Dear editor,
We read with interest the paper by Menichetti and colleagues recently published in the European Journal of Internal Medicine, which allows us to make some considerations [1].
Convalescent plasma (CP) has been for decades a frontline treatment against emerging pathogens, and coronavirus disease 2019 (COVID-19) is no exception. Many randomized controlled trials (RCT) have been reported in the last year, with mixed results, but a general consensus is emerging regarding efficacy in seronegative patients transfused within three days from onset of symptoms [2]. The randomized controlled trial TSUNAMI (TranSfUsion of coNvalescent plAsma for the early treatment of pneuMonIa due to SARS-CoV-2), recently published in JAMA Network Open and with Menichetti as first author and principal investigator, is no exception [3]. The trial was conducted in Italy at 27 clinical centers enrolling 487 patients hospitalized for COVID-19 (241 randomized to CP and standard therapy and 246 to standard treatment [ST] alone). No benefit was observed for CP versus ST on the primary outcome, which was a composite of worsening respiratory failure or death within 30 days of randomization. In addition, a statistically significant higher incidence of adverse events was observed in the CP treatment arm. After a thorough reading of the paper, we noticed some concerns that we want to point out.
First, the long period between data collection and publication. The study was designed in April 2020 as single-center, transformed as nation-wide and approved by the Institutional Review Board of the Istituto Superiore di Sanità (ISS) on May 18 2020. The 18 months elapsed between study conception and publication are poorly explained by the reduced number of hospitalizations for COVID-19 and the shortage of CP collected in Italy during the summer of 2020. This time lag was evident in some obsolete aspects of the study protocol, such as the enrollment of patients up to 10 days from symptom onset. Our understanding of pathogenesis and treatment of COVID-19 has changed rapidly over the past two years, including the CP use. We have known since August 2020 that the highest effect of CP occurs when it is given at high titer (>160) and early (within 72 h from symptom onset) [1]. In addition, the AIFA (Agenzia Italiana del Farmaco)-ISS joint press release on the disappointing results of the TSUNAMI trial was published on 8 April 2021 in Italian and English, circulated to all the main Italian and international press agencies, and was largely retweeted by national opinion leaders [4]. Unfortunately, the final work was published almost 8 months later [3] and during this time it was not possible to have access to the raw data, not even in a pre-published form, such as a preprint. There was great expectation from the scientific community on the results of TSUNAMI, the first CP RCT to be conducted and completed in the first western country hit by the pandemic: the early press release of TSUNAMI, which was promptly embedded within the revised recommendations issued by the Italian Society of Transfusion Medicine and Immunohematology (SIMTI) [5], had the deleterious effect of stopping CP collection and its clinical use in Italy and abroad.
Other more technical aspects deserve attention. In particular, an important issue regards the TSUNAMI sample size calculation reported in the study protocol. A 40% reduction in the primary endpoint was chosen to calculate the number of patients to be enrolled (n=474). It has been well known for more than one year that the “Lazarus effect” is not present neither for CP nor for any other of the agents used against COVID-19. For instance, the more realistic 20% reduction in the primary outcome was selected for sample size calculation in the RECOVERY trial for both dexamethasone and monoclonal antibodies casirivimab and imdevimab in hospitalized patients [6,7]. Thus, reanalyzing the data of TSUNAMI trial using an alpha = 0.05 and a power = 0.8, we note that a sample size of 724 patients would be requested to detect a risk ratio (RR) = 0.67, if the treatment could reduce the incidence rate by 1/3; a sample size of 1304 patients would be needed to detect an RR = 0.75, if the treatment could reduce the incidence rate by 1/4 and a sample size of 2080 patients would be needed to detect an RR = 0.80 if the treatment could reduce the incidence rate by 1/5 (Table 1 ). Thus, in light of this recalculation, the study was clearly underpowered, with the number of patients included in the efficacy analysis (modified intention-to-treat [mITT], N = 473) being insufficient to detect a relative risk reduction in the range 0.20–0.33.
Table 1.
Power analysis of TSUNAMI trial.
| RR | Relative incidence reduction (1-RR) | Control incidence | Experimental incidence | Estimated sample size |
|---|---|---|---|---|
| 0.5 | 0.5 | 0.26 | 0.13 | 290 |
| 0.6 | 0.4 | 0.26 | 0.156 | 476 |
| 0.67 | 0.33 | 0.26 | 0.1742 | 724 |
| 0.75 | 0.25 | 0.26 | 0.195 | 1304 |
| 0.8 | 0.2 | 0.26 | 0.208 | 2080 |
The control incidence was the mean basal incidence rate of the event in the control arm of all sites. The risk ratio (RR) was used to calculate the experimental incidence for power. The relative incidence reduction was expressed as 1-RR. The estimated sample size was the number of subjects to include in the study, with a ratio 1:1 between experimental arm and control arm.
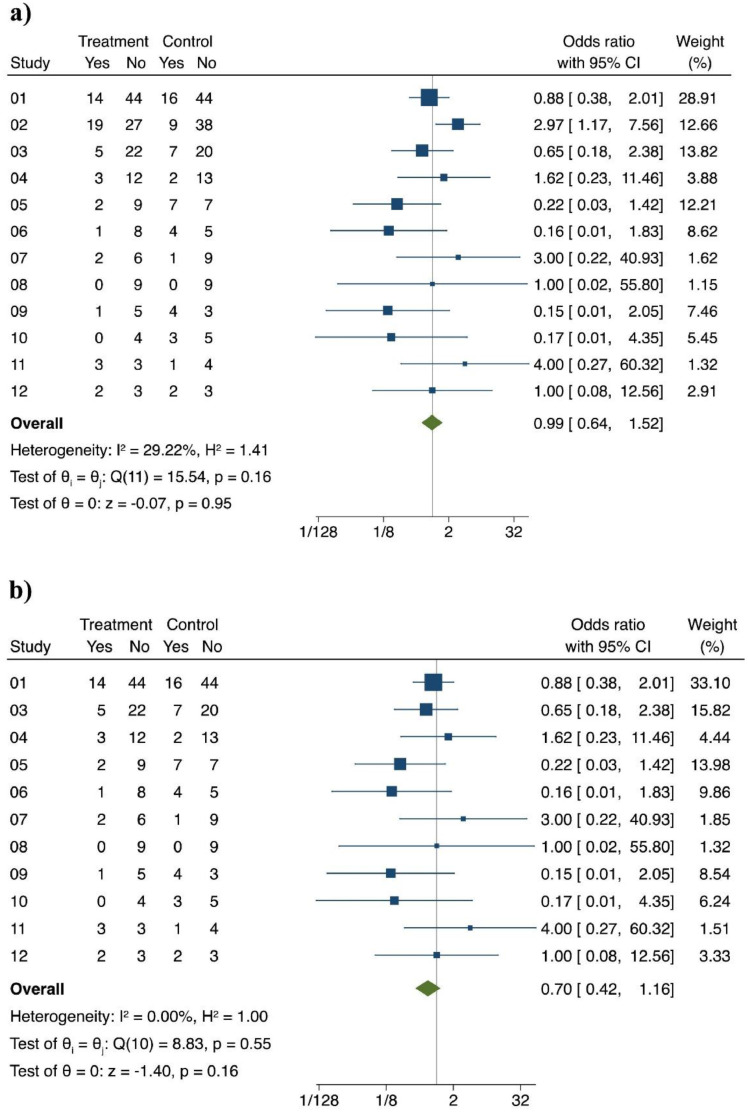
Another major concern regards the centers who enrolled the patients. Although 27 centers participated to the study, 12 clinical sites enrolled more than 80% of patients. Twenty percent of the mITT population (94/473) was enrolled at a single center (clinical site 02), which surprisingly had nearly three times the mortality in the CP group than in the ST group ( Fig. 1a). Even more surprisingly, removing the results of site 02, heterogeneity in outcomes disappeared, and the mean effect sizes were near significance (Fig. 1b). But the most intriguing result is certainly the clear trend towards efficacy that just missed statistical significance (P = 0.06) of CP versus ST in the subgroup of patients with milder disease. This finding is not surprising as it is precisely the group that would be expected to benefit from CP, as it has happened with monoclonal antibodies [8]. This evidence, together with information from other worldwide RCTs and carefully controlled retrospective data, should have provided the basis for the design of a more relevant RCTs focusing on treatment of earlier phases of COVID-19 with CP, but this did not happen, and national authorities did not promote any additional usage of CP other than delivery to plasma manufacturers.
Fig. 1.
Primary endpoint outcome according to clinical site.
All sites. Pooling of OR, fixed-effects, Mantel-Haenszel.
All sites, except #02. Pooling of OR, fixed-effects, Mantel-Haenszel.
Regarding the safety concerns on CP use raised by the authors and highlighted in the abstract, only 5 (2.1%) adverse events were severe and only two of them (0.8%) had a high causality association with CP. This rate is similar to that reported in our previous meta-analysis on CP-related adverse reactions, which documented the overall high safety of this passive immunotherapy [9]. All concerns about CCP safety have been swept away by the recent results of the US Expanded Access Program (EAP) which reported an incidence of serious adverse events of less than 1% in over 100,000 CCP-treated patients [10].
In summary, TSUNAMI reported a negative result for CP but that result is not definitive because of problems of design, power, site concerns and inability to study the population most likely to benefit Finally, we note that plasma from recovered individuals is the only antiviral agent and cure available in low-income countries which have no or limited access to expensive monoclonal antibodies or small-chemical antivirals. TSUNAMI missed a great opportunity to encourage the proper use of CP (early and at high dosage).
References
- 1.Menichetti F., Falcone M., Tiseo G. Management of COVID patients with convalescent plasma: do we have the final word? Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Focosi D., Franchini M., Pirofski L., Burnouf T., Paneth N., Joyner M.J., Casadevall A. COVID-19 convalescent plasma and randomized clinical trials: rebuilding confidence by explaining failures and finding signals of efficacy. MedRxiv https://www.medrxiv.org/content/10.1101/2021.09.07.21263194v3.full.pdf+html 2021.
- 3.Menichetti F., Popoli P., Puopolo M., Spila Alegiani S., Tiseo G., Bartoloni A., De Socio G.V., Luchi S., Blanc P., Puoti M., Toschi E., Massari M., Palmisano L., Marano G., Chiamenti M., Martinelli L., Franchi S., Pallotto C., Suardi L.R., Luciani Pasqua B., Merli M., Fabiani P., Bertolucci L., Borchi B., Modica S., Moneta S., Marchetti G., d'Arminio Monforte A., Stoppini L., Ferracchiato N., Piconi S., Fabbri C., Beccastrini E., Saccardi R., Giacometti A., Esperti S., Pierotti P., Bernini L., Bianco C., Benedetti S., Lanzi A., Bonfanti P., Massari M., Sani S., Saracino A., Castagna A., Trabace L., Lanza M., Focosi D., Mazzoni A., Pistello M., Falcone M., TSUNAMI Study group Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.36246. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agenzia Italiana del Farmaco (AIFA). https://www.aifa.gov.it/en/-/covid-19-studio-tsunami-il-plasma-non-riduce-il-rischio-di-peggioramento-respiratorio-o-morte (Published on 8 April 2021).
- 5.Prati D., Fiorin F., Berti P., De Silvestro G., Accorsi P., Ostuni A. Italian society for transfusion medicine and immunohaematology (SIMTI), and the Italian society for haemapheresis and cell manipulation (SIdEM). position paper on the use of COVID-19 convalescent plasma: an update. Blood Transf. 2021;19(4):277–280. doi: 10.2450/2021.0150-21. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1. [DOI] [PMC free article] [PubMed]
- 8.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. Trial investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M., Cruciani M. How safe is COVID-19 convalescent plasma? Mayo Clin Proc. 2021;96(8):2279–2281. doi: 10.1016/j.mayocp.2021.06.011. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senefeld J.W., Johnson P.W., Kunze K.L., Bloch E.M., van Helmond N., Golafshar M.A., Klassen S.A., Klompas A.M., Sexton M.A., Diaz Soto J.C., Grossman B.J., Tobian A.A.R., Goel R., Wiggins C.C., Bruno K.A., van Buskirk C.M., Stubbs J.R., Winters J.L., Casadevall A., Paneth N.S., Shaz B.H., Petersen M.M., Sachais B.S., Buras M.R., Wieczorek M.A., Russoniello B., Dumont L.J., Baker S.E., Vassallo R.R., Shepherd J.R.A., Young P.P., Verdun N.C., Marks P., Haley N.R., Rea R.F., Katz L., Herasevich V., Waxman D.A., Whelan E.R., Bergman A., Clayburn A.J., Grabowski M.K., Larson K.F., Ripoll J.G., Andersen K.J., Vogt M.N.P., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., Buchholtz Z.A., Pletsch M.C., Wright K., Greenshields J.T., Joyner M.J., Wright R.S., Carter R.E., Fairweather D. Access to and safety of COVID-19 convalescent plasma in the United States expanded access program: a national registry study. PLoS Med. 2021;18(12) doi: 10.1371/journal.pmed.1003872. Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]