD-10 code G40–41) | Antidepressants (n = 12,438) vs unexposed (n = 715,319) | 1.27 | 1.05 to 1.54 | Child’s sex, family income at birth, maternal age at birth, education, marital status at birth, parity, parental epilepsy diagnosis history before birth, smoking |
| Momen et al 201861 | < 15 y | Childhood cancer (ICD-10: C00-C97) | Antidepressants (n = 21,488) vs unexposed (n = 863,033) | 1.15 | 0.78 to 1.72 | Maternal age at delivery, primiparity, maternal history of psychiatric disorders at delivery, inpatient psychiatric treatment from 2 years before pregnancy up to delivery, dispensation of other psychotropic prescriptions during pregnancy, smoking during pregnancy, maternal highest education, calendar year of delivery, parental cancer history at time of delivery |
| Antidepressants (n = 21,488) vs prior antidepressant users (n = 30,607) | 1.03 | 0.63 to 1.68 |
Values in bold are statistically significant. Adjusted outcomes are adjusted relative risks.
Abbreviations: BMI = body mass index, LGA = large for gestational age, SGA = small for gestational age, SSRI = selective serotonin reuptake inhibitor.
Liu et al55 did not find an association between antidepressant use and asthma in a large Danish population-based cohort. Only use of older antidepressants (mainly TCAs) was associated with an increased risk of asthma when treated individuals were compared to an untreated control group. However, this finding could reflect confounding by the severity of maternal depression, which cannot be assessed directly in register-based studies.
Momen et al61 examined the association between in utero antidepressant exposure and childhood cancer. No association was found between antidepressant use during pregnancy and childhood cancer in general, leukemia, and nervous system tumors.
Mao et al59 examined the association between antidepressant exposure and epilepsy. Children exposed to antidepressants in utero (n = 12,438) had a higher overall risk of epilepsy compared to unexposed children, but this difference was not significant when compared to children of mothers who discontinued antidepressants shortly before pregnancy, indicating that the underlying maternal disease plays a role in the observed association.
Neurodevelopmental Outcomes
Cognition.
None of the 5 studies43,45,51,52,62 investigating cognitive-neurophysiologic outcomes following in utero antidepressant exposure found a significant association between prenatal exposure to antidepressants and cognitive performance in a total of 166 antidepressant-exposed children, 460 children exposed to untreated maternal depression, and 5,545 unexposed children (Table 3).
Table 3.
Results for Neurodevelopmental Outcomes
| Study | Child Age at Testing | Outcome | Comparison Groups | Adjusted Outcomea | 95% CI | Covariates | ||
|---|---|---|---|---|---|---|---|---|
| Brown et al 201639 | 0–14 y | Specific developmental disorders of speech and language (ICD-10 code F80) | SSRIs (n = 15,596) vs unexposed (n = 31,207) | 1.53 | 1.26 to 1.86 | Maternal country of birth, parental death, smoking, marital status, socioeconomic status maternal substance abuse, maternal and paternal age, place of residence, maternal and paternal history of psychiatric diagnosis, parity, child’s sex, gestational age, exposure to antiepileptic drugs, anxiolytics/sedatives, entitlement to chronic diseases | ||
| SSRIs (n = 15,596) vs unmedicated psychiatric illness (n = 9,537) | 1.20 | 0.97 to 1.49 | ||||||
| Specific developmental disorders of scholastic skills (ICD-10 code F81) | SSRIs vs unexposed | 1.09 | 0.69 to 1.73 | |||||
| SSRIs vs unmedicated psychiatric illness | 1.00 | 0.63 to 1.59 | ||||||
| Specific developmental disorder of motor function (ICD-10 code F82) | SSRIs vs unexposed | 1.26 | 0.90 to 1.77 | |||||
| SSRIs vs unmedicated psychiatric illness | 1.18 | 0.81 to 1.72 | ||||||
| El Marroun et al 201442 | 4–9 y | Pervasive developmental problems (CBCL score, 1.5–5) | SSRIs (n = 69) vs unexposed (n = 5,531) | 1.91 | 1.13 to 3.47 | Maternal age at intake, child’s sex, maternal education, ethnicity, smoking, gestational age maternal depressive symptoms at 3 y | ||
| SSRIs (n = 69) vs unmedicated depression (n = 376) | 1.33 | 0.68 to 2.57 | ||||||
| Autistic traits (SRS) | SSRIs vs unexposed | β = 0.15 | 0.08 to 0.22 | |||||
| SSRIs vs unmedicated depression | β = 0.10 | 0.02 to 0.18 | ||||||
| Affective problems (CBCL score, 1.5–5) | SSRIs vs unexposed | 1.37 | 0.87 to 2.16 | |||||
| El Marroun et al 201743 | 4 y | Executive functioning (BRIEF) | SSRIs (n = 71) vs unexposed (n = 5,427) | 5.58 | 0.98 to 10.25 | Maternal education, cognitive ability, ethnicity, smoking, child’s age and sex, birth weight, maternal depressive symptoms at 3 y | ||
| SSRIs vs unexposed (corrected for depressive symptoms during pregnancy) | 3.43 | −1.29 to 8.16 | ||||||
| 6 y | Nonverbal IQ (SON-R) | SSRIs (n = 71) vs unexposed (n = 5,427) | −0.48 | −4.13 to 3.17 | ||||
| SSRIs (n = 71) vs unmedicated depression (n = 385) | 0.12 | −4.11 to 4.36 | ||||||
| 7 y | Attention and executive functioning (NEPSY-II) | SSRIs vs unexposed | −0.06 | −0.34 to 021 | ||||
| SSRIs vs unexposed (corrected for depressive symptoms during pregnancy) | −0.05 | −0.33 to 0.23 | ||||||
| Language (NEPSY-II) | SSRIs vs unexposed | -0.40 | -0.65 to −0.15 | |||||
| SSRIs vs unexposed (corrected for depressive symptoms during pregnancy) | -0.45 | -0.70 to −0.19 | ||||||
| Memory and learning (NEPSY-II) | SSRIs vs unexposed | 0.04 | −0.21 to 0.30 | |||||
| SSRIs vs unexposed (corrected for depressive symptoms during pregnancy) | 0.02 | −0.24 to 0.28 | ||||||
| Visuospatial functioning (NEPSY-II) | SSRIs vs unexposed | 0.02 | −0.23 to 0.27 | |||||
| SSRIs vs unexposed (corrected for depressive symptoms during pregnancy) | 0.02 | −0.24 to 0.27 | ||||||
| Sensory motor functioning (NEPSY-II) | SSRIs vs unexposed | 0.16 | −0.10 to 0.42 | |||||
| SSRIs vs unexposed (corrected for depressive symptoms during pregnancy) | 0.17 | −0.09 to 0.44 | ||||||
| Grzeskowiak et al 201648 | 7 y | Total difficulties score (SDQ)—abnormal score | Antidepressants (n = 395) vs unexposed (n = 79,238) | 0.68 | 0.34 to 1.36 | Child’s sex, maternal age at birth, parity, smoking, alcohol use, socioeconomic status, maternal antenatal mood | ||
| Antidepressants (n = 395) vs unmedicated depression (n = 474) | 0.84 | 0.31 to 2.31 | ||||||
| Johnson et al 201628 | 25–5.5 y | Expressive language skills (TELD) | SRIs (n = 102) vs unexposed (n = 76) | β = −0.15a | -0.19 to −0.02 | Maternal education, prenatal depressive symptoms, prenatal mood episodes, prenatal caffeine, alcohol, smoking, gestational age, Apgar score, child age, cognitive ability | ||
| Cognitive functioning (DAS) | SRIs vs unexposed | … | NSb | NA | ||||
| Pervasive Developmental Disorder Subscale (alternate caregiver-rated CBCL) | SRIs vs unexposed | β = 0.17a | <0.01 to < 0.01 c | Maternal epilepsy, no. of children in the home | ||||
| Pervasive Developmental Disorder Subscale (mother-rated CBCL) | SRIs vs unexposed | β = 0.16a | <0.01 to < 0.01c | Preschool maternal depressive symptoms, postpartum antidepressants, prenatal tobacco | ||||
| Kragholm et al 201853 | < 7 y | Special education needs in elementary school | SSRIs (n = 3,214) vs unexposed (history of SSRI use) (n = 3,536) | 1.12 | 0.82 to 1.55 | Maternal age at birth, maternal education, birth year, diabetes prior to pregnancy, gestational diabetes, maternal hypertension, preeclampsia, smoking, Apgar score, birth weight relative to gestational length/age, preterm birth | ||
| Delayed entry in elementary school | SSRIs vs unexposed (history of SSRI use) | 1.17 | 0.99 to 1.38 | |||||
| Lupattelli et al 201856 | 5 y | Internalizing behavior (CBCL) | SSRIs late pregnancy (n = 290) vs unexposed with a history of SSRI use (n = 3,775) | 0.39 | −0.25 to 1.02 | Maternal BMI, parity, maternal education, gross yearly income, marital status, folic acid use, smoking, alcohol use, illicit substance use, paternal education, analgesics, anxiolytics and sedatives, antipsychotics, non-SSRI antidepressants, severity of maternal depressive and anxiety symptoms in pregnancy, lifetime history of major depression | ||
| Emotionally reactive (CBCL) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.05 | −0.50 to 0.61 | |||||
| Anxious/depressed (CBCL) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.50 | 0.04 to 0.95 | |||||
| Somatic complaints (CBCL) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.27 | −0.30 to 0.84 | |||||
| Externalizing behavior (CBCL) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.21 | −0.40 to 0.82 | |||||
| Attention problems (CBCL) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.26 | −0.64 to 1.17 | |||||
| Aggressive behavior (CBCL) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.07 | −0.30 to 0.45 | |||||
| Activity (EAS) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.13 | −0.31 to 0.57 | |||||
| Emotionality (EAS) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.12 | −0.60 to 0.85 | |||||
| Shyness (EAS) | SSRIs late pregnancy vs unexposed with a history of SSRI use | −0.08 | −0.79 to 0.63 | |||||
| Sociability (EAS) | SSRIs late pregnancy vs unexposed with a history of SSRI use | 0.25 | −0.42 to 0.92 | |||||
| Misri et al 200660 | 4–5 y | Internalizing behavior (parent-rated CBCL) | SSRIs (n = 22) vs unexposed (n = 14) | 0.99 | 0.13 to 7.88 | Maternal depression | ||
| Internalizing behavior (alternate caregiver-rated CBCL) | SSRIs vs unexposed | 2.85 | 0.26 to 31.20 | |||||
| Child’s irritability (Crowell) | SSRIs vs unexposed | 0.65 | 0.07 to 5.69 | |||||
| Child’s withdrawal (Crowell) | SSRIs vs unexposed | 2.45 | 0.60 to 10.00 | |||||
| Child’s positivity (Crowell) | SSRIs vs unexposed | 0.46 | 0.10 to 1.98 | |||||
| Galbally et al 201545 |
4 y | Full scale IQ (WPPSI) | Antidepressants (n = 20) vs unexposed (n = 21) | 115.40 | … | 115.76 | .92 | None. Authors report that results do not change when controlling for maternal depression in pregnancy and gestational ageb |
| Behavior (BRIEF-P) | Antidepressants vs unexposed | 88.15 | … | 84.95 | .53 | |||
| Movement ABC | Antidepressants vs unexposed | 80.40 | … | 83.43 | .45 | |||
| Internalizing behavior (CBCL) | Antidepressants vs unexposed | 6.05 | … | 6.45 | .81 | |||
| Externalizing behavior (CBCL) | Antidepressants vs unexposed | 9.30 | … | 9.25 | .98 | |||
| Hanley et al 201549 | 6 y | Externalizing behaviors (HBQ) | SRI s (n = 44) vs u nexposed (n = 66) | 0.26 | … | 0.29 | .53 | Concurrent maternal depression during the second and third trimester and 6 mo postpartum, cesarean section, child’s sex, other psychometric medications during pregnancy, alcohol use during pregnancy, education |
| Internalizing behaviors (HBQ) | SRIs vs unexposed | 0.39 | … | 0.29 | .04 | |||
| Overanxious (HBQ) | SRIs vs unexposed | 0.46 | … | 0.38 | .13 | |||
| Inattention (HBQ) | SRIs vs unexposed | 0.53 | … | 0.61 | .31 | |||
| Hermansen et al 201651 | 5–6 y | Reasoning (WPPSI-R) | SSRIs (n = 28) vs unmedicated depression (n=42) vs unexposed (n = 33) | 10.92 | 11.55 | 11.06 | .35 | None |
| Similarities (WPPSI-R) | SSRIs vs unmedicated depression vs unexposed | 11.36 | 11.48 | 10.94 | .70 | |||
| Vocabulary (WPPSI-R) | SSRIs vs unmedicated depression vs unexposed | 12.19 | 12.62 | 12.78 | .66 | |||
| Block design (WPPSI-R) | SSRIs vs unmedicated depression vs unexposed | 9.14 | 9.79 | 9.82 | .66 | |||
| Visual attention (NEPSY-II) | SSRIs vs unmedicated depression vs unexposed | 4.52 | 4.44 | 4.58 | .55 | |||
| Statue (NEPSY-II) | SSRIs vs unmedicated depression vs unexposed | 3.68 | 3.69 | 3.58 | .90 | |||
| Behavior (CBCL) | SSRIs vs unmedicated depression vs unexposed | 50.93 | 49.46 | 43.63 | .06 | |||
| Hermansen et al 201752 | 4–7 y | Interference suppression (reaction time on a flanker task) | SSRIs (n = 21) vs unmedicated depression (n = 33) vs unexposed (n = 26) | 1,271 | 1,230 | 1,295 | NSb | None |
| Interference suppression (LSW amplitude and latency—flanker task) | SSRIs vs unmedicated depression vs unexposed | … | … | … | NSb | |||
| Conflict monitoring (N2 latency and amplitude—flanker task) | SSRIs vs unmedicated vs unexposed | … | … | … | NSb | |||
| Nulman et al 201264 | 3–7 y | Full scale IQ (WPPSI) | SSRIs (n = 62) vs unexposed (n = 62) | 105 | … | 112 | < .05 | Antidepressant dose, duration of antidepressant treatment during pregnancy, severity of depression during depression and at the time of testing, maternal IQ, child’s age and sex |
| SSRIs (n = 62) vs unmedicated (n = 54) | 105 | 108 | … | NSb | ||||
| Venlafaxine (n = 62) vs unexposed (n = 62) | 105 | … | 112 | < .01 | ||||
| Verbal IQ (WPPSI) | SSRIs vs unexposed | 107 | … | 113 | < .05 | |||
| SSRIs vs unmedicated | 107 | 109 | … | NSb | ||||
| Venlafaxine vs unexposed | 106 | … | 113 | < .01 | ||||
| Performance IQ (WPPSI) | SSRIs vs unexposed | 102 | … | 108 | < .05 | |||
| SSRIs vs unmedicated | 102 | 105 | … | NSb | ||||
| Venlafaxine vs unexposed | 103 | … | 108 | NSb | ||||
| Behavior (CBCL) | Antidepressants vs unexposed | … | … | … | NSb | |||
| Nulman et al 201563 | 3–7 y | Full scale IQ (WPPSI) | SRIs (n = 45) vs unexposed siblings (n=45) | 103 | … | 106 | .30 | Child’s age, birth order, severity of depression during pregnancy |
| Verbal IQ (WWPPSI) | SRIs vs unexposed siblings | 104 | … | 107 | .25 | |||
| Performance IQ (WPPSI) | SRIs vs unexposed siblings | 101 | … | 103 | .28 | |||
| Internalizing problems (CBCL) | SRIs vs unexposed siblings | 5 | … | 3 | .46 | |||
| Externalizing problems (CBCL) | SRIs vs unexposed siblings | 5 | … | 5 | 1.00 | |||
| Total problems (CBCL) | SRIs vs unexposed siblings | 6 | … | 4 | .48 | |||
| Oberlander et al 200765 | 4–5 y | Externalizing problems (CBCL) | SSRIs (n = 22) vs unexposed (n = 14) | 49.8 | … | 48.4 | NSb | None |
| ADHD problems (CBCL) | SSRIs vs unexposed | 52.3 | … | 53.1 | NSb | |||
| Oppositional defiant problems (CBCL) | SSRIs vs unexposed | 56.1 | … | 50.8 | NSb | |||
| Attention problems (CBCL) | SSRIs vs unexposed | 51.5 | … | 53.8 | NSb | |||
| Aggressive behavior (CBCL) | SSRIs vs unexposed | 55.6 | … | 53.7 | NSb | |||
| Externalizing problems (TRF) | SSRIs vs unexposed | 49.6 | … | 48.2 | NSb | |||
| ADHD problems (TRF) | SSRIs vs unexposed | 52.9 | … | 53.1 | NSb | |||
| Oppositional defiant problems (TRF) | SSRIs vs unexposed | 54.0 | … | 54.5 | NSb | |||
| Attention problems (TRF) | SSRIs vs unexposed | 52.8 | … | 52.3 | NSb | |||
| Aggressive behavior (TRF) | SSRIs vs unexposed | 53.2 | … | 54.2 | NSb | |||
| Movement (Crowell) | SSRIs vs unexposed | 110.8 | … | 103.2 | NSb | |||
| Aggressiveness (Crowell) | SSRIs vs unexposed | 8.1 | … | 7.6 | NSb | |||
| Attention (Crowell) | SSRIs vs unexposed | 11.9 | … | 12.4 | NSb | |||
| Emotion (Crowell) | SSRIs vs unexposed | 6.0 | … | 5.9 | NSb | |||
| Partridge et al 201666 | 4–5 y | Visual-motor coordination (no. of MUs) | SRIs (n = 15) vs unmedicated (n = 10) vs unexposed (n = 15) | 2.63 | 2.54 | 2.40 | .66 | Maternal level of depression at study visit, child’s mean age at study visit, gestational age |
| Visual-motor coordination (Proportion of time to the end of first MU) | SRIs vs unmedicated vs unexposed | 0.22 | 0.23 | 0.25 | .13 | |||
| Visual-motor coordination (peak velocity in cm/s) | SRIs vs unmedicated vs unexposed | 64.2 | 61.0 | 66.3 | .38 | |||
| Visual-motor coordination (proportion of maximum aperture) | SRIs vs unmedicated vs unexposed | 0.65 | 0.60 | 0.63 | .20 | |||
| Visual-motor coordination (phase duration in ms) | SRIs vs unmedicated vs unexposed | 449 | 382 | 428 | .29 | |||
| Visual-motor coordination (no. of minor MUs) | SRIs vs unmedicated vs unexposed | 2.71 | 2.72 | 2.75 | 1.00 | |||
| Visual-motor coordination (straightness) | SRIs vs unmedicated vs unexposed | 1.57 | 1.47 | 1.48 | .24 | |||
| Fine motor control (no. of MUs) | SRIs vs unmedicated vs unexposed | 3.16 | 2.90 | 2.91 | .59 | |||
| Fine motor control (proportion of time to the end of first MUs) | SRIs vs unmedicated vs unexposed | 0.19 | 0.17 | 0.18 | .67 | |||
| Fine motor control (peak velocity in cm/s) | SRIs vs unmedicated vs unexposed | 56.9 | 58.2 | 61.8 | .39 | |||
| Fine motor control (proportion of maximum aperture) | SRIs vs unmedicated vs unexposed | 0.66 e | 0.58 e | 0.64 | .003 | |||
| Fine motor control (phase duration in ms) | SRIs vs unmedicated vs unexposed | 655 e | 441 e,f | 581 | < .001 | |||
| Fine motor control (no. of minor MUs) | SRIs vs unmedicated vs unexposed | 3.85 | 2.98 | 3.75 | .19 | |||
| Fine motor control (straightness) | SRIs vs unmedicated vs unexposed | 1.68 e | 1.53 e,f | 1.66 | .004 | |||
| Weikum et al 201362 | 6 y | Internalizing symptoms (HBQ) | SRIs (n = 26) vs unexposed (n = 38) | 0.33 | … | 0.33 | .62 | Child’s age, prenatal (third trimester) and postnatal (6 y) maternal mood |
| Externalizing symptoms (HBQ) | SRIs vs unexposed | 0.23 | … | 0.29 | .02 | |||
| ADHD symptoms (HBQ) | SRIs vs unexposed | 0.47 | … | 0.67 | .03 | |||
| Executive functioning—reaction time and accuracy (H&F) | SRIs vs unexposed | … | … | … | NSb |
Values for Johnson et al28 are standardized. Other adjusted outcomes are relative risks unless otherwise noted.
Exact numbers not reported.
Unstandardized.
AD = antidepressant group.
SRI vs unmedicated group, P < .05.
Unmedicated vs control group, P < .05.
Abbreviations: ADHD = attention-deficit/hyperactivity disorder; BMI = body mass index; BRIEF = Behavior Rating Inventory of Executive Functioning–Preschool Version; CBCL = Child Behavior Checklist; Crowell = Crowell procedure; DAS = Differential Ability Scales; EAS = Emotionality, Activity and Sociability Temperament Questionnaire; HBQ = MacArthur Health and Behavior Questionnaire; H&F = Hearts & Flowers flanker task; LSW = late slow wave; Movement ABC = Movement Assessment Battery for Children; MU = movement unit; NA = not applicable; NEPSY-II = Developmental NEuroPSYchological Assessment; NS = not specified; SDQ = Strengths and Difficulties Questionnaire; SON-R = Snijders-Oomen Niet-verbale intelligentie Test-Revisie; SRI = serotonin reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TELD = Test of Early Language Development; TRF = Child Behavior Checklist-Teacher Report Form; WPPSI = Wechsler Preschool and Primary Scale of Intelligence.
Internalizing and externalizing behavior.
We identified 7 studies45,48,49,56,62–64 examining both externalizing and internalizing behavior, 4 studies28,42,51,65 investigating only externalizing behavior only, and 1 study60 assessing only internalizing behavior following in utero antidepressant exposure (Table 3). Except for 3 case-control studies,45,49,60 all studies were longitudinal cohort studies.28,42,48,51,56,62–65 Two analyses49,62 used the Health and Behavior Questionnaire to assess externalizing and internalizing behavior, 1 study48 used the Strength and Difficulties Questionnaire (SDQ), and 9 studies28,42,45,51,56,60,63–65 assessed externalizing and internalizing behavior using the Child Behavior Checklist (CBCL).
A positive association between antidepressant exposure and externalizing behavior was reported in 3 studies,28,42,62 including a total of 197 individuals prenatally exposed to SRIs and 5,645 unexposed individuals. One study,49 including 44 individuals exposed to SRIs in utero and 66 unexposed individuals, showed a positive association between antidepressant exposure and internalizing behavior as assessed by the CBCL. Eight other studies,45,48,51,56,60,63–65 including a total of 946 children exposed to antidepressants, 570 children exposed to untreated depression, and 83,202 unexposed children, did not show a relationship between in utero antidepressant exposure and internalizing and/or externalizing behavior.
Intelligence quotient.
Of the 5 prospective studies that examined IQ scores,43,45,51,63,64 only 1 study showed a small significant difference in IQ scores in children exposed to antidepressants in utero64 (Table 3). Nulman et al64 compared children of depressed women taking venlafaxine during pregnancy (n = 62) and children of depressed women taking SSRIs during pregnancy (n = 62) to children of untreated depressed pregnant women (n = 54) and children of nondepressed, healthy pregnant women (n = 62). Children of nondepressed mothers had significantly higher full-scale and verbal IQs than those in the venlafaxine and SSRI groups. However, after correcting for covariates, dose and duration of antidepressant use during pregnancy did not predict child IQ score. This finding suggests that factors other than antidepressant exposure during pregnancy predict children’s intellect, including maternal IQ and sex of the child. The other 4 studies,43,45,51,63 together reporting on 164 children exposed to antidepressants in utero, observed no association between antidepressant exposure and IQ scores in children between 3 and 7 years of age.
Viktorin et al33 examined the association between antidepressant exposure in utero and intellectual disability (ICD-10 codes F70–F79). No significant association was observed after adjustment for potential confounding factors.
Motor development.
Five studies examined motor development in the offspring; 4 prospective studies43,45,65,66 and 1 retrospective study39 (Table 3). Brown et al39 used an ICD-10 diagnosis of specific developmental disorders of motor development as outcome, while the prospective studies used a kinematic task of visual motor and fine motor function, the Movement Assessment Battery for Children, the sensorimotor function of the Developmental NEuroPSYchological Assessment, and the movement subscale of the Crowell procedure.43,45,65,66 Of the 4 prospective studies, together reporting on 128 antidepressant-exposed children between the ages of 4 and 7 years, only 1 study66 reported significant findings. Partridge et al66 performed a kinematic task of visual motor and fine motor functions at ages 4–5 years in SRI-exposed children (n = 15), children of untreated depressed pregnant women (n = 10), and a control group (n = 15). Children with prenatal SRI exposure had poorer fine motor control compared with children who were not exposed to SRIs.
Speech, language, and scholastic outcomes.
One prospective28 and 2 retrospective studies39,53 reported on speech, language, and scholastic outcomes (Table 3). Johnson et al28 examined 178 mother-child dyads, including 102 dyads with in utero SRI exposure, with the Expressive Language subtest of the Test of Early Language Development, 3rd edition (TELD-3). There was a modest mean difference of approximately 5 points in Expressive Language scores in favor of non-SRI exposure. Brown et al39 examined specific developmental disorders of speech and language and specific developmental disorders of scholastic skills according to ICD-10 diagnosis. Children exposed to SSRIs in utero had a significant increase in the risk of speech-language disorders compared to unexposed children. No overall association was found between in utero SSRI exposure and special education needs or delayed school start.53
Psychiatric Disorders
Autism spectrum disorders.
We identified 7 studies—1 case-control study,50 2 health record analyses,40,41 and 4 register-based cohort studies34,37,57,67—that reported on the association between in utero antidepressant exposure and autism spectrum disorders (ASDs) (Table 4). All 4 register-based cohort studies34,37,57,67 reported a significant association between in-utero antidepressant exposure and risk for ASDs, with relative risks (RRs) ranging from 1.23 to 4.39. Three studies39,41,50 did not find a significant association. All, except 1 study,50 based their outcome definition on the ICD diagnoses of ASD. Harrington et al50 employed the Autism Diagnostic Interview–Revised and the Autism Diagnostic Observation Schedule.
Table 4.
Results for Neurodevelopmental Disordersa
| Study | Child Age at Testing | Outcome | Comparison Groups | Adjusted Outcome | 95% CI | Covariates |
|---|---|---|---|---|---|---|
| Boukhris et al 201637 | 0–11 y | ASD (ICD-9 code 299 and ICD-10 code F84) | ADs in second and/or third trimester (n = 2,532) vs unexposed (n = 142,924) | 1.87 | 1.15 to 3.04 | AD use 1 y before the first day of gestation, antidepressant use in 1 st trimester, chronic/gestational hypertension, chronic/gestational diabetes, other psychiatric disorders, year of birth, child’s sex, maternal age at first day of gestation, level of education recipient of social assistance, living alone |
| Combined use of ADs (≥ 2 classes) in second and/or third trimester (n = 167) vs unexposed (n = 142,924) | 4.39 | 1.44 to 13.32 | ||||
| Boukhris et al 201738 | 0–11 y | ADHD (ICD-9 code 314 and ICD-10 code F90; or ≥ 1 prescription for ADHD medications [ATC codes: N06BA02, N06BA04, N06BA01, N06BA09]) | SSRIs in second and/or third trimester (n = 1,561) vs. unexposed (n = 141,905) | 1.2 | 0.9 to 1.6 | AD use in first trimester, chronic/gestational hypertension, chronic/gestational diabetes, other psychiatric disorders, maternal history of depression/anxiety, maternal history of ADHD, year of birth, child’s sex, maternal age at first day of gestation, level of education, recipient of social assistance, living alone, area of residence on first day of gestation |
| Combined use of ADs (≥ 2 classes) in second and/or third trimester (n = 162) vs unexposed (n = 141,905) | 1.5 | 0.8 to 2.8 | ||||
| Brown et al 201740 | 2–12 y | ASD (≥ 2 outpatient diagnoses by pediatrician/psychiatrist, ≥ 1 diagnosis in hospital databases after age 2 years, or both, using ICD-9 codes 299 and ICD-10 codes F84) | SSRIs (n = 16,906.1)a vs unexposed (n = 18,999.9)b | 1.61 | 0.997 to 2.59 | Inverse probability of treatment weighting based on a high-dimensional propensity score to balance exposure group differences, measuring a battery of surrogate variables (diagnoses, procedures, drug claims) to control for relevant unobserved confounders |
| SSRIs (n = 620) vs unexposed siblings (n = 620) | 1.60 | 0.69 to 3.74 | ||||
| Castro et al 201641 | 2–19 y | ASD (ICD-9 code 299 and ICD-10 code F84) | ASD (n = 1,245) vs healthy controls (n = 3,405) | 0.90 | 0.50 to 1.54 | Past history of maternal depression, year of birth, child’s sex, race/ethnicity, insurance type, median income tertile |
| ADHD (ICD-9 code 314) | ADHD (n = 1,701) vs healthy controls (n = 3,405) | 0.97 | 0.53 to 1.69 | |||
| Figueroa 201044 | >5 y | ADHD (insurance claims with a primary or secondary diagnosis of ADHD and prescription claims for stimulants) | SSRIs (n = 916) vs unexposed (n = 29,329) | 0.91 | 0.51 to 1.60 | Year of birth, child’s sex, maternal age, urbanicity, age at last claim, age at end of eligibility |
| Harrington et al 201450 | 2–5 y | ASD (ADI-Rand ADOS) | ASD (n = 492) vs typically developing controls (n = 320) | 1.55 | 0.59 to 4.08 | Inverse probability weights, child’s birth year, regional center, and mother’s birthplace |
| Developmental delays other than ASD (Vineland Adaptive Behavior Scales and Mullen Scales of Early Learning) | Developmental delays (n = 154) vs typically developing controls (n = 320) | 1.62 | 0.59 to 4.42 | |||
| Laugesen et al 201354 | 0–14 y | ADHD (ICD-8, ICD-9 and ICD-10; or prescription for ADHD medication) | Any AD (n = 15,008) vs unexposed (n = 816,792) | 1.2 | 1.1 to 1.4 | Maternal and paternal psychiatric diagnoses, maternal diseases during pregnancy (infections, epilepsy), maternal anxiolytics, hypnotics, sedatives use, maternal smoking, calendar time at birth, birth order, child’s sex, maternal age at delivery |
| SSRIs (n = 11,721) vs unexposed (n = 816,792) | 1.2 | 1.0 to 1.5 | ||||
| AD (n = 330) vs unexposed siblings (n = 330) | 0.7 | 0.4 to 1.4 | ||||
| Liu et al 201734 | 0–16.5 y | First diagnosis of psychiatric disorders (ICD-10 codes F00-F99) | AD continuation† (n = 17,560) vs unexposed (n = 854,241) | 1.64 | 1.53 to 1.76 | Inverse probability weights, maternal and paternal psychiatric history at delivery, maternal age at delivery, inpatient and outpatient psychiatric treatment from 2 y before pregnancy until delivery, prescriptions for other psychotropic and antiepileptic drugs during pregnancy, no. of nonpsychiatric hospital visits during pregnancy, smoking during pregnancy, primiparity, calendar year of delivery, place of residence, marital status, highest education, income status |
| AD continuation† (n = 17,560) vs AD discontinuation‡ (n = 30,079) | 1.27 | 1.17 to 1.38 | ||||
| ASD (ICD-10 code F84) | AD continuation† vs unexposed | 1.82 | 1.54 to 2.15 | |||
| AD continuation† vs AD discontinuation‡ | 1.23 | 1.01 to 1.51 | ||||
| Mood disorder (F30-F39) | AD continuation† vs unexposed | 3.20 | 2.12 to 4.81 | |||
| AD continuation† vs AD discontinuation‡ | 2.76 | 1.59 to 4.78 | ||||
| Neurotic, stress-related and somatoform disorder (F40-F48) | AD continuation† vs unexposed | 2.40 | 2.08 to 2.76 | |||
| AD continuation† vs AD discontinuation‡ | 1.62 | 1.36 to 1.94 | ||||
| Behavioral and emotional disorder (F90-F98) | AD continuation† vs unexposed | 1.49 | 1.35 to 1.65 | |||
| AD continuation† vs AD discontinuation‡ | 1.13 | 1.01 to 1.27 | ||||
| Mental retardation (F70-F79) | AD continuation† vs unexposed | 1.21 | 0.83 to 1.74 | |||
| AD continuation† vs AD discontinuation‡ | 1.21 | 0.78 to 1.90 | ||||
| Malm et al 201657 | 0–14 y | Depression (ICD-10 codes F32–39) | SSRI continuation† (n = 15,729) vs unexposed (n = 31,394) | 2.61 | 1.59 to 4.28 | Maternal and paternal psychiatric history, maternal history of substance abuse, smoking during pregnancy, child’s sex, socioeconomic status |
| SSRI continuation† (n = 15,729) vs SSRI discontinuation‡ (n = 7,980) | 1.84 | 1.14 to 2.97 | ||||
| SSRI continuation† (n = 15,729) vs untreated mental illness (n = 9,651) | 1.78 | 1.12 to 2.82 | ||||
| Anxiety (ICD-10 codes F40–41) | SSRI continuation† vs unexposed | 1.74 | 1.16 to 2.61 | Maternal psychiatric history, maternal use of antiepileptic drugs, smoking during pregnancy, parental death, preterm birth, low birth weight, child’s sex, marital status at delivery | ||
| SSRI continuation† vs SSRI discontinuation‡ | 1.53 | 0.94 to 2.50 | ||||
| SSRI continuation† vs untreated mental illness | 1.30 | 0.84 to 2.01 | ||||
| ASD (ICD-10 code F84) | SSRI continuation† vs unexposed | 1.40 | 1.02 to 1.92 | Maternal chronic disease, preterm birth, neonatal care unit, child’s sex, maternal age | ||
| SSRI continuation† vs SSRI discontinuation‡ | 1.30 | 0.88 to 1.92 | ||||
| SSRI continuation† vs untreated mental illness | 0.88 | 0.65 to 1.20 | ||||
| ADHD (ICD-10 code F90) | SSRI continuation† vs unexposed | 1.66 | 1.27 to 2.16 | Maternal and paternal history of psychiatric disorder, maternal substance abuse, smoking during pregnancy, parental death, neonatal care unit, child’s sex, socioeconomic status | ||
| SSRI continuation† vs SSRI discontinuation‡ | 0.98 | 0.75 to 1.27 | ||||
| SSRI continuation† vs untreated mental illness | 0.98 | 0.77 to 1.24 | ||||
| Man et al 201758 | 6–14 y | ADHD (ICD-9-CM code 314, or a prescription for methylphenidate or atomoxetine) | AD (n = 1,252) vs unexposed (n = 189,002) | 1.39 | 1.07 to 1.82 | Maternal preexisting diabetes, epilepsy, gestational diabetes, psychiatric conditions, hypertension, use of antipsychotics, birth year, birth hospital, parity, child’s sex, socioeconomic status, maternal age at delivery |
| SSRI (n = 425) vs unexposed (n = 189,002) | 1.11 | 0.77 to 1.60 | ||||
| AD (n = 1,252) vs AD discontinuation‡ (n = 1,486) | 0.75 | 0.51 to 1.10 | ||||
| AD (n = 26,808) vs unexposed sibling (n = 26,808) | 0.54 | 0.17 to 1.74 | ||||
| Sujan et al 201767 | 0–15 y | ASD (ICD-9 codes 299 and ICD-10 codes F84) | First trimester AD (n = 22,544) vs unexposed (n = 1,558,085) | 1.64 | 1.46 to 1.83 | Maternal preexisting diabetes, epilepsy, gestational diabetes, psychiatric conditions, hypertension, use of antipsychotics, birth year, birth hospital, parity, child’s sex, socioeconomic status, maternal age at delivery |
| First trimester SSRI (18,470) vs unexposed (n = 1,558,085) | 1.66 | 1.46 to 1.89 | ||||
| First trimester AD (n = 10,976) vs unexposed sibling (n = 13,994) | 0.83 | 0.62 to 1.13 | ||||
| ADHD (ICD-9 codes 314 and ICD-10 codes F90) | First trimester AD vs unexposed | 1.58 | 1.46 to 1.71 | |||
| First trimester SSRI vs unexposed | 1.60 | 1.47 to 1.75 | ||||
| First trimester AD vs unexposed sibling | 0.99 | 0.79 to 1.25 |
Estimates in bold are statistically significant. Adjusted outcomes are adjusted relative risks.
Pseudopopulation based on a high-dimensional propensity score (observed population: AD users n = 2,837 and nonusers n = 33,069).
Children whose mothers used antidepressant medication both before and during pregnancy.
Children whose mothers used antidepressant medication before but not during pregnancy.
Abbreviations: AD = antidepressant, ADHD = attention-deficit/hyperactivity disorder, ADI-R = Autism Diagnostic Interview–Revised, ADOS = Autism Diagnostic Observation Schedule, ASD = autism spectrum disorder.
The case-control study50 compared children with ASD (n = 492) to typically developing controls (n = 320) on self-reported maternal SSRI use during pregnancy. Children with ASDs were not more likely to have been exposed to SSRIs during pregnancy (5.9%) than typically developing children (3.4%). Restricting the analysis to boys produced a significant association (OR = 2.92; 95% CI, 1.07–7.93), but further reduced the already small number of children exposed to SSRIs in utero (n = 40; for boys, n = 32).
The 2 health record analyses40,41 employed diverging methods. One study41 identified individuals with an ASD diagnosis (n = 1,245) and compared them to typically developing controls (n = 3,405). The other study40 compared individuals prenatally exposed to SSRIs (n = 2,837) both to unexposed controls (n = 33,069) and to an unexposed sibling (n = 620). Neither of these studies reported a significant association between in utero antidepressant exposure and risk for ASDs.
The 4 register-based cohort studies34,37,57,67 reporting a significant association included a total of 58,365 individuals prenatally exposed to antidepressants and compared them to a total of 2,586,644 unexposed individuals. To control for confounding by indication, Liu et al34 and Malm et al57 further compared the antidepressant-exposed individuals to a total of 38,059 individuals whose mothers had taken antidepressant medication before, but not during, pregnancy (discontinuation group). Malm et al57 added an additional comparison group of 9,651 individuals who were exposed to untreated mental illness in utero. Sujan et al67 carried out a discordant sibling analysis. These attempts at controlling for confounding by indication yielded mixed results. While Liu et al34 reported a small significant association between antidepressant exposure and ASDs, Malm et al57 and Sujan et al67 did not find significant associations.
Attention-deficit/hyperactivity disorder.
We identified 7 studies, 1 insurance data analysis,44 2 health record analyses,41,58 and 4 register-based cohort studies,38,54,57,67 that examined the association between attention-deficit/hyperactivity disorder (ADHD) and in utero antidepressant exposure (Table 4). Three54,57,67 of the 4 register-based cohort studies and 1 health record analysis58 reported a significant association between in utero antidepressant exposure and risk for ADHD, with RRs ranging from 1.2 to 1.66. Three studies did not report a significant association.38,41,44 All except 1 study44 based their outcome definition on the ICD diagnoses of ADHD. Figueroa et al44 based ADHD diagnoses on insurance and prescription claims.
Figueroa et al44 investigated the association between in utero SSRI exposures and the presence of ADHD in the child by the age of 5 years (N = 30,245) and found that, while maternal ADHD, depressive disorders, and prenatal bupropion exposure increased the risk for ADHD, in utero SSRI exposure did not.
The 2 health record analyses41,58 employed diverging methods. One study41 identified individuals with an ADHD diagnosis (n = 1,701) and compared them to typically developing controls (n = 3,405). The other study58 compared individuals prenatally exposed to antidepressants (n = 1,252) to unexposed controls (n = 189,002) and a discontinuation group (n = 1,486). In addition, the researchers in each study conducted a post hoc sibling-matched analysis to control for shared genetic and social confounding, including 53,616 children of 26,049 mothers. Castro et al41 did not find a significant association between risk for ADHD and prenatal antidepressant exposure. Man et al58 found a significant association only when comparing the exposed children to unexposed controls, but not when comparing the exposed children to the discontinuation group or in the sibling-matched analyses, which aimed to control for confounding by indication.
The 3 register-based cohort studies54,57,67 reporting a significant association compared a total of 53,281 individuals prenatally exposed to antidepressants to 2,407,290 unexposed individuals. One register-based cohort study38 not showing a significant association included 1,561 individuals exposed to SSRIs in the second and/or third trimester and 141,905 unexposed individuals. To control for confounding by indication, Malm et al57 further compared the antidepressant-exposed individuals to a total of 7,980 individuals whose mothers had used antidepressant medication before, but not during, pregnancy and 9,651 individuals who were exposed to untreated mental illness in utero. In addition, Sujan et al67 and Laugesen et al54 carried out discordant sibling analyses. These attempts at controlling for confounding by indication resulted in the loss of the significant associations between antidepressant exposure and increased risk for ADHD in all 3 studies.
Affective disorders.
Two studies34,57 report on affective disorders diagnosed in adolescence, taking advantage of the long-term follow-up of the Scandinavian national registers (Table 4). Malm et al57 assessed depression and anxiety (ICD diagnoses) in SSRI-exposed offspring compared to unexposed offspring, offspring exposed to untreated mental illness in utero, and offspring whose mothers had used antidepressants before, but not during, pregnancy. Children exposed to SSRIs during gestation (n = 15,729) were at increased risk of developing depression compared to the other exposure groups. There was no association between SSRI exposure and a diagnosis of anxiety disorders.
Liu et al34 investigated the overall risk of psychiatric disorders, as well as affective disorders more specifically (ICD-10 diagnoses), during a maximum follow-up of 16.5 years. Increased risks for affective disorders were seen in children exposed to antidepressants in utero compared to children whose mother discontinued antidepressants before pregnancy.
Quality of Evidence (GRADE Assessment)
The quality of evidence was summarized for all outcomes reported in 5 or more studies. According to GRADE criteria, the overall quality of evidence was considered low for ASD and ADHD and very low for cognition, behavior, IQ, and motor development.
DISCUSSION
On the basis of our comprehensive systematic review of the literature, we found statistically significant associations between in utero exposure to antidepressants and a wide range of physical, neurodevelopmental, and psychiatric outcomes. Yet, the evidence is inconsistent, and the risk of residual confounding, particularly confounding by indication, is high. Across all identified and included studies, the quality of evidence of the examined outcomes was rated low to very low (GRADE36).
In studying the effects of prenatal antidepressant exposure, it is crucial to consider that the indication for antidepressant use, namely maternal psychopathology, has also been shown to increase the risk of adverse outcomes in the child.68 This issue is known as confounding by indication. Maternal psychopathology may assert its effect on the offspring via shared genetic susceptibility, environmental stress, and/or parenting practices.69–71 All but 9 studies44–46,51–53,55,59,65 statistically controlled for some form of maternal psychopathology. Because these statistical adjustments are unlikely to fully control for the source of confounding, some studies have used additional strategies to disentangle the effects of antidepressant use from the effects of the underlying maternal illness. These strategies include comparing antidepressant-exposed individuals to individuals born to mothers who discontinued antidepressants or to individuals born to mothers with untreated mental illness. In addition, discordant sibling designs, which compare exposed individuals to an unexposed sibling, aim to address this issue by controlling for all genetic and environmental factors shared between siblings. In general, attempts to control for confounding by indication led to a decrease in the magnitude of the association between in utero antidepressant exposure and adverse child outcomes. We discuss studies in which the association persisted as follows.
Physical Health Outcomes
Two small studies46,47 found sex-related decreases and increases in the risk of childhood overweight following in utero antidepressant exposure. Prenatal SSRI exposure can alter postnatal components of central serotonergic signaling, a pathway that plays a critical role in regulating mammalian energy homeostasis.72 In animals, fetal and neonatal SSRI exposure has been shown to result in changes consistent with type 2 diabetes and its comorbidities.73 Previous studies on short-term outcomes in humans have also repeatedly found associations of in utero exposure to antidepressants and decreased birth weight.18 Decreased birth weight, in turn, may reflect altered programming of organ structures and associated functions, which can lead to changes that carry into adulthood, including increased risk of diabetes and heart disease.74,75
Interestingly, both animal and human studies indicate a sex difference. In theory, this sex difference could be related to sex hormones, because estrogen is known to influence the serotonergic system76,77 and regulates serotonin transporter expression, enhancing serotonergic signaling pathways.78,79 Indeed, a study in rats80 demonstrated that genes that regulate serotonin signaling and action in the ovary are altered in prenatally SSRI-exposed offspring. These rats had impaired reproductive cycling and an increased number of follicles in the ovary. In humans, menstrual cycling and fecundity have not yet been investigated.
Psychiatric Disorders
Childhood psychiatric disorders, particularly ADHD and ASDs, have received the majority of attention from researchers. At least a dozen systematic reviews and meta-analyses have attempted to examine a causal link between exposure to antidepressants in utero and ADHD and/or ASDs in the offspring, often with inconclusive results (eg, references81 and 82). This popularity very likely results from methodological considerations since ADHD and ASDs usually manifest early in life and may thus be assessed after a relatively short follow-up period.83 An increased risk for ADHD following prenatal exposure to antidepressants was found in 4 large cohort studies38,54,57,67 when comparing exposed to unexposed individuals. This significant association disappeared when these studies attempted to address confounding by indication in their study designs. Moreover, 4 large register-based cohort studies34,37,57,67 reported a significant association between in utero antidepressant exposure and risk for ASDs. Only 1 of these studies34 found that a small but significantly increased risk for ASDs remained in individuals exposed to antidepressants in utero when comparing them to individuals born to mothers who discontinued antidepressants before pregnancy. For all other studies, the significant association between prenatal antidepressant exposure and ASDs disappeared when exposed individuals were compared to individuals born to mothers who discontinued antidepressants, to individuals born to mothers with untreated mental illness, or to unexposed siblings. Moreover, none of these studies was able to take the severity of the underlying maternal illness into account. These results, therefore, suggest that the underlying maternal disorder, rather than in utero antidepressant exposure, may be driving the association between in utero exposure to antidepressants and childhood psychiatric disorders. This association is conceivable, given that psychiatric disorders are highly heritable84 and maternal psychopathology may assert its effects via environmental stress and suboptimal parenting practices.69–71
The average age at onset of most psychiatric disorders, including depression, anxiety, psychosis, and mania, is around late adolescence or early adulthood. This makes it harder to assess these disorders, because longitudinal and register-based studies in existence today have limited follow-up durations. Consequently, only 2 studies34,57 thus far have reported on affective disorders. Interestingly, both studies found that the risk for depression was increased in individuals prenatally exposed to antidepressants, even compared to individuals born to mothers who discontinued antidepressants before pregnancy or individuals born to mothers with untreated mental illness. These findings point to a putative causal relationship between in utero antidepressant exposure and depressive disorder in the offspring. However, comparing antidepressant-exposed individuals to individuals born to mothers who discontinued antidepressants before pregnancy or individuals born to mothers with untreated mental illness may still not be sufficiently addressing confounding by indication, because women who continue antidepressants throughout pregnancy may be fundamentally different from women who discontinue or women who have never used antidepressants. In particular, mothers with severe symptoms are more likely to continue treatment during pregnancy,85 and the severity of symptoms may differ between pregnancies. To address residual confounding by indication, randomized controlled trials, in which women are randomly assigned to an antidepressant continuation or discontinuation (plus cognitive therapy) group, are needed. This approach will ensure that all potential confounding factors are distributed equally among the groups to be compared.
Strengths and Limitations
The literature search was performed by an experienced medical information specialist. Study selection, data extraction, and risk of bias assessments were conducted by two reviewers, ensuring validity and accuracy. However, due to resource limitations, we were unable to include articles published in languages other than English. Moreover, the clinical implications of our findings are limited by the quality of the reviewed studies, including their selection of confounding factors, and the heterogeneity of the investigated outcome measures.
Implications
Approximately 50% of women who take antidepressants before pregnancy decide to discontinue their antidepressants, either before or during pregnancy, due to concerns about the negative consequences for the child. However, evidence indicating a causal relationship between in utero exposure to antidepressants and long-term health of the offspring is limited. Concerns remain given the significant associations of prenatal antidepressant exposure with BMI and affective disorders, the heterogeneity of the literature, and the scarcity of findings on long-term outcomes. As potential negative consequences of psychiatric illness during pregnancy may transcend potential negative consequences of in utero exposure to antidepressants, women and their health care providers must carefully weigh the risks and benefits of antidepressant (dis)continuation during pregnancy. Treatment decisions will need to be tailored to the individual patient, taking her disorder severity, course of illness and psychiatric history, previous experiences with antidepressant discontinuation, and treatment preferences into account. Substantial evidence points to the efficacy of alternative non-pharmacologic interventions, such as cognitive-behavioral therapy and interpersonal therapy, in preventing perinatal depression in at-risk women.86 Women may, therefore, capitalize on these treatment options to minimize risks of exposure to antidepressants and untreated illness and maximize treatment of the disorder. However, judicious use of pharmacotherapy is recommended for women with severe mental illness or a high risk of relapse based on psychiatric history to avoid undertreatment and the resulting exposure to untreated illness.
To date, most clinical guidelines do not give any clear recommendations on antidepressant management during pregnancy.87 It is challenging to formulate such guidelines when the evidence base is weak and inconsistent, and confounding by indication is difficult to avoid. Consequently, future research may use randomization to not only limit confounding by indication but also identify women who may discontinue antidepressant use during pregnancy with a low risk of relapse and women for whom antidepressant treatment is indicated to remain euthymic.88
CONCLUSION
Our comprehensive systematic review of the literature revealed statistically significant associations between in utero exposure to antidepressants and a wide range of physical, neurodevelopmental, and psychiatric outcomes. However, rather than reflecting a causal relationship, most of these associations seem to be driven by the underlying maternal disorder. After limiting confounding by indication, in utero exposure to antidepressants remained significantly associated only with BMI and increased risk for affective disorders.
The literature is heterogeneous, and findings on long-term outcomes are scarce. As time goes on, more data will become available on the long-term effects of prenatal antidepressant exposure, including on physical and mental health outcomes. These data will help to clarify the relationship between prenatal antidepressant exposure and BMI. Future research must also substantiate the association between in utero antidepressant exposure and affective disorders in adolescence and adulthood and investigate the associations between prenatal antidepressant exposure and other psychopathologies arising later in life.
Supplementary Material
Clinical Points.
There are no clear recommendations on antidepressant management during pregnancy because the evidence base, especially on long-term outcomes, is weak and inconsistent. Evidence indicating a causal relationship between in utero exposure to antidepressants and long-term health of the offspring is limited, but concerns remain.
Treatment decisions regarding pregnant depressed patients must take disorder severity, the course of illness, and patient preferences into account.
Acknowledgments:
The authors thank Wichor Bramer, MSc, from Erasmus MC Medical Library, Rotterdam, the Netherlands, for developing the search strategies and Rachel Cohen, BSc, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York, for help with the study selection. Mr Bramer and Mrs Cohen have no conflicts of interest to declare.
Funding/support:
Dr Munk-Olsen is supported by the Lundbeck Foundation (iPSYCH), grant number, R155-2012-11280; and Fabrikant Vilhelm Pedersen og Hustrus Mindelegat. Dr Liu is supported by the Danish Council for Independent Research (DFF-5053-00156B).
Role of the sponsor:
The funders had no role in the conduct and interpretation of this review.
Footnotes
Bergink, Liu, Munk-Olsen, and Molenaar have no conflicts of interest to report.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
- 1.World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2.Ko JY, Rockhill KM, Tong VT, et al. MMWR Morb Mortal Wkly Rep. 2017;66(6):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alder J, Fink N, Bitzer J, et al. J Matern Fetal Neonatal Med. 2007;20(3):189–209. [DOI] [PubMed] [Google Scholar]
- 4.Faisal-Cury A, Menezes PR. Br J Psychiatry. 2012;34(4):446–450. [DOI] [PubMed] [Google Scholar]
- 5.Jarde A, Morais M, Kingston D, et al. JAMA Psychiatry. 2016;73(8):826–837. [DOI] [PubMed] [Google Scholar]
- 6.Verhaak PF, van Dijk CE, Nuijen J, et al. Scand J Prim Health Care. 2012;30(3):156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olfson M, Blanco C, Marcus SC. JAMA Intern Med. 2016;176(10):1482–1491. [DOI] [PubMed] [Google Scholar]
- 8.Pratt LA, Brody DJ, Gu Q. NCHS Data Brief. 2017;(283):1–8. [PubMed] [Google Scholar]
- 9.Jimenez-Solem E, Andersen JT, Petersen M, et al. PLoS One. 2013;8(4):e63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlton RA, Jordan S, Pierini A, et al. BJOG. 2015;122(7):1010–1020. [DOI] [PubMed] [Google Scholar]
- 11.Cooper WO, Willy ME, Pont SJ, et al. Am J Obstet Gynecol. 2007;196(6):544.e1–544.e5. [DOI] [PubMed] [Google Scholar]
- 12.Bakker MK, Kölling P, van den Berg PB, et al. Br J Clin Pharmacol. 2008;65(4):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewing G, Tatarchuk Y, Appleby D, et al. Clin Pharmacokinet. 2015;54(4):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawluski JL. Neuroendocrinology. 2012;95(1):39–46. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima T Neurosci Res. 2018;129:32–39. [DOI] [PubMed] [Google Scholar]
- 16.Lam DD, Garfield AS, Marston OJ, et al. Pharmacol Biochem Behav. 2010;97(1):84–91. [DOI] [PubMed] [Google Scholar]
- 17.Hanley GE, Mintzes B. BMC Pregnancy Childbirth. 2014;14(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Liu Q, Cao S, et al. J Affect Disord. 2018;241:563–570. [DOI] [PubMed] [Google Scholar]
- 19.Zhou XH, Li YJ, Ou JJ, et al. Mol Autism. 2018;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viuff AC, Pedersen LH, Kyng K, et al. Clin Epigenetics. 2016;8(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suri R, Lin AS, Cohen LS, et al. J Clin Psychiatry. 2014;75(10):e1142–e1152. [DOI] [PubMed] [Google Scholar]
- 22.Prady SL, Hanlon I, Fraser LK, et al. Arch Women Ment Health. 2018;21(2):127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell J, Goodman J. Arch Women Ment Health. 2018;21(5):505–516. [DOI] [PubMed] [Google Scholar]
- 24.Masarwa R, Bar-Oz B, Gorelik E, et al. Am J Obstet Gynecol. 2019;220(1):57.e1–57.e13. [DOI] [PubMed] [Google Scholar]
- 25.Grove K, Lewis AJ, Galbally M. Pediatrics. 2018;142(1):e20180356. [DOI] [PubMed] [Google Scholar]
- 26.Casper RC, Fleisher BE, Lee-Ancajas JC, et al. J Pediatr. 2003;142(4):402–408. [DOI] [PubMed] [Google Scholar]
- 27.Oberlander TF, Papsdorf M, Brain UM, et al. Arch Pediatr Adolesc Med. 2010;164(5):444–451. [DOI] [PubMed] [Google Scholar]
- 28.Johnson KC, Smith AK, Stowe ZN, et al. J Clin Psychiatry. 2016;77(2):e176–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross LE, Grigoriadis S, Mamisashvili L, et al. JAMA Psychiatry. 2013;70(4):436–443. [DOI] [PubMed] [Google Scholar]
- 30.Bérard A, Sheehy O, Zhao JP, et al. Br J Clin Pharmacol. 2017;83(5):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udechuku A, Nguyen T, Hill R, et al. Aust N Z J Psychiatry. 2010;44(11):978–996. [DOI] [PubMed] [Google Scholar]
- 32.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. J Clin Psychiatry. 2013;74(4):e293–e308. [DOI] [PubMed] [Google Scholar]
- 33.Viktorin A, Uher R, Kolevzon A, et al. JAMA Psychiatry. 2017;74(10):1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Agerbo E, Ingstrup KG, et al. BMJ. 2017;358:j3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberati A, Altman DG, Tetzlaff J, et al. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyatt GH, Oxman AD, Vist G, et al. J Clin Epidemiol. 2011;64(4):407–415. [DOI] [PubMed] [Google Scholar]
- 37.Boukhris T, Sheehy O, Mottron L, et al. JAMA Pediatr. 2016;170(2):117–124. [DOI] [PubMed] [Google Scholar]
- 38.Boukhris T, Sheehy O, Bérard A. Paediatr Perinat Epidemiol. 2017;31(4):363–373. [DOI] [PubMed] [Google Scholar]
- 39.Brown AS, Gyllenberg D, Malm H, et al. JAMA Psychiatry. 2016;73(11):1163–1170. [DOI] [PubMed] [Google Scholar]
- 40.Brown HK, Ray JG, Wilton AS, et al. JAMA. 2017;317(15):1544–1552. [DOI] [PubMed] [Google Scholar]
- 41.Castro VM, Kong SW, Clements CC, et al. Transl Psychiatry. 2016;6(1):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Marroun H, White TJH, van der Knaap NJF, et al. Br J Psychiatry. 2014;205(2):95–102. [DOI] [PubMed] [Google Scholar]
- 43.El Marroun H, White TJ, Fernandez G, et al. J Psychopharmacol. 2017;31(3):346–355. [DOI] [PubMed] [Google Scholar]
- 44.Figueroa R J Dev Behav Pediatr. 2010;31(8):641–648. [DOI] [PubMed] [Google Scholar]
- 45.Galbally M, Lewis AJ, Buist A. Aust N Z J Psychiatry. 2015;49(7):642–650. [DOI] [PubMed] [Google Scholar]
- 46.Grzeskowiak LE, Gilbert AL, Morrison JL. J Dev Orig Health Dis. 2012;3(4):253–261. [DOI] [PubMed] [Google Scholar]
- 47.Grzeskowiak LE, Gilbert AL, Sørensen TIA, et al. Ann Epidemiol. 2013;23(11):681–687. [DOI] [PubMed] [Google Scholar]
- 48.Grzeskowiak LE, Morrison JL, Henriksen TB, et al. BJOG. 2016;123(12):1919–1928. [DOI] [PubMed] [Google Scholar]
- 49.Hanley GE, Brain U, Oberlander TF. Pediatr Res. 2015;78(2):174–180. [DOI] [PubMed] [Google Scholar]
- 50.Harrington RA, Lee LC, Crum RM, et al. Pediatrics. 2014;133(5):e1241–e1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermansen TK, Røysamb E, Augusti EM, et al. Psychopharmacology (Berl). 2016;233(8):1523–1535. [DOI] [PubMed] [Google Scholar]
- 52.Hermansen TK, Yrttiaho S, Røysamb E, et al. Psychopharmacology (Berl). 2017;234(3):339–351. [DOI] [PubMed] [Google Scholar]
- 53.Kragholm K, Andersen MP, Mortensen RN, et al. Acta Psychiatr Scand. 2018;137(6):481–490. [DOI] [PubMed] [Google Scholar]
- 54.Laugesen K, Olsen MS, Telén Andersen AB, et al. BMJ Open. 2013;3(9):e003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Olsen J, Pedersen LH, et al. Pediatrics. 2015;135(4):e911–e917. [DOI] [PubMed] [Google Scholar]
- 56.Lupattelli A, Wood M, Ystrom E, et al. J Am Acad Child Adolesc Psychiatry. 2018;57(3):200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malm H, Brown AS, Gissler M, et al. J Am Acad Child Adolesc Psychiatry. 2016;55(5):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Man KKC, Chan EW, Ip P, et al. BMJ. 2017;357:j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao Y, Pedersen LH, Christensen J, et al. Pharmacoepidemiol Drug Saf. 2016;25(11):1320–1330. [DOI] [PubMed] [Google Scholar]
- 60.Misri S, Reebye P, Kendrick K, et al. Am J Psychiatry. 2006;163(6):1026–1032. [DOI] [PubMed] [Google Scholar]
- 61.Momen NC, Munk-Olsen T, Li J, et al. Pharmacoepidemiol Drug Saf. 2018;27(1):114–118. [DOI] [PubMed] [Google Scholar]
- 62.Weikum WM, Brain U, Chau CMY, et al. Front Cell Neurosci. 2013;7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nulman I, Koren G, Rovet J, et al. J Clin Psychiatry. 2015;76(7):e842–e847. [DOI] [PubMed] [Google Scholar]
- 64.Nulman I, Koren G, Rovet J, et al. Am J Psychiatry. 2012;169(11):1165–1174. [DOI] [PubMed] [Google Scholar]
- 65.Oberlander TF, Reebye P, Misri S, et al. Arch Pediatr Adolesc Med. 2007;161(1):22–29. [DOI] [PubMed] [Google Scholar]
- 66.Partridge MCAE, Salisbury AL, LaGasse LL. J Pediatr. 2016;175:144–149.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sujan AC, Rickert ME, Öberg AS, et al. JAMA. 2017;317(15):1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gentile S. Neuroscience. 2017;342:154–166. [DOI] [PubMed] [Google Scholar]
- 69.Dean K, Stevens H, Mortensen PB, et al. Arch Gen Psychiatry. 2010;67(8):822–829. [DOI] [PubMed] [Google Scholar]
- 70.McLearn KT, Minkovitz CS, Strobino DM, et al. Arch Pediatr Adolesc Med. 2006;160(3):279–284. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor TG, Ben-Shlomo Y, Heron J, et al. Biol Psychiatry. 2005;58(3):211–217. [DOI] [PubMed] [Google Scholar]
- 72.Donovan MH, Tecott LH. Front Neurosci. 2013;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Long NE, Barry EJ, Pinelli C, et al. Toxicol Appl Pharmacol. 2015;285(1):32–40. [DOI] [PubMed] [Google Scholar]
- 74.Zhao H, Song A, Zhang Y, et al. Endocr J. 2018;65(9):923–933. [DOI] [PubMed] [Google Scholar]
- 75.Barker DJ, Eriksson JG, Forsén T, et al. Int J Epidemiol. 2002;31(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 76.Bouali S, Evrard A, Chastanet M, et al. Eur J Neurosci. 2003;18(8):2203–2212. [DOI] [PubMed] [Google Scholar]
- 77.Uçeyler N, Schütt M, Palm F, et al. Int J Obes. 2010;34(4):701–711. [DOI] [PubMed] [Google Scholar]
- 78.Brown LM, Clegg DJ. J Steroid Biochem Mol Biol. 2010;122(1–3):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rivera HM, Oberbeck DR, Kwon B, et al. Brain Res. 2009;1259:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore CJ, DeLong NE, Chan KA, et al. Reprod Sci. 2015;22(10):1297–1311. [DOI] [PubMed] [Google Scholar]
- 81.Morales DR, Slattery J, Evans S, et al. BMC Med. 2018;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uguz F J Clin Psychopharmacol. 2018;38(3):254–259. [DOI] [PubMed] [Google Scholar]
- 83.Emerson ND, Morrell HER, Neece C. J Autism Dev Disord. 2016;46(1):127–138. [DOI] [PubMed] [Google Scholar]
- 84.Anttila V, Bulik-Sullivan B, Finucane HK, et al. ; Brainstorm Consortium. Science. 2018;360(6395):eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petersen I, Gilbert RE, Evans SJ, et al. J Clin Psychiatry. 2011;72(7):979–985. [DOI] [PubMed] [Google Scholar]
- 86.O’Connor E, Senger CA, Henninger ML, et al. JAMA. 2019;321(6):588–601. [DOI] [PubMed] [Google Scholar]
- 87.Molenaar NM, Kamperman AM, Boyce P, et al. Aust N Z J Psychiatry. 2018;52(4):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molenaar NM, Brouwer ME, Bockting CL, et al. BMC Psychiatry. 2016;16(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


