Abstract
We evaluated the Meridian IC-STAT direct fecal and broth culture antigen detection methods with samples from children infected with Escherichia coli O157:H7 and correlated the antigen detection results with the culture results. Stools of 16 children who had recently had stool cultures positive for this pathogen (population A) and 102 children with diarrhea of unknown cause (population B) were tested with the IC-STAT device (direct testing). Fecal broth cultures were also tested with this device (broth testing). The results were correlated to a standard of the combined yield from direct culture of stools on sorbitol-MacConkey (SMAC) agar and culture of broth on SMAC agar. Eleven (69%) of the population A stool specimens yielded E. coli O157:H7 when plated directly on SMAC agar. Two more specimens yielded this pathogen when the broth culture was similarly plated. Of these 13 stool specimens, 8 and 13 were positive by direct and broth testing (respective sensitivities, 62 and 100%). Compared to the sensitivity of a simultaneously performed SMAC agar culture, the sensitivity of direct testing was 73%. Three (3%) of the population B stool specimens contained E. coli O157:H7 on SMAC agar culture; one and three of these stool specimens were positive by direct and broth testing, respectively. The direct and broth IC-STAT tests were 100% specific with samples from children from population B. Direct IC-STAT testing of stools is rapid, easily performed, and specific but is insufficiently sensitive to exclude the possibility of infection with E. coli O157:H7. Performing the IC-STAT test with a broth culture increases its sensitivity. However, attempts to recover E. coli O157:H7 by culture should not be abandoned but, rather, should be increased when the IC-STAT test result is positive.
Escherichia coli O157:H7 causes diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (HUS) (8). Present methods for detection of this pathogen exploit its inability to ferment sorbitol after overnight incubation on sorbitol MacConkey (SMAC) agar (6). Sorbitol-nonfermenting colonies that test positive for the O157 lipopolysaccharide antigen are then confirmed biochemically to be E. coli. Although economical, this methodology requires overnight incubation to identify a positive colony and is not as sensitive as more labor-intensive techniques (e.g., immunomagnetic bead separation) (5).
Accelerated and accurate diagnosis of E. coli O157:H7 infections can avert unnecessary or harmful therapies, focus outbreak investigations, and potentially increase the sensitivity of SMAC agar screening. We report here on the evaluation of a device that identifies E. coli O157:H7 rapidly by immunochromatography.
(These data were presented in part at the 99th Annual Meeting of the American Society for Microbiology, Chicago, Ill., 30 May to 3 June 1999.)
MATERIALS AND METHODS
Patients and initial microbiology.
Population A consisted of 16 children under age 10 years whose stools were known to have recently contained E. coli O157:H7. To identify this population, a network of 46 clinical laboratories in Washington, Idaho, and Oregon notified one of the authors (usually P.I. Tarr) whenever a patient's stool culture was positive for E. coli O157:H7 by SMAC agar screening (10). After obtaining informed consent, fresh stools from these patients were sent on wet ice as unpreserved self-collected specimens to the Children's Hospital and Regional Medical Center (CHRMC) Microbiology Laboratory, arriving within 24 h of production. Study stools were obtained within 8 days of the onset of illness (day 1 is the first day of diarrhea) and within 4 days of submission of the index positive culture.
Population B consisted of 102 children with diarrhea evaluated at the CHRMC Emergency Department or at a pediatric practice in Seattle and represented all patients who submitted ≥5 g of stool between November 1998 and July 1999. These children were also taking part in a prospective study to determine the etiology of childhood diarrhea in Seattle. Stools from population B were evaluated by the laboratorian for the presence of gross blood by visual inspection.
Sample flow.
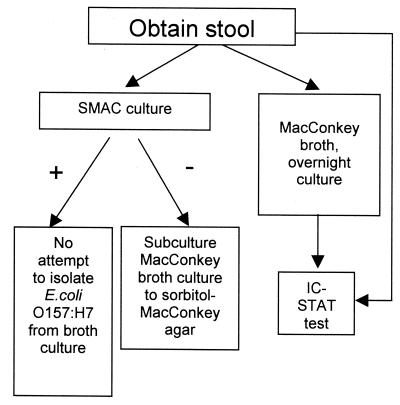
Figure 1 demonstrates sample flow, which encompasses direct stool and broth antigen testing for E. coli O157:H7 and attempts to isolate E. coli O157:H7 from the stool and, if appropriate, from the broth.
FIG. 1.
Specimen flow chart. Upon receipt in the laboratory, stools were subjected to the procedures outlined in the text.
Culture.
All stools were streaked onto SMAC agar (Prepared Media Laboratories, Tualatin, Oreg.) plates, which were then incubated overnight (35°C, aerobic conditions). If the day-old SMAC agar culture was negative for E. coli O157:H7, the MacConkey broth was streaked onto SMAC agar to attempt to isolate the pathogen. Three to five colorless colonies were tested for the expression of the O157 lipopolysaccharide antigen by latex particle agglutination (Oxoid, Basingstoke, United Kingdom) or until a positive result was observed. This reactive colony was confirmed to be E. coli by standard biochemical reactions.
Additional pathogens sought in population B stools included Campylobacter, Salmonella, Shigella, and Yersinia by standard culture techniques and non-O157:H7 Shiga toxin (Stx)-producing E. coli via assay of an overnight MacConkey broth culture by the Premier EHEC (Meridian) test (7). If Stx was detected in this broth and E. coli O157:H7 was not identified on SMAC agar, lactose-fermenting Stx-producing E. coli was recovered from the MacConkey agar cultures of these stools and serotyped by the Centers for Disease Control and Prevention.
Direct IC-STAT testing.
Upon receipt, stools from both populations were tested directly with the IC-STAT (Meridian Diagnostics, Cincinnati, Ohio) device. Approximately 25 μl of stool was suspended in 350 μl of diluent supplied with the device. A total of 150 μl of this suspension was placed into the device port. A test was read as positive if a band was apparent at the designated site, after migration at room temperature. A negative control band confirms specimen migration.
IC-STAT testing of overnight broth culture.
The IC-STAT test was performed with broth cultures of all stools. Approximately 50 μl of specimen was inoculated into 5-ml tubes of MacConkey broth (Binax Laboratories, Portland, Maine), and the tubes were incubated overnight (35°C, aerobic conditions). A total of 350 μl of broth culture was added to 350 μl of diluent. A total of 150 μl of the mixture was introduced into the device port, and positive and negative results were read as described above.
Statistics.
The sensitivities of the IC-STAT tests with samples from population A were related to the culture results (direct SMAC agar plating of the stool and of the broth culture). The specificities of the IC-STAT tests with samples from population B were related to the number of negative tests by direct SMAC agar screening. Fisher's exact test and the chi-square test for linear trend (1) were used to evaluate the impact of length of time after symptoms began to the time to a positive culture.
RESULTS
Population A.
Eleven of 16 recently positive stool specimens that were sent to the CHRMC Microbiology Laboratory for analysis contained E. coli O157:H7 on direct culture on SMAC agar; 2 more yielded this pathogen from culture of the broth (Table 1). Eight of these 13 specimens were positive by the direct IC-STAT test (sensitivity, 62%); all were positive when the IC-STAT test was applied to the broth cultures (sensitivity, 100%). The sensitivity of the direct antigen test was 73% when the results were compared only to those for the 11 stool specimens for which direct SMAC culture of the stool yielded E. coli O157:H7.
TABLE 1.
Culture and IC-STAT results for population A stoolsa
| Patient | Day of illness with:
|
IC-STAT test result by
|
Broth culture result | ||
|---|---|---|---|---|---|
| First (index) positive culture | Second (study) culture (result) | Direct testing of stool | Testing of broth | ||
| G | 1 | 3 (+) | Pos | Pos | Not done |
| L | 2 | 4 (+) | Pos | Pos | Not done |
| A | 2 | 4 (+) | Pos | Pos | Not done |
| O | 2 | 5 (+) | Pos | Pos | Not done |
| J | 4 | 5 (+) | Pos | Pos | Not done |
| C | 4 | 6 (+) | Pos | Pos | Not done |
| E | 3 | 7 (+) | Pos | Pos | Not done |
| I | 5 | 7 (+) | Pos | Pos | Not done |
| B | 2 | 4 (+) | Neg | Pos | Not done |
| H | 2 | 5 (+) | Neg | Pos | Not done |
| F | 2 | 4 (−) | Neg | Pos | + |
| N | 3 | 6 (−) | Neg | Pos | − |
| P | 5 | 8 (+) | Neg | Pos | Not done |
| M | 3 | 7 (−) | Neg | Pos | + |
| K | 3 | 6 (−) | Neg | Neg | − |
| D | 5 | 7 (−) | Neg | Neg | − |
| Percent | 100b | 69b | 50b | 88b | |
| Yield | 85c | 62c | 108c | ||
| 100d | 73d | 127d | |||
Symbols and abbreviations: +, positive result; −, negative result; Pos, positive result; Neg, negative result.
Number of positive stool specimens/number of stool specimens studied (n = 16).
Number of positive stool specimens/number of stool specimens for which study culture from stool or from broth was positive (n = 13).
Number of positive stool specimens/number of specimens for which direct study culture from stool was positive (n = 11).
Population A stools that were negative on direct culture, on average, were collected later than those that were positive (6.2 ± 1.5 versus 5.0 ± 1.4 days [P = 0.28 by Fisher's exact test]).
Population B (prospective diarrhea etiology study).
Three (3%) of 102 population B stool specimens yielded E. coli O157:H7 on direct SMAC agar culture. One of these stool specimens, a specimen collected on day 5 of illness, was positive by the direct IC-STAT test. The IC-STAT broth culture test detected all three stool specimens from population B that yielded E. coli O157:H7 by SMAC culture. Nine (9%) population B stool specimens were obviously bloody, including each of the three specimens with E. coli O157:H7 and one with Shigella group B. The remaining 93 (91%) nonbloody population B stool specimens included 1 stool specimen with Stx-producing E. coli O103:H2 and 1 stool specimen with Salmonella C1. None of the 99 stool specimens that were negative for E. coli O157:H7 on direct plating on SMAC agar produced a positive direct or broth culture IC-STAT test result. The IC-STAT tests were therefore 100% specific with samples from population B compared to the results of standard culture.
DISCUSSION
The IC-STAT test rapidly and specifically identifies human stool specimens that contain E. coli O157:H7 (5a). The sensitivity of broth antigen testing for the recovery of E. coli O157:H7 exceeds (population A) that of direct plating of the stool on SMAC agar plates. The sensitivity of the IC-STAT test directly applied to stools is substantially less than that of a culture of the stool on SMAC agar.
The increased sensitivity of antigen testing of a broth culture might be attributed to preferential enrichment of E. coli O157:H7 over other fecal flora by the broth or to the resuscitation of this pathogen in the broth. This situation probably applied to the stools of patients F and M, for whom the broth culture yielded E. coli O157:H7, although the primary stool culture and the IC-STAT direct antigen test were negative, and patient N, for whom testing was negative for E. coli O157:H7 by all methods except IC-STAT testing of the broth culture.
Few population B patients had E. coli O157:H7 in their stools, so it is not possible to calculate reliably the sensitivity of the direct IC-STAT test as applied to stools when the microbiologist has no a priori reason to suspect E. coli O157:H7. However, the overall diminished sensitivity of direct fecal antigen testing compared to the results of cultures of simultaneously plated SMAC agar plates demonstrates that the IC-STAT test cannot replace traditional SMAC agar screening. In contrast, applying the IC-STAT antigen test to an overnight broth culture might actually increase the E. coli O157:H7 recovery rate by one of two mechanisms. First, as discussed above, broth culture might enrich the culture for E. coli O157:H7 over other flora. Second, a positive IC-STAT broth test result might prompt a microbiologist to find E. coli O157:H7 that is not initially apparent on a SMAC agar culture of the directly plated stool or on a culture of the enrichment broth. Enrichment cultures, a technique rarely used by microbiologists when seeking E. coli O157:H7, should be evaluated for their effect on the recovery of this pathogen.
The rapid diagnosis of E. coli O157:H7 infection via application of the direct antigen IC-STAT test to stool specimens would be valuable in several situations. First, if a therapeutic agent ameliorates the course of E. coli O157:H7 infection (2), it will be important to diagnose as expeditiously as possible all infected patients. Second, the rapid identification of patients presumptively infected with E. coli O157:H7 can reinforce the inadvisability of using therapies such as antimotility agents (3) or antibiotics (10) that might increase the likelihood of developing HUS. However, we strongly believe that such agents should always be withheld pending complete microbiologic (i.e., culture) evaluation. Third, the high degree of specificity of a positive IC-STAT test with stool specimens can bring diagnostic clarity to a subset of patients with acute bloody diarrhea. Such clarity might obviate unnecessary tests such as colonoscopy or laparotomy in advance of diagnosis of infection by standard culture. Fourth, direct IC-STAT testing of stools could be useful in E. coli O157:H7 outbreaks, in which the early and accurate identification of probable infected patients can accelerate outbreak investigations. Fifth, if a patient presents late in the course of illness, when cultures are frequently negative (i.e., patients with HUS [9]), and E. coli O157:H7 was not previously sought, the use of the IC-STAT broth test might increase somewhat the ability to determine the etiologic agent.
The IC-STAT test is highly specific, but we nevertheless urge that microbiologists attempt to isolate the infecting organism from the stool when the IC-STAT test is positive. In fact, such recovery efforts should be enhanced when the IC-STAT test is positive. Public health surveillance and control measures for this infection depend critically on routine molecular subtyping of the recovered organism (4).
In summary, the Meridian IC-STAT test is rapid, easily performed, and specific for the detection of fecal E. coli O157:H7 (5a). Direct testing of stools with the IC-STAT device is accurate when it is positive, but this test as applied directly to stools is not sufficiently sensitive to exclude the possibility of infection with E. coli O157:H7. Testing of an overnight broth culture with the IC-STAT device could actually increase the rate of detection of E. coli O157:H7 over direct culture of the stool alone. Finally, because monitoring of emerging pathogens and molecular analyses of infecting strains are critical components of modern disease surveillance and outbreak detection efforts, standard culture techniques for the recovery of this and other pathogens should not be abandoned in favor of nonculture detection techniques.
ACKNOWLEDGMENTS
This work was supported by grant RO1DK52081 from the National Institutes of Health, contract CCU015040 from the Centers for Disease Control and Prevention, and Meridian Laboratories.
We thank Christine Merrikin for expert secretarial assistance, Rachelle Whitman for assistance with manuscript preparation, Evangeline Sowers for serotyping the E. coli O103:H2 isolates, Joe Rutledge for reviewing the manuscript, participating laboratories for notifying us of positive patients, and the CHRMC Microbiology Laboratory staff for the high standards of testing used in this study.
REFERENCES
- 1.Altman D G. Practical statistics for medical research. 1st ed. New York, N.Y: Chapman & Hall; 1991. [Google Scholar]
- 2.Armstrong G D, Rowe P C, Goodyer P, Orrbine E, Klassen T P, Wells G, MacKenzie A, Lior H, Blanchard C, Auclair F, et al. A phase I study of chemically synthesized Verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to Chromosorb for preventing hemolytic-uremic syndrome. J Infect Dis. 1995;171:1042–1045. doi: 10.1093/infdis/171.4.1042. [DOI] [PubMed] [Google Scholar]
- 3.Bell B P, Griffin P M, Lozano P, Christie D L, Kobayashi J M, Tarr P I. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics. 1997;100:E121–E126. doi: 10.1542/peds.100.1.e12. [DOI] [PubMed] [Google Scholar]
- 4.Bender J B, Hedberg C W, Besser J M, Boxrud D J, MacDonald K L, Osterholm M T. Surveillance by molecular subtype for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N Engl J Med. 1997;337:388–394. doi: 10.1056/NEJM199708073370604. [DOI] [PubMed] [Google Scholar]
- 5.Karch H, Janetzki-Mittmann C, Aleksic S, Datz M. Isolation of enterohemorrhagic Escherichia coli O157 strains from patients with hemolytic-uremic syndrome by using immunomagnetic separation, DNA-based methods, and direct culture. J Clin Microbiol. 1996;34:516–519. doi: 10.1128/jcm.34.3.516-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Mackenzie A, Orrbine E, Hyde L, Benoit M, Chan F, Park C, Alverson J, Lembke A, Hoban D, Kennedy W. Performance of the ImmunoCard STAT! E. coli O157:H7 test for detection of Escherichia coli O157:H7 in stools. J Clin Microbiol. 2000;38:1866–1868. doi: 10.1128/jcm.38.5.1866-1868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.March S, Ratnam S. Sorbitol-MacConkey medium for detection of Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1986;23:869–872. doi: 10.1128/jcm.23.5.869-872.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park C H, Gates K M, Vandel N M, Hixon D L. Isolation of Shiga-like toxin producing Escherichia coli (O157 and non-O157) in a community hospital. Diagn Microbiol Infect Dis. 1996;26:69–72. doi: 10.1016/s0732-8893(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 8.Tarr P I. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–10. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Tarr P I, Neill M A, Clausen C R, Watkins S L, Christie D L, Hickman R O. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J Infect Dis. 1990;162:553–556. doi: 10.1093/infdis/162.2.553. [DOI] [PubMed] [Google Scholar]
- 10.Wong C S, Jelacic S, Habeeb R L, Watkins S L, Tarr P I. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]