Abstract
Background
Although several self-injectable preventive treatments for migraine have become available, they are not yet widely used. Thus, understanding patients’ perceptions towards them is limited.
Objective
This study aimed to inform the design of a preference-elicitation instrument, which is being developed to quantify preventive treatment preferences of people with migraine.
Methods
We conducted a qualitative study involving nine in-person focus groups (three per country) in the United States, the United Kingdom, and Germany. Participants were adults (n = 47) with episodic or chronic migraine who were currently using or had used a prescription preventive treatment for migraine within the previous 5 years. During the focus groups, participants described their experiences of migraine and preventive treatments; handled and simulated self-injection using five different unbranded, fired demonstration auto-injectors and prefilled syringes; and ranked different aspects of preventive treatments by importance. Focus groups were analyzed with a focus on themes that would be feasible or meaningful to include in a subsequent preference-elicitation instrument.
Results
Reducing the frequency and severity of migraine attacks was consistently ranked as the most important aspect of preventive treatment. Participants expressed dissatisfaction with available daily oral preventive treatments for migraine they had previously used because they were ineffective or caused intolerable adverse events. Many participants were willing to self-inject a treatment that was effective and tolerable. When presented with devices for self-injecting a preventive treatment for migraine, participants generally preferred autoinjectors over prefilled syringes. Participants especially valued safety features such as the unlocking step and automated needle insertion, and audible and visual dose confirmation increased confidence in autoinjector use. Autoinjector needle protection mechanisms were also appreciated, especially by participants averse to needles, as the needles are not visible.
Conclusions
This study highlights the fact that many people with migraine still lack access to a preventive treatment that is effective and tolerable. In addition to efficacy and safety considerations, treatment decisions may be guided by the mode of administration. In the case of self-injectable preventive treatments, key device characteristics affecting these decisions may be ease of use, comfort, and confidence in self-injection. Insights gained from this study were used to help develop a preliminary set of attributes and levels for a preference-elicitation instrument.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40271-021-00525-z.
Key Points for Decision Makers
| People with migraine who participated in focus groups consistently ranked reducing the frequency and severity of migraine attacks as the most important aspect of treatment, and also ranked avoiding adverse events as important. |
| People with migraine are dissatisfied with currently available preventive oral (non-calcitonin gene-related peptide targeting) medications due to a lack of efficacy and tolerability concerns; most of the focus group participants would be willing to self-inject a preventive treatment that is effective and tolerable. |
| People with migraine have different preferences for device characteristics that may affect the treatment burden of self-injectable treatments, such as the injection time, method of dose confirmation, mechanism of needle insertion and retraction, and storage requirements. |
Introduction
Migraine affects more than 1 billion people worldwide [1], most of whom are working-age adults [2]. Among neurological disorders, it is the second-most common cause of disability [3]. Migraine can be episodic or chronic [4–6], and common symptoms include throbbing pain, photo- or phonophobia, nausea, and aura [7]. Many people with migraine experience a negative impact on functioning, including decreased productivity at work, school, and home, restricting family and social activities and resulting in an overall decrease in health-related quality of life [8–10]. In addition to the personal impact, the direct and indirect costs of migraine are high [11].
Individuals who experience at least four migraine headache days per month should be considered for preventive treatment [12]. Until recently, the main preventive treatment options were daily oral agents, such as beta-blockers, antidepressants, and antiepileptics. However, adherence and persistence to these treatments is low [13], primarily due to adverse events and a perceived lack of efficacy [14]. Monoclonal antibodies (mAbs) targeting calcitonin gene-related peptide (CGRP) that bind either CGRP itself or the CGRP receptor provide a novel alternative for people with migraine, especially for those in whom standard (non-CGRP-targeting) oral agents have failed. Erenumab, galcanezumab, and fremanezumab are self-injectable CGRP-targeting mAbs indicated for migraine prevention [15]. The CGRP-targeting mAbs appear to have similar efficacy and safety [16], although they have not been compared in controlled head-to-head studies.
Patients are expected to prefer treatments with favorable clinical properties; however, their preferences may be influenced by other factors, such as frequency of administration, ease of use, and storage, especially if different treatment options have comparable efficacy and safety [17]. Understanding the experiences and perspectives of people with migraine pertaining to preventive treatment characteristics, including delivery mechanisms (e.g., injection devices), is needed to help guide decision-making about preventive treatments and, for injectable treatments, the devices for administering them [18, 19]. This study aimed to inform the design of a preference-elicitation instrument, which is being developed to quantify preventive treatment preferences of people with migraine [20]. To this end, we explored what people with episodic or chronic migraine value as key characteristics of preventive treatments, including attributes of devices for subcutaneously administering self-injectable treatments.
Methods
Study Design
An in-person focus group study was conducted to identify patient-relevant treatment aspects. The focus groups used a discussion guide, whose design was informed by a targeted literature review (Online Resource 1, see the electronic supplementary material). The targeted literature review was conducted in May 2019 using the Ovid platform (which includes key medical literature databases such as EMBASE and MEDLINE). It yielded a total of 14 studies to be reviewed (six qualitative and eight quantitative). Data for outcomes and results were extracted, including patient and clinician experiences and treatment perception; themes and subthemes from patient-reported outcomes for qualitative studies; and preference-elicitation methods, attributes and levels, and trade-off measures for quantitative studies. A review of the extracted data identified 28 potentially relevant characteristics of migraine treatments: thirteen benefits (mostly related to symptom relief), eleven risks (e.g., loss of function, fatigue, drowsiness, and weight gain), and four regimen-related measures (e.g., mode and frequency of administration and ease of use). Only two out of the 14 studies considered preventive treatments for migraine [21, 22]; other studies addressed acute treatments or did not specify a treatment of interest. Manuscripts describing clinical trials of self-injectable preventive treatments for migraine were also reviewed to augment the targeted literature review. Additional risk measures pertinent to CGRP-targeting mAbs that were identified from clinical trials included nausea and injection site reaction [23–25].
Development of the focus group discussion guide was also based on concepts covered in the Subcutaneous Administration Assessment Questionnaire, a self-administered instrument that evaluates patient experiences of using devices such as a prefilled syringe or autoinjector [26]. The discussion guide (Online Resource 2, see the electronic supplementary material) included questions to elicit participant feedback about device characteristics.
Nine focus groups were conducted at five locations across the United States (US), the United Kingdom (UK), and Germany in October and November 2019, with the aim of having three focus groups per country, each with approximately seven participants (total n = 63). These countries were targeted to recruit individuals who, given the approval status in the countries and current treatment guidelines/recommendations from payers or physician societies, would be eligible for a prescription of a CGRP-targeting mAb.
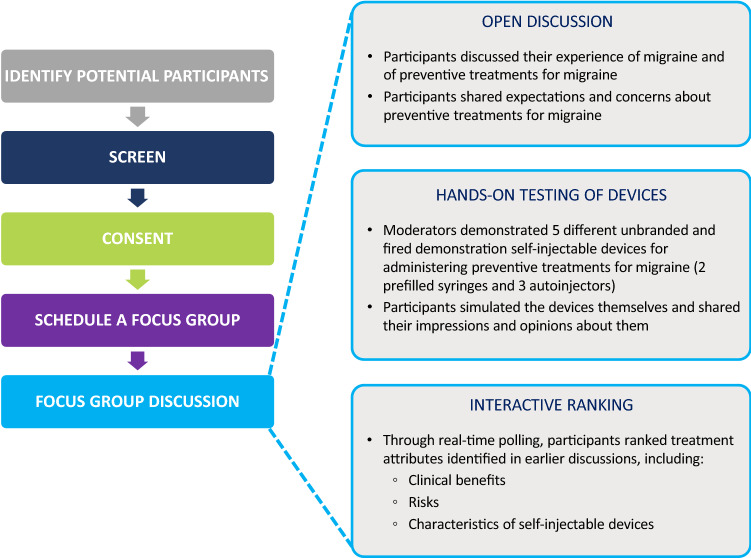
A previous study on health-seeking behaviors found that 80% of the themes emerged within the first two to three focus groups, and that 90% emerged within the first three to six focus groups [27]. Nine focus groups conducted across three countries were therefore considered as sufficient for the purpose of this study. Saturation grids were used to analyze theme saturation within the target sample rather than determine sample size. The study also aimed to recruit an approximately equal number of participants with episodic and chronic migraine. The overall study design and format of the focus group discussions are illustrated in Fig. 1.
Fig. 1.
Overall study design and format of the focus group discussions
Participants
The study included adults (≥ 18 years) with a self-reported diagnosis of migraine by a clinician, at least 4 headache days a month, and a Migraine Symptom Severity Score (MSSS) [28] indicating at least moderate severity. Participants were also currently using or had used a prescription preventive treatment for migraine within the last 5 years. Participants were considered to have episodic migraine if they had up to 14 headache days per month during the previous 3 months and chronic migraine if they had ≥ 8 days with the features of migraine out of a total of ≥ 15 headache days per month for > 3 months [6]. Eligibility was ascertained using a self-completed online screening questionnaire, which included questions about age, country of residence, health conditions, number of headache days, and treatment history, as well as the MSSS. To reach the maximum number of eligible participants at the desired locations within a recruitment period lasting 1-month, potential participants were recruited by convenience sampling through referrals from healthcare professionals, databases, social media, and patient association groups and were approached via email or telephone.
Focus Groups
Focus groups were held in conference rooms or market research facilities. Each focus group discussion lasted approximately 90 min and was conducted by a moderator [SM and ZB (both female) in the US; AH (female) in the UK; NP (female) in Germany] and co-moderator [JS (female) in the US; CT (female) and CAS (male) in the UK; TB (male) in Germany] in the country’s local language with the help of the discussion guide. The moderators and co-moderators were patient-centered outcome research professionals. They were trained in qualitative interview techniques, held a minimum of a master’s degree, and were fluent in the local language. Participants were given the moderator’s name and employer, as well as their reason for doing the research. Before the focus groups, there had been no contact between the moderators/co-moderators and the participants.
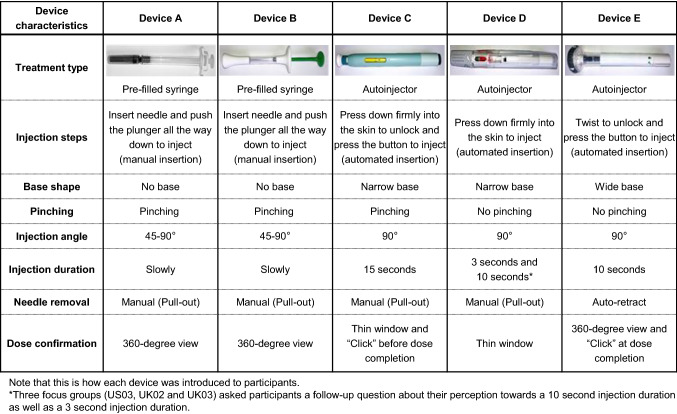
The focus groups began with an open discussion about experiences with migraine and preventive treatments (Fig. 1). Participants were encouraged to share their expectations and concerns about preventive treatments for migraine, to help inform development of benefit and risk attributes for the planned preference study. The moderator then presented the participants with five different demonstration single-use medical devices for subcutaneously administering CGRP-targeting mAbs (Fig. 2) and demonstrated how they should be used per the package insert instructions for use. The devices comprised two prefilled syringes and three autoinjectors that were unbranded, had already been discharged, and had their needles removed. The devices were presented to different focus groups in different orders to eliminate the possibility of ordering effects. The participants were then encouraged to simulate self-injection with each medical device. During and after handling and simulating self-injection using the five devices, participants were asked to share their perspectives on the aspects of the medical devices most important to them in making treatment decisions. This discussion identified device characteristics that mattered to patients.
Fig. 2.
Medical devices presented to the focus groups
Next, participants were asked to rank treatment attributes identified in previous discussions by importance in a live polling exercise. This exercise was designed to help select candidate attributes for inclusion in the planned preference study [29]. The attributes included in the exercise were selected by the co-moderator and were ranked by participants anonymously using their cell phones. The number of attributes varied between six and 11 for the different focus groups. The polling results were shared and discussed within the focus group.
The focus group discussions were audio recorded digitally and then transcribed. The transcripts from the focus groups in Germany were subsequently translated into English. In addition, the co-moderator took field notes. Confidentiality was ensured by assigning each participant a unique participant number at enrolment and by removing any personally identifiable information from the transcripts before coding. Transcripts were not returned to participants for comment or correction.
Data Analysis
The analysis focused on identifying the themes that explain patients’ valuation of key characteristics of preventive treatments for migraine, as well as their perceptions of differences between treatments and the medical devices for administering them [30–33]. A combined approach consisting of inductive (bottom-up) and deductive (top-down) methods was used to capture data-driven themes while ensuring that specific topics of interest included in the semi-structured discussion guide were covered. This approach initially involved two researchers (CAS and EB [female]) independently double-coding the same two transcripts inductively using the qualitative data analysis program ATLAS.ti version 8.4.22 [34]. Next, a wider team meeting was held to compare and discuss the coding by reviewing the transcripts in conjunction with the initial codes; identifying, modifying, and merging codes of similar meaning; and identifying discrepancies and resolving them through discussion. In addition, the aims of the study were reviewed to inform the inclusion of additional codes to deductively capture data of interest that were not already obtained from the initial transcripts. This process of coding the first two transcripts yielded a coding framework (Online Resource 3, see the electronic supplementary material), which was used by one researcher (CAS) to code the remaining transcripts. The coding framework was updated iteratively to reflect new themes that emerged during coding and regular discussions with the research team. Focus group participants were not invited to provide feedback on the coding.
A conceptual map was produced to illustrate the main outcomes of the focus group study in a hierarchical framework [35]. Saturation grids were produced to evaluate whether new themes emerged toward the end of data collection [27].
Results
Participants
Forty-seven participants took part in nine focus groups (14 participants in the US, 17 in the UK, and 16 in Germany). Saturation analysis indicated that many of the higher-level themes reached saturation (i.e., no substantially new information would be expected to emerge from additional focus groups) (Online Resource 4, see the electronic supplementary material).
Of the 47 participants, 28 (59.6%) had episodic migraine and 19 (40.4%) had chronic migraine (Table 1). Most participants (n = 40; 85.1%) were female, and the median age was 50.0 years (interquartile range 37.5–56.0). Most participants (n = 28; 59.6%) scored ≥ 41 on the Migraine Disability Assessment, indicating very severe disability due to migraine [8]. The mean MSSS was 28.6, indicating that, on average, participants experienced migraine-related symptoms half the time or more during their most severe type of headache [28]. The most commonly used type of preventive treatment for migraine over the previous 5 years was antiepileptics (n = 20; 42.6%), followed by antidepressants and beta-blockers (n = 17 [36.2%] each). Twelve participants (25.5%) had experience using self-injectables (for migraine or another indication), and five participants (10.6%) were currently using self-injectable CGRP-targeting mAbs for migraine prevention. Most participants (n = 34; 72.3%) had no or a low-level fear of needles.
Table 1.
Participant demographic and clinical characteristics
| Characteristic | N = 47 |
|---|---|
| Sex, n (%) | |
| Female | 40 (85.1) |
| Male | 7 (14.9) |
| Age (years) | |
| Mean (SD) | 46.8 (13.0) |
| Age group (years), n (%) | |
| 18–34 | 7 (14.9) |
| 35–64 | 37 (78.7) |
| ≥ 65 | 3 (6.4) |
| Highest-level of education, n (%) | |
| Elementary/primary school | 1 (2.1) |
| High school | 21 (44.7) |
| Some college/university | 7 (14.9) |
| College/University (B.A., B.Sc.) | 7 (14.9) |
| Postgraduate degree | 11 (23.4) |
| Migraine type, n (%) | |
| Episodic | 28 (59.6) |
| Chronic | 19 (40.4) |
| MIDAS total score | |
| Mean (SD) | 72.0 (51.9) |
| MIDAS category, n (%) | |
| Little or no disability (score 0–5) | 2 (4.3) |
| Mild disability (score 6–10) | 2 (4.3) |
| Moderate disability (score 11–20) | 4 (8.5) |
| Severe disability (score 21–40) | 11 (23.4) |
| Very severe disability (score ≥ 41) | 28 (59.6) |
| MSSSa | |
| Mean (SD) | 28.6 (3.8) |
| First migraine experience ≥ 20 years previously, n (%) | 22 (46.8) |
| Migraine diagnosis ≥ 20 years previously, n (%) | 17 (36.2) |
| Number of preventive medications used for migraine, mean (SD) | 2.2 (1.5) |
| Use of preventive medications for migraine within the previous 5 yearsb, n (%) | |
| Antidepressants | 17 (36.2) |
| Antiepileptics | 20 (42.6) |
| Beta-blockers | 17 (36.2) |
| CGRP-targeting monoclonal antibodies | 5 (10.6) |
| Experience with self-injectables (for migraine or another indication)c, n (%) | |
| No | 35 (74.5) |
| Currently using | 7 (14.9) |
| Used in the previous 6 months | 5 (10.6) |
| Fear of self-injectingd, n (%) | |
| None | 18 (38.3) |
| Low | 16 (34.0) |
| Moderate | 5 (10.6) |
| High | 4 (8.5) |
| Extremely high | 4 (8.5) |
CGRP calcitonin gene-related peptide, MIDAS Migraine Disability Assessment, MSSS Migraine Symptom Severity Score, SD standard deviation
aTo be included in one of the focus groups, individuals had to have at least moderate migraine severity based on MSSS
bMedications were not mutually exclusive
cOne of the five participants who reported having used CGRP-targeting monoclonal antibodies in the previous 5 years also reported that they were not currently using a self-injectable and had not used a self-injectable in the previous 6 months
dAssessed with the question “How afraid are you of injecting yourself with a medicine?”
Themes Arising from Focus Group Discussions
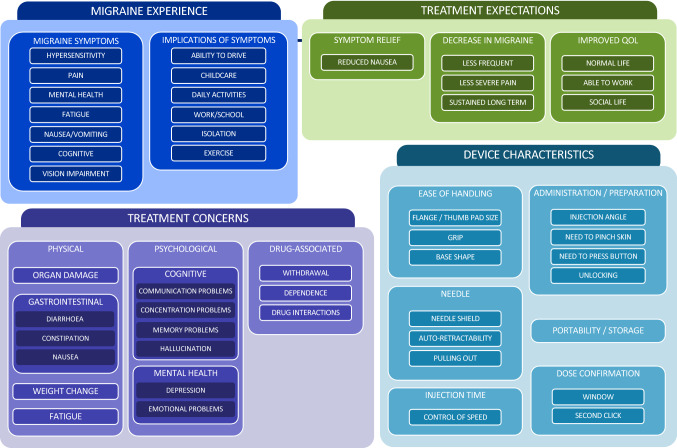
Fourteen key themes with 43 subthemes were identified across four different areas of discussion. These are illustrated as a conceptual map in Fig. 3.
Fig. 3.
Conceptual map summarizing identified themes and subthemes
Migraine Experience
Themes and illustrative quotations related to migraine symptoms are provided in Online Resource 5 (see the electronic supplementary material). Migraine affected participants’ ability to work or attend school, causing them to “have many times off school,” feel “half-functioning,” switch from full-time to part-time, change to a day or night shift, be simply “not able to work,” or “quit” their online job. Severe migraine left some participants struggling to get out of bed due to pain, vomiting, or suicidal feelings and limited one participant to function just enough to “go to the toilet.” Participants also reported that migraine made it hard for them to care for their children. Some, particularly those from more suburban areas in the US, expressed an inability or unwillingness to drive because they would “lose vision” while driving.
Treatment Expectations and Concerns
Themes and illustrative quotations related to preventive treatments for migraine are provided in Table 2. The most discussed expectation of any preventive treatment for migraine was reduced severity and frequency of migraine. Participants in five of the nine focus groups indicated that they would like a preventive treatment that allows them to have “normal functions” and live a “normal life,” enabling them to work, leave the house, and perform daily activities that they were otherwise unable to do during a migraine attack. Participants anticipated that this would be achieved by the preventive treatment substantially reducing the frequency and severity of migraine attacks.
Table 2.
Themes and illustrative quotes related to expectations and concerns about preventive treatments for migraine
| Theme | Illustrative quotation [participant ID] | |
|---|---|---|
| Expectations about effectiveness | Reducing the frequency and severity of migraine attacks | “I don’t think anything would make it completely gone but as less frequent and less severe as possible” [US01-001] |
| “I would say that it should address the days, that means fewer attacks, but especially less intensity” [DE03-042] | ||
| Improved quality of life | “I think there needs to be just an expectation that if there’s less days of headache and there’s more days that we can actually participate in life and have our normal functions and maybe even be able to schedule things without worrying oh if I schedule that in advance what if I have a migraine that day?” [US01-006] | |
| “You can’t take all these days off work like your manager’s not going to understand, so really kind of traps your life.” [UK01-005] | ||
| “Being able to be together with other people, being able to join in nice activities.” [DE01-033] | ||
| “I would prefer, if I had almost no attacks and that in between my life would be normal. I wish to have a normal life. I mean not standing up and making a complete body check in the morning. Not thinking: ‘Oh, there is something coming again.’” [DE02-043] | ||
| Concerns about adverse events | Physical adverse events include weight change, stomach problems and fatigue | “I mean they talk about constipation but that is some serious constipation. I didn’t know it was a big deal to use a suppository every day until the doctor was like ‘you’re going to forget how to go naturally’, you will not want to do that long term” [US01-006] |
| “With the side effects it’s always like one in ten and one in 100 and I’m always that one. I always get all the symptoms and it’s usually fatigue and all that” [UK01-005] | ||
| “It made me very drowsy during the day, so almost unable to function.” [UK03-103] | ||
| “For me it is the gain of weight. I have gained 15 kg weight […] I cannot lose it again” [DE02-038] | ||
| “[M]y weight dropped all of a sudden” [DE03-004] | ||
| Psychological adverse events interfere with work and school and make it hard to communicate with others | “I had no concentration, I couldn’t work, I couldn’t think, I couldn’t communicate properly” [US01-004] | |
| “[I]t makes you as a zombie […] the lack of being able to converse properly so my brain was just not firing so if somebody asked a question you’ve almost forgotten what they asked before you’ve even answered…” [UK01-004] | ||
| “I was on it in high school and I had to come off it because I just couldn’t concentrate, I’d just fall asleep in class and I wouldn’t have done my exams otherwise” [UK01-005] | ||
| Drug-associated side effects include withdrawal effects, dependence, contraindications, and drug-drug interactions | “It [your body] is trying to like flush it out of the system and you just feel really weird” [UK01-001] | |
| “Like with the antidepressants you would get the side effects for six weeks which would be horrendous and then they’d start and then you’d have to wean them off it so you’d get like the what’s it called like withdrawal symptoms and then they’d start you on a new one and it’s like I’ve got to go to work, I can’t keep saying to my boss oh I’m not coming in for six weeks” [UK01-005] | ||
| “I’ve had problems with addiction, so I need something that’s a little less habit forming” [US03-101] | ||
| “It turned out that [the preventive treatment for migraine] has something in it that if you have some sort of a glaucoma, narrow angled glaucoma, that’s contraindicated” [US01-004] | ||
| “Interactions with acute therapy. We must not forget that. I know; we did not discuss that; we found out that the effect of triptans is decreased by some prophylactic therapies; therefore, it’s very important to take a prevention that does not decrease the effect of acute medicine” [DE03-042] | ||
| “[W]on’t have interactions with other things that I’m on high blood pressure medicine, so just things that won’t interact with normal medication, like cholesterol medication…” [US03-100] |
Participants from all focus groups had tried multiple daily (non-CGRP-targeting) oral preventive treatments for migraine. They expressed dissatisfaction with these treatments because of ineffectiveness or intolerable adverse events, which in some cases had caused them to switch or discontinue treatment. Participants wanted a preventive treatment to be completely free of adverse events, although discussions revealed that they would be willing to make trade-offs between effectiveness and adverse events. The most frequently discussed physical adverse event associated with preventive treatment (emerging in six focus groups) was weight change, with which participants from all three countries expressed their dissatisfaction. One participant who had used a self-injectable CGRP-targeting mAb reported constipation, which led to them switching to another CGRP-targeting mAb.
Participants across four focus groups reported experiencing depression and mood-related adverse events associated with preventive treatment. In four focus groups, participants discussed cognitive adverse events that they attributed to preventive treatments, such as impaired memory and concentration, which had interfered with work and school and made it difficult for them to communicate with others.
Treatment-associated adverse events that were discussed included concerns about withdrawal effects (with antidepressants), dependence, contraindications, and drug–drug interactions. One participant with past addiction behavior expressed a general wish for treatments not to be habit forming. Preventive treatment use also raised concerns about contraindication in patients with glaucoma and about drug–drug interactions with acute treatments for migraine.
Review of Medical Devices
Themes and illustrative quotations related to the devices are provided in Table 3. After simulating self-injection with the five different medical devices, participants generally preferred autoinjectors over prefilled syringes, describing them with words such as “robust,” “stable,” and “easy to use.” Some participants from three of the focus groups preferred prefilled syringes because they expected them to provide greater “control” of the administration process than autoinjectors. These participants generally preferred a large thumb rest and flange for providing a good grip of the prefilled syringe. Participants who had experience with self-injecting insulin pens were not opposed to using prefilled syringes because they would “get used to it” despite any fear of needles. However, most participants disliked prefilled syringes, describing them as “old-fashioned,” “creepy,” “scary,” “clumsy,” and “cheap and nasty.” Some felt that they were fragile and unreliable, believing that they might break during use and would leave “room for error.”
Table 3.
Themes and illustrative quotes related to impressions of the five medical devices
| Theme | Summary | Illustrative quotation [participant ID] |
|---|---|---|
| Ease of handling | Certain device characteristics help you to maintain a good grip, which facilitates stable administration | “I do like the bigger surface area, I feel like in case you’re fidgeting with E it’ll get the spot” [US01-006] |
| “B compared to A is better for the elderly because it’s thicker you know, bigger…” [US01-007] | ||
| “I have issues sometimes with shaking and I think that if you are challenged, if you’re older or have issues with mobility getting a hold of A is going to be significantly harder than getting a hold of B because it’s got a much bigger” [US03-103] | ||
| “I think if somebody had got like arthritis in their hands or something, this one [device B] would be more beneficial, the second one because it’s larger whereas that first one is really quite difficult to get hold of” [UK01-002] | ||
| “It [device B] is nicer to hold this than that, that’s a bit fiddly, this is like I’ve got arthritis in my fingers and that is a lot easier for me to hold” [UK02-010] | ||
| “I have a lot of arthritis and I felt more control on this [device B] than the other syringe” [UK02-013] | ||
| “Maybe E; you do not have to be aware that much of the angle, if I understood it correctly. You can simply hold and inject” [DE01-033] | ||
| “I think that E would be the most suitable one. Because it has this small attachment at the bottom, which defines simply this 90-degree angle” [DE02-038] | ||
| “For D I have simply the impression that the material is most suitable, because for the others I got the impression that my hands could slip, as soon as they are a little bit sweaty” [DE02-050] | ||
| Administration/preparation | Pressing the button, automatic insertion, unlocking feature, injection angle, and need for skin pinching are important considerations | “I do like that you never see the needle […] and that you can put it in at any angle and you don’t have to push hard.” [US01-002] |
| “I don’t know the amount of pressure that I have to do against the skin for it to fire. […] I think it’s like less one anxiety step because it’s like the button, the first time I tried to do it I was still holding it four hours later trying to push the button so…” [US01-006] | ||
| “As opposed to E, D seems more secure if you have children in your house [remove the end numbered 2 of device D to unlock] […] I don’t have children in my house but it’s a little harder to just get to” [US03-103] | ||
| “Just any autoinjector I think it’s easier to teach people how to use an autoinjector than anything else and there’s less room for error. I can’t see a huge amount of difference error wise between the three autoinjectors” [UK01-004] | ||
| “[O]n [device E] it says you don’t have to pinch; […] I find that it’s easier to get this thing to fire if I pinch.” [US01-006] | ||
| “Because you have to twist it and you have to click something off, it's not going to accidentally fire, which would be my concern. Especially if I kind of don't realise and I grab something wrong, I'm forever getting caps of things that should be secure—make-up and whatever—finding them in the bottom of my bag. The last thing you want to do is accidentally stick your hand on a needle, because that's not a very nice thing” [UK03-032] | ||
| “To be honest, I did minimal pinching and it's a little bit sore already” [UK03-102] | ||
| “As a beginner you might otherwise wrongly apply it angle-wise. […] with [prefilled syringe] I am in doubt whether the needle has entered correctly and is it inside deep enough; you think too often about what you might do wrong or right.” [DE01-033] | ||
| Needle | Auto-retractors avoid problems caused by moving the device with the needle inserted or retracting the needle incorrectly, and mean that you never have to see the needle | “I do like that you never see the needle [with device E]” [US01-002] |
| “No, because I just think that that [device A] is not a good fit especially if there’s children in the household” [US03-100] | ||
| “The reason I like A the least is because I don’t see any benefit for people holding onto it, I think it would be just as dangerous as this one if a kid got a hold of it and also I forgot but you’re going to have to deal with sharps disposal if you have a needle sticking out somewhere whereas those things you can just dump at your pharmacy” [US03-103] | ||
| “[Participants were discussing the auto-retractability] It would just come out and you just move it anywhere you want to.” [UK02-010] | ||
| “But because E is auto-retract and it’s just putting the pressure on I would feel a lot more comfortable with that. My concern with those that don’t auto-retract is me pulling it out wrong or something. So I would choose E” [UK02-014] | ||
| Injection time | Opinions vary as to the optimal injection time | “With [prefilled syringe] A I would be afraid of like the speed, pressing in like how fast I’m doing it, same thing with [prefilled syringe] B because I might accidentally push it on all the way too fast” [US01-001] |
| “Sorry, 15 seconds is too long” [US01-004] | ||
| “I like this one better […] three seconds, number one” [US01-007] | ||
| “[T]he slowness that’s one of the things that creeps me out about […] any kind of shot that you need, I can’t stand the feeling of it going into me so that the direction to do it slowly would really creep me out” [US01-002]. | ||
| “Ten seconds and three seconds, the time was not what bothered me” [UK03-032] | ||
| Dose confirmation | Dose confirmation is advantageous, although the confirmation window could be better placed on some devices | “I would suggest on this one [device D] though it looked like the red thing was closer to your stomach and you had to go like this, it would make sense to have it at the other end so you don’t have to see what’s going on there and you know, contort yourself. It would be better if it could be at the other end and then it’s easier just holding it and you see the red and then you’re done” [US01-002] |
| “I think the counting is to … in the event that it doesn’t click that you don’t pull it out sooner than the time…” [US01-006] | ||
| ”I think the window should be a little bit further away […] because I don’t know, I mean I’m fatter but I, you know, you need to see it further away and then the needle is covered” [US01-007] | ||
| “I also like the fact that it [device E] clicks a second time when the full dose is administered because I think it just takes away from the counting, like you can still count but you know when it’s done and it tells you that, and I think I would prefer that” [US02-014] | ||
| “There's just so many seconds of waiting for one part to inform you you've passed that stage and then another part … I just think I would get that one and I'd be concerned, ‘am I getting the correct dosage for that treatment?’” [UK03-102] | ||
| “You're going to hear one click and then you have to wait 15 seconds and then you're going to hear another click and then if it hasn't clicked right, then you don't … You're going to hear a ding to hear that […] it seemed like I need to focus and pay attention to it” [UK03-032] | ||
| “[I]f you have placed it wrongly, you do not have placed the window in the right position. […] then you cannot see it so well. I think the window is the most important indicator, more important than the click, to see that the medicament is really inside.” [DE02-043] | ||
| “[T]here was no click at all, and I was like: ‘what did I wrong…’ But I did not want to stop; also, I had the window not directed to the top, so I twisted my head in the hope of being able to see whether the administration has taken place or not” [DE03-042] | ||
| “[I]f you hold it wrongly you cannot see the window. That’s quite complicated in the beginning. […]. And the first time I have hold it in a way that you could not see the window. And I waited for a second click, but there was none, because I held on to the tip with my finger, the first time I applied” [DE03-042] | ||
| “Then you are waiting for a click that does not come. That’s a nightmare.” [DE03-046] | ||
| Portability/storage | Some but not all participants valued a smaller device because of portability and ease of storage | “I have to sometimes carry a box this big of medicine that is embarrassing because you can’t check that so you’re there with your carry on…” [US03-103] |
| “Sometimes I travel and so if it were a patch then that would be great. If it were injectable then I would have … it would have to be small enough where I could take some of them, I could get enough” [US03-104] | ||
| “I will say that like you know most of the preventatives have to be stored in the fridge or like in a cool place like in your cupboard or whatever but like in the box, so I guess I could take it out of the box, my only concern would be like the size of the box because it’s [location] and my fridge space is limited so just to make sure that the packaging it comes in is minimal” [US02-034] |
Many participants who were asked if they would be willing to try any of the self-injectable treatments they had handled indicated their willingness to try one that was effective and tolerable. In discussions of dosing frequency, willingness to self-inject depended on the effectiveness of the preventive treatment. As long as a treatment worked, some participants would be willing to self-inject it “every day” or “daily,” including participants who would otherwise be unhappy to have daily injections. One participant declared that they would be willing to administer an effective medicine “twice a day just to be able to function properly” and another “three times a day would be the maximal number that is still good to handle when [the medicine was] working”; other participants indicated that monthly administration would be ideal.
Device characteristics discussed by participants could be divided into six main themes: ease of handling, administration/preparation, needle, injection time, portability/storage, and dose confirmation. Device characteristics often judged to contribute to a stable grip included size, material, and shape, with participants across five focus groups expressing concern that they may experience an accident (i.e., the devices might “slip”) during administration. In particular, the bases of certain autoinjectors mattered, especially the large flat surface of device E. This was largely because it allowed the device to be held in place at the correct angle, thereby ensuring stable administration. Size and shape were felt to be particularly helpful for “the elderly” and people with “shaky” hands or “arthritis,” who would want a device that provides good grip and control.
Participants disliked overcomplicated or multistep administration and appreciated features that, in their view, made the process easier, reducing the risk of mistakes and accidents. Some participants specifically liked the automatic injection of autoinjectors, which was perceived to give “less room for error” and to avoid concerns about manual needle insertion and retraction. On the other hand, one participant was not convinced that the injection would be initiated when pressing a button on an autoinjector. The need to unlock autoinjectors was perceived as “more secure if you have children in your house” by one participant and a useful way of preventing accidental skin penetration or device activation by another. Some participants expressed concern that the requirement for a 45° angle with the prefilled syringes would make them “see the needle” against their will and might result in administration errors.
Although most participants preferred not having to pinch the skin before needle insertion, opinions about skin pinching were diverse, with some participants favoring pinching and others expressing indifference. Advantages of pinching the skin included feeling less anxious about accidentally injecting at the wrong site. Discomfort was mentioned as a disadvantage.
Participants considered prefilled syringes “not a good fit” and “dangerous,” especially for children, because of the exposed needle. Needle protection features of autoinjectors, which include auto-retraction or a needle shield/guard, were appreciated by participants, especially those averse to needles, because they contributed to safe, patient-friendly administration with the needle hidden. Needle auto-retraction offered by one of the autoinjectors was valued because people do not have to manually retract the needle from their skin and because it avoids errors and accidents caused by moving the device with the needle inserted or incorrectly retracting the needle from the skin. Similarly, some participants were worried about the possibility of such accidents if they were to move the device while the needle is inserted.
The respective injection times of the three autoinjectors were 3, 10, and 15 s. A shorter duration of injection was predominantly preferred. Several participants were not comfortable with an injection lasting 15 s or longer, especially one participant who disliked injections. Notably, one participant was concerned that a long injection time might cause them to miss the dose confirmation.
Although participants expressed differing views about whether control of injection speed was better achieved with prefilled syringes or autoinjectors, one participant indicated that using autoinjectors would eliminate concerns about how quickly they were injecting the drug.
Autoinjectors have two mechanisms to confirm dose completion: by making an audible click sound and visually via a confirmation window, which can be either a narrow window that changes color on dose completion or transparent housing providing a 360-degree view. Some autoinjectors click shortly before the full dose is administered, which concerned some participants who thought they might “pull it out sooner than the time.” However, one autoinjector clicks after dose completion; this was valued because it removed the need to count how many seconds had elapsed to confirm dose completion.
Although a narrow confirmation window that changes color was found to be reassuring, some participants felt it could be better positioned because it was obstructed by their breasts or adipose tissue or because they did not want to see the injection. A transparent housing was preferred by some because it allowed dose completion to be observed from any angle and because it could serve as a back-up confirmation if audible clicking did not work or if they missed the click sound. One participant considered visual confirmation more important than audible confirmation.
Portability was important for frequent travelers, especially for one participant who felt embarrassed about having to fly with a large box of medicines. However, portability was not a concern for everyone.
Storage requirements were also considered, because all the prefilled syringes and autoinjectors must be stored in a refrigerator prior to use. One participant explained that the size of the device and its packaging was important to them because of having limited refrigerator space.
Concerns about the impact of single-use devices on the environment were raised, particularly by participants from the UK and Germany, due to the size of certain devices and the amount of non-recyclable plastic they contain.
Ranking of Treatment Characteristics
Across focus groups, reducing the frequency of migraine and reducing the severity of migraine were consistently ranked as the most important characteristics (Online Resource 6, see the electronic supplementary material). However, some differences emerged among countries. Adverse events were deemed important by four of the nine focus groups (two in the UK and two in Germany). Two of the three US focus groups highlighted speed of injection as being important. Also, vision problems due to migraine were ranked as the most important attribute by participants of the focus group conducted in a US suburb, where it is necessary to drive. Environmental impact was a notable device concern among German focus group participants, although they ranked it as less important than other attributes.
Preference-Elicitation Instrument
Insights gained from the focus groups were used to develop a preliminary set of attributes and levels for a preference-elicitation instrument (Table 4). The levels of the device attributes were derived from the characteristics of the autoinjectors used to administer CGRP-targeting mAbs (Emgality® [galcanezumab], Aimovig® [erenumab], and Ajovy® [fremanezumab]), which are approved in the target countries [36–41].
Table 4.
Attributes and levels included in the preliminary preference-elicitation instrument
| Attribute | Levels | Rationale for inclusion |
|---|---|---|
| Dosing schedule |
Daily Once a month, one injection Once every 3 months, three injections |
Frequency of administration differs between oral medications (daily) and CGRP-targeting mAbs (one injection monthly or three injections once every 3 months) [36, 38, 40] |
| Storage requirements |
None Outside of fridge up to 14 days Outside of fridge up to 7 days Outside of fridge up to 1 day |
Storage was raised by focus group participants who traveled often or had limited refrigeration space. CGRP-targeting mAbs need refrigeration; oral medications do not. For CGRP-targeting mAbs, the amount of time they can be stored at room temperature varies from 1 to 14 days [36, 38, 40] |
| Base and pinching |
Wide base, no pinching Narrow base, no pinching Narrow base, pinching |
Some focus group participants preferred a wide base, as it helped them to hold the device in place, at the correct angle. Opinions about pinching were diverse: some participants preferred not having to pinch their skin, while others were indifferent. Still others preferred to pinch as they thought it might minimize injection site pain |
| Injection steps |
Press down firmly into the skin to inject Twist to unlock and press the button to inject Press down firmly into the skin to unlock and press the button to inject |
Some focus group participants disliked having several steps to administer the injection, while others appreciated the device being locked and requiring unlocking steps before injection for reasons of safety. Additionally, some participants preferred having a button to press to inject, while others preferred not having to press a button to inject, as it was an extra step |
| Injection duration |
5 s 10 s 20 s 30 s |
Attitudes towards injection duration varied among the focus group participants. A shorter injection duration was generally preferred, but some participants were indifferent. The levels were based on the injection durations of available CGRP-targeting mAbs (10–30 s) [36, 38, 40] and for prefilled syringes (5 s) |
| Needle removal |
Auto-retract Pull-out |
The needle auto-retraction of one of the autoinjectors used in the focus groups was valued by participants for reasons of ease of use and safety. For the other two autoinjectors, the needle is removed by manual pull-out, although the needle remains hidden by a needle shield |
| Dose confirmation |
360° view and “click” after dose completion Thin window and “click” before dose completion |
Many focus group participants would want to know if they had administered the full dose of treatment correctly. Autoinjectors for CGRP-targeting mAbs confirm dose completion through both a “click” sound and visual confirmation via of a window on the device. The timing of the click sound in relation to dose completion and nature of the window vary between autoinjectors |
| Monthly migraine headache days |
Episodic migraine: 5 days 6 days 7 days Chronic migraine: 10 days 12 days 14 days |
All focus groups ranked reducing the frequency and severity of migraine as the most important aspect of treatment. Monthly migraine headache days is a commonly used outcome in clinical trials of preventive treatments for migraine [44–46]. The levels reflect 30%, 40%, and 50% reductions in migraine frequency from a hypothetical starting point of 10 monthly migraine headache days for episodic migraine and 20 monthly migraine headache days for chronic migraine. These levels were designed to balance the need for the efficacy levels to be clinically plausible while at the same time ensuring that the efficacy attribute does not dominate the preference-elicitation exercise |
| Side effects | Levels (encompassing various adverse events) fixed separately for self-injectable and oral medications | Many focus group participants had experienced adverse events from daily oral non-CGRP-targeting preventive treatments for migraine, including weight change, nausea, fatigue, and emotional, mood, and cognitive problems. Four focus groups also ranked adverse events as an important aspect of treatment. Adverse events for daily oral preventive treatments for migraine depend on the class of medicine: the adverse events that are most common differ markedly between classes |
CGRP calcitonin gene-related peptide, mAb monoclonal antibody
Discussion
This focus group study examined which characteristics of preventive treatments for migraine matter to people with migraine and why, with a focus on gaining insight on preferences for devices used to inject CGRP-targeting mAbs indicated for migraine prevention. During the focus group discussions, participants were given the opportunity to handle and simulate self-injection using several devices and were queried about which characteristics mattered to them. The study confirmed that reducing the frequency and severity of migraine attacks was a critical benefit expected from preventive treatments and that migraine symptoms greatly interfere with normal daily activities, including the ability to work [42, 43]. This is consistent with clinical guidelines that recommend change in migraine headache days per month as a primary outcome measure in clinical trials [44–46]. The focus group study also identified a number of adverse events of concern that are linked to oral and self-injectable preventive migraine treatments, including weight change, cognitive problems, and psychological problems; these were anticipated based on the results of the targeted literature review. The study also revealed adverse events and lack of effectiveness to be principal reasons for changing or discontinuing previous preventive treatments. This aligns with other studies showing that effectiveness and tolerability matter greatly to people with migraine and substantially affect adherence and persistence [13, 47, 48]. The focus group discussions identified only two new themes relating to treatment expectations and concerns (nausea and injection site pain) that had not been identified from the targeted literature review but from supplemental review with CGRP-targeting mAb clinical trial studies. By contrast, various device characteristics beyond mode and frequency of administration and out-of-pocket costs were highlighted as important, including ease of holding, specific injection steps (twisting to unlock and pressing the button), number of injection steps, shape, size and weight of the device, needle protection or visibility, disposal, portability, storage, injection time, dose confirmation, and pinching. Comparisons of the clinical findings were limited by the fact that only two papers identified during the targeted literature review considered preventive treatment for migraine. It is also possible that some participants may not have been fully able to disentangle treatment-related adverse events from migraine symptoms.
Patients’ valuation of self-injectable preventive treatments for migraine may be driven by the characteristics of the device used to administer them [49]. The current study discovered additional attributes explaining ease of use of devices that were not identified from the targeted literature review. The participants in this study generally found autoinjectors to be easy and safe to use, and they valued characteristics such as the unlocking step, automated insertion and retraction, and needle protection. Audible and visual dose confirmation also increased confidence in administering the full dose with an autoinjector. Although instructions for using autoinjectors were initially overwhelming to some participants, they generally believed this would not be a barrier once they became accustomed to using the devices.
The ranking exercise results further helped confirm the potential of the identified aspects to be attributes in the planned preference study. While ranked benefit attributes did not differ across the focus groups, it was difficult to consolidate risk attributes, as many discrete side effects were included in the ranking exercise. Another challenge was to materialize device characteristics into attributes. If only plausible pairs of device characteristics are to be considered based on actual devices, some characteristics can form a single attribute rather than being separate attributes. For instance, base of autoinjectors and skin pinching can be a single attribute for having three plausible pairs together: an autoinjector that has a narrow base and requires skin pinching, an autoinjector that has a narrow base and does not require pinching, and an autoinjector that has a wide base and does not require pinching. Furthermore, some device characteristics may overlap, such as size of the device, portability, storage, and disposal. This issue may be overcome by considering whether levels of attributes can be differentiated and represent a reasonable improvement from a base level. For instance, variation in storage requirements may include different numbers of days that the device can be stored at room temperature for the size of the device would be different dimensions of the package or the device itself.
This study had some limitations that should be considered when interpreting the results. Because this study had a relatively small sample size and was conducted in a limited number of geographic regions, its generalizability may be limited. Also, although the study included people with both episodic and chronic migraine spanning a wide age range (20–69 years) across different countries, the sample may not have been large or diverse enough to fully understand the treatment preferences of people with migraine or to explore any potential differences based on participant characteristics [19]. Notably, the study did not capture the perspectives of people with undiagnosed migraine or who have not previously received preventive treatments. However, the study included some participants who had experienced self-injecting treatments, which helped broaden the range of experiences and perspectives captured. A further potential limitation is that it was difficult to determine whether perceptions or experiences differed between participants with chronic and episodic migraine because all the UK focus groups and two of the German focus groups included both types of patients.
Describing attributes and levels can pose a challenge when transferring the results of a focus group study to a subsequent preference study. How to communicate device characteristics to participants in the planned preference study has to be carefully considered because, unlike the focus group participants, participants in the preference study will not be given the opportunity to physically handle and simulate device usability. Without tangible experience of self-injectable devices, people might find it difficult to understand how the devices look and function. We plan to leverage the focus group findings to develop visual aids such as illustrations and videos to help participants make informed choice decisions in the preference study.
Conclusion
In conclusion, this focus group study identified relevant attributes that might influence the decisions people with migraine make when selecting a preventive treatment for migraine. The study suggests that many people with migraine are willing to take a self-injectable preventive treatment. Faced with multiple self-injectable treatments with comparable efficacy and safety, patient preferences for injection time, dose confirmation, needle protection, storage requirements, and other factors that may affect the treatment burden can help guide treatment selection. This study will serve as a foundation for future preference studies that aim to quantify the trade-offs that people with migraine are willing to make when choosing between different preventive treatments for migraine, including self-injectables.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding for this study was provided by Eli Lilly. Sally Mannix, B.A. (Evidera), and Zaneta Balantac, B.S. (Evidera), helped to conduct focus group discussions in the United States. Nicole Petersohn, M.A., and Tobias Basedow, M.A. (pharma-consult Petersohn), helped conduct focus groups in Germany. Thibaud Prawitz, M.Sc. (Evidera), provided statistical support by conducting quantitative analysis, and Ella Brooks, M.Sc. (Evidera), by conducting qualitative coding. Medical writing was provided by Stephen Gilliver, Ph.D., and Phillip S. Leventhal, Ph.D. (Evidera), and was paid for by Eli Lilly.
Declarations
Funding
The study was funded by Eli Lilly & Co.
Conflicts of interest/Competing interests
Jaein Seo, Charlie A. Smith, Caitlin Thomas, Tommi Tervonen, and Asha Hareendran are employees of Evidera, which was paid by Eli Lilly & Co. for work related to this study. Janet H. Ford, Virginia L. Stauffer, Robert A. Nicholson, Kevin Harrison Duffy, and Antje Tockhorn-Heidenreich are employees of Eli Lilly & Co.
Ethics approval
Before any study procedures were initiated, the study was approved by the US central institutional review board of Ethical & Independent Review Services (Independence, MO, USA) (institutional review board reference number 19144-01). This study does not require review by a local ethics committee in the UK and Germany according to the NHS Health Research Authority and the German Medicinal Products Act (Arzneimittelgesetz –AMG). The study was conducted in accordance with the International Council on Harmonization Privacy Statement, the Health Insurance Portability and Accountability Act, and the European General Data Protection Regulation and is reported in accordance with the recommendations of the COnsolidated criteria for REporting Qualitative research (COREQ) Checklist and Standards for Reporting Qualitative Research (SRQR) reporting guidelines and Good Publication Practice 3 guidelines.
Consent to participate
All participants provided verbal and online consent to participate.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available as no consent was sought from participants to allow sharing of data with third parties.
Code availability
Not Applicable.
Author contributions
JS contributed to conception and planning of the work, acquisition and interpretation of the data, and drafting and critical revision of the manuscript. CAS contributed to the acquisition, analysis, and interpretation of the data and drafting the manuscript. CT contributed to the conception and planning of the work, acquisition and analysis of the data, and drafting the manuscript. TT contributed to the conception and planning of the work, interpretation of the data, and drafting the manuscript. AH contributed to the conception and planning of the work, data acquisition, and the drafting and critical revision of the manuscript. JHF contributed to the conception and planning of the work and critical revision of the manuscript. VLS contributed to the conception and planning of the work and critical revision of the manuscript. RAN contributed to the conception and planning of the work and critical revision of the manuscript. KHD contributed to the conception and planning of the work and critical revision of the manuscript. AT-H contributed to the conception and planning of the work and critical revision of the manuscript. All participated sufficiently in the work to take public responsibility for the entire content of the manuscript.
References
- 1.Collaborators GBDH Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stang PE, Osterhaus JT. Impact of migraine in the United States: data from the National Health Interview Survey. Headache. 1993;33(1):29–35. doi: 10.1111/j.1526-4610.1993.hed3301029.x. [DOI] [PubMed] [Google Scholar]
- 3.Group GBDNDC Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16(1):86–92. doi: 10.1007/s11916-011-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headache Classification Committee. Olesen J, Bousser MG, Diener HC, Dodick D, First M, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26(6):742–746. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 6.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. [DOI] [PubMed]
- 7.Gil-Gouveia R, Oliveira AG, Martins IP. The impact of cognitive symptoms on migraine attack-related disability. Cephalalgia. 2016;36(5):422–430. doi: 10.1177/0333102415604471. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31(3):301–315. doi: 10.1177/0333102410381145. [DOI] [PubMed] [Google Scholar]
- 9.Lanteri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31(7):837–850. doi: 10.1177/0333102411398400. [DOI] [PubMed] [Google Scholar]
- 10.Abu Bakar N, Tanprawate S, Lambru G, Torkamani M, Jahanshahi M, Matharu M. Quality of life in primary headache disorders: a review. Cephalalgia. 2016;36(1):67–91. doi: 10.1177/0333102415580099. [DOI] [PubMed] [Google Scholar]
- 11.Leonardi M. Burden of migraine: what should we say more? Neurol Sci. 2015;36(Suppl 1):1–3. doi: 10.1007/s10072-015-2188-z. [DOI] [PubMed] [Google Scholar]
- 12.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 13.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33. doi: 10.18553/jmcp.2014.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawata A, Shah N, Poon J, Shaffer S, Sapra S, Wilcox T, et al. G24: treatment patterns and healthcare resource use in migraine patients newly initiating a preventive treatment: interim Results from the Assessment of TolerabiliTy and Effectiveness in MigrAINeurs using Preventive Treatment (ATTAIN) Study. J Manag Care Spec Pharm. 2017;23(10a):S54. [Google Scholar]
- 15.Scuteri D, Adornetto A, Rombola L, Naturale MD, Morrone LA, Bagetta G, et al. New trends in migraine pharmacology: targeting calcitonin gene-related peptide (CGRP) with monoclonal antibodies. Front Pharmacol. 2019;10:363. doi: 10.3389/fphar.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Li GG, Nie H, Feng YY, Guo GY, Guo WL, et al. Efficacy and safety of calcitonin-gene-related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine—an updated systematic review and meta-analysis. BMC Neurol. 2020;20(1):57. doi: 10.1186/s12883-020-01633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD, et al. European Headache Federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6. doi: 10.1186/s10194-018-0955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purks JL, Wilhelm EE, Shoulson I, Creveling J, Dorsey ER, Irony T, et al. Inaugural conference on incorporating patient-reported outcomes and patient preference information into clinical research, clinical care, and risk-benefit assessments for neurodegenerative diseases. Patient. 2017;10(5):541-4. [DOI] [PMC free article] [PubMed]
- 19.Benz HL, Lee TJ, Tsai JH, Bridges JFP, Eggers S, Moncur M, et al. Advancing the use of patient preference information as scientific evidence in medical product evaluation: a summary report of the patient preference workshop. Patient. 2019;12(6):553–557. doi: 10.1007/s40271-019-00396-5. [DOI] [PubMed] [Google Scholar]
- 20.Hollin IL, Craig BM, Coast J, Beusterien K, Vass C, DiSantostefano R, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient. 2020;13(1):121–136. doi: 10.1007/s40271-019-00401-x. [DOI] [PubMed] [Google Scholar]
- 21.Mansfield C, Gebben DJ, Sutphin J, Tepper SJ, Schwedt TJ, Sapra S, et al. Patient Preferences for preventive migraine treatments: a discrete-choice experiment. Headache. 2019;59(5):715–726. doi: 10.1111/head.13498. [DOI] [PubMed] [Google Scholar]
- 22.Peres MF, Silberstein S, Moreira F, Corchs F, Vieira DS, Abraham N, et al. Patients' preference for migraine preventive therapy. Headache. 2007;47(4):540–545. doi: 10.1111/j.1526-4610.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 23.Ashina M, Kudrow D, Reuter U, Dolezil D, Silberstein S, Tepper SJ, et al. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: a pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia. 2019;39(14):1798–1808. doi: 10.1177/0333102419888222. [DOI] [PubMed] [Google Scholar]
- 24.Silberstein SD, McAllister P, Ning X, Faulhaber N, Lang N, Yeung P, et al. Safety and tolerability of fremanezumab for the prevention of migraine: a pooled analysis of phases 2b and 3 clinical trials. Headache. 2019;59(6):880–890. doi: 10.1111/head.13534. [DOI] [PubMed] [Google Scholar]
- 25.Bangs ME, Kudrow D, Wang S, Oakes TM, Terwindt GM, Magis D, et al. Safety and tolerability of monthly galcanezumab injections in patients with migraine: integrated results from migraine clinical studies. BMC Neurol. 2020;20(1):25. doi: 10.1186/s12883-020-1609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callis Duffin K, Bukhalo M, Bobonich MA, Shrom D, Zhao F, Kershner JR, et al. Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience. Med Devices (Auckl). 2016;9:361–369. doi: 10.2147/MDER.S113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guest G, Namey E, McKenna K. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods. 2017;29(1):3–22. doi: 10.1177/1525822X16639015. [DOI] [Google Scholar]
- 28.Serrano D, Buse DC, Reed ML, Runken MC, Lipton RB. Development of the Migraine Symptom Severity Score (MSSS): a latent variable model for migraine definition: PO-86. Headache:40. 2010;50(suppl 1):40.
- 29.Louis E, Ramos-Goni JM, Cuervo J, Kopylov U, Barreiro-de Acosta M, McCartney S, et al. A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient. 2020;13(3):317–325. doi: 10.1007/s40271-019-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obermann K, Scheppe J, Glazinski B. More than figures? Qualitative research in health economics. Health Econ. 2013;22(3):253–257. doi: 10.1002/hec.2906. [DOI] [PubMed] [Google Scholar]
- 31.Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398–405. doi: 10.1111/nhs.12048. [DOI] [PubMed] [Google Scholar]
- 32.Patton MQ. Qualitative research & evaluation methods. Integrating theory and practice. 4. Los Angeles: SAGE Publications, Inc.; 2015. [Google Scholar]
- 33.Ikenwilo D, Heidenreich S, Ryan M, Mankowski C, Nazir J, Watson V. The best of both worlds: an example mixed methods approach to understand men's preferences for the treatment of lower urinary tract symptoms. Patient. 2018;11(1):55–67. doi: 10.1007/s40271-017-0263-7. [DOI] [PubMed] [Google Scholar]
- 34.ATLAS.ti Scientific Software Development GmbH. ATLAS.ti 7 User Guide and Reference. 2013. https://atlasti.com/wp-content/uploads/2014/05/atlasti_v7_manual_201312.pdf?q=/uploads/media/atlasti_v7_manual_201312.pdf. Accessed 26 Feb 2020.
- 35.Wheeldon J, Faubert J. Framing experience: concept maps, mind maps, and data collection in qualitative research. Int J Qual Methods. 2009;8(3):68–83. doi: 10.1177/160940690900800307. [DOI] [Google Scholar]
- 36.Eli Lilly and Company. Emgality (galcanezumab-gnlm) injection [package insert]. U.S. Food and Drug Administration. Revised September, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf. Accessed 9 Apr 2021.
- 37.Eli Lilly Nederland B.V. Emgality (galcanezumab). Summary of product characteristics. 2018. https://www.ema.europa.eu/en/documents/product-information/emgality-epar-product-information_en.pdf. Accessed 9 Apr 2021.
- 38.Amgen Inc. Aimovig™ (erenumab-aooe) injection [package insert]. U.S. Food and Drug Administration. Revised May, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf. Accessed 9 Apr 2021.
- 39.Amgen, Inc. Aimovig (erenumab-aooe) injection. Summary of product characteristics. 2018. https://www.ema.europa.eu/en/documents/product-information/aimovig-epar-product-information_en.pdf. Accessed 9 Apr 2021.
- 40.Teva Pharmaceuticals USA, Inc. AJOVY™ (fremanezumab-vfrm) injection [package insert]. U.S. Food and Drug Administration. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761089s002lbl.pdf. Accessed 9 Apr 2021.
- 41.Teva Pharmaceuticals Europe B.V. AJOVY (fremanezumab-vfrm) injection. Summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/ajovy-epar-product-information_en.pdf. Accessed 9 Apr 2021.
- 42.Kryst S, Scherl E. A population-based survey of the social and personal impact of headache. Headache. 1994;34(6):344–350. doi: 10.1111/j.1526-4610.1994.hed3406344.x. [DOI] [PubMed] [Google Scholar]
- 43.Stewart WF, Lipton RB, Simon D. Work-related disability: results from the American migraine study. Cephalalgia. 1996;16(4):231–238. doi: 10.1046/j.1468-2982.1996.1604231.x. [DOI] [PubMed] [Google Scholar]
- 44.Silberstein S, Tfelt-Hansen P, Dodick DW, Limmroth V, Lipton RB, Pascual J, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28(5):484–495. doi: 10.1111/j.1468-2982.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 45.American HS. The American headache society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18. doi: 10.1111/head.13456. [DOI] [PubMed] [Google Scholar]
- 46.Tassorelli C, Diener HC, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38(5):815–832. doi: 10.1177/0333102418758283. [DOI] [PubMed] [Google Scholar]
- 47.Mitsikostas DD. Nocebo in headaches: implications for clinical practice and trial design. Curr Neurol Neurosci Rep. 2012;12(2):132–137. doi: 10.1007/s11910-011-0245-4. [DOI] [PubMed] [Google Scholar]
- 48.Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–488. doi: 10.1177/0333102414547138. [DOI] [PubMed] [Google Scholar]
- 49.Stauffer VL, Sides R, Lanteri-Minet M, Kielbasa W, Jin Y, Selzler KJ, et al. Comparison between prefilled syringe and autoinjector devices on patient-reported experiences and pharmacokinetics in galcanezumab studies. Patient Prefer Adherence. 2018;12:1785–1795. doi: 10.2147/PPA.S170636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.